Abstract
BCG vaccines are a family of closely related daughter strains of an attenuated isolate of Mycobacterium bovis derived by in vitro passage from 1908 to 1921. During subsequent laboratory propagation of the vaccine strain until its lyophilization in 1961, BCG Pasteur underwent at least seven further genomic mutations. The impact of these mutations on the properties of the vaccine is currently unknown. One mutation, a glycine-to-aspartic acid substitution in the mmaA3 gene, occurred between 1927 and 1931 and impairs methoxymycolic acid synthesis in BCG strains obtained from the Pasteur Institute after this period. Mycolic acids of the cell wall are classified into three functional groups (alpha-, methoxy-, and ketomycolic acids), and together these lipids form a highly specialized permeability barrier around the bacterium. To explore the impact of methoxymycolic acid production by BCG strains, we complemented the functional gene of mmaA3 into BCG Denmark and tested a number of in vitro and in vivo phenotypes. Surprisingly, restoration of methoxymycolic acids alone had no effect on cell wall permeability, resistance to antibiotics, or growth in cultured macrophages and C57BL/6 mice. Our results demonstrate that the loss of methoxymycolic acid production did not apparently affect the virulence of BCG strains.
Bacille Calmette-Guérin (BCG) vaccines are live attenuated strains of Mycobacterium bovis derived by in vitro passage from 1908 to 1921. BCG vaccines are given to millions of infants each year as antituberculosis vaccines, although their capacity to prevent tuberculosis in clinical trials has ranged from 80% protection to no detectable benefit (11). Several hypotheses have been proposed to explain this variable protection, including exposure to environmental mycobacteria (26) and differences between BCG vaccine strains (8).
From genomic analyses, it is now known that during in vitro passage, M. bovis lost a genomic region called RD1 (19), which has been shown to contribute to the observed attenuation of virulence of M. bovis BCG strains (16, 27). However, complementation of RD1 in the Pasteur strain of BCG did not completely restore pathogenicity in immunocompetent mice (27), suggesting that further mutations contribute to the observed phenotype of BCG strains. Because BCG stocks were propagated for another 40 to 50 years in various vaccine production laboratories, it has been hypothesized that ongoing evolution of BCG in vitro may have resulted in additional attenuation to the detriment of protective efficacy (5). Early reports on BCG suggest a second phase of attenuation in the late 1920s (24), with a decrease in BCG virulence in animal models (15) and reduced persistence of BCG in the mesenteric lymph nodes of vaccinated children (35). These observations are consistent with recent genomic analysis of existing BCG strains that revealed numerous mutations occurring after 1921 (6, 23).
Further evidence of evolution of BCG strains followed from studies demonstrating the loss of methoxymycolic acid production in BCG Pasteur and other strains (1, 20, 22). Prompted by these observations, it was determined that the production of methoxymycolates by BCG strains corresponded to their pattern of distribution from the Pasteur Institute, as strains obtained prior to 1927 (Birkhaug, Brazil, Japan, Russia, and Sweden) produce methoxymycolates, whereas strains distributed after this time (Connaught, Denmark, Frappier, Glaxo, Pasteur, Phipps, Prague, and Tice) do not (4).
A single-nucleotide nonsynonymous point mutation in mmaA3 causes a glycine-to-aspartic acid substitution at position 293 that impairs methoxymycolic acid production. Mycolic acids are long-chain α-alkyl, β-hydroxy fatty acids that are characteristic of the mycobacterial cell wall and are classified according to their functional group; the Mycobacterium tuberculosis complex consists of alpha-, keto-, and methoxymycolic acids (3, 21). The role of subclasses of mycolates has been explored in previous work, in which disruption of the hma gene in M. tuberculosis impaired both methoxy- and ketomycolic acid production and decreased cell wall permeability to the small molecules chenodeoxycholic acid and glycerol (10). The hma mutant also manifested reduced growth in a mouse model. Another study showed that heterologous promoter-driven overexpression of mmaA3 in BCG strains increased the production of methoxymycolates but, interestingly, impaired production of ketomycolates (34). Paradoxically, resistance to hydrophobic antibiotics increased in this mutant, whereas the uptake of the permeability marker chenodeoxycholate was unaffected. Overexpression of mmaA3 also appeared to reduce virulence, in this case assessed by growth inhibition in the human monocytic THP-1 cell line.
To further explore the importance of methoxymycolic acid production by BCG strains, we expressed the functional mmaA3 gene from its native promoter in a late strain of BCG and tested the impact on a number of in vitro and in vivo phenotypes.
MATERIALS AND METHODS
Bacterial cultures.
Unless otherwise stated, all BCG strains were grown at 37°C in Middlebrook 7H9 medium (Difco Laboratories, Detroit, Mich.) containing 0.05% Tween 80 (Sigma-Aldrich, St. Louis, Mo.) and 10% albumin-dextrose-catalase (Becton Dickinson and Co., Sparks, Md.) supplement on a rotating platform (Wheaton). Transformed BCG strains were grown to an optical density at 600 nm (OD600) of 0.4, pelleted by centrifugation, and resuspended in 7H9 containing 15% glycerol; 1-ml aliquots were frozen at −80°C until needed. Frozen bacteria were thawed and diluted in fresh 7H9 medium containing 10% albumin-dextrose-catalase and grown with rotation at 37°C.
Complementation of mmaA3 in BCG.
Primers were designed to PCR amplify the wild-type mmaA3 gene from BCG Russia (Tuberculist Rv0643c), including 250 bp of upstream sequence to obtain promoter activity. The PCR primer sequences were 5′-ATAAAAGCTTTCCGAAGAGGTCTACGAGCGG-3′ and 5′-ATAAGCTAGCCTTGGCCAGCGTGAACTGGTT-3′. Restriction sites for HindIII and NheI were added to facilitate cloning into the vector pGH1 (provided by D. R. Sherman). pGH1 is an integrating vector that inserts into the attB site in the mycobacterial genome. The sequence of the insert was confirmed by DNA sequencing. The final construct was designated mmaA3::pGH1. For transformations, rolling cultures of BCG Denmark 1331 (ATCC 35733) and BCG Pasteur (ATCC 35734) were grown to an OD600 of 1 and pelleted by centrifugation at 2,000 × g for 10 min at room temperature. Bacteria were washed three times in 20 ml of 10% glycerol and resuspended at 1/100 of the initial volume in 10% glycerol (200 μl). BCG strains were electroporated (Bio-Rad Laboratories, Hercules, Calif.) with settings of 2,500 mV, 1,000 Ω, and 25 μF in 2-mm gap cuvettes (VWR, West Chester, Pa.) with 1 μg (5 μl) of either pGH1 vector or mmaA3::pGH1. Warm 7H9 (1 ml) was quickly added to the cells after electroporation. The cells were incubated for 2 h, plated on 7H10 containing 50 μg of hygromycin B (Wisent Inc., St.-Bruno, Canada) per ml, and incubated for approximately 3 weeks at 37°C.
Mycolic acid analysis.
For mycolic acid analysis, BCG strains were grown at 37°C in Middlebrook 7H9 supplemented with 0.2% glycerol and 10% oleic acid-albumin-dextrose-catalase supplement to an OD600 of >1. For each strain, 50 ml of culture was grown and 5 ml was used for mycolic acid analysis.
Mycolic acid methyl ester derivatives were prepared and analyzed by thin-layer chromatography by a standard protocol (7). Briefly, mycolic acid methyl ester derivatives were dissolved in 20 μl of benzene, and 5 μl was spotted onto Whatman silica gel 60 plates (Whatman Inc., Clifton, N.J.). Thin-layer chromatography plates were resolved in petroleum ether-diethyl ether (95:5 [vol/vol]) four times and charred by spraying with 5% phosphomolybdic acid, followed by heating at 110°C for 5 min.
Permeability assays.
Cell wall permeability was determined by following a modified protocol described in reference 18. Bacteria were grown in 7H9 containing 0.2% glycerol and 10% oleic acid-albumin-dextrose-catalase (without Tween 80) to an OD600 of 0.6 to 0.8 and harvested by centrifugation. After being washed three times in basal salts solution (pH 7), the cells were separated into 1-ml aliquots in basal salts solution. One aliquot was lyophilized to determine cell mass (approximately 30 mg, dry mass). The remaining 1-ml aliquots of bacteria were incubated with 10 μM [14C]chenodeoxycholate (0.5 μCi/μl; ICN Biomedicals, Irvine, Calif.) for the indicated times. The cell suspensions were vortexed, and 75 μl of suspension was removed and applied to 0.22-μm cellulose acetate filters (Millipore Corporation, Bedford, Mass.) by vacuum filtration (Hoefer Scientific Instruments, San Francisco, Calif.). The filters were washed with 10 ml of basal salts solution, and the radioactivity on the filter was determined by liquid scintillation counting.
Sensitivity to antibacterial agents.
BCG strains were grown to an OD600 of 0.3 to 0.5 and then diluted to an OD600 of 0.01 in fresh growth medium. Bacteria were grown for 7 days at 37°C in 96-well plates in the presence of vehicle, twofold serial dilutions of antibiotics (rifampin, isoniazid, or ampicillin; Sigma-Aldrich Co.), or bovine bile solution (ox gall powder; Sigma-Aldrich Co.). Growth was measured as the increase in OD630 in an EL-800 Universal microplate reader (Bio-Tek Instruments Inc., Winooski, Vt.).
Microarray expression analysis of BCG strains.
BCG strains grown to an OD600 of 0.3 to 0.5 were pelleted by centrifugation, resuspended in 1 ml of wash buffer (0.5% Tween 80, 0.8% sodium chloride), and transferred to 1.5-ml screw-cap cryovials. Bacteria were pelleted by centrifugation, snap frozen in liquid nitrogen and stored at −80°C until processed. RNA was extracted by a modified phenol-chloroform extraction protocol. Bacterial pellets were resuspended in 400 μl of lysis buffer (20 mM sodium acetate [pH 4.0], 0.5% sodium dodecyl sulfate [SDS], 1 mM EDTA), 800 μl of acidified phenol:chloroform (pH 4.5; Ambion Inc., Austin, Tex.), and 0.8 g of glass beads (Sigma-Aldrich Co.). Cells were lysed by agitation in a FastPrep FP120 (Savant Bio 101) set to a speed of 6.5 for 15 s. The aqueous phase was removed to a fresh tube, and 400 μl of lysis buffer was added to the mixture and pulsed for another 45 s. The pooled aqueous phase (approximately 800 μl) was mixed with chloroform-isoamyl alcohol (24:1) by vigorous vortexing for 1 min at high speed. RNA was precipitated by adding an equal volume of isopropanol to the aqueous phase and placed overnight at −80°C. RNA was pelleted by centrifugation, washed with 75% ethanol, dried for 10 min at room temperature, and resuspended in diethylpyrocarbonate-treated water (Ambion). Genomic DNA contamination was removed by RNAeasy on-column digestion, following the manufacturer's protocol (Qiagen Inc., Mississauga, Canada). The quality of RNA was confirmed by denaturing gel electrophoresis (formaldehyde). RNA (10 μg) was labeled with monoreactive indocarbocyanine (Cy3) or indodicarbocyanine (Cy5) dyes (Amersham Biosciences, Baie D'Urfe, Canada) by coupling to incorporated amino-allyl nucleotides as described in the protocol from the Institute for Genomic Research (http://pfgrc.tigr.org/protocols/M007.pdf).
The Chipwriter (model SDDC2; Virtek, Waterloo, Canada) was used to print oligonucleotides on Sigmascreen microarray slides (Sigma-Aldrich Co.). Lyophilized 70-mers from the TB Array-Ready Oligo Set (Qiagen) were resuspended and printed in duplicate as 16 grids, each 24 by 24. For both clones, duplicate hybridizations were performed for each dye combination (Cy3/Cy5 and Cy5/Cy3), four hybridizations in all. The probe was applied to a postprocessed array, covered with a glass coverslip, and placed in a hybridization chamber overnight at 42°C. The arrays were dipped into 42°C 1× SSC (0.15 M NaCl plus 0.015 M sodium citrate)-0.2% SDS to remove the cover glass and then washed in 1× SSC-0.2% SDS for 5 min, 0.1× SSC-0.2% SDS for 5 min (repeated two times), and 0.1 × SSC for 5 min. The arrays were scanned with Scanarray 5000XL (PerkinElmer, Freemont, Calif.). Hybridization results were quantified with Scanalyze software (http://rana.Stanford.EDU/software/).
Misrepresentative spots (array artifacts, etc.) were flagged and omitted from the analysis. Total spot intensity minus the surrounding background produced a corrected spot intensity. Negative corrected spot intensities were set to +1. Spots producing a corrected intensity below the 95th percentile of negative controls were excluded from analysis. Intensity ratios (Cy3/Cy5 or Cy5/Cy3) were calculated by log10 transforming the corrected spot intensities. Values for each gene were obtained in duplicate for each array and averaged. A representative Z score, indicative of how many standard deviations a data point lies from the population mean, was calculated for each gene. Z scores for each gene were averaged across four replicates within each experiment to minimize the probability of observing such variation by chance alone. Genes with average Z scores of 2 or greater for both clones are presented, and the change was calculated from a normalized log ratio of that gene.
Growth of BCG in THP-1 cells.
The human leukemic monocytic cell line THP-1 (ATCC TIB-202) was maintained in RPMI medium containing 10% fetal bovine serum in 75-mm2 tissue culture flasks (Becton Dickinson Labware, Franklin Lakes, N.J.). Cells were grown at 37°C in 5% CO2, and fresh medium was added every 3 to 4 days. To cause THP-1 monocytes to differentiate into macrophages, cells were plated at a density of 106/well in six-well plates in complete medium containing 10 nM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) and incubated for 2 days. The supernatants were removed, and fresh medium (2 ml) was added to adherent monolayers and incubated for another 24 h. The following day, the cells were infected with BCG strains (OD600 of 0.3 to 0.5). The bacteria were resuspended in complete RPMI to obtain a multiplicity of infection of 1 (106 bacteria/ml/well) and then added to the THP-1 monolayer and incubated for 4 h at 37°C. Extracellular bacteria were removed by washing each well three times in warm phosphate-buffered saline (PBS). Complete RPMI (3 ml) was added to each well and incubated for the indicated times. To quantify the intracellular growth of bacteria, the medium was removed and the monolayers were washed three times with PBS. THP-1 cells were lysed by adding 7H9 containing 1% Triton X-100 (Sigma-Aldrich). Serial dilution plating was performed on 7H10 agar.
Growth of BCG strains in C57BL/6 mice.
Mice were housed in the conventional rodent facility of the Montreal General Hospital. Procedures were approved by the Facility Animal Care Committee as recommended by the Canadian Council on Animal Care. Bacteria (OD600 of 0.3 to 0.5) were diluted in PBS to 105 CFU/ml. Inoculum doses of 104 CFU were confirmed by serial dilution plating on 7H10 agar containing 50 μg of hygromycin per ml (Denmark::pGH1, 7.1 × 104 CFU; Denmark::mmaA3, 1.1 × 104 CFU). Mice (Jax Mice, Bar Harbor, Maine) were placed in a restraining cone and infected by injecting 100 μl in the caudal tail vein with a 0.5-ml insulin syringe. On the indicated days, mice were sacrificed by CO2 inhalation. Weight gain was monitored throughout the experiment. The thoracic cavity was opened, and the lungs and spleen were removed and placed in 0.025% saponin-PBS on ice. Tissue homogenates were prepared with a Polytron PT 2100 bench-top homogenizer (Kinematica AG, Lucerne, Switzerland). The Polytron was washed sequentially in distilled H2O-0.05% SDS-70% ethanol-PBS before the next organ was processed; 10-fold serial dilutions of the organ homogenates were plated on 7H10 agar containing hygromycin B. For histopathologic evaluations, the renal vein was cut, and 20 ml of PBS followed by 20 ml of 10% formalin was perfused via the left atrium with a 30-ml syringe. Organs were embedded in paraffin, mounted, and stained with hematoxylin and eosin.
Statistical analysis.
Data are presented as means ± standard error of the mean and were analyzed by the Student t test where indicated. A P value of <0.05 was considered significant.
RESULTS
Functional complementation of mmaA3.
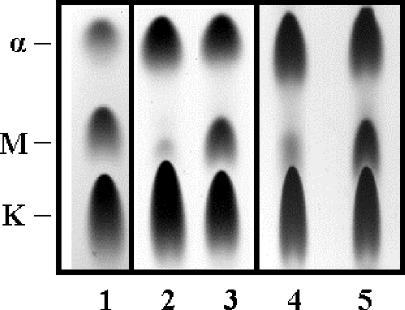
To assess functional expression of mmaA3, mycolate profiles were determined for the mmaA3-complemented strains, vector control strains, and BCG Japan, a strain that has the wild-type mmaA3 gene. As shown in Fig. 1, the parental strains of BCG transformed with the vector alone (lanes 2 and 4) exhibited impaired methoxymycolate production compared to BCG Japan (lane 1). Transformation with pGH1::mmaA3 increased methoxymycolate production in Denmark::mmaA3 (lane 3) and Pasteur::mmaA3 (lane 5), comparable to the levels in BCG Japan. However, the mycolate profiles of the mmaA3-complemented strains and BCG Japan were not identical. We consistently found that the relative proportion of alpha-mycolates was greater in the Denmark and Pasteur strains.
FIG. 1.
Restoration of methoxymycolic acid production by BCG strains. Mycolic acid classes produced by the various strains were resolved by thin-layer chromatography. Strains BCG Japan (lane 1), Denmark::pGH1 (lane 2), Denmark::mmaA3 (lane 3), Pasteur::pGH1 (lane 4), and Pasteur::mmaA3 (lane 5) were tested. The identities of the alpha- (α), methoxy- (M), and ketomycolic acids (K) are indicated on the left.
In the following experiments, we studied the isogenic pair Denmark::pGH1 and Denmark::mmaA3. Although transformation with pGH1::mmA3 restored methoxymycolate production to both the Denmark and Pasteur strains, BCG Demark has fewer known genomic mutations than BCG Pasteur and may better represent the genome of BCG 1921. Initial characterization of growth in culture at 37°C revealed that Denmark::pGH1 had a growth rate similar to that of Denmark::mmaA3 in liquid culture and on solid media (data not shown).
Microarray expression profiling.
Microarray expression profiling was used to contrast the gene expression patterns in Denmark::pGH1 and Denmark::mmaA3 to determine if the presence of methoxymycolates in the cell wall resulted in compensatory gene expression. Overall, the in vitro global expression patterns of Denmark::mmaA3 and Denmark::pGH1 were nearly identical. No genes were consistently upregulated in Denmark::mmaA3 compared to Denmark::pGH1 with a Z score cutoff of ≥2. Only Rv1815 was consistently downregulated in Denmark::mmaA3 compared to Denmark::pGH1 (Z score, 2.08). Rv1815 encodes a conserved hypothetical protein of unknown function. Rv1816, which flanks Rv1815, had a Z score just below the cutoff (Z score, 1.86) and is a putative transcriptional regulator, whereas Rv1814 (erg3), putatively involved in lipid desaturation, showed no evidence of altered regulation. These results demonstrate that restoration of methoxymycolic acids in the cell wall causes minimal compensatory modulation of gene expression during in vitro growth.
Cell wall permeability.
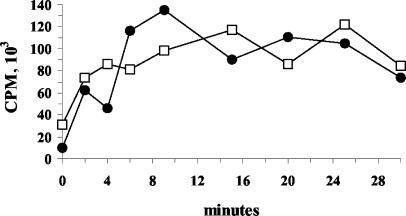
The mycolate composition of the cell wall has been demonstrated to influence the fluidity of this barrier, which in turn regulates the rate of passive diffusion of hydrophobic molecules (17). To determine whether the presence of methoxymycolates in the cell wall affects the uptake of a hydrophobic molecule, we measured the uptake of the permeability marker [14C]chenodeoxycholate. Both strains showed time-dependent increases in uptake of the marker (Fig. 2). However, no overt differences in uptake between the two strains were observed in two independent experiments. Thus, restoration of methoxymycolate production alone did not influence the passive diffusion of chenodeoxycholate through the membrane and may have had only a subtle effect on membrane permeability in Denmark::mmaA3.
FIG. 2.
Time-dependent uptake of [14C]chenodeoxycholic acid by Denmark::pGH1 and Denmark::mmaA3. Permeability was measured as uptake of the marker [14C]chenodeoxycholic acid over time. At each time point, aliquots of Denmark::pGH1 (•) and Denmark::mmaA3 (□) cell suspensions were applied to cellulose filters by vacuum filtration and washed, and the incorporated radioactivity was quantified by liquid scintillation counting. Data shown are from an experiment repeated twice with similar results.
Resistance to antibacterial agents.
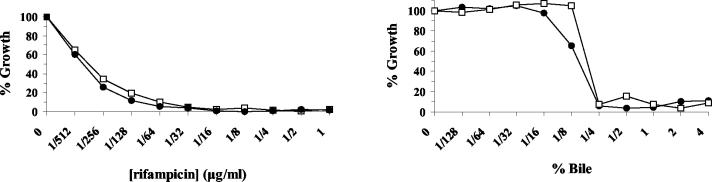
In a previous study, overexpression of mmaA3 in BCG Pasteur increased the sensitivity of the mutant to rifampin and ampicillin (34). To address whether altered antibiotic sensitivity occurs in Denmark::mmaA3, we compared the growth of Denmark::mmaA3 to that of Denmark::pGH1 in the presence of rifampin. As shown in Fig. 3A, increasing concentrations of rifampin completely inhibited the growth of the BCG strains over a 7-day period. Growth of both strains demonstrated that production of methoxymycolates did not alter the sensitivity to rifampin. Similar results were obtained for ampicillin and isoniazid (data not shown). Originally, BCG was cultivated on medium containing bile. Because this may have promoted the selection of the mmaA3 mutant, we also determined whether loss of methoxymycolate production could have facilitated growth in bile-containing medium. As shown in Fig. 3B, Denmark::pGH1 did not have any growth advantage over Denmark::mmaA3 in the presence of increasing concentrations of bile. These experiments demonstrate that methoxymycolates do not influence antibiotic or bile sensitivity.
FIG. 3.
Sensitivity of isogenic BCG strains to antibacterial agents. Denmark::pGH1 (•) and Denmark::mmaA3 (□) were grown in the presence of increasing concentrations of rifampin (A) or bile (B) for 7 days at 37°C. Growth was measured as the increase in OD630 and plotted as a percentage of the growth of controls grown in the presence of vehicle. Data shown are from an experiment repeated four times with similar results.
Growth of BCG strains in THP-1 macrophages.
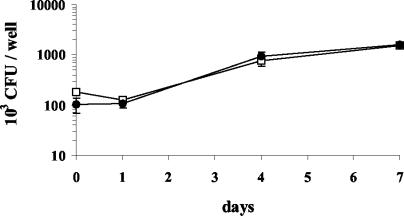
The target cell for intracellular growth of BCG is the macrophage. Many studies have used PMA-differentiated THP-1 cells as a surrogate to study intracellular growth of mycobacteria in vitro. In this study, we determined whether production of methoxymycolates imparted a growth advantage to Denmark::mmaA3 over Denmark::pGH1 in PMA-differentiated THP-1 cells. Initially, we determined that a multiplicity of infection of 1 significantly reduced THP-1 monolayer destruction over the 7-day period compared to a multiplicity of infection of 5 (data not shown). In all experiments, methoxymycolic acid production did not affect the uptake of Denmark::mmaA3, as similar numbers of CFU for both strains were consistently enumerated following the 4-h incubation to allow phagocytosis (data not shown). An approximately 1 log increase above the initial CFU was observed for both strains by day 7 (Fig. 4). Thus, the intracellular growth profile was similar for both strains over this time period. We conclude that the presence of methoxymycolic acids in the cell wall does not provide any measurable advantage for uptake or growth in the macrophage-like THP-1 cell line.
FIG. 4.
Growth of isogenic BCG strains in THP-1-derived macrophages. THP-1 cells were forced to differentiate into macrophage-like cells with 10 nM PMA for 2 days at 37°C and then rested overnight. Monolayers were infected at a multiplicity of infection of 1 for 4 h, washed, and lysed at the indicated time points. Intracellular growth of Denmark::pGH1 (•) and Denmark::mmaA3 (□) was determined by serial dilution plating. The data shown represent the mean CFU ± standard error of the mean from an experiment repeated four times with similar results (P > 0.05).
Growth of BCG strains in C57BL/6 mice.
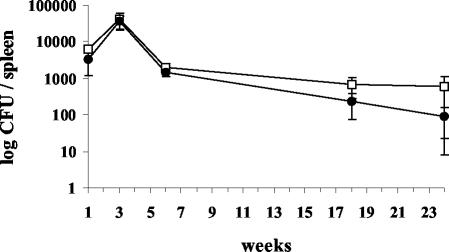
To determine whether methoxymycolic acid production could affect the growth of Denmark::mmaA3 in the murine model of infection, C57BL/6 mice were infected by lateral tail vein injection, and growth in lung and spleen was followed over time. As expected, mice infected with 104 CFU of Denmark::pGH1 or Denmark::mmaA3 gained weight and showed no obvious manifestations of disease during infection. Weight gain was not significantly different between the strains (data not shown). At this dose, no significant bacterial growth or pathology occurred in the lung (data not shown). In contrast, equivalent amounts of Denmark::pGH1 and Denmark::mmaA3 were recovered from the spleen (Fig. 5). Both strains had increased in number by approximately 0.5 to 1 log by week 3. With the onset of acquired immunity at this time, the number of CFU in the spleen decreased approximately 20-fold by week 6, and organisms were progressively eliminated from the spleens of infected mice over the following 18 weeks. From weeks 18 to 24, the average number of CFU of Denmark::mmaA3 was approximately twofold to fourfold higher than that of Denmark::pGH1, although these differences never achieved statistical significance (P > 0.05).
FIG. 5.
Growth of isogenic BCG strains in C57BL/6 mice. Mice were infected by the lateral tail vein with approximately 104 CFU of Denmark::pGH1 (•) or Denmark::mmaA3 (□). Mice were sacrificed at the indicated times, and the number of CFU in homogenized spleens was determined by serial dilution plating. Each point represents the average CFU ± standard error of the mean for four mice (P > 0.05).
DISCUSSION
Understanding the biological consequences of mutations in the BCG genome provides a window into understanding the determinants of a successful antituberculosis vaccine. Indeed, of the candidate vaccines developed to replace BCG, two that have performed better than BCG in animal models are modified forms of BCG (14, 28). It is now known that loss of RD1 contributed to the attenuation of M. bovis, as an RD1 deletion mutant of M. tuberculosis had reduced growth, virulence, and pathogenicity in human macrophages and mice (16). Furthermore, disruption of the RD1 gene esat-6 decreased the virulence of M. bovis in guinea pigs (32). What is still unclear is whether subsequent mutations in the BCG genome contributed to further attenuation or compromised the efficacy of the vaccine.
Methoxymycolate production was restored in Denmark::mmaA3 and Pasteur::mmaA3, demonstrating that transcriptional promoter activity is encoded in the 250-bp region upstream of this gene. Notably, these strains consistently produced a larger amount of alpha-mycolates than BCG Japan and other early strains (4). Little is known about how mycolic acid production is regulated. However, it has been shown that mycolic acid composition ratios vary at different growth phases in vitro, during intracellular growth, and at temperature shifts (2, 9, 17, 31, 34). Whether feedback inhibition mechanisms maintain the mycolate composition ratio in the cell wall (alpha to methoxy) in wild-type strains remains to be determined. Perhaps a repressor-type element binds to a sequence further upstream of the 250 bp that were included in the mmaA3 amplicon and therefore the regulatory mechanism in Denmark::mmaA3 may not be responsive to metabolic signals.
BCG was initially cultivated on medium containing potatoes and glycerinated bile. It is possible that some mutations incurred by BCG provided a selective advantage for growth on this medium. In our hands, Denmark::pGH1 and Denmark::mmA3 grew similarly in liquid and solid culture media and in medium containing bile, indicating that loss of methoxymycolic acid did not contribute to the penetrance of the mutant. That loss of methoxymycolate production did not change the growth rate of the organism suggests that RD2 or some other uncharacterized mutation contributed to the selection of mutant forms of BCG over the wild type during in vitro culture.
Microarray expression profiling was used to determine if methoxymycolate production resulted in compensatory gene expression in Denmark::mmaA3. With the exception of Rv1815, encoding a hypothetical protein of unknown function, no differences were observed at the level of gene expression. Rv1816, a putative transcriptional regulator, had a Z score below our cutoff but may also be downregulated in Denmark::mmaA3. Further analysis will be needed to understand the significance of the expression differences of these genes. While this technique provides a good approach to contrast global expression patterns in isogenic bacterial mutants, it is possible that the conditions used to explore expression patterns (log-phase growth in a nutrient-rich liquid broth) were unrevealing about the importance of methoxymycolic acids.
Growth in macrophages and resistance to innate killing mechanisms are crucial for bacterial virulence. PMA-differentiated THP-1 cells have been shown to be a good model for intracellular infection (29) and were permissive for intracellular growth in this study. Loss of methoxymycolates in the cell wall did not alter the growth profile of Denmark::pGH1 in PMA-differentiated THP-1 macrophages compared to Denmark::mmaA3. This is in agreement with the results for the hma mutant of M. tuberculosis, in which loss of keto- and methoxymycolic acid production did not affect intracellular growth (10). Furthermore, restoration of methoxymycolic acids in the cell wall did not affect resistance to the antibacterial agents rifampin, isoniazid, and ampicillin. We did not observe changes in permeability to chenodeoxycholate in Denmark::mmaA3 as was described for the hma disruption mutant. Perhaps ketomycolates compensate for the loss of methoxymycolates in Denmark::pGH1 and maintain membrane fluidity. In contrast, ketomycolate production was impaired in BCG strains overexpressing mmaA3, but these mutants did not exhibit increased permeability to chenodeoxycholate and were more resistant to antibiotics (34). It is difficult to interpret our results and those from the hma mutant with those from strains overexpressing mmaA3, as these strains exhibited an uncommon mycolate profile with increased methoxymycolates and impaired ketomycolate production.
Methoxymycolate production is conserved in all members of the M. tuberculosis complex (M. tuberculosis, M. bovis, M. microti, and M. africanum) (3). However, production by other pathogenic mycobacteria varies. What is striking is that virtually all pathogenic mycobacteria produce ketomycolates and that ketomycolate production is increased in vivo (9). It was recently shown that inhalation exposure of guinea pigs to purified methyl ketomycolates but not methyl methoxymycolates or methyl alpha-mycolates induced pulmonary granulomas (30). Loss of keto- and methoxymycolic acid production in the hma knockout M. tuberculosis mutant resulted in growth attenuation in C57BL/6 mice. Our findings demonstrated that restoration of methoxymycolate production alone did not increase the growth or pathogenicity of Denmark::mmaA3 in mice. Growth of Denmark::mmaA3 displayed a trend towards increased persistence over Denmark::pGH1 during longer growth in mouse spleens (between 18 and 24 weeks), but these differences did not achieve statistical significance. Although these results may perhaps allude to a persistence phenotype of Denmark::mmaA3 during very long term infection (>24 weeks), vaccine challenge experiments may be more revealing in distinguishing the in vivo significance of methoxymycolate production. As has been proposed, the methoxy group of methoxymycolates may be more chemically inert than the cis double bonds of ketomycolates and may be important for maintaining membrane integrity during long-term growth in vivo (9).
It is interesting to speculate how the growth, tissue distribution, or virulence of BCG strains can influence protection against M. tuberculosis challenge. It has been suggested that the persistence of BCG strains in the host may be important for the generation and maintenance of long-term immunity (25). Disruption of pcaA, which codes for an enzyme that introduces the proximal cyclopropane ring structure into alpha-mycolic acids, had a dramatic effect on the persistence of BCG in mice (12), whereas in our study, loss of methoxymycolic acid did not. Loss of pcaA activity also shifted the proportion of mycolic acids in the cell wall, so that ketomycolate production became more predominant in the mutant strain compared to wild-type M. tuberculosis. Although it is possible that an essential role for the methoxymycolates is fulfilled by the residual, albeit very low levels produced by the mmaA3 mutants, we prefer other explanations.
In a detailed study of mycolate distribution, the proportion of nonoxygenated alpha-mycolates was shown to be roughly equal to the sum of oxygenated methoxy- and ketomycolates in M. tuberculosis and M. bovis (33). This suggests that an effective cell wall requires a balance of nonoxygenated and oxygenated mycolic acids and that there may be interchangeability between keto- and methoxymycolates (9). Perhaps ketomycolates can compensate for the loss of methoxymycolates in BCG strains. In infection studies, growth phenotypes were only found when oxygenated mycolate (i.e., hma mutant) or nonoxygenated mycolate (i.e., pcaA mutant) production was impaired. Although BCG Denmark and Pasteur are deficient in methoxymycolate production, both produce oxygenated (ketomycolates) and nonoxygenated (alpha-mycolates) forms and may retain the required mycolate composition ratio (oxygenated to nonoxygenated) for growth in vivo.
It has been difficult to associate a clear phenotype with the mmaA3 mutation of BCG. Recent work on recombinant BCG strains expressing antigen 85 demonstrated a dissociation between growth and vaccine efficacy, as an increased capacity to protect against M. tuberculosis pathogenesis was observed despite similar growth profiles in guinea pigs (13, 14). In light of these findings, it appears that formal challenge experiments with our isogenic mutants are required to definitively test whether loss of methoxymycolate production affects the protective efficacy of the vaccine.
Acknowledgments
We thank Serge Mostowy and Cynthia Cleto for assistance with microarray analysis, Maria Harrell for constructing the pGH1 vector, and Danielle Charlet for helpful discussions.
A.B. is supported by a postdoctoral fellowship award of the Canadian Institutes for Health Research (CIHR), and M.A.B. is a New Investigator of the CIHR. The work was funded by CIHR grant MOP-36054 and NIH grant HL068533.
Editor: V. J. DiRita
REFERENCES
- 1.Abou-Zeid, C., G. A. W. Rook, D. E. Minnikin, J. H. Parlett, T. W. Osborn, and J. M. Grange. 1987. Effect of the method of preparation of bacille Calmette-Guérin (BCG) vaccine on the properties of four daughter strains. J. Appl. Bacteriol. 63:449-453. [DOI] [PubMed] [Google Scholar]
- 2.Baba, T., K. Kaneda, E. Kusunose, M. Kusunose, and I. Yano. 1989. Thermally adaptive changes of mycolic acids in Mycobacterium smegmatis. J. Biochem. 106:81-86. [DOI] [PubMed] [Google Scholar]
- 3.Barry, C. E., III, R. E. Lee, K. Mdluli, A. E. Sampson, B. G. Schroeder, R. A. Slayden, and Y. Yuan. 1998. Mycolic acids: structure, biosynthesis and physiological functions. Prog. Lipid Res. 37:143-179. [DOI] [PubMed] [Google Scholar]
- 4.Behr, M. A., B. G. Schroeder, J. N. Brinkman, R. A. Slayden, and C. E. Barry. 2000. A point mutation in the mma3 gene is responsible for impaired methoxymycolic acid production in Mycobacterium bovis BCG strains obtained after 1927. J. Bacteriol. 182:3394-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behr, M. A., and P. M. Small. 1997. Has BCG attenuated to impotence? Nature 389:133-134. [DOI] [PubMed] [Google Scholar]
- 6.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 7.Besra, G. S. 2003. Preparation of cell-wall fractions from mycobacteria, p. 91-108. In T. Parrish and N. G. Stoker (ed.), Methods in molecular biology: mycobacteria protocols. Humana Press, Totowa, Iowa. [DOI] [PubMed]
- 8.Comstock, G. W. 1994. Field trials of tuberculosis vaccines: how could we have done them better? Control. Clin. Trials 15:247-276. [DOI] [PubMed] [Google Scholar]
- 9.Davidson, L. A., P. Draper and, D. E. Minnikin. 1982. Studies on the mycolic acids from the walls of Mycobacterium microti. J. Gen. Microbiol. 128:823-828. [DOI] [PubMed] [Google Scholar]
- 10.Dubnau, E., J. Chan, C. Raynaud, V. P. Mohan, M. A. Laneelle, K. Yu, A. Quemard, I. Smith, and M. Daffe. 2000. Oxygenated mycolic acids are necessary for virulence of Mycobacterium tuberculosis in mice. Mol. Microbiol. 36:630-637. [DOI] [PubMed] [Google Scholar]
- 11.Fine, P. E. M. 1995. Bacille Calmette-Guérin vaccines: a rough guide. Clin. Infect. Dis. 20:11-14. [DOI] [PubMed] [Google Scholar]
- 12.Glickman, M. S., J. S. Cox, and W. R. Jacobs, Jr. 2000. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5:717-727. [DOI] [PubMed] [Google Scholar]
- 13.Horwitz, M. A., and G. Harth. 2003. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect. Immun. 71:1672-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz, M. A., G. Harth, B. J. Dillon, and S. Maslesa-Galic'. 2000. Recombinant bacillus Calmette-Guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc. Natl. Acad. Sci. USA 97:13853-13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen, K. A. 1946. Practice of the Calmette vaccination. Acta Tuberc. Scand. 20:1-45. [Google Scholar]
- 16.Lewis, K. N., R. Liao, K. M. Guinn, M. J. Hickey, S. Smith, M. A. Behr, and D. R. Sherman. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guérin attenuation. J. Infect. Dis. 187:117-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, J., C. E. Barry III, G. S. Besra, and H. Nikaido. 1996. Mycolic acid structure determines the fluidity of the mycobacterial cell wall. J. Biol. Chem. 271:29545-29551. [DOI] [PubMed] [Google Scholar]
- 18.Liu, J., H. E. Takiff, and H. Nikaido. 1996. Active efflux of fluoroquinolones in Mycobacterium smegmatis mediated by LfrA, a multidrug efflux pump. J. Bacteriol. 178:3791-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minnikin, D. E., J. H. Parlett, M. Magnusson, M. Ridell, and A. Lind. 1984. Mycolic acid patterns of representatives of Mycobacterium bovis BCG. J. Gen. Microbiol. 130:2733-2736. [DOI] [PubMed] [Google Scholar]
- 21.Minnikin, D. E., and M. Goodfellow. 1980. Lipid composition in the classification and identification of acid-fast bacteria, p. 189-256. In M. Goodfellow and R. G. Board (ed.), Microbiological classification and identification. Academic Press, London, England. [PubMed]
- 22.Minnikin, D. E., S. M. Minnikim, G. Dobson, M. Goodfellow, F. Portaels, L. Van Den Breen, and D. Sesardic. 1984. Mycolic acid patterns of four vaccine strains of Mycobacterium bovis BCG. J. Gen. Microbiol. 129:889-891. [DOI] [PubMed] [Google Scholar]
- 23.Mostowy, S., A. G. Tsolaki, P. M. Small, and M. A. Behr. 2003. The in vitro evolution of BCG vaccines. Vaccine 21:4270-4274. [DOI] [PubMed] [Google Scholar]
- 24.Oettinger, T., M. Jorgensen, A. Ladefoged, K. Haslov, and P. Andersen. 1999. Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuberc. Lung Dis. 79:243-250. [DOI] [PubMed] [Google Scholar]
- 25.Orme, I. M., D. N. McMurray, and J. T. Belisle. 2001. Tuberculosis vaccine development: recent progress. Trends Microbiol. 9:115-118. [DOI] [PubMed] [Google Scholar]
- 26.Palmer, C. E., and M. W. Long. 1966. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am. Rev. Respir. Dis. 94:553-568. [DOI] [PubMed] [Google Scholar]
- 27.Pym, A. S., P. Brodin, R. Brosch, M. Huerre, and S. T. Cole. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46:709-717. [DOI] [PubMed] [Google Scholar]
- 28.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and S. T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9:533-539. [DOI] [PubMed] [Google Scholar]
- 29.Stokes, R. W., and D. Doxsee. 1999. The receptor-mediated uptake, survival, replication, and drug sensitivity of Mycobacterium tuberculosis within the macrophage-like cell line THP-1: a comparison with human monocyte-derived macrophages. Cell. Immunol. 197:1-9. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara, I., T. Udagawa, S. C. Hua, M. Reza-Gholizadeh, K. Otomo, Y. Saito, and H. Yamada. 2002. Pulmonary granulomas of guinea pigs induced by inhalation exposure of heat-treated BCG Pasteur, purified trehalose dimycolate and methyl ketomycolate. J. Med. Microbiol. 51:131-137. [DOI] [PubMed] [Google Scholar]
- 31.Toriyama, S., I. Yano, M. Masui, E. Kusunose, M. Kusunose, and N. Akimori. 1980. Regulation of cell wall mycolic acid biosynthesis in acid-fast bacteria. I. Temperature-induced changes in mycolic acid molecular species and related compounds in Mycobacterium phlei. J. Biochem. 88:211-221. [PubMed] [Google Scholar]
- 32.Wards, B. J., G. W. de Lisle, and D. M. Collins. 2000. An esat6 knockout mutant of Mycobacterium bovis produced by homologous recombination will contribute to the development of a live tuberculosis vaccine. Tuberc. Lung Dis. 80:185-189. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe, M., Y. Aoyagi, M. Ridell, and D. E. Minnikin. 2001. Separation and characterization of individual mycolic acids in representative mycobacteria. Microbiology 147:1825-1837. [DOI] [PubMed] [Google Scholar]
- 34.Yuan, Y., Y. Zhu, D. D. Crane, and C. E. Barry III. 1998. The effect of oxygenated mycolic acid composition on cell wall function and macrophage growth in Mycobacterium tuberculosis. Mol. Microbiol. 29:1449-1458. [DOI] [PubMed] [Google Scholar]
- 35.Zeyland, J., and E. Piasecka-Zeyland. 1936. Sur la vitalité du BCG dans l'organisme vacciné. Ann. Inst. Pasteur 56:46-51. [Google Scholar]