Abstract
Vaccine development and our understanding of the pathology of bovine tuberculosis in cattle would be greatly facilitated by definition of the immunological correlates of protection and/or pathology. In this study we analyzed humoral immune responses in Mycobacterium bovis BCG-vaccinated and control cattle (in particular, the relationship between the intradermal comparative tuberculin skin test and serum immunoglobulin G [IgG] responses) against a range of mycobacterial antigens (MPB59, MPB64, MPB70, MPB83, ESAT-6, CFP-10, Acr1, and PstS-1) by multiantigen print immunoassay and conventional enzyme-linked immunosorbent assay. Following M. bovis infection, the comparative tuberculin skin test strongly boosted IgG, IgG1, and IgG2 antibody responses, particularly against MPB83 and MPB70, in unvaccinated cattle but failed to boost these responses, or did so only weakly, in BCG-vaccinated calves. In addition, the skin test-induced increases in MPB83-specific IgG responses correlated positively with bacterial loads and ESAT-6-induced in vitro gamma interferon responses. In conclusion, both the negative correlation of skin test-enhanced MPB83-specific antibody responses with BCG-induced protection and their positive correlation with bacterial loads can serve as useful markers for vaccine efficacy after challenge.
Worldwide more than 50 million cattle are infected with Mycobacterium bovis, the causative agent of bovine tuberculosis (BTB) (28). Human tuberculosis caused by M. bovis is also still a public health problem in developing countries (13). The implementation of a (tuberculin skin) test and slaughter control strategy resulted in a dramatic reduction in BTB in Great Britain, although the incidence of BTB in cattle has been rising exponentially since the mid-1980s (14). An independent scientific review commissioned for the United Kingdom government in 1997 concluded that the development of a cattle vaccine would offer the best long-term prospect for BTB control in British herds (14).
M. bovis bacillus Calmette-Guérin (BCG), the vaccine against human tuberculosis, has also been tested in cattle. However, the protection imparted by BCG vaccination of cattle over the past 70 years has been as variable as that observed in human trials, ranging from none to about 70% protection (reviewed by Francis [9] and Hewinson et al. [12]). Studies conducted in New Zealand (1, 2) and in our own laboratory (30) have reported efficacies as high as 75% for BCG vaccination of cattle against experimental intratracheal M. bovis infection. The analysis of humoral responses to BCG vaccination and M. bovis challenge described in this report is based on one such experiment (30).
BTB is a spectral disease with predominantly cellular responses during early and intermediate disease stages that are complemented, and in severe cases displaced, by humoral immune responses in the later stages of disease (24). In particular, the mycobacterial antigens MPB70 and MPM83 have been identified as serodominant antigens (7, 8, 17, 19, 22). However, precise correlates of protection and disease severity in cattle (and in other systems) are not well defined. Recently it was reported that gamma interferon (IFN-γ) responses induced by the M. bovis- and M. tuberculosis-specific antigens ESAT-6 and CFP-10 after M. bovis infection correlated negatively with the protective efficacy of BCG in cattle and positively with disease severity and pathology (30). Identification of these and other such correlates in cattle would greatly enhance vaccine development against BTB because it would facilitate the field testing of promising vaccine candidates.
In the present study, we analyzed humoral immunity in BCG-vaccinated calves challenged with a virulent Great Britain field strain of M. bovis (30) to determine whether the antibody responses correlated with parameters of cellular immunity and disease severity as well as with the protection conferred by BCG vaccination. Our data show that skin testing increased MPB83-specific immunoglobulin G (IgG) responses in control animals, but not in BCG-vaccinated animals, and that this increase correlated positively with disease severity, bacterial loads, and in vitro IFN-γ production induced by ESAT-6.
MATERIALS AND METHODS
Cattle.
Calves (ca. 6 months old; Friesian or Friesian crosses; castrated males) were obtained from herds free of BTB and were kept in the Animal Services Unit at the Veterinary Laboratories Agency (VLA), Weybridge, Addlestone, United Kingdom, in category 3 biosafety accommodations.
Experimental schedule.
Six calves were vaccinated with M. bovis BCG Pasteur by subcutaneous injection of 106 CFU into the side of the neck, followed 6 weeks later by a booster injection using the same route and dose. A group of six unvaccinated calves served as controls. Seven weeks after the second BCG vaccination, both vaccinated and unvaccinated animals were infected with an M. bovis field strain from Great Britain (AF 2122/97) by endobronchial instillation of 4 × 104 CFU as described previously (30). Blood samples were collected at regular intervals throughout the vaccination and challenge periods. Animals were skin tested with the single intradermal comparative cervical tuberculin test 14 weeks after M. bovis infection. The skin tests were performed as specified in European Economic Community directive 80/219, amending directive 64/422, annex B (6). Animals were slaughtered 2 weeks later, and postmortem examinations were performed to assess the protective efficacy of vaccination (see below).
Postmortem examination.
At the end of the experimental period (18 weeks postinfection), the calves were euthanatized by intravenous injection of sodium pentobarbital, and postmortem examinations (30) were performed. The personnel performing the postmortems were unaware of the vaccination statuses of the animals examined. Lungs were first examined externally for lesions and then sliced into 0.5- to 1-cm-thick slices, which were individually examined for lesions. In addition, lymph nodes of the head and pulmonary regions were removed and weighed. They were sliced into thin sections (1 to 2 mm thick) and examined for the presence of visible lesions. Tissue samples, removed from the central parts of the lymph nodes, were taken for M. bovis culture and for histopathological examination (Ziehl-Neelsen staining for acid-fast bacilli and hematoxylin-eosin staining). The samples taken for culture were weighed to allow an estimation of the bacterial burden per lymph node. These samples were homogenized and plated onto 7H10 agar plates (see below). The severity of the gross pathological changes was scored by using the semiquantitative systems described below (30).
(i) Lungs.
Lung lobes (left apical, left cardiac, left diaphragmatic, right apical, right cardiac, right diaphragmatic, and right accessory) were examined individually. For each lobe, the following scoring system was applied: 0, no visible lesions; 1, no gross lesions, but lesions apparent upon slicing; 2, <5 gross lesions with diameters of <10 mm; 3, >6 gross lesions with diameters of <10 mm, or a single distinct gross lesion with a diameter of >10 mm; 4, >1 distinct gross lesion with diameters of >10 mm; 5, gross coalescing lesions. The scores of the individual lobes were added up to calculate the lung score.
(ii) Lymph nodes.
The severity of the observed gross pathology in individual lymph nodes was scored by use of the following scoring system: 0, no necrosis or visible lesions; 1, small focus (1 to 2 mm in diameter); 2, several small foci, or a necrotic area of at least 5 by 5 mm; 3, multiple necrotic areas of at least 5 by 5 mm distributed throughout the node, or one necrotic area affecting >5% of the node. Individual lymph node scores were added up to calculate the lymph node score. Both lymph node and lung pathology scores were added to determine the total pathology score per animal. All scoring was performed by the same operator for all animals to ensure scoring consistency.
Bacterial enumeration.
Tissue sections collected from lymph nodes at postmortem were individually homogenized in 5 ml of sterile distilled water by using a rotating-blade macerator system. Viable counts were performed on serial dilutions of the macerate in water containing 0.05% (vol/vol) Tween 80 to maintain dispersion. Suspensions were plated onto 7H10 agar containing sodium pyruvate (4.16 mg/ml) and 10% (vol/vol) Middlebrook OADC (oleic acid, albumin, dextrose, catalase) enrichment.
Antigens.
The following recombinant antigens of M. bovis were purified to near-homogeneity as polyhistidine-tagged proteins (with Rv numbers according to the classification of Cole et al. [4] given in parentheses): ESAT-6 (Rv3875) and CFP-10 (Rv3874), produced at the Statens Serum Institut, Copenhagen, Denmark; MPB59 (Rv1886c), MPB64 (Rv1980c), MPB70 (Rv2875), and MPB83 (Rv2873), produced at the Veterinary Sciences Division, Stormont, Belfast, United Kingdom (16); and alpha-crystallin (Acr1; Rv3391) and the 38-kDa protein (PstS1; Rv0934), purchased from Standard Diagnostics, Seoul, South Korea. M. bovis culture filtrate (MBCF) was obtained from a field strain of M. bovis (T/91/1378; Veterinary Sciences Division, Belfast) cultured in synthetic Sauton's medium for 21 days. Bovine purified protein derivative (PPD-B) was produced by VLA Weybridge.
MAPIA.
Multiantigen print immunoassay (MAPIA) was performed as described previously (18). Briefly, antigens were immobilized on a nitrocellulose membrane (Schleicher & Schuell, Keene, N.H.) at a protein concentration of 0.05 mg/ml by using a semiautomated airbrush-printing device (Linomat IV; Camag Scientific Inc, Wilmington, Del.). The membrane was cut perpendicular to the antigen bands into 4-mm-wide strips. Strips were blocked for 1 h with 1% nonfat skim milk in phosphate-buffered saline with 0.05% Tween 20 and then incubated for 1 h with serum samples diluted 1:40 in blocking solution. After being washed, strips were incubated for 1 h with alkaline phosphatase-conjugated anti-bovine IgG antibody (Sigma Chemical Co., St. Louis, Mo.) diluted 1:2,000, followed by another washing step. Bovine antibodies bound to printed antigens were visualized with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium (BCIP-NTB) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.). Results were also evaluated by semiquantitative densitometry using Scion Image (version Beta 4.0.2).
IFN-γ assay.
For the IFN-γ assay (30), whole-blood cultures were performed in 96-well plates in 0.20-ml/well aliquots by mixing 0.1 ml of heparinized blood with an equal volume of antigen-containing solution. Supernatants were harvested after 24 h of culture, and IFN-γ levels were determined by using the BOVIGAM enzyme immunoassay kit (CSL, Melbourne, Australia).
ELISAs.
MPB70 and MPB83-specific serum IgG, IgG1, and IgG2 levels were determined as described previously (31). Nunc Maxisorb enzyme-linked immunosorbent assay (ELISA) plates (Nunc, Roskilde, Denmark) were coated with 0.1 μg of recombinant protein/well. Serial dilutions of serum samples were added to the plates. Total IgG was detected by using a horseradish peroxidase-conjugated sheep anti-bovine IgG antibody (Serotec, Oxford, United Kingdom). The IgG1 and IgG2 subclasses were assessed with a mouse-anti-bovine IgG1 or IgG2 serum (Serotec) followed by incubation with a horseradish peroxidase-conjugated sheep anti-mouse IgG antibody (Serotec). Data are expressed as optical densities at 450 nm (OD450) obtained at a serum dilution of 1:100.
Statistical analysis.
Statistical analysis was performed with Instat (version 3; GraphPad, San Diego, Calif.). Increases in antibody responses due to skin testing (MAPIA and ELISA) were assessed by use of the unpaired two-tailed t test, since these data sets were normally distributed as determined by F tests. In cases where data sets were of unequal variance, the unpaired two-tailed t test was used with Welch corrections. Correlations between antibody responses, IFN-γ responses, bacterial loads, and disease severity were assessed by nonparametric analysis (Spearman rank test).
RESULTS
Antigen recognition by serum IgG antibodies in BCG-vaccinated and unvaccinated cattle during experimental tuberculosis.
As previously reported by Vordermeier et al. (30), in this experiment BCG conferred about 75% protection as defined by reductions in disease severity and in the numbers of viable bacilli cultured from lymph node samples (for details of results and statistical analysis, see reference 30). Sera were collected regularly following BCG vaccination, as well as following M. bovis challenge of BCG-vaccinated cattle and unvaccinated controls. To assess the specificity of the humoral immunity induced after vaccination and/or infection, MAPIAs detecting IgG responses were performed with a range of defined mycobacterial antigens (ESAT-6, CFP-10, MPB70, MBP59, MPB64, MPB83, Acr1, and PstS-1). In addition, the complex antigen MBCF was also included. No responses to the defined antigens were detected in uninfected or BCG-vaccinated calves prior to infection (data not shown). However, following infection, more-frequent IgG responses to MPB83 (six of six), ESAT-6 (three of six), MPB70 (two of six), and PstS-1 (one of six) were found in unvaccinated animals than in BCG-vaccinated cattle (two of six for MPB83, one of six for ESAT-6, none of six for MPB70, and none of six for Pst-1). CFP-10, MPB59, MPB64, and Acr1 were recognized neither by serum antibodies from controls nor by those from BCG-vaccinated animals (data not shown).
The most pronounced differences in serum IgG responses between BCG-vaccinated and control animals were observed after the comparative tuberculin skin test, performed at 14 weeks postinfection. Intradermal injection of PPD-B strongly boosted the antibody responses measured 2 weeks later, particularly those to ESAT-6, MPB83, and MBP70 and mainly in nonvaccinated infected animals. Weak increases in responses against CFP-10, PstS-1, and MPB64 were also observed in some control animals but not in BCG-vaccinated animals (data not shown). The differences between the pre- and post-skin test IgG responses of all control and BCG-vaccinated calves were analyzed quantitatively by densitometry of MAPIA band intensities for ESAT-6, MPB83, MPB70, and MBCF. The results shown in Table 1 demonstrate significantly higher serum IgG responses to MPB83 in control animals than in BCG-vaccinated animals. Responses against MPB70 were also substantially, though not statistically significantly, boosted after skin testing in control animals.
TABLE 1.
Increase in antigen-specific IgG responses following skin test application
| Animal | Increase in IgG responsea to:
|
|||
|---|---|---|---|---|
| ESAT-6 | MPB70 | MPB83 | MBCF | |
| Controls | ||||
| C1 | 0.00 | 12.00 | 149.24 | 28.40 |
| C2 | 0.00 | 2.32 | 35.95 | 0.50 |
| C3 | 18.18 | 0.00 | 36.91 | 6.38 |
| C4 | 6.26 | 2.30 | 49.59 | 0.00 |
| C5 | 2.23 | 2.00 | 98.77 | 16.00 |
| C6 | 3.46 | 12.20 | 69.68 | −12.50 |
| Mean | 5.02 | 5.14 | 73.36 | 6.46 |
| BCG vaccinated | ||||
| B1 | 20.68 | 0.00 | 41.89 | 4.18 |
| B2 | 0.00 | 0.00 | −7.61 | 0.86 |
| B3 | 0.00 | 1.27 | 0.00 | 4.06 |
| B4 | 0.00 | 0.00 | 0.00 | −3.86 |
| B5 | 0.00 | 0.00 | 0.00 | 14.70 |
| B6 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mean | 3.45 | 0.21 | 5.71 | 3.32 |
Data are expressed as relative density units and were calculated by subtracting values obtained by MAPIA analysis of sera taken before the skin test (week 27) from values obtained post-skin test (week 31). The tuberculin skin test was performed during week 28. P values for differences between control and BCG-vaccinated animals were 0.730 for ESAT-6 (by an unpaired, two-tailed t test), 0.079 for MPB70, 0.013 for MPB83, and 0.495 for MBCF. For the latter three P values, an unpaired, two-tailed t test with the Welch correction was used.
Both IgG1 and IgG2 contribute to the increase in MPB83-specific serum IgG responses after skin testing.
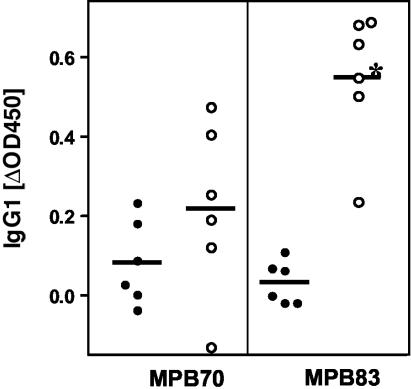
To determine whether the increases in IgG responses to MPB70 and MPB83 were due to increases in particular IgG isotypes, ELISAs were performed with bovine IgG1 and IgG2 isotype-specific antisera. Both MPB83-specific IgG1 (Fig. 1) and IgG2 (data not shown) responses were significantly increased as a result of tuberculin skin testing in control animals compared to BCG-vaccinated calves (P, 0.0009 for IgG1 and 0.045 for IgG2 by the t test). MPB70-specific IgG1 (Fig. 1) and IgG2 responses post-skin test were also more elevated following the tuberculin test in control animals than in BCG-vaccinated cattle, although this difference did not reach statistical significance. IgG1 responses contributed to a larger proportion to the increase in total IgG responses post-skin test (for example, mean increases in MPB83-specific responses [in OD450 units, at a serum dilution of 1:100] were 0.548 for IgG1 and 0.257 for IgG2 in control animals and 0.219 for IgG1 and 0.083 for IgG2 in BCG-vaccinated animals).
FIG. 1.
Boost of MPB70 and MPB83 IgG1 responses due to tuberculin skin testing. Sera were collected before and 14 days after tuberculin skin testing, and IgG1 responses were determined by ELISA. OD450 before skin testing were subtracted from those obtained post-skin test. Sera were diluted 1:100 for the ELISA. Filled circles, BCG-vaccinated, M. bovis-infected animals; open circles, unvaccinated, M. bovis-infected animals; horizontal bars, means. *, P < 0.001 (by an unpaired, two-tailed t test).
Correlation of pathology, humoral immune responses, and cellular immune responses.
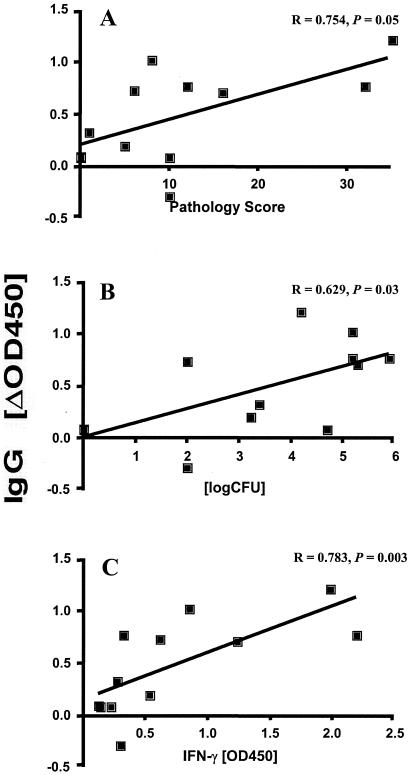
As reported previously, ESAT-6-specific IFN-γ levels were lower in BCG-vaccinated animals and correlated positively with the extent of disease observed at postmortem (30). Here we assessed whether the MPB83-specific increases in IgG responses observed after boosting with the tuberculin skin test also correlated with the observed gross pathology. Considerable correlation was observed with MPB83-specific IgG (Fig. 2A) and IgG1 responses (Spearman's r, 0.754 and 0.747, respectively), although these correlations narrowly failed to be statistically significant (P = 0.05 for both). Neither MPB83-specific IgG2 responses nor antibody responses (IgG, IgG1, or IgG2) against MPB70 correlated with pathology (data not shown). However, bacterial loads in lymph nodes, determined by culture of samples collected at postmortem, positively correlated significantly with increases in MPB83-specific IgG (Fig. 2B) and IgG1 responses post-skin test (for IgG, Spearman's r = 0.629 and P = 0.03; for IgG1, r = 0.6215 and P = 0.031) but not with MPB83-specific IgG2 responses or with MPB70-specific IgG, IgG1, or IgG2 responses (data not shown). Importantly, when we analyzed the correlation between the increases in MPB83-specific IgG (Fig. 2C), IgG1, and IgG2 responses 2 weeks post-skin test and ESAT-6-specific in vitro IFN-γ production measured at the same time point, we found a highly significant relationship between these parameters (for IgG and IFN-γ, Spearman's r = 0.7832 and P = 0.003; for IgG1 and IFN-γ, Spearman's r = 0.859 and P = 0.008; for IgG2 and IFN-γ, Spearman's r = 0.9 and P = 0.02). Thus, a positive relationship exists between disease severity, as measured by gross pathology and bacterial loads, ESAT-6-specific IFN-γ production, and serum anti-MPB83 IgG responses.
FIG. 2.
Positive correlations between skin test-induced increases in MPB83-specific IgG levels and disease severity (A), bacterial loads (B), and in vitro IFN-γ responses to ESAT-6 (C). Sera were collected before and 14 days after tuberculin skin testing, and IgG responses were determined by ELISA. OD450 determined before skin testing were subtracted from those obtained post-skin test. (A) Increases in MPB83-specific IgG responses, determined by ELISA (serum dilution, 1:100), are shown in relation to total pathology scores (30). (B) Serum responses were correlated with total bacterial loads in the lymph nodes of vaccinated and unvaccinated animals (30). (C) Serum responses were correlated with in vitro IFN-γ production induced by ESAT-6. IFN-γ levels were determined by enzyme immunoassays of supernatants from ESAT-6-stimulated (5 mg/ml) whole-blood cultures performed 14 days post-skin test. Statistical analysis for all panels was conducted by using the Spearman rank test.
DISCUSSION
Humoral responses to MBP83 and MPB70 were found to be strongly boosted after tuberculin skin testing in unvaccinated, M. bovis-infected control animals. In contrast, antibody responses in BCG-vaccinated, M. bovis-infected calves, which were protected against BTB, were boosted only marginally, or not at all, by skin testing. These data complement previous studies demonstrating that ESAT-6-specific IFN-γ responses were also lower in vaccinated animals (30), suggesting that IFN-γ responses in vitro can be used to predict the efficacies of new vaccines against BTB in experiments with cattle (27). The present finding, i.e., that humoral responses to MPB83, in particular, are boosted by skin testing to a lower degree in protected animals than in diseased animals, may also have very important practical implications. Our results suggest that vaccine efficacy could now be monitored in the field by assessing this serological parameter, particularly in developing countries, where in vitro cytokine assays may be difficult to perform.
MPB70 and MPB83 are both expressed constitutively in M. bovis yet are expressed only at very low levels in BCG strains that have lost the RD2 regions, such as BCG Pasteur, which was used in the present study (20, 33). MPB70 is a secreted protein (21), whereas MPB83 is a cell wall-associated glycolipoprotein that is also found in culture filtrates (11). The proteins are highly homologous at the amino acid level (11) and are major constituents of PPD-B (K. Lyashchenko, A. O. Whelan, and H. M. Vordermeier, unpublished data), which would explain why the immune responses to these antigens were boosted by tuberculin skin testing. Of note, it has been reported that skin testing also boosts cellular responses in infected cows, in particular tuberculin- or specific antigen-induced in vitro IFN-γ responses (25, 26). While in previous experiments we have occasionally observed this boosting in individual animals, in the present study neither PPD-B nor ESAT-6 could elicit significant anamnestic IFN-γ responses (H. M. Vordermeier, unpublished data).
The tuberculin-induced increase in MPB83-specific IgG levels correlated with overall disease severity and bacterial loads. Because both parameters also correlated with in vitro IFN-γ responses to ESAT-6 (30), we determined whether ESAT-6-induced IFN-γ levels also correlated with the boost in anti-MPB83 IgG responses following tuberculin testing, and we found a close and significant association between these parameters of cellular and humoral immunity. However, the kinetics of IgG and IFN-γ production were different, with cellular responses preceding antibody responses by several weeks on average, and in some cases by months (30; Vordermeier, unpublished). These observations are therefore consistent with the notion of BTB as a spectral disease with predominantly cellular responses at the early stages of disease, complemented during disease progression by humoral responses, which may replace cellular immune responses in animals with far advanced disease (23, 24). In particular, the BCG-vaccinated animals that presented with reduced pathology and bacterial loads, i.e., that were partially protected, did not develop serum IgG responses either before or after skin testing (animals BCG-3 and BCG-4, for example). This is not surprising, since vaccinated animals had lower pathology scores. However, this observation also highlights a major drawback of using serology to diagnose BTB in vaccinated cattle. These infected animals would not be identified by measuring antibody responses, yet both BCG-3 and BCG-4 tested positive with the IFN-γ whole-blood assay using the specific antigens ESAT-6 and CFP-10 (3, 32).
Surprisingly, both IgG1 and IgG2 responses correlated with the amount of IFN-γ produced in vitro. In cattle, the results of in vitro experiments have suggested an association of IFN-γ with IgG2 production, whereas IgG1 is related to interleukin-4 production (reviewed by Estes and Brown [5]). Therefore, our observation of a correlation between IFN-γ and IgG1 responses seems at first glance paradoxical. However, recent data have shown that the regulation of immunoglobulin class switching in cattle in vivo is more complex, and other, as yet unidentified factors, could play a role (5). For example, cattle vaccinated with a surface protein of Anaplasma marginale developed strong type 1 IFN-γ CD4+ T-cell responses, yet most calves had increased IgG1 responses, and only one-fourth also had increased IgG2 levels (29).
The first demonstration that tuberculin skin testing could boost antibody responses in cattle was reported by Harboe and coworkers (10), who showed that skin testing of cattle with PPD-B was a potent stimulator of anti-MPB70 antibody formation in experimental and natural M. bovis infections. Subsequently, Lightbody and coworkers (15, 16) demonstrated that anti-MPB70 IgG1 responses were particularly boosted by tuberculin skin testing in cattle presenting with visible lesions at postmortem, i.e., in animals with severe disease. This observation was confirmed in the present study, in which tuberculin-induced boosting of IgG and IgG1 responses against MPB70 was particularly profound in unvaccinated control animals with more-advanced disease. Furthermore, the use of MAPIA has enabled us to identify several other proteins of M. bovis (MPB83, ESAT-6, CFP-10, and MPB64) that appear to be involved in skin test-induced antibody boosting.
In conclusion, the present study describes an inverse relationship between the humoral immune responses, especially following tuberculin skin testing, and BCG vaccine efficacy, as well as a positive relationship between MPB83-specific IgG levels, bacterial load, disease severity, and cellular immunity. Thus, the degree of tuberculin skin test-boosted antibody responses against MPB83 may be a useful immunological marker predicting both vaccine efficacy and disease severity. This parameter could therefore serve as a simple and valuable tool to assist in the future development of novel vaccines for BTB, particularly in field trials.
Acknowledgments
This work was funded by the Department for Environment, Food and Rural Affairs of Great Britain. We express our appreciation to the staff of the Animal Services Unit at the VLA for their dedication to animal welfare.
Editor: A. D. O'Brien
REFERENCES
- 1.Buddle, B. M., G. W. de Lisle, A. Pfeffer, and F. E. Aldwell. 1995. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 13:1123-1130. [DOI] [PubMed] [Google Scholar]
- 2.Buddle, B. M., D. Keen, A. Thomson, G. Jowett, A. R. McCarthy, J. Heslop, G. W. De Lisle, J. L. Stanford, and F. E. Aldwell. 1995. Protection of cattle from bovine tuberculosis by vaccination with BCG by the respiratory or subcutaneous route, but not by vaccination with killed Mycobacterium vaccae. Res. Vet. Sci. 59:10-16. [DOI] [PubMed] [Google Scholar]
- 3.Buddle, B. M., N. A. Parlane, D. L. Keen, F. E. Aldwell, J. M. Pollock, K. Lightbody, and P. Andersen. 1999. Differentiation between Mycobacterium bovis BCG-vaccinated and M. bovis-infected cattle by using recombinant mycobacterial antigens. Clin. Diagn. Lab. Immunol. 6:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 5.Estes, D. M., and W. C. Brown. 2002. Type 1 and type 2 responses in regulation of Ig isotype expression in cattle. Vet. Immunol. Immunopathol. 90:1-10. [DOI] [PubMed] [Google Scholar]
- 6.European Economic Community. 1980. EEC directive 80/219, amending directive 64/432 annex B. Official J. L047:25-32. [Google Scholar]
- 7.Fifis, T., L. A. Corner, J. S. Rothel, and P. R. Wood. 1994. Cellular and humoral immune responses of cattle to purified Mycobacterium bovis antigens. Scand. J. Immunol. 39:267-274. [DOI] [PubMed] [Google Scholar]
- 8.Fifis, T., C. Costopoulos, L. A. Corner, and P. R. Wood. 1992. Serological reactivity to Mycobacterium bovis protein antigens in cattle. Vet. Microbiol. 30:343-354. [DOI] [PubMed] [Google Scholar]
- 9.Francis, J. 1947. Bovine tuberculosis. Staples Press, London, United Kingdom.
- 10.Harboe, M., H. G. Wiker, J. R. Duncan, M. M. Garcia, T. W. Dukes, B. W. Brooks, C. Turcotte, and S. Nagai. 1990. Protein G-based enzyme-linked immunosorbent assay for anti-MPB70 antibodies in bovine tuberculosis. J. Clin. Microbiol. 28:913-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewinson, R. G., S. L. Michell, W. P. Russell, R. A. McAdam, and W. R. Jacobs, Jr. 1996. Molecular characterization of MPT83: a seroreactive antigen of Mycobacterium tuberculosis with homology to MPT70. Scand. J. Immunol. 43:490-499. [DOI] [PubMed] [Google Scholar]
- 12.Hewinson, R. G., H. M. Vordermeier, and B. M. Buddle. 2003. Use of the bovine model of tuberculosis for the development of improved vaccines and diagnostics. Tuberculosis 83:119-130. [DOI] [PubMed] [Google Scholar]
- 13.Kidane, D., J. O. Olobo, A. Habte, Y. Negesse, A. Aseffa, G. Abate, M. A. Yassin, K. Bereda, and M. Harboe. 2002. Identification of the causative organism of tuberculous lymphadenitis in Ethiopia by PCR. J. Clin. Microbiol. 40:4230-4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs, J. R. 1997. Bovine tuberculosis in cattle and badgers. Ministry of Agriculture, Fisheries and Food Publications, London, United Kingdom.
- 15.Lightbody, K. A., J. McNair, S. D. Neill, and J. M. Pollock. 2000. IgG isotype antibody responses to epitopes of the Mycobacterium bovis protein MPB70 in immunised and in tuberculin skin test-reactor cattle. Vet. Microbiol. 75:177-188. [DOI] [PubMed] [Google Scholar]
- 16.Lightbody, K. A., R. A. Skuce, S. D. Neill, and J. M. Pollock. 1998. Mycobacterial antigen-specific antibody responses in bovine tuberculosis: an ELISA with potential to confirm disease status. Vet. Rec. 142:295-300. [DOI] [PubMed] [Google Scholar]
- 17.Lyashchenko, K. P., J. M. Pollock, R. Colangeli, and M. L. Gennaro. 1998. Diversity of antigen recognition by serum antibodies in experimental bovine tuberculosis. Infect. Immun. 66:5344-5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyashchenko, K. P., M. Singh, R. Colangeli, and M. L. Gennaro. 2000. A multi-antigen print immunoassay for the development of serological diagnosis of infectious diseases. J. Immunol. Methods 242:91-100. [DOI] [PubMed] [Google Scholar]
- 19.McNair, J., D. M. Corbett, R. M. Girvin, D. P. Mackie, and J. M. Pollock. 2001. Characterization of the early antibody response in bovine tuberculosis: MPB83 is an early target with diagnostic potential. Scand. J. Immunol. 53:365-371. [DOI] [PubMed] [Google Scholar]
- 20.Miura, K., S. Nagai, M. Kinomoto, S. Haga, and T. Tokunaga. 1983. Comparative studies with various substrains of Mycobacterium bovis BCG on the production of an antigenic protein, MPB70. Infect. Immun. 39:540-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagai, S., J. Matsumoto, and T. Nagasuga. 1981. Specific skin-reactive protein from culture filtrate of Mycobacterium bovis BCG. Infect. Immun. 31:1152-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Loan, C. J., J. M. Pollock, J. Hanna, and S. D. Neill. 1994. Immunoblot analysis of humoral immune responses to Mycobacterium bovis in experimentally infected cattle: early recognition of a 26-kilodalton antigen. Clin. Diagn. Lab. Immunol. 1:608-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollock, J. M., J. McNair, M. D. Welsh, R. M. Girvin, H. E. Kennedy, D. P. Mackie, and S. D. Neill. 2001. Immune responses in bovine tuberculosis. Tuberculosis (Edinburgh) 81:103-107. [DOI] [PubMed] [Google Scholar]
- 24.Ritacco, V., B. Lopez, I. N. De Kantor, L. Barrera, F. Errico, and A. Nader. 1991. Reciprocal cellular and humoral immune responses in bovine tuberculosis. Res. Vet. Sci. 50:365-367. [DOI] [PubMed] [Google Scholar]
- 25.Rothel, J. S., S. L. Jones, L. A. Corner, J. C. Cox, and P. R. Wood. 1992. The gamma-interferon assay for diagnosis of bovine tuberculosis in cattle: conditions affecting the production of gamma-interferon in whole blood culture. Aust. Vet. J. 69:1-4. [DOI] [PubMed] [Google Scholar]
- 26.Ryan, T. J., B. M. Buddle, and G. W. De Lisle. 2000. An evaluation of the gamma interferon test for detecting bovine tuberculosis in cattle 8 to 28 days after tuberculin skin testing. Res. Vet. Sci. 69:57-61. [DOI] [PubMed] [Google Scholar]
- 27.Skinner, M., B. M. Buddle, N. Wedlock, D. Keen, G. W. de Lisle, R. E. Tascon, J. C. Ferraz, D. B. Lowrie, P. J. Cockle, H. M. Vordermeier, and R. G. Hewinson. 2003. A DNA prime-Mycobacterium bovis BCG boost vaccination strategy for cattle induces protection against bovine tuberculosis. Infect. Immun. 71:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steele, J. H. 1995. Regional and country status report, p. 169-172. In C. O. Thoen and J. H. Steele (ed.), Mycobacterium bovis infection in animals and human. Iowa State University Press, Ames.
- 29.Tuo, W., G. H. Palmer, T. C. McGuire, D. Zhu, and W. C. Brown. 2000. Interleukin-12 as an adjuvant promotes immunoglobulin G and type 1 cytokine recall responses to major surface protein 2 of the ehrlichial pathogen Anaplasma marginale. Infect. Immun. 68:270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vordermeier, H. M., M. A. Chambers, P. J. Cockle, A. O. Whelan, J. Simmons, and R. G. Hewinson. 2002. Correlation of ESAT-6-specific gamma interferon production with pathology in cattle following Mycobacterium bovis BCG vaccination against experimental bovine tuberculosis. Infect. Immun. 70:3026-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vordermeier, H. M., P. J. Cockle, A. O. Whelan, S. Rhodes, M. A. Chambers, D. Clifford, K. Huygen, R. Tascon, D. Lowrie, M. J. Colston, and R. G. Hewinson. 2000. Effective DNA vaccination of cattle with the mycobacterial antigens MPB83 and MPB70 does not compromise the specificity of the comparative intradermal tuberculin skin test. Vaccine 19:1246-1255. [DOI] [PubMed] [Google Scholar]
- 32.Vordermeier, H. M., A. Whelan, P. J. Cockle, L. Farrant, N. Palmer, and R. G. Hewinson. 2001. Use of synthetic peptides derived from the antigens ESAT-6 and CFP-10 for differential diagnosis of bovine tuberculosis in cattle. Clin. Diagn. Lab. Immunol. 8:571-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiker, H. G., S. Nagai, R. G. Hewinson, W. P. Russell, and M. Harboe. 1996. Heterogenous expression of the related MPB70 and MPB83 proteins distinguish various substrains of Mycobacterium bovis BCG and Mycobacterium tuberculosis H37Rv. Scand. J. Immunol. 43:374-380. [DOI] [PubMed] [Google Scholar]