Abstract
Mycoplasma hyopneumoniae is an economically significant swine pathogen that colonizes the respiratory ciliated epithelial cells. Cilium adherence is mediated by P97, a surface protein containing a repeating element (R1) that is responsible for binding. Here, we show that the cilium adhesin is proteolytically processed on the surface. Proteomic analysis of strain J proteins identified cleavage products of 22, 28, 66, and 94 kDa. N-terminal sequencing showed that the 66- and 94-kDa proteins possessed identical N termini and that the 66-kDa variant was generated by cleavage of the 28-kDa product from the C terminus. The 22-kDa product represented the N-terminal 195 amino acids of the cilium adhesin preprotein, confirming that the hydrophobic leader signal sequence is not cleaved during translocation across the membrane. Comparative studies of M. hyopneumoniae strain 232 showed that the major cleavage products of the cilium adhesin are similar, although P22 and P28 appear to be processed further in strain 232. Immunoblotting studies using antisera raised against peptide sequences within P22 and P66/P94 indicate that processing is complex, with cleavage occurring at different frequencies within multiple sites, and is strain specific. Immunogold electron microscopy showed that fragments containing the cilium-binding site remained associated with the cell surface whereas cleavage products not containing the R1 element were located elsewhere. Not all secreted proteins undergo multiple cleavage, however, as evidenced by the analysis of the P102 gene product. The ability of M. hyopneumoniae to selectively cleave its secreted proteins provides this pathogen with a remarkable capacity to alter its surface architecture.
Mycoplasma hyopneumoniae, the etiological agent of enzootic pneumonia, significantly impacts swine production (28). During colonization, M. hyopneumoniae forms an intricate association with the ciliated epithelial lining of the porcine respiratory tract, leading to chronic respiratory disease. Colonization disrupts the normal function of the mucociliary escalator through ciliostasis, loss of cilia, epithelial cell death, and acute inflammation. This results in a purulent exudate (composed primarily of neutrophils and mononuclear cells) in the airways (17). Disease resolution occurs only after a prolonged period (if at all). M. hyopneumoniae colonization also predisposes the host to more-severe infections from secondary pathogens (2). For example, it is now clear that colonization by M. hyopneumoniae leads to more-severe and longer-lasting disease with the porcine respiratory and reproductive syndrome virus (34). Thus, the impact of M. hyopneumoniae on swine production has not been fully realized.
It is known that the initial event in colonization by M. hyopneumoniae is binding to swine respiratory cilia (19, 32). In the absence of binding activity, colonization does not occur (38). Identification of the molecules involved in cilium binding occurred only after the discovery of adherence-blocking monoclonal antibodies (MAbs) (36) and development of appropriate binding assays (37). These studies led to the cloning of the gene for the cilium adhesin of virulent strain 232 and identification of the cilium-binding region (9, 10, 21). (For clarity, subscripts will be used to distinguish proteins or adhesin fragments from different mycoplasma strains; i.e., P97232 designates the P97 cilium adhesin fragment of strain 232.) These studies also showed that the initial 126-kDa preprotein product of the cilium adhesin gene underwent a major cleavage event at amino acid 195 to generate P97232 (9). The cilium-binding motif of P97232, which resides in the carboxy-terminal R1 repeat region, consists of fifteen copies of the repeated 5-amino-acid motif AAKPV/E (9, 21). In geographically diverse strains of M. hyopneumoniae, the cilium adhesin possesses variable numbers of R1 repeat units, ranging from 8 in strain C1735/2 to 15 in strain 232; the strain J adhesin possesses 9 copies of the R1 repeat units (35). A second repeat region, R2, consists of the 10-amino-acid motif GTPNQGKKAE that ranges from 3 to 5 in the number of copies in strains of M. hyopneumoniae and that is located downstream of R1 in the C terminus of the adhesin (9, 35). This sequence differs slightly in strain J (GAPSQGKKAE). In addition, other proteins (such as P102, the second gene in the two-gene cilium adhesin operon) (10) may play crucial roles in adherence.
One of the more perplexing observations with the cilium adhesin has been the multiple immunoblot banding pattern observed with whole-cell antigen and adherence-blocking MAbs that recognize the R1 repeat sequence of the P97 adhesin (36). Size variation of surface lipoproteins is now recognized as a common mechanism used by mycoplasmas to generate antigenic diversity (27). This is usually demonstrated as an evenly spaced ladder pattern resulting from a change in the number of repetitive units in gene sequences during DNA replication. The immunoblot profile represented by the cilium adhesin of M. hyopneumoniae is atypical and has not been observed in other mycoplasma species, suggesting that a different mechanism might be responsible. Also, P97 size variation in the immunoblot profiles among different M. hyopneumoniae strains is common (36). To examine this phenomenon in more detail, we studied the laboratory-adapted, nonadherent J strain and the virulent 232 strain, which binds cilia and causes disease in pigs. Here we show that multiple posttranslational cleavage events are responsible for the size variation of the cilium adhesin protein revealed in immunoblot analysis. Immunogold electron microscopy showed that the R1 cilium-binding fragment remained closely associated with the external surface of the M. hyopneumoniae membrane, while other fragments of P97 had different staining patterns. Finally, examination of P102 gene products showed that selective cleavage of translocated proteins was occurring in M. hyopneumoniae.
MATERIALS AND METHODS
Bacterial strains and plasmids.
M. hyopneumoniae strains 232 (36) and J (NCTC 10110) were grown in modified Friis broth and harvested as described by Zhang et al. (36) and Djordjevic et al. (7), respectively, at the mid- to late log phase when the pH of the media reached 6.8. All broth media were filter sterilized through 0.22-μm-pore-size filters. Mycoplasmas were harvested by centrifugation and extensively washed with phosphate-buffered saline (PBS) to remove the remaining medium contaminants. Escherichia coli TOP10 [F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80lac ΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rspL endA1 nupG] containing pISM405 was grown on Luria- Bertani agar or in Luria-Bertani broth (29) containing 100 μg of ampicillin/ml. Isopropyl-β-d-thiogalactopyranoside induction was carried out by addition to achieve a final concentration of 1 mM. Bacterial cultures were routinely grown at 37°C, and liquid cultures were aerated by shaking at 200 rpm.
M. hyopneumoniae growth studies.
Sterile tubes (each containing 6 ml of Friis broth) were simultaneously inoculated with 300 μl of M. hyopneumoniae strain J culture and incubated as described previously (7). M. hyopneumoniae cells were harvested at 8, 16, 20, 24, 28, 40, 48, 52, and 56 h postinoculation, and the cell pellets were used for immunoblotting studies. Growth of M. hyopneumoniae was monitored spectrophotometrically at an optical density of 560 nm as described previously (36). Optical density values at 560 nm versus time (in hours) after inoculation were plotted to estimate the growth phase of M. hyopneumoniae at each of the time points.
Construction and expression of mycoplasmal proteins.
Hexahistidyl P97 and P102 fusion proteins were constructed using pTrcHisA (Invitrogen, Carlsbad, Calif.) and pQE9 (Qiagen, Alameda, Calif.) cloning vectors. Primers FMhp3 (5′-GAACAATTTGATCACAAGATCCTGAATATACC) and RMhp4 (5′-AATTCCTCTGATCATTATTTAGATTTTAATTCCTG) (the underlined sequences represent BclI) were used to amplify a 3,013-bp fragment representing bp 315 to 3321 of the P97 gene sequence containing amino acids 105 to 1107. The template for the amplification of P97 sequences was pMYCO161 (14). This plasmid contained the entire open reading frame (ORF) of P97 minus the N-terminal 74 amino acids. In addition, the five TGA codons had been modified to TGGs by site-directed mutagenesis (14). The PCR fragment was digested with BclI and inserted into the BamHI site of vector pTrcHisA. A construct with the proper fragment orientation was identified by restriction digestion and DNA sequencing and was designated pISM405. The plasmid was transformed into E. coli TOP10 cells for expression of recombinant proteins. The induction and purification procedure used for recombinant mycoplasma proteins has been described previously (20). The resulting 116-kDa recombinant P97-polyhistidine fusion protein contained the R1 and R2 repeat regions as well as the major cleavage site at amino acid 195 in the P97 sequence.
Two hexahistidyl fusion proteins were constructed in pQE9. One of these, p97 N, spanned nucleotides 462 to 2421 of the P97 gene sequence (amino acids 150 to 802 that lacked R1 and R2 sequences). To construct p97 N, primers p97-NF (5′- GGGTCGACCAAGATCCTGAATATACC) and p97-NR (5′-GGCTGCAGTTAGGCTGCTTTTAAGAAAAATGC) (the underlined sequences represent SalI and PstI, respectively) were used to amplify a 1,959-bp fragment of the P97 gene from pMYCO161. This hexahistidyl fusion protein was used to generate P97 N-terminal serum in rabbits (see below). To construct pR2, a fragment spanning nucleotides 2936 to 3342 (amino acids 979 to 1114) of the J strain cilium adhesin homologue p94 (accession number AF001398) was amplified using primers pR2F (5′-GGGGATCCCAGGAAGTCAAGGTAACTAGT) and pR2R (5′-GGCTGCAGCCCGGGTTAGGATCACCGGATTTTGAATC) (the underlined sequences represent BamHI and PstI, respectively). This fragment was cloned into pQE9, and the hexahistidyl fusion protein was used to generate R2 serum.
To clone the gene for P102, primers TH130 (5′-GCTTTATTGGATCCGAGTCAGCTAAAAGTAGC) and TH131 (5′-AAAATTCTGCAGTTATTTAACATAGTTTCTAATCAACCC) (the underlined sequences represent BamHI and PstI, respectively) were used to amplify a 2,568-bp fragment from plasmid pISM1217 (11) representing base pairs 144 to 2712 of the P102 gene sequence. The fragment was digested with BamHI and PstI and ligated into BamHI/PstI-digested, dephosphorylated pTrcHisA plasmid DNA. Site-directed mutagenesis was performed on the resulting plasmid (pISM1249) through the use of a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, Calif.) to remove the five TGA codons in the cloned gene sequence, converting them to TGG. pISM1316.6, the final plasmid, was sequenced to confirm the sequence modifications and proper frame of the gene.
Antisera.
The MAb F1B6 has been described previously (36). It binds to the R1 region of the cilium adhesin that has at least three repeat sequences (21). Peptides with sequences TSSQKDPST (ΔNP97) and VNQNFKVKFQAL (NP97) were used to raise antibodies against P97/P66 and P22, respectively. An Imject maleimide-activated immunogen conjugation kit (Pierce Chemical Co., Rockford, Ill.) was used to bind the peptides to keyhole limpet hemocyanin. P97 N-terminal and R2 sera were each generated by subcutaneous immunization of a New Zealand White rabbit with hexahistidyl products purified by nickel-affinity chromatography. Rabbits were each immunized on two occasions 1 month apart, and immune responses to the immunized antigen were monitored by immunoblotting. Prebleed sera were collected prior to immunization with hexahistidyl antigens for the preparation of control serum. Rabbits were euthanized, and serum was collected as described previously (35). Anti-P102 antiserum was prepared from purified recombinant P102. The peptide conjugates and recombinant P102 were then used to generate mouse hyperimmune antisera by the method of Luo and Lin (18). The resulting antisera were tested by enzyme-linked immunosorbent assay using ovalbumin-peptide conjugate and purified recombinant P97 or P102 antigens and by immunoblot analysis with the recombinant antigens (data not shown). Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin heavy-plus-light-chain [Ig(H+L)] antibodies were purchased commercially (Southern Biotechnology Associates, Inc., Birmingham, Ala.). Goat anti-mouse IgG plus IgM labeled with 10-nm-diameter colloidal gold particles (EY Laboratories, Inc., San Mateo, Calif.) were used in immunogold electron microscopy studies.
Immunoblot analysis.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (16) or using Criterion Tris-HCl gels of various percentages and gradients with SDS-PAGE sample buffer (Bio-Rad Laboratories, Inc., Hercules, Calif.). For the medium control experiments, purified recombinant P97 was incubated with fresh and spent Friis media. Spent medium was prepared from a mid- to late-log-phase culture harvested at 36 h that had been centrifuged and filtered through a 0.1-μm-pore-size filter. Purified recombinant P97 (2.5 μg) in 20 μl of PBS was diluted 1:1 in fresh or spent medium and incubated overnight at 37°C. A total of 10 μl of the mixture was then loaded onto SDS-PAGE gels, blotted to nitrocellulose, and developed with F1B6 MAb.
Trypsin treatment of M. hyopneumoniae.
Mycoplasmas (0.5 g) were treated with trypsin essentially as described previously (35). Briefly, trypsin was added to cell suspensions of M. hyopneumoniae at concentrations of trypsin of 0, 0.3, 0.5, 1, 3, 10, 50, and 300 μg/ml and incubated at 37°C for 15 min. Cell suspensions were then immediately lysed in sample buffer and heated to 95°C for 10 min. Lysates were analyzed by SDS-PAGE and immunoblotting using F1B6 MAb.
2-DGE.
Two-dimensional (2-D) gel electrophoresis (2-DGE) was carried out essentially as described by Cordwell et al. (3). First, dimension-immobilized pH gradient (IPG) strips (Amersham Pharmacia Biotech, Uppsala, Sweden) (180 mm in length; linear pH 6 to 11) were prepared for focusing by submersion in 2-DGE-compatible sample buffer (5 M urea, 2 M thiourea, 0.1% carrier ampholytes 3 to 10, 2% [wt/vol] CHAPS, 2% [wt/vol] sulfobetaine 3 to 10, 2 mM tributyl phosphine [Bio-Rad]) overnight. M. hyopneumoniae whole-cell protein (250 μg) was diluted with sample buffer to a volume of 100 μl for application to the anodic end of each IPG strip via an applicator cup. Isoelectric focusing was performed with a Multiphor II electrophoresis unit (Amersham Pharmacia Biotech) for 85 kV · h at 20°C. IPG strips were detergent exchanged, reduced, and alkylated in buffer containing 6 M urea, 2% SDS, 20% glycerol, 5 mM tributyl phosphine, 2.5% (vol/vol) acrylamide monomer, a trace amount of bromophenol blue dye, and 375 mM Tris-HCl (pH 8.8) for 20 min prior to loading the IPG strip onto the top of an 8 to 18% T-2.5% C (piperazine diacrylamide) 20-cm by 20-cm polyacrylamide gel. Second-dimension electrophoresis was carried out at 4°C using 3 mA/gel for 2 h followed by 20 mA/gel until the bromophenol blue dye had run off the end of the gel. Gels were fixed in 40% methanol-10% acetic acid for 1 h and then stained overnight in Sypro Ruby (Molecular Probes, Eugene, Oreg.). Images were acquired using a Molecular Imager Fx apparatus (Bio-Rad). Gels were then double stained in Coomassie blue G-250.
Postseparation analyses.
Protein spots were excised from gels with a sterile scalpel and placed in a 96-well tray (8). Gel pieces were washed with 50 mM ammonium bicarbonate-100% acetonitrile (60:40 [vol/vol]) and then dried in a Speed Vac (Savant Instruments, Holbrook, N.Y.) for 25 min. Gel pieces were then hydrated in 12 μl of a 12-ng/ml concentration of sequencing-grade modified trypsin (Promega, Madison, Wis.) for 1 h at 4°C. Excess trypsin solution was removed, and the gel pieces were immersed in 50 mM ammonium bicarbonate and incubated overnight at 37°C. Eluted peptides were concentrated and desalted using C18 Zip-Tips (Millipore Corp., Bedford, Mass.). The peptides were washed on the column with 10 μl of 5% formic acid. The bound peptides were eluted from the Zip-Tip in matrix solution (10 mg of α-cyano-4-hydroxycinnamic acid [Sigma]/ml in 70% acetonitrile) directly onto the target plate. Matrix-assisted laser desorption ionization-time-of-flight (mass spectrometry) [MALDI-TOF (MS)] mass spectra were acquired using either a Voyager DE-STR apparatus (PerSeptive Biosytems, Framingham, Mass.) or a TofSpec2E apparatus (Micromass, Manchester, United Kingdom). Both instruments were equipped with 337-nm nitrogen lasers. All spectra were obtained in reflectron-delayed extraction mode, averaging 256 laser shots per sample. Two-point internal calibration of spectra was performed on the basis of the use of internal porcine trypsin autolysis peptides (842.5 and 2211.10 [M+H]+ ions). A list of monoisotopic peaks corresponding to the mass of generated tryptic peptides was used to search a modified translated version of the M. hyopneumoniae genome (F. C. Minion, unpublished data). Successful identifications were made on the basis of the number of matching peptide masses and the percentage of sequence coverage afforded by those matches. N-terminal Edman sequencing was performed as previously described (24).
For electrospray-ionization (ESI) tandem MS-MS peptide sequencing of the P102 C-terminal fragment, peptides were eluted from microcolumns in 1 to 2 μl of 50% methanol-1% formic acid directly into borosilicate nanoelectrospray needles (Micromass). Tandem ESI MS was performed using a Q-Tof of hybrid quadrupole/orthogonal-acceleration TOF mass spectrometer (Micromass). Nanoelectrospray needles containing the sample were mounted in the source, and stable flow was obtained using capillary voltages of 900 to 1,200 V. Precursor ion scans were performed to detect mass:charge (m/z) values for peptides within the mixture. The m/z of each individual precursor ion was selected for fragmentation and subjected to collision with argon gas at collision energies of 18 to 30 eV. Fragment ions (corresponding to the loss of amino acids from the precursor peptide) were recorded and processed using MassLynx version 3.4 software (Micromass). Amino acid sequences were deduced (using MassSeq software) (Micromass) according to the mass differences between y- or b-ion ladder series and confirmed by manual interpretation. Peptide sequences were then used to search the M. hyopneumoniae genome (Minion, unpublished).
Immunoelectron microscopy.
M. hyopneumoniae strain 232 cells were grown to mid-log phase, pelleted by centrifugation, and washed with PBS. The final cell pellets were fixed with 3% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) at 4°C overnight. The pellets were then washed three times (15 min between changes) with 0.1 M sodium cacodylate buffer and postfixed with 1% osmium tetroxide in 0.1 M sodium cacodylate buffer for 2 h at room temperature. The pellets were then washed with distilled water, passed through an acetone series, and embedded in Embed 812 and Araldite (Electron Microscopy Sciences, Fort Washington, Pa.). Thin sections (80 to 90 nm in thickness) were then washed six times with TS buffer (10 mM Tris, 150 mM sodium chloride, pH 7.4) and then reacted with F1B6 ascites fluid (diluted 1:50), anti-ΔNP97 ascites fluid (1:10), anti-NP97 ascites fluid (1:10), or anti-P102 ascites fluid (1:10) overnight at 4°C. The grids were washed five times with TS buffer and then reacted with goat anti-mouse IgG plus IgM labeled with 10-nm-diameter colloidal gold particles (EY Laboratories, Inc.) diluted 1:25 for 30 min at room temperature. The cells were then washed five times with TS buffer, dried, contrasted with osmium vapors for 2 min, and stained with uranyl acetate-lead citrate. The sections were examined on a Hitachi 500 electron microscope at 75 kV.
RESULTS
Immunoblot analysis.
Zhang et al. showed multiple banding patterns of the cilium adhesin by SDS-PAGE using MAbs F1B6 and F2G5 (36). Subsequent studies showed that those MAbs reacted to the R1 repeat region of the P97232 adhesin of strain 232 near the carboxy terminus of the protein (10). To extend these studies, antisera were raised to peptides located at two additional regions of the unprocessed P97232 gene product located on either side of the major cleavage site at amino acid 195 (Fig. 1A). The peptide N-terminal sequencing results obtained with fragments of P97232 (identified on 2-D gels) are also shown in Fig. 1A. The results obtained with M. hyopneumoniae strain J cell lysates reacted with F1B6 are shown in Fig. 1B (lane 1). The most strongly reactive proteins had masses of 94, 66, 45, 33, and 29 kDa, although other more faintly reacting proteins are evident, especially between 120 and 66 kDa. The largest strongly reactive protein (94 kDa) represented what has been thought to be the mature adhesin, as described previously (35). Evidence for processing of the cilium adhesin is shown with peptide antisera ΔNP97 and NP97. Peptide antiserum ΔNP97 reacted with a subset of F1B6-reactive proteins with masses of 94, 72, and 66 kDa in J strain lysates (Fig. 1B, lane 2). Although proteins of 94 and 66 kDa were predicted to react with the ΔNP97 serum, we did not expect a protein with a mass of approximately 72 kDa to be identified by this serum. A faintly staining F1B6-reactive protein which migrates to the same position of the blot as the 72-kDa fragment recognized by ΔNP97 serum was also identified.
FIG. 1.
Map of the cilium adhesin of M. hyopneumoniae and immunoblot analysis using MAb F1B6 and anti-peptide, P97 N-terminal, and R2 antisera. (A) The map shows antibody epitope locations, repeat regions, and selected cleavage sites identified by peptide mass fingerprint analysis, fragment masses, and N-terminal sequences. The major cleavage event at amino acid 195 (large arrow) and another event at amino acid 891 (small arrow) are shown. The locations of the R1 and R2 repeat regions are represented by gray-shaded boxes. The locations of the epitope for MAb F1B6, ΔNP97 peptide, and NP97, P97 N-terminal (P97 N-term), and P28 antisera are shown as bars above or below the map. The coiled-coil regions are represented by the black boxes. The cleavage products of strains J and 232 are shown as gray-shaded bars below the map. Their molecular masses and N-terminal sequences are shown to the right of the map. ND, not determined. *, the peptide TSSQKDPSTLR was identified by peptide mass fingerprinting P70 from strain 232, suggesting that this molecule has the same N-terminal sequence as P97. (B) Immunoblot patterns of M. hyopneumoniae strain J reacted with F1B6 (1:3,000), ΔNP97 (1:20), and NP97 (1:20). An SDS-12% polyacrylamide gel was used to resolve J strain cell lysates. Molecular mass markers (MBI Fermentas) are shown on the left. Equivalent amounts of M. hyopneumoniae cell lysate were loaded in lanes reacted with ΔNP97 (lane 2) and NP97 (lane 3) antibodies. The amount of protein loaded in lane 1 (reacted with MAb F1B6) was approximately one-third of that loaded in lanes 2 and 3. (C) Immunoblot patterns (12% polyacrylamide gel) of synchronized M. hyopneumoniae strain J cultures harvested at different times postinoculation. The blot was reacted with MAb F1B6 (1:1,000). Equivalent amounts of protein were loaded in each lane. Lane 1, 8 h of growth (late lag phase); lane 2, 16 h of growth (early exponential phase); lane 3, 20 h of growth (mid-log phase); lane 4, 24 h of growth (mid-log phase); lane 5, 28 h of growth (mid-log phase); lane 6, 40 h of growth (late log-early stationary phase); lane 7, 48 h of growth (stationary phase); lane 8, 52 h of growth (stationary phase); lane 9, 56 h of growth (stationary phase). (D) Immunoblot patterns (12% polyacrylamide gel) of synchronized M. hyopneumoniae strain J (left panel) and 232 (right panel) cultures harvested at different times postinoculation. The blot was reacted with P97 N-terminal serum (1:100). Equivalent amounts of protein were loaded in each lane. Lane 1, 20 h of growth (mid-log phase); lane 2, 28 h of growth (mid log phase); lane 3, 40 h of growth (late log-early stationary phase); lane 4, 56 h of growth (stationary phase). (E) Immunoblot patterns (10% polyacrylamide gel) of synchronized M. hyopneumoniae strain J (left panel) and 232 (right panel) cultures harvested at different times postinoculation. The blot was reacted with R2 serum (1:100). Equivalent amounts of protein were loaded in each lane. (Left panel) Lane 1, 8 h of growth (late log phase); lane 2, 16 h of growth (early exponential phase); lane 3, 20 h of growth (mid-log phase); lane 4, 24 h of growth (mid-log phase); lane 5, 28 h of growth (mid-log phase); lane 6, 40 h of growth (late log-early stationary phase); lane 7, 48 h of growth (stationary phase). (Right panel) Lane 1, 8 h of growth (late log phase); lane 2, 16 h of growth (early exponential phase); lane 3, 24 h of growth (mid-log phase); lane 4, 28 h of growth (mid-log phase); lane 5, 32 h (mid-log phase); lane 6, 40 h of growth (late log-early stationary phase); lane 7, 48 h of growth (stationary phase). (F) Comparison (using NP97 and ΔNP97 antisera) of immunoblot patterns of strains 232 and J. Molecular weight markers (Bio-Rad Precision Proteins Standards) (broad range) are shown on the right of the panel. An 8 to 16% Bio-Rad Criterion gradient gel was used to separate the proteins.
J strain lysates reacted with peptide antiserum NP97 showed two proteins, one that migrated just above P94J and a second of 72 kDa (Fig. 1B, lane 3). In consistency with our model for processing of the cilium adhesin in strain J (Fig. 1A), NP97 serum did not react with P66J or P94J. Our data suggests that the 72-kDa protein is a processing product of the cilium adhesin recognized by both peptide antisera. Although the only protein with a mass greater than P94J predicted by our model is the cilium adhesin preprotein (with a predicted mass of 123 kDa), we cannot rule out the possibility that other minor cleavage products may be generated. Consistent with this is the presence of two faintly staining, F1B6-reactive proteins with masses greater than 94 kDa (lane 1), one of which most likely represents P123J, the preprotein. Although P22 was not observed with NP97 serum (Fig. 1B, lane 3), a protein with this mass was identified using antisera raised to a recombinant, polyhistidine-tagged N-terminal fragment of P97 that did not contain R1 or R2 (Fig. 1D). Furthermore, this antiserum identified other fragments of the cilium adhesin (Fig. 1D), indicating that processing of this molecule is more complex than is currently understood (see below).
To examine the effect of growth cycle on cilium adhesin processing, synchronous cultures of strain J were harvested at early log (8 h), mid-log (16 to 28 h), and stationary (40 to 56 h) phases and cell lysates were examined by immunoblotting with MAb F1B6 (Fig. 1C), P97 N-terminal serum (Fig. 1D), and P28 serum (Fig. 1E). With F1B6, two strongly staining proteins of 94 and 66 kDa were observed at all time points (Fig. 1C). Interestingly, a strongly reactive protein at 37 kDa appeared to increase in intensity between 8 to 28 h but waned in intensity thereafter. Two F1B6-reactive proteins of 35 and 33 kDa steadily increased in intensity at each time point, reaching maximum intensity at 56 h. A 30-kDa protein first appeared at 20 h and continued to increase in intensity until 56 h (Fig. 1C). Faintly staining proteins of 120 (possibly representing the preprotein), 100, 75 to 90, and 60 to 40 kDa were evident at time points between 16 and 56 h, but their intensity appeared to increase only slightly within this period. These data suggest that P94J and P66J and three proteins with masses between 33 and 37 kDa represent the predominant F1B6-reactive adhesin cleavage fragments that are present throughout the growth cycle of M. hyopneumoniae strain J.
Similar blotting experiments using M. hyopneumoniae J (left panel) strain lysates collected at different times during the growth phase and reacted with P97 N-terminal serum identified strongly reactive proteins with masses of 94, 82, 72, 66, 24, and 22 kDa and faintly reactive proteins with masses of approximately 50 and 120 kDa (Fig. 1D, left panel, lanes 1 to 4). The two proteins with masses of 24 and 22 kDa (possibly P22) failed to react with F1B6. None of the strongly straining F1B6-reactive proteins with masses between 33 and 40 kDa reacted with P97 N-terminal serum (Fig. 1D, lanes 1 to 4), suggesting that these represent processing products of the cilium adhesin that possess R1- and R2-containing fragments. Blot analysis of cell lysates of strain 232 (Fig. 1D, right panel) revealed a similar pattern, indicating that the N-terminal half of the cilium adhesin is processed similarly in both strains. Similar blots reacted with R2 serum identified strongly reactive J strain proteins with masses of 94 (P94J), 60, and 28 (P28J) and several faintly staining proteins with masses of 45, 35, and 33 kDa (Fig. 1E, left panel). A protein of 28 kDa (possibly P28232) was only faintly visible on blots containing cell lysates of strain 232 reacted with R2 serum, and a 60-kDa protein was not observed. Furthermore, cell lysates of strain 232 reacted with R2 serum recognized two proteins of 45 and 36 kDa (Fig. 1E, right panel). These observations provide further evidence that the C-terminal third of the cilium adhesin is processed differently in these two M. hyopneumoniae strains.
Collectively, our immunoblotting data suggest that that the cilium adhesin of M. hyopneumoniae undergoes complex, strain-specific processing. Although cleavage events that remove P22J and P28J from the cilium adhesin appear to be fairly common events, other cleavage events also occur within P66J (although perhaps not with the same frequency). This suggests that ΔNP7 and NP97 serum should recognize more cleavage fragments than were observed as indicated in Fig. 1B. To examine this more carefully, we used gradient gels to resolve cell lysates of strains J and 232 and reacted the blots with these two sera (Fig. 1F). The pattern of reactivity with NP97 and ΔNP97 sera showed that there are at least four proteins with masses greater than 60 kDa and proteins with masses of approximately 37 to 39 kDa and 50 kDa that are recognized. Our model (see Fig. 7) indicates how some of these NP97- and ΔNP97-reactive proteins might be generated. The pattern of reactivity observed across multiple immunoblots with F1B6 MAb showed that there are at least four proteins with masses greater than 60 kDa and other fragments between 30 and 50 kDa that are recognized. Minor mass differences between some of these fragments from strains J and 232 are probably due to differences in the numbers of 5-amino-acid repeats in R1 and 10-amino-acid repeats in R2. Some high-mass fragments may not possess R1 and R2 and are not observed with F1B6 MAb or R2 antisera. Although some of these products may represent transient, low-abundance cleavage products, these complex patterns of reactivity are consistent with immunoblot profiles generated using MAb F1B6 and P97 N-terminal and R2 antisera.
FIG. 7.
Adhesin cleavage products identified by peptide mass mapping and N-terminal sequence analysis and those predicted from immunoblotting studies. Large arrows identify confirmed cleavage sites; smaller arrows indicate predicted but unconfirmed cleavage events. The locations of the epitopes for MAb F1B6, peptide ΔNP97 and NP97 antisera, and P97 N-terminal (P97 N-term) and P28 antisera are shown as black bars.
2-DGE and mass spectrometry.
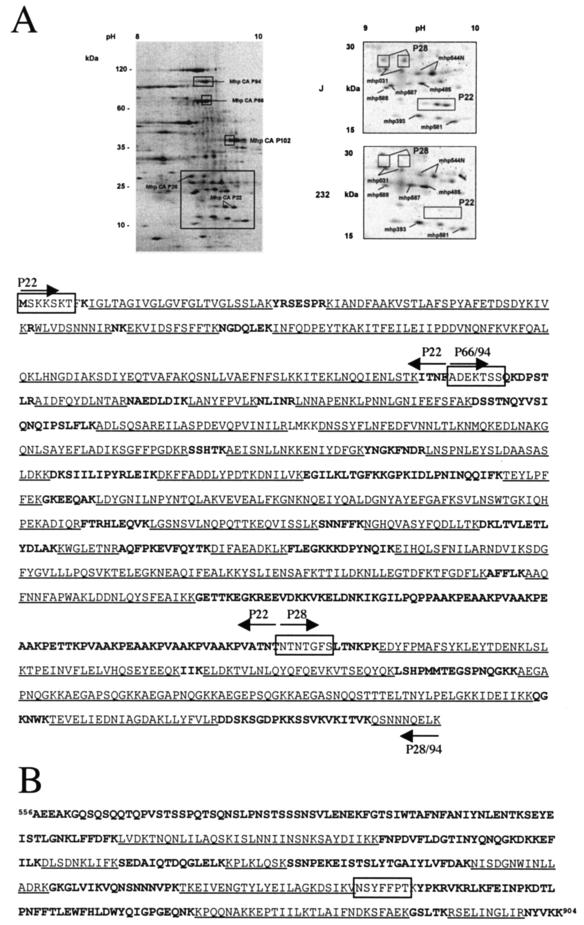
Previous studies have demonstrated that the gene product for the cilium adhesin of strain 232 (126-kDa preprotein, 1,036 amino acids) undergoes a cleavage event at amino acid 195 (9) (Fig. 1A). During peptide mass mapping studies of J strain proteins, four spots of 22, 28, 66, and 94 kDa were identified that represented different fragments of the adhesin (Fig. 2). The cilium adhesin of strain J is smaller than that of strain 232; thus, its fragment sizes are sometimes slightly smaller. The N-terminal sequences for these proteins allowed unequivocal alignment with the cilium adhesin protein sequence. P94J mapped to a region that begins immediately downstream of amino acid 195 and continues to the end of the ORF.
FIG. 2.
Peptide mass fingerprint analysis of the cilium adhesin. (A) (Upper panel) 2-D electrophoresis was used to resolve M. hyopneumoniae proteins, which were subsequently analyzed by peptide mass fingerprinting. (Upper left panel) Analysis of total J strain proteins. A region of the gel representing pH 8 to 10 is shown. The upper boxes indicate the proportion of the spots containing either P94J, P66J, or the 42-kDa fragment of P102 as indicated. The lower larger boxed area is shown in expanded form on the right for a comparison of strains J and 232 in the region of P28. (Lower panel) MALDI-TOF (MS) was used to analyze tryptic digests of indicated spots. The resulting fingerprints were matched to a database containing theoretical tryptic digests of M. hyopneumoniae ORFs derived from genome sequencing analysis (Minion, unpublished). Each cleavage product (P22, P28, P66, and P94) is shown by the arrows above and below the sequence. Underlined sequences were matched by MALDI-TOF (MS). Unmatched sequences are shown in bold characters. Boxed sequences represent N-terminal protein sequences of isolated spots obtained by Edman degradation. (B) Peptide mass mapping of a 42-kDa C-terminal fragment of P102. Underlined sequences were matched by MALDI-TOF (MS). Unmatched sequences are boldface. ESI MS-MS analysis was used to identify the sequence NSYFFPT (underlined). The N-terminal sequence AEEAKG indicates the beginning of the 42-kDa P102 fragment.
Two closely spaced proteins at 66 kDa had identical mass maps and corresponded to a region beginning immediately downstream of amino acid 195 of the adhesin and ending near the R1 repeat. N-terminal sequence analysis of P66 showed a sequence (ADEKTSS) that is identical to that of P94J (Fig. 1 and 2). Immunoblotting results with MAb F1B6 suggested that P66J contains R1 (Fig. 1 and data not shown). Thus, the cleavage event must occur immediately downstream of the R1 repeat region. These data suggest that a fragment approximately 28 kDa in size had been removed from the C terminus in some (but not all) of the P94J molecules. This observation was confirmed when a 28-kDa fragment was identified that mapped to the C terminus of P94J. Previously Wilton et al. showed that antiserum raised against the 28-kDa C-terminal recombinant peptide of the adhesin containing the R2 but not the R1 repeat regions recognized the largest adhesin band (94 to 97 kDa) in different strains of M. hyopneumoniae and recognized a 28-kDa fragment only in strain J (35). In the present studies, 2-D immunoblots of J strain proteins probed with the anti-28-kDa peptide antiserum recognized both P28 and P94 proteins (data not shown).
Tryptic peptide mass mapping showed that peptides from P22J mapped to the first 190 amino acids of the strain J 123-kDa adhesin preprotein (Fig. 2). The N-terminal sequence of P22J (SKKSKTF) aligned to amino acids 2 to 8 in the N terminus (Fig. 2), suggesting that cleavage of the hydrophobic leader peptide (amino acids 8 to 22) is not necessary for translocation of the cilium adhesin across the membrane. Similar studies were performed with strain 232, and although the N-terminal sequence was not obtained for the P97-related peptides, the mass spectrometry data confirmed the identities of P97232 and P70232 (data not shown). P28232 and P22232 were not identified on 2-D (pI, 6 to 11) gels of strain 232 in regions in which they appear in strain J (Fig. 2), suggesting that these cleavage products undergo further processing in this strain.
Peptide mass mapping studies also identified a protein with a predicted mass of 42 kDa (Fig. 2A) that matched the C-terminal region of P102 (Fig. 2B). To determine whether this protein represented a bona fide C-terminal cleavage product of P102 and not the product of a P102 paralog (Minion, unpublished), we performed ESI MS-MS of the 42-kDa protein. The peptide sequence NSYFFPTK unequivocally determined that the 42-kDa protein represented the C-terminal fragment of P102, since this peptide was not predicted from P102 paralog sequences (Fig. 2B). The N-terminal sequence (AEEAKG) of P42 was confirmed by Edman sequencing.
Medium effects on P97.
To rule out the possibility that cleavage of the cilium adhesin resulted from a proteolytic activity in the medium used for growing M. hyopneumoniae in culture, purified recombinant P97232 was incubated with fresh and spent medium and then examined for proteolytic cleavage by immunoblot analysis. Recombinant P97232 was obtained by induction of strain TOP10 pISM405 and purification of the histidine-tagged protein through the use of metal chelate chromatography. Purity was confirmed by SDS-PAGE and immunoblot analysis. Following elution from the column, P97-containing fractions were dialyzed against PBS. The soluble recombinant P97232 was then incubated with fresh and spent modified Friis broth. The results of the study are shown in Fig. 3. Because the medium contained 20% swine serum, large quantities of swine Igs were present in the protein samples, causing some background staining with the anti-mouse conjugate. However, it was still clear that neither fresh nor spent medium contained proteolytic activity capable of cleaving soluble recombinant P97232 after 12 h of incubation at 37°C. Thus, cleavage of the cilium adhesin in mycoplasma cells was mediated by mycoplasma-encoded activities that are not secreted into the medium and was not due to medium components.
FIG. 3.
Growth medium does not contain recombinant P97232 cleavage activity. Recombinant P97232 was incubated in fresh and spent media overnight, and the products were resolved by immunoblotting using MAb F1B6. Lanes: 1, molecular weight standards; 2, fresh medium; 3, spent medium; 4, fresh medium plus recombinant P97232; 5, spent medium plus recombinant P97232. The arrow indicates the position of purified recombinant P97232.
Trypsin sensitivity of R1-containing cleavage products.
Immunoblot analyses of strain J and 232 cells digested with different concentrations of trypsin were used to investigate the cellular location of R1-containing cleavage fragments. The F1B6 MAb typically recognized proteins with masses of 35, 66, 88, 94, and 123 kDa in strain J (Fig. 4), and a similar pattern was observed for strain 232 (data not shown). Exposure of intact M. hyopneumoniae to concentrations of trypsin ranging from 0.1 to 10 μg/ml showed a gradual loss of the higher-mass proteins. Concentrations between 10 and 50 μg/ml resulted in the loss of all the immunoreactive proteins (except one of 35 kDa), indicating that R1-containing adhesin fragments are surface accessible. The pattern of digestion of R1-containing adhesin fragments was consistent in repeat experiments except that the 35-kDa fragment was not reliably resistant to trypsin at concentrations above 10 μg/ml. Analyses of identical blots reacted with antiserum raised to recombinant M. hyopneumoniae lactate dehydrogenase (previously shown to reside in the cytosol) (31) and to antisera raised to recombinant fragments of pyruvate dehydrogenase subunits A and D showed that these proteins remained detectable with trypsin concentrations up to 500 μg/ml (data not shown). In control experiments in which lysed cells were exposed to trypsin, lactate dehydrogenase and pyruvate dehydrogenase subunit D were rapidly degraded (data not shown).
FIG. 4.
Trypsin digestion of M. hyopneumoniae strain J cells and immunoblotting with MAb F1B6 (1:5,000). Each lane contains approximately 10 μg of J strain protein. Approximately 50 mg of mycoplasma whole-cell protein was treated with the indicated concentration (in micrograms per milliliter) of trypsin for 15 min at 37°C. Molecular mass markers (Bio-Rad) are shown on the left. Lanes: 1, 0 μg/ml; 2, 0.3 μg/ml; 3, 0.5 μg/ml; 4, 1 μg/ml; 5, 3 μg/ml; 6, 10 μg/ml; 7, 50 μg/ml; 8, 300 μg/ml.
Immunogold electron microscopy.
Antisera generated against specific regions of the adhesin enabled analysis (using immunogold electron microscopy) of cleavage in vivo. Virulent strain 232 was used in these studies, because the results would have the most impact on our understanding of pathogenic mechanisms. R1-specific MAb F1B6 and antisera raised to peptides TSSQKDPST (ΔNP97 antiserum) and VNQNFKVKFQAL (NP97 antiserum) were used in these studies. The MAb F1B6 remained associated with the mycoplasma membrane but not intimately associated with the cell (Fig. 5B and C), confirming a previous report (36) and our trypsin studies described above. ΔNP97 antiserum reacted with membrane epitopes like MAb F1B6 did (Fig. 5D), but it also reacted with protein aggregates at locations distal to the membrane in association with extracellular material of unknown composition (Fig. 5D to F). NP97 antibodies clustered in small aggregates in the cytosol or on the membrane surface. Some aggregates were also observed outside the cell (Fig. 5G and H). Anti-P102 antiserum reacted only with epitopes in the extracellular matrix and was never observed in association with the membrane surface (Fig. 6).
FIG. 5.
Immunolocalization of the cilium adhesin and cleavage products on the surface of M. hyopneumoniae strain 232. Thin sections were successively labeled with normal mouse (A), mouse MAb F1B6 (B and C), mouse anti-peptide ΔNP97 (D to F), or mouse anti-peptide NP97 (G and H) sera and 10-nm-diameter colloidal gold-conjugated goat anti-mouse Ig and negatively stained with 1% phosphotungstic acid. Arrows indicate gold particles. Bars, 1 μm.
FIG. 6.
Analysis of the surface protein P102 by immunoblotting and immunoelectron microscopy. (A) Analysis of recombinant P102 with a Coomassie blue-stained SDS-PAGE gel. Lane 1, uninduced E. coli culture; lane 2, induced E. coli culture; lane 3, purified polyHis-P102 fusion. Molecular weight markers (in thousands) are shown on the left. (B) Immunoblot of M. hyopneumoniae whole-cell lysate with anti-P102 antiserum. Molecular weight markers are shown on the left. (C) Immunoelectron microscopy of M. hyopneumoniae cells with anti-P102 antiserum. Bar, 1 μm.
P102 cleavage.
To determine whether all surface proteins were subject to the same extent of proteolytic digestion as P97, the fate of P102 was studied. The gene for P102 lies just downstream of that for P97, generating a two-gene operon with P97. Purification of recombinant P102 required two rounds of chromatography because of the low-level yield of the mycoplasma gene product in E. coli (Fig. 6A). This is often the case with mycoplasma proteins (Minion, unpublished). P102-specific antiserum was prepared from the recombinant protein and used in immunoblot and immunogold studies. Anti-P102 antibodies recognized proteins of 102, 72, and 42 kDa (according to the results of immunoblot analysis) (Fig. 6B). Immunogold labeling of M. hyopneumoniae showed that P102 was distributed in the extracellular matrix and was not cell associated (Fig. 6C).
DISCUSSION
The cilium adhesin is a critical component of the M. hyopneumoniae virulence repertoire. It is the only molecule in the genome that possesses the R1 cilium-binding domain (11), an essential element for adherence to porcine epithelial cells. The molecule is not a lipoprotein, and it contains a single N-terminal transmembrane domain. Given its function in colonization, one could hypothesize that the molecule is translocated to the cell surface through the general secretory pathway, where it is locked into the membrane by the transmembrane domain, exposing the cilium-binding motif to the extracellular milieu. Previous studies, however, clearly showed that the adhesin is cleaved at amino acid 195, separating the transmembrane domain from the cilium-binding epitope (9, 36) and complicating this model for placement of the cilium-binding activity in association with the cell surface. Obviously, the translocation of the adhesin and its maintenance on the cell surface are more complicated than originally thought. In addition, R1-specific MAbs (according to the results of immunoblot analysis) recognize multiple proteins, further clouding our understanding of this molecule.
Our data demonstrate that the cilium adhesin is cleaved not at one site, as previously reported (9, 36), but at multiple sites, generating a family of peptides that remain in association with the cell or extracellular matrix proteins (Fig. 1 and 5). Since virtually all fragments of the adhesin recognized by MAb F1B6 and the anti-28-kDa antiserum are surface accessible (Fig. 4) (35), the simplest model would argue that cleavage occurs primarily on the extracellular side of the membrane. Cleavage at amino acid 195 may occur during translocation, since little uncleaved preprotein (P123) was identified on immunoblots with MAb F1B6 (Fig. 1). The cleavage event that removes P28 from the preprotein may occur prior to the removal of P22 in a proportion of adhesin molecules (Fig. 7). This is shown by experiments that identify proteins (other than P97232 and P94J) with masses greater than 66 kDa that react with antisera NP97, ΔNP97, MAb F1B6, P97 N terminal, and R2 (Fig. 1B to F). We have not identified these proteins on 2-D gels by peptide mass mapping analysis to confirm this. Cleavage at other sites was a slower process, resulting in a collection of partially cleaved fragments of P97232 in the cell population (Fig. 1C). This suggests that cleavage at most sites and membrane translocation are not integrated events. To eliminate the possibility that cleavage of the cilium adhesin is an artifact arising from the medium, we tested fresh and spent mycoplasma media with recombinant full-length P97232 and found no proteolytic activity (Fig. 3), confirming that cleavage is a mycoplasma-associated activity.
Many secreted proteins undergo proteolytic processing that is essential to their function. Processing typically occurs at the bacterial cell surface and usually comprises one or two proteolytic cleavage events (4). We also examined whether another surface protein undergoes extensive proteolytic cleavage in M. hyopneumoniae. Results with anti-P102 hyperimmune sera show that complex processing is protein specific. Cleavage of P102 may be occurring at most at one or two sites (Fig. 6B). Peptide mass matching and ESI MS-MS confirmed that a 42-kDa protein represented the C-terminal cleavage fragment of P102 (Fig. 2B). Further studies are required to confirm the identity of the remaining protein with a predicted mass of 72 kDa (Fig. 6B). Additionally, previous studies of a 65-kDa lipoprotein did not indicate proteolytic cleavage of that molecule while grown in culture (13). Thus, it seems likely that proteolytic activity on the mycoplasma membrane surface is selective. How the decision is made respecting which proteins to digest is unclear.
Comparative peptide mass mapping studies demonstrated complex, differential processing between strains J and 232. In some aspects, processing was similar. Figure 2 shows the results obtained with strain J, and parallel studies with strain 232 identified two proteins of 70 and 97 kDa whose mass maps were virtually identical to those of P94J and P66J. The presence of six extra copies of the R1 repeat in the strain 232 proteins could account for the size differences of P70232 and P97232 (Fig. 2). Immunoblots probed with antiserum raised against a recombinant 28-kDa fragment of P94J containing R2 but not R1 (Fig. 1E) (35) recognized P97232 but not P70232, suggesting that cleavage between the R1 and R2 regions generates P70232 from P97232. That the same antisera recognized P28232 very poorly and did not recognize it at all stages of the growth cycle (Fig. 1E) and that we were unable to locate P28232 or P22232 on 2-D gels of strain 232 in regions in which they were identified in strain J (Fig. 2A, upper right panel) suggests that further processing is occurring in strain 232 and in other strains in which P28 is not observed by immunoblotting analysis (35). P22 sequences were identical in the two strains and should have been present in the 2-D gel, but the P28 sequences differed in the two strains; the predicted mass and pI values for P28232 were 24.6 kDa and 5.88 and for P28J were 26.0 kDa and 8.39, respectively. Whether this can explain the lack of cilium-binding activity with strain J and its inability to colonize and infect is still unclear.
The single most important processing event in the cilium adhesin is likely to be cleavage at amino acid 195, because it occurs immediately after translation, possibly in concert with membrane translocation. This cleavage results in the formation of P94J, P22J, and other potential cleavage fragments (Fig. 2 and 7). The fate of P22J was unknown until peptide mass mapping identified the protein in 2-D gels. A 22-kDa band in strains J and 232 was observed in immunoblot studies with P97 N-terminal serum (Fig. 1D), but we could not confirm that this protein was P22232. To confirm that processing fragments of the cilium adhesin that possess sequences upstream of amino acid 195 persist in association with the cell after cleavage, immunogold electron microscopy with peptide-specific antiserum NP97 localized P22232 (and other fragments) intracellularly, in association with the surface of the bacterium, and in the extracellular milieu (Fig. 5G and H). One would expect these fragments to be completely membrane bound, since they possess a transmembrane domain that is clearly not removed by a signal peptide peptidase, a component of the type II secretion apparatus (Fig. 1 and 2).
Numerous particles are also found intracellularly; while some of these may represent tangential thin sections through membranes, not all of the particles can be accounted for in this way. Other particles are found outside the cell, but they seem to be closely associated with the cell surface. Further studies will be required to better understand the role of P22 and other fragments that possess this N-terminal sequence at these sites. The immunogold data also indicate that P22232 and other N-terminal fragments may self-associate, because small aggregates of gold particles are evident throughout (unlike the observations obtained with MAb F1B6 and ΔNP97 antiserum) (Fig. 5). This hypothesis is strengthened by the presence of a statistically significant coiled-coil domain (14-, 21-, and 28-amino-acid window settings), as predicted (by the program COILS) (http://www.ch.embnet.org) between amino acids 180 and 195 within P22232 just upstream of the major cleavage site at amino acid 195. Coiled-coil domains are increasingly being identified in secreted bacterial virulence effector molecules, and functional studies suggest that these motifs play an important role in subunit assembly and translocation and in flexible interactions with multiple bacterial and host proteins (6).
Given the location of F1B6 and ΔNP97 epitopes in the P70 fragment, it is reasonable to conclude that the immunogold staining patterns would be similar. Indeed, this was observed in some instances (Fig. 5B to D). Interestingly, however, immunogold patterns of F1B6 and ΔNP97 differed in many cases (Fig. 5E and F). The clustering of gold particles in an extracellular matrix at a considerable distance from the cell surface was never observed with MAb F1B6. This correlates with our model, according to which P66J is subject to further proteolytic cleavage (Fig. 7). Further analyses are required to identify and map these proteins. In our model (Fig. 7), both epitopes are present in some molecules for at least a portion of time. Once the two epitopes are separated by cleavage, however, the N-terminal ΔNP97 epitope-containing peptides (P58 and possibly others) are released from the cell membrane, possibly aggregating or associating with other proteins (Fig. 5E and F), while the R1 region containing the F1B6 epitope remains cell associated (Fig. 5B and C). A second coiled-coil domain at amino acids 364 to 394 found in the N-terminal region of P66J and P70232 may enable these ΔNP97 epitope-specific proteins to form interactions with themselves or other molecules.
A number of potential adhesin cleavage products could be generated from the cleavage events that are hypothesized in our model (Fig. 7). Several F1B6 and P97 N-terminal serum-reactive fragments with masses greater than 48 kDa are evident (Fig. 1). Several of these are also reactive with R2 serum (Fig. 1E). F1B6-reactive fragments with masses between 26 and 37 kDa are present (Fig. 1C), and several of these may also react with R2 serum (Fig. 1E). Although our model shows how F1B6-reactive fragments of about 50 kDa might be generated, we cannot predict the presence of R2-reactive fragments with masses 47 to 49 kDa without proposing a further cleavage event(s) in the middle of P123. Consistent with this is the presence of P97 N-terminal-reactive fragments with masses of approximately 24 kDa that are also not predicted by our model. The observation of R2, F1B6, and P97 N-terminal-reactive fragments with masses of 47 to 50 kDa might be consistent with the generation of C-terminal cleavage fragments that contain R1 and R2. We have observed that the recombinant adhesin proteins containing R1 and or R2 migrate atypically during SDS-PAGE (Wilton et al., unpublished data), further complicating the generation of models that predict complex cleavage patterns.
Cleavage of the adhesin, however, presents a paradox. How does the R1 cilium-binding domain remain associated with the mycoplasma surface even though the hydrophobic N-terminal sequence has been removed? No other portion of the molecule seems capable of direct membrane anchorage, so we hypothesize that protein-protein interactions prevent the loss of the R1-binding domain to the environment. The proposed anchorage protein could be either mycoplasma or host derived. Many pathogenic bacteria have evolved surface molecules or receptors capable of binding host proteins that enhance adherence, colonization, and invasion of host epithelial surfaces (15, 26, 33). For instance, binding to fibronectin by pathogenic bacteria can enhance initial colonization of the epithelial cells on mucosal surfaces (12, 22, 25, 30) and trigger cellular invasion (33). In any event, these observations have radically altered the way we think about the surface architecture of M. hyopneumoniae.
We now have evidence that other high-molecular-weight proteins undergo proteolytic processing on the surface (S. P. Djordjevic, unpublished data), suggesting that this is an important phenotype of this species. It has not been observed in any other species of mycoplasmas (or not, at least, to this extent). In support of this model, several potential proteases have been identified in the M. hyopneumoniae genome sequence (Minion, unpublished). There have been reports of cleavage of mycoplasma lipoproteins to form immunoreactive lipopeptides (1, 5, 23), but the cilium adhesin is not a lipoprotein, and there is no evidence that it is lipid modified. Even more interesting is the fact that the cleavage products remain associated with the cell during growth in vitro and are not lost during extensive cell washing. This suggests that active mechanisms exist for binding these fragments and maintaining them in association with the cell. Not all translocated proteins undergo this extensive cleavage, as evidenced by the studies with P102 (Fig. 6). Thus, it appears that cleavage is selective and complex and that it is controlled by unknown mechanisms and mycoplasma components. Given the importance of the cilium adhesin to virulence, its posttranslational cleavage may play an important role in the disease process.
Acknowledgments
We thank Sreekumar A. Menon, Tsungda Hsu, and Cary Adams for cloning and site-directed mutagenesis and Jean Olsen for assistance with electron microscopy. The skillful technical assistance of Nelson Guerreiro and Wendy Forbes is also acknowledged.
These studies were supported in part with funds from the Iowa Livestock Health Advisory Council and from the Healthy Livestock Initiative from the College of Veterinary Medicine, Iowa State University.
Editor: J. T. Barbieri
REFERENCES
- 1.Calcutt, M. J., M. F. Kim, A. B. Karpas, P. F. Muhlradt, and K. S. Wise. 1999. Differential posttranslational processing confers intraspecies variation of a major surface lipoprotein and a macrophage-activating lipopeptide of Mycoplasma fermentans. Infect. Immun. 67:760-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciprian, A., C. Pijoan, T. Cruz, J. Camacho, J. Tortora, G. Colmenares, R. R. Lopez, and M. de la Garza. 1988. Mycoplasma hyopneumoniae increases the susceptibility of pigs to experimental Pasteurella multocida pneumonia. Can. J. Vet. Res. 52:434-438. [PMC free article] [PubMed] [Google Scholar]
- 3.Cordwell, S. J., D. J. Basseal, B. Bjellqvist, D. C. Shaw, and I. Humphery-Smith. 1997. Characterisation of basic proteins from Spiroplasma melliferum using novel immobilised pH gradients. Electrophoresis 18:1393-1398. [DOI] [PubMed] [Google Scholar]
- 4.Coutte, L., E. Willery, R. Antoine, H. Drobecq, C. Locht, and F. Jacob-Dubuisson. 2003. Surface anchoring of bacterial subtilisin important for maturation function. Mol. Microbiol. 49:529-539. [DOI] [PubMed] [Google Scholar]
- 5.Davis, K. L., and K. S. Wise. 2002. Site-specific proteolysis of the MALP-404 lipoprotein determines the release of a soluble selective lipoprotein-associated motif-containing fragment and alteration of the surface phenotype of Mycoplasma fermentans. Infect. Immun. 70:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delahay, R. M., and G. Frankel. 2002. Coiled-coil proteins associated with type III secretion systems: a versatile domain revisited. Mol. Microbiol. 45:905-916. [DOI] [PubMed] [Google Scholar]
- 7.Djordjevic, S. P., G. J. Eamens, L. F. Romalis, and M. M. Saunders. 1994. An improved enzyme linked immunosorbent assay (ELISA) for the detection of porcine serum antibodies against Mycoplasma hyopneumoniae. Vet. Microbiol. 39:261-273. [DOI] [PubMed] [Google Scholar]
- 8.Gobom, J., E. Nordhoff, E. Mirgorodskaya, R. Ekman, and P. Roepstorff. 1999. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 34:105-116. [DOI] [PubMed] [Google Scholar]
- 9.Hsu, T., S. Artiushin, and F. C. Minion. 1997. Cloning and functional analysis of the P97 swine cilium adhesin gene of Mycoplasma hyopneumoniae. J. Bacteriol. 179:1317-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu, T., and F. C. Minion. 1998. Identification of the cilium binding epitope of the Mycoplasma hyopneumoniae P97 adhesin. Infect. Immun. 66:4762-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu, T., and F. C. Minion. 1998. Molecular analysis of the P97 cilium adhesin operon of Mycoplasma hyopneumoniae. Gene 214:13-23. [DOI] [PubMed] [Google Scholar]
- 12.Jaffe, J., S. Natanson-Yaron, M. G. Caparon, and E. Hanski. 1996. Protein F2, a novel fibronectin-binding protein from Streptococcus pyogenes, possesses two binding domains. Mol. Microbiol. 21:373-384. [DOI] [PubMed] [Google Scholar]
- 13.Kim, M. F., M. B. Heidari, S. J. Stull, M. A. McIntosh, and K. S. Wise. 1990. Identification and mapping of an immunogenic region of Mycoplasma hyopneumoniae p65 surface lipoprotein expressed in Escherichia coli from a cloned genomic fragment. Infect. Immun. 58:2637-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King, K. W., D. H. Faulds, E. L. Rosey, and R. J. Yancey. 1997. Characterization of the gene encoding Mhp1 from Mycoplasma hyopneumoniae and examination of Mhp1's vaccine potential. Vaccine 15:25-35. [DOI] [PubMed] [Google Scholar]
- 15.Kronvall, G., and K. Jonsson. 1999. Receptins: a novel term for an expanding spectrum of natural and engineered microbial proteins with binding properties for mammalian proteins. J. Mol. Recognit. 12:38-44. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 17.Livingston, C. W., E. L. Stair, N. R. Underdahl, and C. A. Mebus. 1972. Pathogenesis of mycoplasmal pneumonia in swine. Am. J. Vet. Res. 33:2249-2258. [PubMed] [Google Scholar]
- 18.Luo, W., and S.-H. Lin. 1997. Generation of moderate amounts of polyclonal antibodies in mice. BioTechniques 23:630-632. [DOI] [PubMed] [Google Scholar]
- 19.Mebus, C. A., and N. R. Underdahl. 1977. Scanning electron microscopy of trachea and bronchi from gnotobiotic pigs inoculated with Mycoplasma hyopneumoniae. Am. J. Vet. Res. 43:1249-1254. [PubMed] [Google Scholar]
- 20.Menon, S. A., M. J. Wannemuehler, G. G. Mahairas, and F. C. Minion. 2002. Mycobacterial ESAT-6 protein enhances mouse IFN-γ responses to Mycoplasma hyopneumoniae P71 protein. J. Interferon Cytokine Res. 22:807-813. [DOI] [PubMed] [Google Scholar]
- 21.Minion, F. C., C. Adams, and T. Hsu. 2000. R1 region of P97 mediates adherence of Mycoplasma hyopneumoniae to swine cilia. Infect. Immun. 68:3056-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mongodin, E., O. Bajolet, J. Cutrona, N. Bonnet, F. Dupuit, E. Puchelle, and S. de Bentzmann. 2002. Fibronectin-binding proteins of Staphylococcus aureus are involved in adherence to human airway epithelium. Infect. Immun. 70:620-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhlradt, P. F., M. Kiess, H. Meyer, R. Sussmuth, and G. Jung. 1998. Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis. Infect. Immun. 66:4804-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nouwens, A. S., S. J. Cordwell, M. R. Larsen, M. P. Molloy, M. Gillings, M. D. P. Willcox, and B. J. Walsh. 2000. Complementing genomics with proteomics: the membrane subproteome of Pseudomonas aeruginosa PA01. Electrophoresis 21:3797-3809. [DOI] [PubMed] [Google Scholar]
- 25.Pasula, R., P. Wisniowski, and W. J. Martin. 2002. Fibronectin facilitates Mycobacterium tuberculosis attachment to murine alveolar macrophages. Infect. Immun. 70:1287-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patti, J. M., B. L. Allen, M. J. McGavin, and M. Hook. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585-617. [DOI] [PubMed] [Google Scholar]
- 27.Razin, S., D. Yogev, and Y. Naot. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiol. Mol. Biol. Rev. 62:1094-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross, R. F. 1992. Mycoplasmal disease, p. 537-551. In A. D. Leman, B. E. Straw, W. L. Mengeling, S. D'Allaire, and D. J. Taylor (ed.), Diseases of swine. Iowa State University Press, Ames.
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Secott, T. E., T. L. Lin, and C. C. Wu. 2002. Fibronectin attachment protein is necessary for efficient attachment and invasion of epithelial cells by Mycobacterium avium subsp. paratuberculosis. Infect. Immun. 70:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strasser, M., J. Frey, G. Bestetti, M. Kobisch, and J. Nicolet. 1991. Cloning and expression of a species-specific early immunogenic 36-kilodalton protein of Mycoplasma hyopneumoniae in Escherichia coli. Infect. Immun. 59:1217-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tajima, M., and T. Yagihashi. 1982. Interaction of Mycoplasma hyopneumoniae with the porcine respiratory epithelium as observed by electron microscopy. Infect. Immun. 37:1162-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talay, S. R., A. Zock, M. Rhode, G. Molinari, M. Oggioni, G. Pozzi, C. A. Guzman, and G. S. Chatwal. 2000. Co-operative binding of human fibronectin to Sfb1 protein triggers streptococcal invasion into respiratory epithelial cells. Cell. Microbiol. 2:521-535. [DOI] [PubMed] [Google Scholar]
- 34.Thacker, E. L., P. G. Halbur, R. F. Ross, R. Thanawongnuwech, and B. J. Thacker. 1999. Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J. Clin. Microbiol. 37:620-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilton, J. L., A. L. Scarman, M. J. Walker, and S. P. Djordjevic. 1998. Reiterated repeat region variability in the ciliary adhesin gene of Mycoplasma hyopneumoniae. Microbiology 144:1931-1943. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, Q., T. F. Young, and R. F. Ross. 1995. Identification and characterization of a Mycoplasma hyopneumoniae adhesin. Infect. Immun. 63:1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, Q., T. F. Young, and R. F. Ross. 1994. Microtiter plate adherence assay and receptor analogs for Mycoplasma hyopneumoniae. Infect. Immun. 62:1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zielinski, G. C., T. Young, R. F. Ross, and R. F. Rosenbusch. 1990. Adherence of Mycoplasma hyopneumoniae to cell monolayers. Am. J. Vet. Res. 51:339-343. [PubMed] [Google Scholar]