Abstract
Several salivary proteins exhibit fungicidal activity against the opportunistic oral pathogen Candida albicans when they are tested as pure proteins in vitro. However, salivary secretions that are examined by the same assays either lack or exhibit very low candidacidal activity. Since ionic strength is known to have an inhibitory effect on the fungicidal activities of some proteins, parotid secretion was subjected to dialysis with membranes having molecular weight cutoffs (MWCOs) of 500, 1,000, 10,000, and 25,000. Dialysis with membranes with MWCOs of ≥1,000 promoted fungicidal activity of parotid secretion, and this activity was dose dependent. The addition of sodium chloride to dialyzed, fungicidal parotid secretion abolished this activity, indicating that the fungicidal component was salt sensitive. Similar results were obtained with submandibular and sublingual secretions. Polyacrylamide gel electrophoresis under native and denaturing conditions was used to analyze the composition of the dialysate. Unexpectedly, proteins with MWs much lower than the nominal MWCOs of the membranes were not lost during dialysis. Among the retained proteins, the two fractions with MWs of approximately 17,000 and 4,000 exhibited fungicidal activity. These results are consistent with the presence of lysozyme and histatins, respectively, which may represent the major candidacidal capacity of dialyzed parotid secretion.
Saliva is a complex biological fluid that plays an important role in the maintenance of the integrity of the hard and soft tissues in the oral cavity (19). Saliva is comprised mostly of water (99% of the total volume) and contains both inorganic and organic components. Although the contribution of proteins to the composition of saliva is less than 1% by weight, they are believed to be responsible for the important antimicrobial functional properties of saliva.
Candida albicans is a yeast associated with humans and other mammalian species and is frequently encountered as a harmless commensal microorganism of the digestive system and vaginal tract. It is considered to be an opportunistic pathogen, since it causes infection when the immune system of the host is compromised (22, 27). For example, oral candidiasis and other C. albicans infections are commonly found in human immunodeficiency virus-positive patients and in subjects treated with immunosuppressive drugs (8). Furthermore, oral candidiasis is frequently seen in otherwise healthy elderly individuals. The etiology in those cases has been related to reduced salivary flow rates and possibly to an age-associated increase in Candida adhesion sites on oral keratinocytes (29).
Both innate resistance and acquired immunity in the oral cavity play a role in maintaining C. albicans in the commensal state. Cell-mediated immunity conferred by CD4+-T-helper cells is considered the most important host defense against C. albicans on mucosal surfaces (9). However, in saliva, no correlation has been found between the Th1/Th2 cytokine profiles and the susceptibility to denture stomatitis in immunocompetent individuals (16). Oral humoral immunity is represented by secretory immunoglobulin A, which is the major component of the salivary immune host defense. It may act primarily as a preventive defense factor against oral candidiasis by aggregating C. albicans cells in saliva, thereby preventing their adherence to mucosal epithelium (4, 8). In addition to acquired immune systems, saliva also contains a number of innate host defense factors, which provide a first line of defense against oral infections. The lactoperoxidase-thiocyanate-H2O2 system exhibits antibacterial and also antifungal activities (17). Lactoferrin is a cationic iron-binding glycoprotein that is synthesized by acinar epithelial cells and polymorphonuclear leukocytes and exerts candidacidal activity in vitro in its iron-free form (apo-lactoferrin) (20). Another salivary antifungal agent is lysozyme, which has a bimodal action on C. albicans. At higher concentrations, it has a direct killing effect, while at lower concentrations, it inhibits the proteolytic activity of secreted aspartyl proteinases, enzymes that facilitate the penetration of keratinocytes and are therefore considered important virulence factors of C. albicans (30). Furthermore, saliva contains histatins, a family of small cationic, histidine-rich peptides which display fungistatic (23) and fungicidal (7, 11, 21) activities against C. albicans and other fungi and inhibit the conversion of C. albicans from blastospores into the more virulent germinated form (27, 31).
Despite the fact that all of the antimicrobial systems described above exert fungicidal or fungistatic activity against C. albicans when they are tested as purified proteins in vitro, salivary secretions that contain all of these antifungal factors in adequate amounts lack or show very little antifungal activity. The objective of the present investigation was to assess the factors that mask the activity of the antifungal components in a salivary environment.
MATERIALS AND METHODS
Proteins and peptides.
Histatins 1, 3, and 5 were isolated from parotid secretion by using the recently developed zinc precipitation method (10). Lysozyme was obtained from Sigma (St. Louis, Mo.).
Saliva collection.
Parotid or submandibular-sublingual (SMSL) secretions were collected from two healthy female subjects (aged 30 and 36 years) under conditions of gustatory stimulation by using sour lemon candies (Regal Crown; Trebor Sharps Ltd., London, England) as previously described (13). Samples were collected into a polypropylene graduated cylinder chilled on ice with the aid of a Carlson-Crittenden device positioned over the Stenson's duct. SMSL secretions were collected by placing a custom-fitted collecting device at the opening of the Wharton's and Bartholin's ducts. The first milliliter that was collected was discarded. The protocol for this investigation was approved by the Institutional Review Board of Boston University Medical Center, and informed consent was obtained from both subjects.
Dialysis of saliva.
Parotid and SMSL salivary secretions were collected as described above, and 7 ml was dialyzed with membrane tubing with molecular weight cutoffs (MWCOs) of 500, 1,000, 10,000, and 25,000 (Spectra/Por; Spectrum Laboratories, Inc., Rancho Dominguez, Calif.). Dialysis was performed for 20 h at 4°C against 20 liters of deionized water with two water volume changes. After dialysis, the secretions were removed from the tubing and the sample volume was determined. With all membranes, the sample volume typically increased to about 7.5 ml. Aliquots of undialyzed parotid and SMSL secretions were stored on ice during the dialysis time of 20 h.
Gel electrophoresis.
Gel electrophoresis was performed using a mini-gel electrophoresis system (Novex, San Diego, Calif.). Dialyzed and undialyzed parotid and SMSL secretions were concentrated 10-fold using a speed-vac (Vacufuge; Eppendorf), and their protein profiles were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Tris-Tricine PAGE, or cationic PAGE. The SDS-separating gel contained 15% (wt/vol) acrylamide and 0.4% (wt/vol) biacrylamide, and electrophoresis was carried out as described in reference 15. Tris-Tricine SDS-PAGE was performed as described in reference 26, with a separating gel containing 9.6% acrylamide, 0.3% bisacrylamide, and 3.6 M urea. Cationic PAGE was performed to separate the positively charged proteins and was carried out on a separating gel containing 15% acrylamide and 0.4% bisacrylamide (1, 21).
Protein concentration determination.
The protein concentrations in saliva were estimated using the bicinchoninic acid method (Pierce, Rockford, Ill.) by reference to a standard curve generated with bovine serum albumin (0 to 50 μg/ml) according to the manufacturer's instructions. The absorbance at 562 nm was determined by using a Cary 50 spectrophotometer (Varian Instruments, Walnut Creek, Calif.). The data were plotted, and the protein concentrations of diluted (1:20 or 1:60) saliva samples were determined from the linear part of the bovine serum albumin standard curve.
Candida killing assay.
Dose-dependent candidacidal activities of salivary secretions were determined in a microtiter assay. In a 96-well polypropylene microtiter plate (Costar, Cambridge, Mass.), serial dilutions of dialyzed and undialyzed salivary samples were prepared in water. C. albicans (ATCC 10231) was grown for 48 h at 30°C on Sabouraud dextrose agar. Several colonies were suspended in water to a final optical density at 620 nm (OD620) of approximately 40. The suspension was diluted in water to an OD620 of 0.5 and added to the dilution series of saliva in water or to water only (the control sample) to a final OD620 of 0.25 (approximately 2.6 × 106 CFU/ml). The killing activities of undiluted salivary samples were tested by suspending the yeasts directly from the concentrated stock suspension into saliva to a final OD620 of 0.25. Microtiter plates were sealed with plastic covers to prevent evaporation and incubated at 37°C for 1.5 h with shaking. After incubation, 50-μl aliquots from selected wells were diluted 180-fold in phosphate-buffered saline (10 mM sodium phosphate buffer, pH 7.0, containing 0.9% NaCl). Twenty-five-microliter aliquots of these diluted suspensions were spread on Sabouraud dextrose agar plates and incubated for 48 h at 30°C, and the percentage of viable cells was determined by comparing the number of colonies in the control sample to those in the treated samples. Typically, the plates contained 250 to 350 colonies.
Atomic absorption spectroscopy.
Sodium levels in undialyzed and dialyzed glandular secretions were determined by atomic absorption spectroscopy (AAnalyst 200; Perkin Elmer). A linear absorption curve was obtained with solutions containing sodium at 1 to 10 ppm (R2, 0.9986), which were prepared by diluting a reference solution containing sodium at 1,000 ppm (Fisher Scientific, Pittsburgh, Pa.) with deionized water. Measurements were performed in the presence of 0.2% LaCl and 0.2% KCl. Undialyzed salivary secretions were diluted until the absorption was within the linear part of the sodium standard curve; dialyzed secretions were measured undiluted.
Extraction of proteins from the gels.
Proteins in dialyzed salivary samples were separated by electrophoresis in a Tris-Tricine gel. The first and last lanes of each gel slab were loaded with a prestained standard, See Blue 2 (Bio-Rad Laboratories, Richmond, Calif.). All other lanes were loaded with 15 μl of 10-fold-concentrated dialyzed parotid saliva samples (MWCO, 10,000). After electrophoretic separation of the proteins, the gel was cut into two parts, of which one was stained with Coomassie brilliant blue (Fisher). Gel slices were excised from the unstained gel to correspond with the positions of the major proteins identified in the stained gel. As a control, a slice of gel containing no saliva sample was excised and treated the same way. Each gel piece was placed in a microtube and was manually crushed into smaller pieces. Proteins were eluted using a mixture of formic acid, water, and 2-propanol (1:3:2, vol/vol/vol) which just covered the gel pieces, and the mixture was left for 15 h at room temperature (5). Samples were subsequently centrifuged for 10 min at 16,000 × g, and the supernatant was collected. The crushed gel was washed again with the extraction solution, and the supernatants were combined. Sterile water was added to the supernatant to a final volume of 1 ml, and the sample was dialyzed with membrane microtubing with an MWCO of 1,000 (Amika Corp., Columbia, Md.). Dialyzed samples were concentrated to 120 μl in a Speed-Vac (Savant, Farmingdale, N.Y.). From this solution, 10-μl samples were analyzed on a Tris-Tricine gel to verify protein extraction, and 100-μl samples were used in a killing assay.
RESULTS
The effect of dialysis on the fungicidal activity of parotid secretion.
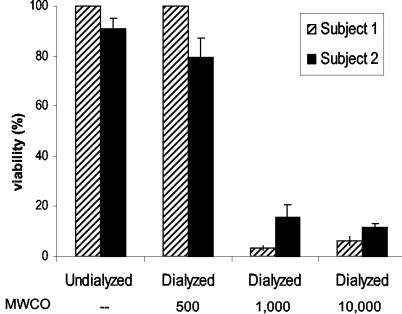
To investigate the effect of dialysis on the fungicidal activity of parotid saliva, parotid secretions were collected from two healthy subjects and dialyzed with membrane tubings with various MWCOs. Incubation of C. albicans cells with undialyzed parotid secretion as well as with parotid secretion that had been dialyzed with membrane tubing with an MWCO of 500 did not result in cell killing (Fig. 1). However, when C. albicans cells were incubated with parotid secretion dialyzed with membrane tubing with an MWCO of 1,000 or higher, virtually 100% cell killing was observed (Fig. 1). The percentage of dead cells was corroborated by the percentage of cells that stained blue with trypan blue, a dye which is excluded from live cells (data not shown).
FIG. 1.
Candidacidal activities of undialyzed and dialyzed parotid secretions. Parotid secretions were collected from two healthy individuals (subject 1 and subject 2) and dialyzed with membrane tubings with MWCOs of 500, 1,000, and 10,000. A sample of 50 μl of dialyzed saliva was added to 50 μl of C. albicans cells in water (final OD620, 0.25). The mixture was incubated for 1.5 h at 37°C, and the viability of the cells was determined by colony counting after plating. Results shown are the averages of results from three experiments performed in duplicate.
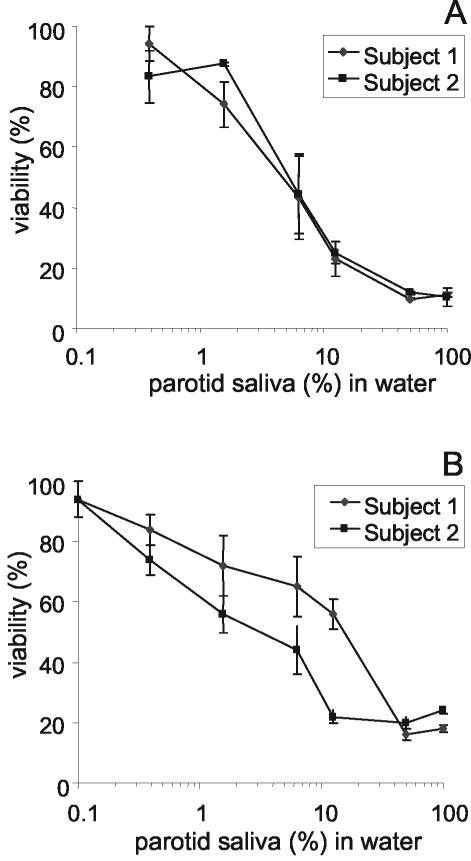
To assess whether the observed killing activity was concentration dependent, a dilution series of dialyzed parotid secretions (MWCO, 10,000) from two subjects was prepared. Samples from both subjects showed a dose-dependent killing activity against C. albicans. A 5% dialyzed parotid saliva solution caused a 50% reduction in viable counts (Fig. 2A). It was established that such a 20-fold dilution of parotid saliva contains approximately 40 μg of salivary protein/ml. When parotid saliva samples were dialyzed with membrane tubing with an MWCO of 25,000, a similar concentration-dependent killing effect was observed (Fig. 2B). Fungicidal activities were also observed upon dialysis of secretions from the SMSL glands. From the obtained killing curves, the 50% inhibitory concentrations were determined. This value is the concentration of parotid or SMSL secretion in water that leads to a 50% reduction of the C. albicans inoculum. The results expressed in Table 1 show that dialyzed parotid and SMSL secretions from both donors show candidacidal activities.
FIG. 2.
Candidacidal activities of dialyzed parotid secretions. Parotid secretions were collected from two subjects and dialyzed with membrane tubings with MWCOs of 10,000 (A) and 25,000 (B). The percentage of viability is plotted against the dilution of dialyzed parotid secretion in water, with 100% representing the viability with undiluted dialyzed secretion. Graphs represent the averages and errors from killing assays performed in duplicate for each subject.
TABLE 1.
Fungicidal activities of undialyzed and dialyzed parotid and SMSL salivary secretions
| Salivary secretion | Subject | IC50a of saliva (%) in water
|
|
|---|---|---|---|
| Dialyzedb | Undialyzed | ||
| Parotid | 1 | 4.9 ± 2.0 | >100 |
| 2 | 5.1 ± 1.2 | >100 | |
| SMSL | 1 | 7.9 ± 3.1 | >100 |
| 2 | 27.5 ± 0.5 | >100 | |
Fifty percent inhibitory concentrations (IC50) are concentrations of parotid secretions (expressed as percentages) in water that lead to killing of 50% of the inoculum. Values are averages and errors of the results of an experiment performed in duplicate.
Secretions were dialyzed with a membrane with an MWCO of 10,000.
One possible explanation for the fungicidal effects that arise after dialysis is that an inhibitor of fungicidal activity is removed during this procedure. A known inhibitor of the antifungal action of salivary cationic peptides is salt (11, 31). However, if salt was the inhibitor, one would expect secretions that were dialyzed with tubing with an MWCO of 500 to also display fungicidal activity. To investigate the efficiency of the dialysis procedure, we measured the concentrations of sodium ions in parotid secretions after dialysis with membrane tubings with various MWCOs. Table 2 shows that dialysis with tubing with an MWCO of 500 indeed leads to incomplete removal of sodium ions, suggesting the incomplete dialysis of salts in general. This finding is of importance since salts, in particular those containing divalent cations such as Ca2+ and Mg2+, are known to strongly inhibit the activities of cationic antifungal peptides (7, 28, 31).
TABLE 2.
Sodium concentrations in undialyzed and dialyzed parotid salivary secretions
| Dialysis procedure | Subject | Sodium concn (mM)a |
|---|---|---|
| Not dialyzed | 1 | 23.8 ± 1.1 |
| MWCO 500 | 1.58 ± 0.01 | |
| MWCO 1,000 | <0.001 | |
| MWCO 10,000 | <0.001 | |
| Not dialyzed | 2 | 103.1 ± 1.2 |
| MWCO 500 | 0.47 ± 0.01 | |
| MWCO 1,000 | <0.001 | |
| MWCO 10,000 | <0.001 |
Sodium levels were determined by atomic absorption spectroscopy. Values are the averages and standard deviations of results from two experiments performed in triplicate.
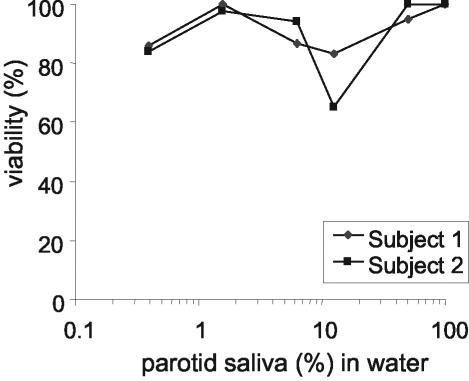
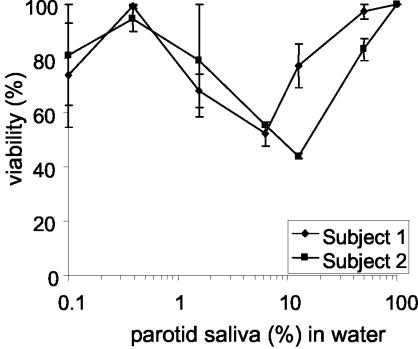
To further assess whether the killing activity that emerges upon dialysis is related to the removal of salt, sodium chloride was added to dialyzed (MWCO, 10,000) parotid saliva samples to a final concentration of 40 mM, and the candidacidal activity of this sample was compared to that of dialyzed samples to which no salt was added after dialysis (Fig. 1A). It was observed that the addition of sodium chloride to dialyzed parotid secretion abolishes the fungicidal activity of the sample (Fig. 3). Some activity could be recovered if such samples were diluted. At a 10-fold dilution, samples showed a maximum fungicidal activity of about 25%. Because of this observed activity, we investigated whether undialyzed parotid saliva would also become active upon dilution. For this purpose, a serial dilution series of undialyzed parotid salivary secretion in water was prepared. As expected, undialyzed parotid secretion was incapable of killing C. albicans; however, approximately 50 to 60% killing of the C. albicans inoculum was observed upon 10-fold dilution of the sample (Fig. 4). Also, the diluted undialyzed SMSL secretions showed a trend towards being more active at a 10-fold dilution than the undiluted secretion (data not shown). The observed killing activity in diluted parotid and SMSL samples disappeared upon further dilution of the samples.
FIG. 3.
Candidacidal activities of dialyzed parotid secretions supplemented with NaCl. Parotid secretions were collected from two subjects, dialyzed with membrane tubing with an MWCO of 10,000, and supplemented with 40 mM NaCl. The percentage of viability is plotted against the dilution of dialyzed, salt-supplemented parotid secretion in water, with 100% representing the viability with undiluted dialyzed secretion.
FIG. 4.
Candidacidal activities of undialyzed parotid secretions. Parotid secretions were collected from two subjects, serially diluted in water, and tested for fungicidal activity. The percentage of viability is plotted against the dilution of parotid secretion in water, with 100% being the viability with undiluted secretion. Graphs show the averages and errors from killing assays performed in duplicate for each subject.
Electrophoretic analysis of dialyzed and undialyzed parotid secretion.
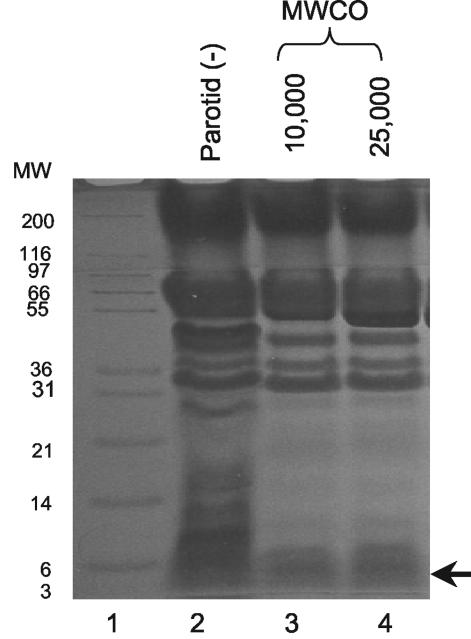
The fact that secretions that were dialyzed with membrane tubings with MWCOs of up to 25,000 were fungicidal suggested that the MW of the fungicidal component was higher than 25,000. To investigate the MWs of the proteinaceous components that remained in the tubing after dialysis, the dialyzed secretions were analyzed by gel electrophoresis. Dialysis of parotid secretions with tubings with MWCOs of 10,000 and 25,000 did not remove all proteins with MWs smaller than the nominal MWCOs of the tubings (Fig. 5). Interestingly, proteins with MWs much below the MWCO of the membrane (e.g., with MWs of about 4,000) were still retained after dialysis (arrow). Figure 6A shows that both the dialyzed parotid and SMSL secretions seem to retain the low-MW proteins, some of which have MWs close to those of histatins (MW, <4,000) and lysozyme (MW, 17,000). Increases in dialysis times and the number of volume changes did not improve the dialysis efficiency (data not shown). The retention of low-MW proteins was of interest since the fungicidal activity of the dialyzed secretions could possibly be attributed to these proteins. In order to determine whether the retained low-MW proteins were histatins, undialyzed and dialyzed secretions were subjected to cationic PAGE. Comparison of the retained proteins with histatin standards confirmed that histatins 1, 3, and 5 were indeed still present after dialysis (Fig. 6B).
FIG. 5.
Tris-Tricine PAGE analysis of the protein compositions of parotid secretions before and after dialysis. Lane 1, MW marker; lane 2, undialyzed (−) parotid secretion; lane 3, parotid secretion dialyzed with tubing with an MWCO of 10,000; lane 4, parotid secretion dialyzed with tubing with an MWCO of 25,000. In lanes 2 to 4, proteins contained in 20-μl samples were loaded.
FIG. 6.
SDS-PAGE and cationic PAGE of parotid and SMSL secretions before and after dialysis with tubing with an MWCO of 10,000. (A) SDS-PAGE of undialyzed (−) and dialyzed (+) parotid and SMSL secretions. Lane 1, MW (in thousands) marker; lane 2, histatin 1 (3 μg); lane 3, histatin 3 (5 μg); lane 4, histatin 5 (5 μg); lane 5, lysozyme (5 μg); lane 6, undialyzed parotid secretion (100 μg); lane 7, dialyzed parotid secretion (100 μg); lane 8, dialyzed SMSL secretion (50 μg); lane 9, undialyzed SMSL secretion (50 μg). (B) Cationic PAGE of samples identical to those used to obtain the results in panel A.
The observed fungicidal activity of dialyzed parotid secretion could, in principle, be attributed to any of the proteins remaining in the tubing after dialysis. To test which of the retained proteins displayed candidacidal activity, the major proteins in the dialysate with MWs of 4,000, 17,000, 25,000, and 60,000 were extracted from the gel. A qualitative assessment of their candidacidal activities indicated that proteins with MWs of approximately 17,000 and 4,000 exhibited killing activity. In contrast to these low-MW proteins, two higher-MW proteins that were extracted did not show fungicidal activity. This result suggested that either the 4,000-MW protein, the 17,000-MW protein, or both proteins may have been responsible for the observed fungicidal activity of dialyzed parotid secretion.
DISCUSSION
Several proteins present in human saliva have been demonstrated to display in vitro fungicidal or fungistatic activity against C. albicans. For example, histatins, present in human parotid and submandibular secretions, are highly fungicidal in vitro. Besides histatins, other well-known antifungal agents, such as lactoferrin, lysozyme, and peroxidase, are also present in human saliva. It is therefore surprising that salivary secretions containing these proteins do not display fungicidal activity when they are tested in the same assays for candidacidal activity. Some studies have even shown that C. albicans is able to grow in whole saliva and to grow well in glucose-supplemented whole saliva supernatant (18, 24). From these observations, it is evident that the antifungal components present in salivary secretions are inactive, or at least less active than might be expected from their in vitro killing activities.
C. albicans killing by parotid, SMSL, and whole salivary secretions cannot be detected by direct assessment of yeast cell killing in saliva samples. However, it has been demonstrated that when secretions are subjected to acidification and boiling, reduction in blastospore viability and inhibition of blastospore-to-germ tube conversion can be observed (25). The use of these treatments to reveal the antifungal activity of salivary secretions suggests that only a fraction of the salivary antifungal components are available in their free form. By acidification and boiling, complexed proteins would be released and would then be able to exert their fungicidal activities.
Histatins exhibit fungicidal activity against C. albicans in vitro, but their activity has been demonstrated to be strongly reduced under high-ionic-strength conditions (11, 31). As parotid secretions contain salt at concentrations over 50 mM and histatins are inactive at such concentrations, we examined whether dialysis of saliva could unmask the fungicidal activity of parotid secretion. The results indicated that the fungicidal activity of parotid saliva appears upon dialysis of the samples with tubing with an MWCO of ≥1,000. Since the MWs of the major histatins range from 3,000 to 5,000, it was expected that dialysis of salivary secretions with tubings with MWCOs of 10,000 and 25,000 would not retain the histatins. Electrophoretic analysis of the samples dialyzed with tubings with MWCOs of 10,000 and 25,000 revealed, however, that histatins were still retained after dialysis, indicating that the size of the proteins remaining in the tubing after dialysis did not correspond to the MWCO of the membrane.
One explanation for the fact that histatins were retained upon dialysis is that histatins form complexes with other proteins with higher MWs. For example, it has been demonstrated that histatins form a heterotypic complex with the gel-forming mucin MG1 (12), which is present in SMSL secretions. Although mucins are not present in parotid saliva, the same phenomenon of complex formation may occur between histatins and other higher-MW proteins. Another explanation for histatin retention is that proper dialysis is prevented by other macromolecules present in saliva. Large proteins such as amylase may clog the membrane pores, thereby preventing dialysis of low-MW proteins. Alternatively, histatins could have been trapped by a higher-MW protein, preventing them from reaching the pores.
Interestingly, our results demonstrated that the addition of salt reverses the fungicidal activity of parotid secretion dialyzed with membranes with an MWCO of 10,000. While the promotion of fungicidal activity by dialysis may, theoretically, be related to the removal of inhibitors other than inorganic cations and anions, the reversal of this activity by the addition of sodium chloride after dialysis makes this possibility unlikely. Furthermore, it was demonstrated that when salts are not efficiently removed by dialysis (with tubing with an MWCO of 500), the parotid secretion's fungicidal properties are not revealed. The facts that salts inhibit histatin activity and that histatins are still present in the dialyzed secretions suggest that the fungicidal effects may be attributable to histatins.
Theoretically, any individual protein or combination of proteins retained in the dialyzed parotid saliva samples could be responsible for the observed fungicidal activity of this secretion. To identify which protein was responsible for this activity, major proteins were extracted from a gel and tested in a candidacidal assay. Our preliminary results indicated that of the proteins excised, the ones with MWs of approximately 4,000 and 17,000 exhibited killing activity, indicating that one or both of these proteins could account for the observed fungicidal activity of dialyzed parotid secretion. The proteins with a MW of 4,000 may very well represent the histatins, and the 17,000-MW protein likely represents lysozyme.
Another interesting observation made during this study was that certain dilutions of undialyzed parotid secretion in water exhibited significant fungicidal activity. This is consistent with the fact that parotid secretion becomes active when it is dialyzed against water. The 50 to 60% killing that was observed with 10-fold-diluted undialyzed parotid saliva samples could be explained by the dilution of ions, which may lead to the activation of proteins that are active only at low ionic strengths. Upon further dilution of undialyzed parotid saliva, this activity disappeared, likely due to the fact that the proteins that are responsible for the fungicidal activity became too diluted to exert their function. Physiological considerations of protein and ionic compositions are important in this context. It is well known that the protein concentration increases at most twofold as parotid flow increases from the resting state to 1 ml/min (6). In contrast, sodium levels can increase over 30-fold, from a resting 2 to 60 meq/liter or more (6, 14). The increase in sodium levels in stimulated secretion is due to the reduced reabsorption of this ion in the ductal system under these conditions. The reabsorption mechanism selectively diminishes the sodium levels but not the protein concentration. It is feasible that the dialyzed parotid saliva samples used in this study, in which the sodium concentrations were selectively diminished by the dialysis procedure, are more similar to unstimulated parotid secretion with respect to fungicidal activity than to stimulated secretions.
In the present study we addressed the discrepancy in the killing activities of individual salivary proteins and salivary secretions and discovered that dialysis and dilution unmask the fungicidal potential. Since C. albicans can be cultured from the saliva of approximately 50% of healthy individuals (2, 3), it is clear that in in vivo situations whole saliva is not fungicidal in absolute terms. It is feasible that the control of yeast overgrowth in the oral cavity is a delicate process based on salivary fungistatic rather than on fungicidal activities. In other words, growth inhibition rather than outright killing may be sufficient to maintain oral fungal homeostasis. The fact that dialysis unmasks killing activity indicates a hidden salivary antifungal potential, but this activity probably far exceeds the activity required and desired under physiological conditions. Future studies should reveal whether the antifungal potential of dialyzed secretions from different individuals is related to oral yeast carriage and to susceptibility to the development of oral candidiasis.
Acknowledgments
This study was supported by NIH (NIDCR) grants DE05672, DE07652, and DE14950.
We thank H. C. Margolis and A. Esslinger-Litman for their help in the atomic absorption measurements and N. Grand-Pierre for technical assistance.
Editor: T. R. Kozel
REFERENCES
- 1.Baum, B. J., J. L. Bird, and R. W. Longton. 1977. Polyacrylamide gel electrophoresis of human salivary histidine-rich-polypeptides. J. Dent. Res. 56:1115-1118. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Aryeh, H., E. Blumfield, R. Szargel, D. Laufer, and I. Berdicevsky. 1995. Oral Candida carriage and blood group antigen secretor status. Mycoses 38:355-358. [DOI] [PubMed] [Google Scholar]
- 3.Burford-Mason, A. P., J. C. Weber, and J. M. Willoughby. 1988. Oral carriage of Candida albicans, ABO blood group and secretor status in healthy subjects. J. Med. Vet. Mycol. 26:49-56. [DOI] [PubMed] [Google Scholar]
- 4.Challacombe, S. J. 1994. Immunologic aspects of oral candidiasis. Oral Surg. Oral Med. Oral Pathol. 78:202-210. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, S. L., and B. T. Chait. 1997. Mass spectrometry of whole proteins eluted from sodium sulfate-polyacrylamide gel electrophoresis gels. Anal. Biochem. 247:257-267. [DOI] [PubMed] [Google Scholar]
- 6.Dawes, C. 1969. The effects of flow rate and duration of stimulation on the concentrations of protein and the main electrolytes in human parotid saliva. Arch. Oral Biol. 14:277-294. [DOI] [PubMed] [Google Scholar]
- 7.Dong, J., S. Vylkova, X. S. Li, and M. Edgerton. 2003. Calcium blocks fungicidal activity of human salivary histatin 5 through disruption of binding with Candida albicans. J. Dent. Res. 82:748-752. [DOI] [PubMed] [Google Scholar]
- 8.Epstein, J. B., E. L. Truelove, and K. T. Izutzu. 1984. Oral candidiasis: pathogenesis and host defence. Rev. Infect. Dis. 6:96-106. [DOI] [PubMed] [Google Scholar]
- 9.Fidel, P. L., Jr. 2002. Immunity to Candida. Oral Dis. 8(Suppl. 2):69-75. [DOI] [PubMed] [Google Scholar]
- 10.Flora, B., H. Gusman, E. J. Helmerhorst, R. F. Troxler, and F. G. Oppenheim. 2001. A new method for the isolation of histatins 1, 3, and 5 from parotid secretion using zinc precipitation. Protein Expr. Purif. 23:198-206. [DOI] [PubMed] [Google Scholar]
- 11.Helmerhorst, E. J., W. Van't Hof, E. C. I. Veerman, I. Simoons-Smit, and A. V. Nieuw Amerongen. 1997. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem. J. 326:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iontcheva, I., F. G. Oppenheim, and R. F. Troxler. 1997. Human salivary mucin MG1 selectively forms heterotypic complexes with amylase, proline-rich protein, statherin, and histatins. J. Dent. Res. 76:734-743. [DOI] [PubMed] [Google Scholar]
- 13.Jensen, J. L., M. S. Lamkin, and F. G. Oppenheim. 1992. Adsorption of human salivary proteins to hydroxyapatite: a comparison between whole saliva and glandular salivary secretions. J. Dent. Res. 71:1569-1576. [DOI] [PubMed] [Google Scholar]
- 14.Kreusser, W., A. Heidland, H. Hennemann, M. E. Wigand, and H. Knauf. 1972. Mono- and divalent electrolyte patterns, pCO2 and pH in relation to flow rate in normal human parotid saliva. Eur. J. Clin. Investig. 2:398-406. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Leigh, J. E., C. Steele, F. Wormley, and P. L. Fidel, Jr. 2002. Salivary cytokine profiles in the immunocompetent individual with Candida-associated denture stomatitis. Oral Microbiol. Immunol. 17:311-314. [DOI] [PubMed] [Google Scholar]
- 17.Lenander-Lumikari, M. 1992. Inhibition of Candida albicans by the peroxidase/SCN−/H2O2 system. Oral Microbiol. Immunol. 7:315-320. [DOI] [PubMed] [Google Scholar]
- 18.Lenander-Lumikari, M., and I. Johansson. 1995. Effect of saliva composition on the growth of Candida albicans and Torulopsis glabrata. Oral Microbiol. Immunol. 10:233-240. [DOI] [PubMed] [Google Scholar]
- 19.Mandel, I. D. 1987. The functions of saliva. J. Dent. Res. 66:623-627. [DOI] [PubMed] [Google Scholar]
- 20.Nikawa, H., L. P. Samaranayake, J. Tenovuo, K. M. Pang, and T. Hamada. 1993. The fungicidal effect of human lactoferrin on Candida albicans and Candida krusei. Arch. Oral Biol. 38:1057-1063. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheim, F. G., T. Xu, F. M. McMillian, S. M. Levitz, R. D. Diamond, D. Offner, and R. F. Troxler. 1988. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure and fungistatic effects on Candida albicans. J. Biol. Chem. 263:7472-7477. [PubMed] [Google Scholar]
- 22.Pla, J., C. Gil, L. Moneoliva, F. Navarro-Garcia, M. Sanchez, and C. Nombela. 1996. Understanding Candida albicans at the molecular level. Yeast 12:1677-1702. [DOI] [PubMed] [Google Scholar]
- 23.Pollock, J. J., L. Denepitiya, B. J. MacKay, and V. J. Iacono. 1984. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect. Immun. 44:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samaranayake, Y. H., T. W. MacFarlane, L. P. Samaranayake, and T. Aichison. 1994. The in vitro proteolytic and saccharolytic activity of Candida species cultured in human saliva. Oral Microbiol. Immunol. 9:229-235. [DOI] [PubMed] [Google Scholar]
- 25.Santarpia, R. P., L. Xu, K. Lal, and J. J. Pollock. 1992. Salivary anti-candidal assays. Oral Microbiol. Immunol. 7:38-43. [DOI] [PubMed] [Google Scholar]
- 26.Schägger, H., and G. von Jagow. 1987. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 166:368-379. [DOI] [PubMed] [Google Scholar]
- 27.Scully, C., and M. El-Kabir. 1994. Candida and oral candidosis: a review. Crit. Rev. Oral Biol. Med. 5:125-157. [DOI] [PubMed] [Google Scholar]
- 28.Selsted, M. E., D. Szklarek, T. Ganz, and R. I. Lehrer. 1985. Activity of rabbit leukocyte peptides against Candida albicans. Infect. Immun. 49:202-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanida, T., E. Ueta, A. Tobiume, T. Hamada, F. Rao, and T. Osaki. 2001. Influence of aging on candidal growth and adhesion regulatory agents in saliva. J. Oral Pathol. Med. 30:328-335. [DOI] [PubMed] [Google Scholar]
- 30.Wu, T., L. P. Samaranayake, W. K. Leung, and P. A. Sullivan. 1999. Inhibition of growth and secreted aspartyl proteinase production in Candida albicans by lysozyme. J. Med. Microbiol. 48:721-730. [DOI] [PubMed] [Google Scholar]
- 31.Xu, T., S. M. Levitz, R. D. Diamond, and F. G. Oppenheim. 1991. Anticandidal activity of major human salivary histatins. Infect. Immun. 59:2549-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]