Abstract
Context
Androgen deprivation therapy (ADT) is widely used to manage the symptoms of advanced prostate cancer and has been shown to slow the progression of the disease. Previous research investigating racial differences in the use of ADT has reported inconsistent findings.
Objectives
The purpose of this study was to assess use trends for ADT overall and by type (orchiectomy and luteinizing hormone-releasing hormone [LHRH] agonists) and the factors associated with time to receipt for metastatic prostate cancer.
Methods
Data from the Surveillance, Epidemiology, and End Results (SEER) cancer registry and Medicare claims database were obtained for 5,273 men, aged 65 years and older and diagnosed with Stage IV prostate cancer during 1991–1999 from seven SEER regions. An accelerated failure time regression model with lognormal distribution was used to examine factors associated with mean time to receipt of ADT.
Results
African-American men were less likely than white men to receive any ADT after diagnosis (P < 0.001). Differences were noted in the time to receipt of ADT, with African-American men having a longer mean time to receipt of orchiectomy (time ratio [TR] = 1.50; 95% confidence interval [CI] = 1.03, 2.17) or LHRH agonist (TR = 1.42; 95% CI = 1.06, 1.89) than white men.
Conclusion
African-American men with metastatic prostate cancer were significantly less likely to receive ADT and, when treated, had a slightly longer time to receipt than white men, which has implications for patients and physicians involved in the palliative management of metastatic prostate cancer.
Keywords: Prostate cancer, treatment, race/ethnicity, innovation diffusion
Introduction
The incidence and mortality rates for prostate cancer have gradually declined in the United States since 1993.1,2 However, African-American men still have prostate cancer incidence and mortality rates that are acutely higher than those of men from any other racial and ethnic background.2 Several factors have been posited as possible contributors to the racial disparity in prostate cancer incidence and mortality, including the presentation of more African-American men with advanced disease at diagnosis.2,3 Although fewer than 5% of prostate cancer diagnoses are made when the disease has metastasized to other parts of the body, African-American men are more often diagnosed at this advanced stage and have a lower five-year relative survival rate compared with white men diagnosed at this stage.4,5
A curative therapy for metastatic prostate cancer does not exist, although the efficacy of androgen deprivation therapy (ADT) for the palliative management of advanced prostate cancer symptoms was first reported more than 65 years ago and continues to be the standard treatment.6,7 The use of ADT has increased over time for prostate cancer,8,9 even as the incidence of prostate cancer has declined, and as the initial therapeutic modality, bilateral orchiectomy, has been supplanted by luteinizing hormone-releasing hormone (LHRH) agonists that were introduced in 1982.7,10 The two modalities of ADT have comparable side effects, although there are added surgical risks associated with orchiectomy and added time commitments and cost associated with repeated administrations of LHRH agonists. Previous research investigating racial differences in the use of ADT have reported inconsistent findings of both no racial differences2,11,12 and significant racial differences13,14 as well as reports that ADT is less likely to be discussed as a treatment option with African-American men than white men.11
Previous studies of prostate cancer treatment have reported that African-American men with localized prostate cancer have a longer median time between diagnosis and a subsequent medical visit or procedure and receive less medical monitoring than white men.15 Although the most appropriate time to administer ADT to men with metastatic prostate cancer is undetermined,16 underuse or delays in initiation of ADT may affect quality of life and/or survival, because ADT is widely used to manage the symptoms of advanced prostate cancer and has been shown to slow the progression of the disease.17,18 Previous studies have not investigated racial differences in the use of ADT modalities and time to receipt of ADT, and the purpose of this study was to evaluate trends and racial differences in the use and time to receipt of ADT among men with metastatic prostate cancer, with a goal of informing and improving future clinical management of this population.
Methods
This study used data from the Surveillance, Epidemiology, and End Results (SEER)-Medicare Linked Data base. An overview of the SEER-Medicare data has been previously published.19 Briefly, the SEER Program collects population-based cancer registry data consisting of demographics, tumor site, tumor morphology, stage at diagnosis, and treatment for incident cancer cases from state or regional SEER cancer registries. The SEER data are linked to beneficiary claims data from Medicare, which is the primary health insurance program for adults 65 years of age and older and covers more than 97% of the U.S. population.
The study sample included men with a primary diagnosis of prostate cancer during 1991–1999, who were not first diagnosed at autopsy or by death certificate, had no other cancer diagnoses, were aged 65 years and older, and had Medicare coverage (n = 127,056). Participants were excluded if they were not diagnosed with American Joint Committee on Cancer modified Stage IV prostate cancer (n = 114,710), did not have complete claims (n = 3,618), received other types of hormonal therapies (n = 31), had a treatment date beyond 30 days before diagnosis (n = 85), multiple treatments occurring on same date (n = 2), or were missing a diagnosis date (n = 65). Because this study focused on racial differences in treatment, participants who were not African American or white (n = 510) were excluded, and this study was limited to seven SEER sites (Atlanta, Connecticut, Detroit, Los Angeles, San Francisco-Oakland, Seattle-Puget Sound, and San Jose-Monterey), resulting in a final study sample size of 5,273.
Demographic characteristics (age, race, marital status, SEER region), diagnosis year, and histologic grade were obtained from the SEER data. Histologic grade was determined according to Gleason scores: well differentiated (Gleason score: 2–4), moderately differentiated (Gleason score: 5–7), and poorly differentiated (Gleason score: 8–10). Medicare claims were available for 1991–2002 and were used to create a comorbidity index using inpatient and outpatient data.20 Claims data also were used to identify neighborhood socioeconomic status (percent high-school education and household median income) at the census tract level using the participant’s residential address, and to identify claims for orchiectomies and LHRH agonists using the Healthcare Common Procedure Coding System/Current Procedural Terminology (HCPCS/CPT) and International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM). Orchiectomy procedures were identified using HCPCS/CPT codes 54520, 54521, and 54530, and ICD-9-CM codes 62.3, 62.41, and 62.42. The LHRH agonists evaluated were goserelin acetate and leuprolide acetate, identified using HCPCS/CPT codes J9202, J9217, J9218, and J9219. Claims data were available for years 1991 through 2002 for men diagnosed with prostate cancer between 1991 and 1999.
To assess group differences in ADT use, analysis of variance and linear regression were used. The proportional hazards assumption for Cox regression was violated; hence, a time-to-event analysis was done using accelerated failure time (AFT) regression models to examine factors associated with time to receipt of ADT.21 AFT models are parametric regression models similar to linear regression, which allow for modeling of censored data. To select the best distribution for modeling our data, several functions were tested, and the distribution with the best fit (the log normal) was selected. Use of the log normal allows us to model the logarithm of the event time; hence, results are reported as estimates of the expected time ratio (TR). Estimates less than 1 suggest comparatively shorter time to receipt of ADT, and estimates greater than 1 suggest comparatively longer time to receipt of ADT. Time to receipt of ADT was evaluated using three subsets of the original study population based on ADT use: 1) orchiectomy vs. no ADT, 2) LHRH agonist vs. no ADT, and 3) LHRH agonist vs. orchiectomy. In addition to the comparisons with men who received no ADT, time to receipt of LHRH agonist vs. orchiectomy was examined to determine if there were differences in time to receipt of the two modalities between African-American and white men who received ADT. Variables included in the analyses were age, race, marital status, diagnosis year, comorbidities, histologic grade, and SEER region. Neighborhood socioeconomic status variables were evaluated in supplemental analyses but were not included in final models, because the magnitude of the effect estimates were not appreciably affected, and their inclusion resulted in collinearity issues and greater missing data. Observations were censored at death or the end of the surveillance period (December 2002). Minimum follow-up time was three years. All analyses were done using SAS Version 9.1 (SAS Institute, Cary, NC).
Results
The characteristics of study participants are presented in Table 1 by race. Most of the participants were white (79%). African-American participants tended to be younger, not married, have more comorbidities, and be resident in census tracts with lower high-school education levels and median income. The mean time to receipt of any ADT was slightly higher for African-American men (6.2 months) compared with white men (5.7 months), although this difference was not statistically significant (P= 0.395).
Table 1.
Characteristics of Study Participants With Metastatic Prostate Cancer—SEER-Medicare, 1991–1999
| Characteristics | African American |
White |
|---|---|---|
| n | 1,133 | 4,140 |
| Age (years), n (%) | ||
| 65–69 | 308 (27.2) | 941 (22.7) |
| 70–74 | 315 (27.8) | 1,046 (25.3) |
| 75–79 | 229 (20.2) | 923 (22.3) |
| 80+ | 281 (24.8) | 1,230 (29.7) |
| Marital status, n (%) | ||
| Married | 529 (46.7) | 2,769 (66.9) |
| Not married | 533 (47.0) | 1,204 (29.1) |
| Data missing | 71 (6.3) | 167 (4.0) |
| Diagnosis year, n (%) | ||
| 1991 | 168 (14.8) | 707 (17.1) |
| 1992 | 217 (19.2) | 757 (18.3) |
| 1993 | 147 (13.0) | 555 (13.4) |
| 1994 | 127 (11.2) | 419 (10.1) |
| 1995 | 124 (10.9) | 412 (10.0) |
| 1996 | 91 (8.0) | 329 (7.9) |
| 1997 | 98 (8.6) | 312 (7.5) |
| 1998 | 86 (7.6) | 345 (8.3) |
| 1999 | 75 (6.6) | 304 (7.3) |
| Months to ADT receipt, mean (standard deviation) |
6.2 (15.7) | 5.7 (15.0) |
| SEER site, n (%) | ||
| Atlanta | 188 (16.6) | 256 (6.2) |
| Connecticut | 84 (7.4) | 876 (21.2) |
| Detroit | 499 (44.0) | 861 (20.8) |
| Los Angeles | 166 (14.7) | 614 (14.8) |
| San Francisco | 152 (13.4) | 511 (12.3) |
| San Jose | <11* | 264 (6.4) |
| Seattle-Puget Sound | 35 (3.1) | 758 (18.3) |
| Histologic grade, n (%) | ||
| Well differentiated | 36 (3.2) | 114 (2.8) |
| Moderately differentiated | 310 (27.4) | 1,309 (31.6) |
| Poorly differentiated | 534 (47.1) | 1,785 (43.1) |
| Data missing | 253 (22.3) | 932 (22.5) |
| Charlson comorbidity score, n (%) | ||
| 0 | 578 (51.0) | 2,581 (62.3) |
| 1 | 203 (17.9) | 739 (17.9) |
| 2 | 99 (8.7) | 279 (6.7) |
| 3+ | 82 (7.2) | 166 (4.0) |
| Data missing | 171 (15.1) | 375 (9.1) |
| Census tract % high-school education, n (%) | ||
| Lowest tertile: 24%–79% | 777 (68.6) | 686 (16.6) |
| Middle tertile: 80%–89% | 184 (16.2) | 1,166 (28.2) |
| Highest tertile: 90%–100% | 61 (5.4) | 1,527 (36.9) |
| Data missing | 111 (9.8) | 761 (18.4) |
| Census tract median household income, n (%) | ||
| Lowest tertile: $7,657–40,111 |
792 (69.9) | 662 (16.0) |
| Middle tertile: $40,112–57,972 |
170 (15.0) | 1,283 (31.0) |
| Highest tertile: $57,973–200,008 |
60 (5.3) | 1,434 (34.6) |
| Data missing | 111 (9.8) | 761 (18.4) |
Cell sizes less than 11 are not reported for SEER-Medicare data.
Table 2 presents the characteristics of participants by ADT receipt. Overall, 28.3% did not receive any ADT, 29% received an orchiectomy, and 42.7% received an LHRH agonist. A greater proportion of African-American men did not receive ADT (38.8%) compared with white men (25.5%) (P < 0.001). A smaller proportion of men aged 80 years and older received an LHRH agonist (35.3%) than those of younger age groups (P < 0.001). Men with no comorbidities were more likely to receive an LHRH agonist (47.4%), whereas men with three or more comorbidities were more likely to have neither type of ADT (37.9%).
Table 2.
Characteristicsa of Study Participants With Metastatic Prostate Cancer—SEER-Medicare, 1991–1999
| Characteristics | n | No ADT | Orchiectomy | LHRH Agonist |
|---|---|---|---|---|
| n | 5,273 | 1,494 (28.3) | 1,528 (29.0) | 2,251 (42.7) |
| Age, n (%) | ||||
| 65–69 | 1,249 | 373 (29.9) | 276 (22.1) | 600 (48.0) |
| 70–74 | 1,361 | 356 (26.2) | 367 (27.0) | 638 (46.9) |
| 75–79 | 1,152 | 295 (25.6) | 377 (32.7) | 480 (41.7) |
| 80+ | 1,511 | 470 (31.1) | 508 (33.6) | 533 (35.3) |
| Race, n (%) | ||||
| White | 4,140 | 1,054 (25.5) | 1,238 (29.9) | 1,848 (44.6) |
| African American | 1,133 | 440 (38.8) | 290 (25.6) | 403 (35.6) |
| Marital status, n (%) | ||||
| Married | 3,298 | 850 (25.8) | 964 (29.2) | 1,484 (45.0) |
| Not married | 1,737 | 576 (33.2) | 515 (29.6) | 646 (37.2) |
| Data missing | 238 | 68 (28.6) | 49 (20.6) | 121 (50.8) |
| Diagnosis year, n (%) | ||||
| 1991 | 875 | 216 (24.7) | 421 (48.1) | 238 (27.2) |
| 1992 | 974 | 264 (27.1) | 358 (36.8) | 352 (36.1) |
| 1993 | 702 | 174 (24.8) | 221 (31.5) | 307 (43.7) |
| 1994 | 546 | 145 (26.6) | 150 (27.5) | 251 (46.0) |
| 1995 | 536 | 156 (29.1) | 136 (25.4) | 244 (45.5) |
| 1996 | 420 | 145 (34.5) | 89 (21.2) | 186 (44.3) |
| 1997 | 410 | 123 (30.0) | 65 (15.9) | 222 (54.1) |
| 1998 | 431 | 160 (37.1) | 50 (11.6) | 221 (51.3) |
| 1999 | 379 | 111 (29.3) | 38 (10.0) | 230 (60.7) |
| SEER site, n (%) | ||||
| Atlanta | 444 | 116 (26.1) | 133 (30.0) | 195 (43.9) |
| Connecticut | 960 | 222 (23.1) | 284 (29.6) | 454 (47.3) |
| Detroit | 1,360 | 454 (33.4) | 383 (28.2) | 523 (38.5) |
| Los Angeles | 780 | 225 (28.8) | 182 (23.3) | 373 (47.8) |
| San Francisco | 663 | 201 (30.3) | 201 (30.3) | 261 (39.4) |
| San Jose | 273 | 57 (20.9) | 91 (33.3) | 125 (45.8) |
| Seattle-Puget Sound | 793 | 219 (27.6) | 254 (32.0) | 320 (40.4) |
| Histologic grade, n (%) | ||||
| Well differentiated | 150 | 38 (25.3) | 41 (27.3) | 71 (47.3) |
| Moderately differentiated | 1,619 | 447 (27.6) | 438 (27.1) | 734 (45.3) |
| Poorly differentiated | 2,319 | 513 (22.1) | 781 (33.7) | 1,025 (44.2) |
| Data missing | 1,185 | 496 (41.9) | 268 (22.6) | 421 (35.5) |
| Charlson comorbidity score, n (%) | ||||
| 0 | 3,159 | 706 (22.3) | 957 (30.3) | 1,496 (47.4) |
| 1 | 942 | 251 (26.6) | 313 (33.2) | 378 (40.1) |
| 2 | 378 | 124 (32.8) | 100 (26.5) | 154 (40.7) |
| 3+ | 248 | 94 (37.9) | 72 (29.0) | 82 (33.1) |
| Data missing | 546 | 319 (58.4) | 86 (15.8) | 141 (25.8) |
| Census tract % high-school education, n (%) | ||||
| Lowest tertile: 24%–79% | 1,463 | 502 (34.3) | 415 (28.4) | 546 (37.3) |
| Middle tertile: 80%–89% | 1,350 | 359 (26.6) | 426 (31.6) | 565 (41.9) |
| Highest tertile: 90%–100% | 1,588 | 384 (24.2) | 433 (27.3) | 771 (48.6) |
| Data missing | 872 | 249 (28.6) | 254 (29.1) | 369 (42.3) |
| Census tract median household income, n (%) | ||||
| Lowest tertile: $7,657–40,111 | 1,454 | 505 (34.7) | 410 (28.2) | 539 (37.1) |
| Middle tertile: $40,112–57,972 | 1,453 | 388 (26.7) | 458 (31.5) | 607 (41.8) |
| Highest tertile: $57,973–200,008 | 1,494 | 352 (23.6) | 406 (27.2) | 736 (49.3) |
| Data missing | 872 | 249 (28.6) | 254 (29.1) | 369 (42.3) |
All pairwise comparisons of percentages for each non-missing level were statistically significant (P < 0.05) except for orchiectomy vs. no ADT for San Francisco, using Tukey’s multiple comparison test.
Figure 1 presents the proportion of participants who received orchiectomy, LHRH agonists, or no ADT by year of diagnosis. Overall, the use of orchiectomy declined and the use of LHRH agonists increased during the study period from 1991 to 1999. The proportion of participants that did not receive ADT remained relatively stable throughout the period. African-American men were less likely than white men to receive any ADT after diagnosis (P < 0.001), and this racial difference persisted as the months since diagnosis increased (Fig. 2). When evaluating the modality of ADT used among men who received treatment, the proportion of men receiving an LHRH agonist increased over time for African-American and white men and was not significantly different by race (P = 0.117), although in the latter years of the study period, African-American men lagged behind white men in both the adoption of LHRH agonists and the concomitant decrease in orchiectomy (Fig. 3).
Fig. 1.
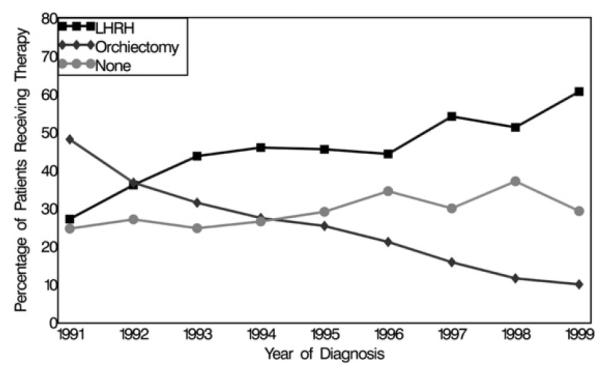
Proportion of men receiving LHRH agonists, orchiectomy, or no ADT, by diagnosis year for African-American and white men with metastatic prostate cancer, SEER-Medicare 1991–1999.
Fig. 2.
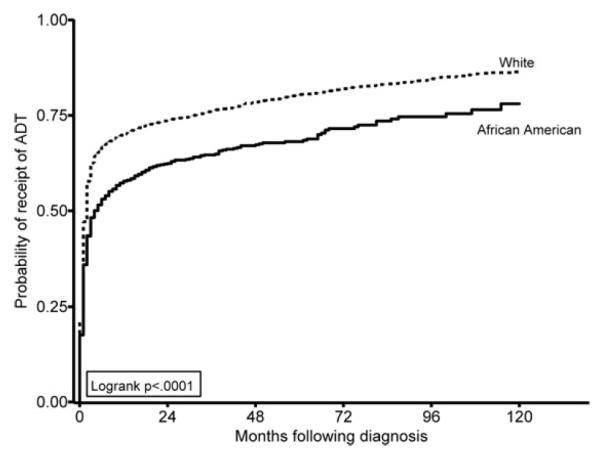
Probability of receipt of ADT during months following diagnosis by race, SEER-Medicare 1991–1999.
Fig. 3.
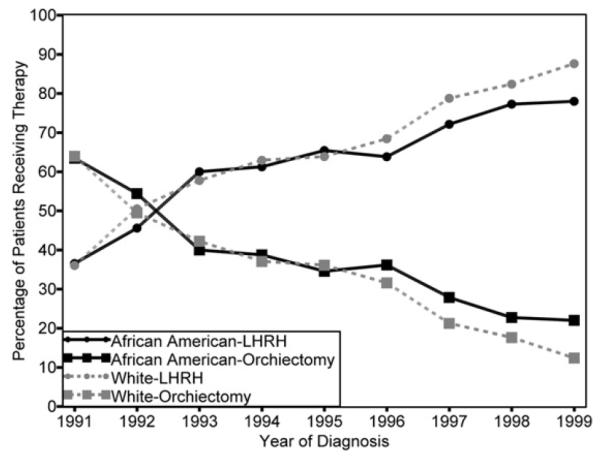
Proportion of men treated with an orchiectomy or an LHRH agonist by race, among those receiving ADT, SEER-Medicare 1991–1999.
Table 3 presents the results of an AFT regression model to determine factors associated with the time to receipt of ADT. In analyses adjusted for age, marital status, diagnosis year, SEER region, histologic grade, and comorbidities, African-American men had a longer mean time to receipt than white men for both orchiectomy (TR = 1.50; 95% confidence interval [CI] = 1.03, 2.17) and LHRH agonist (TR = 1.42; 95% CI = 1.06, 1.89), using no ADT as the reference group. When evaluating the use of an LHRH agonist vs. orchiectomy among men who received ADT, African-American men had a 19% longer mean time to receipt of the newer ADT modality than white men, although this association was not statistically significant (TR = 1.19; 95% CI = 0.97, 1.45). Participants aged 80 years and older had a shorter mean time to receipt than those aged 65–69 years for an orchiectomy (TR = 0.13; 95% CI = 0.09, 0.20) or an LHRH agonist (TR = 0.47; 95% CI = 0.34, 0.64) compared with no ADT. Furthermore, those with poorly differentiated disease had a shorter mean time to receipt of an orchiectomy (TR = 0.26; 95% CI = 0.12, 0.54) or an LHRH agonist (TR = 0.48; 95% CI = 0.28, 0.83) than those with well-differentiated disease, though there was no statistically significant difference when comparing the newer and older modalities.
Table 3.
Mean Time Ratios and 95% CI for Time to Receipt of ADT From Multivariate Accelerated Failure Time Model—SEER-Medicare, 1991–1999
| Ratio of Time to Receipt (95% CI) |
|||
|---|---|---|---|
| Characteristics | Orchiectomy vs. No ADT | LHRH vs. No ADT | LHRH vs. Orchiectomy |
| Race | |||
| White | 1.00 | 1.00 | 1.00 |
| African American | 1.50 (1.03, 2.17) | 1.42 (1.06, 1.89) | 1.19 (0.97, 1.45) |
| Age (years) | |||
| 65–69 | 1.00 | 1.00 | 1.00 |
| 70–74 | 0.52 (0.34, 0.79) | 0.80 (0.61, 1.05) | 0.95 (0.78, 1.15) |
| 75–79 | 0.23 (0.15, 0.35) | 0.54 (0.40, 0.73) | 0.78 (0.64, 0.96) |
| 80+ | 0.13 (0.09, 0.20) | 0.47 (0.34, 0.64) | 0.82 (0.67, 1.01) |
| Marital status | |||
| Married | 1.00 | 1.00 | 1.00 |
| Unmarried | 0.83 (0.61, 1.13) | 1.01 (0.80, 1.28) | 0.94 (0.80, 1.10) |
| Diagnosis year | |||
| 1991 | 0.07 (0.03, 0.16) | 2.78 (1.75, 4.41) | 3.74 (2.73, 5.14) |
| 1992 | 0.11 (0.05, 0.24) | 1.89 (1.25, 2.88) | 2.57 (1.93, 3.43) |
| 1993 | 0.13 (0.06, 0.29) | 1.44 (0.93, 2.22) | 1.91 (1.41, 2.59) |
| 1994 | 0.13 (0.05, 0.29) | 1.28 (0.81, 2.04) | 1.62 (1.18, 2.23) |
| 1995 | 0.17 (0.07, 0.39) | 1.17 (0.74, 1.85) | 1.25 (0.91, 1.72) |
| 1996 | 0.30 (0.12, 0.76) | 1.38 (0.85, 2.25) | 1.03 (0.73, 1.47) |
| 1997 | 0.23 (0.09, 0.59) | 0.69 (0.43, 1.12) | 0.88 (0.63, 1.23) |
| 1998 | 1.42 (0.55, 3.70) | 1.64 (1.04, 2.60) | 1.03 (0.74, 1.45) |
| 1999 | 1.00 | 1.00 | 1.00 |
| SEER site | |||
| Atlanta | 1.20 (0.66, 2.19) | 0.79 (0.50, 1.25) | 0.78 (0.57, 1.07) |
| Detroit | 2.18 (1.38, 3.43) | 1.43 (1.00, 2.04) | 0.99 (0.78, 1.26) |
| Connecticut | 0.76 (0.46, 1.25) | 0.49 (0.34, 0.71) | 0.58 (0.45, 0.74) |
| Los Angeles | 1.17 (0.69, 1.99) | 0.74 (0.51, 1.08) | 0.66 (0.51, 0.85) |
| San Francisco | 1.26 (0.74, 2.14) | 0.82 (0.54, 1.24) | 0.73 (0.55, 0.97) |
| San Jose | 1.47 (0.74, 2.89) | 0.94 (0.55, 1.61) | 0.98 (0.69, 1.39) |
| Seattle-Puget Sound | 1.00 | 1.00 | 1.00 |
| Histologic grade | |||
| Well differentiated | 1.00 | 1.00 | 1.00 |
| Moderately differentiated | 0.76 (0.36, 1.64) | 1.08 (0.62, 1.88) | 1.17 (0.79, 1.73) |
| Poorly differentiated | 0.26 (0.12, 0.54) | 0.48 (0.28, 0.83) | 0.91 (0.62, 1.34) |
| Comorbidity score | |||
| 0 | 1.00 | 1.00 | 1.00 |
| 1 | 0.66 (0.46, 0.94) | 0.97 (0.73, 1.29) | 1.03 (0.85, 1.24) |
| 2 | 0.71 (0.40, 1.27) | 0.72 (0.47, 1.11) | 0.79 (0.60, 1.06) |
| 3+ | 0.73 (0.38, 1.40) | 1.06 (0.62, 1.80) | 0.82 (0.56, 1.20) |
Additional adjustment for neighborhood education and income did not significantly alter these findings and were not included in the final analysis, because there was greater missing data for these covariates, and their inclusion did not appreciably change the results. Sensitivity analyses were done to evaluate the effect of censoring bias using a closed three-year window instead of the open-study window, which could range from 3 to 10 years for participants. The effect estimates were not appreciably altered; hence, only the results for the entire study period are presented.
Discussion
Racial differences in the use of any ADT were noted in this study of elderly men diagnosed with metastatic prostate cancer. African-American men were less likely to receive any ADT and had a longer mean time to receipt of orchiectomy and LHRH agonists than white men.
Previous studies evaluating localized or regional prostate cancer12 and all stages of prostate cancer22 have reported comparable use of ADT by whites and African Americans, but these findings may have been the result of the multiple treatment options available for the earlier stages of prostate cancer. In contrast, our study included only metastatic prostate cancer cases, because ADT has been the primary therapy for advanced prostate cancer for decades.
Lesser adoption of LHRH agonists occurred during the latter years of the study period among African-American men than white men. The relative utilization curves for LHRH agonists and orchiectomy began to diverge around 1996, when, among those who used ADT, a greater proportion of white men used LHRH agonists than African-American men, and a greater proportion of African-American men used orchiectomy than white men. These trends are notable, given innovation diffusion theory that posits that population adoption over time follows an S-shaped curve where adoption increases (represented by an upward slope in the curve) until a saturation inflection point is reached where adoption slows or stops (represented by a flattening of the curve).23 The adoption of LHRH agonists in this study resembles such an S-shaped diffusion curve (Fig. 3), except for the differential adoption of LHRH agonists at the end of the curve (the later years of the study period). Equity in the use of LHRH agonists would be expected during this time, because the proportion of men treated reached saturation, but instead, a racial disparity in LHRH adoption occurred.
The racial differences in mean time to receipt were small, but the overall range in time to receipt was wide during the study period. This could be relevant, given the debate about the most appropriate time to administer ADT for metastatic prostate cancer. The advantages and disadvantages of early or deferred ADT and the factors that should determine the time window, that is, prostate-specific antigen levels or time since diagnosis, have not been clearly defined.24 Results from early studies by the Veterans’ Administration Cooperative Research Group suggested that ADTshould be deferred until the presentation of symptoms,25,26 whereas a later study reported that early receipt of ADT slowed the progression of prostate cancer and associated pain.17 Recently, in its updated clinical practice guidelines, the American Society of Clinical Oncology did not recommend the early use of ADT because of results from a meta-analysis, which found no overall survival advantage for the use of early ADT.27 Although there is still debate about the advantages and disadvantages of early ADT, African-American men were less likely than white men to receive this therapy and had a slightly longer mean time to receipt.
Previous studies have reported that orchiectomy and LHRH agonists have comparable efficacies for the management of metastatic prostate cancer, with no differences in endocrine responses, side effects, and survival.28,29 Thus, the decision to use ADT at all or one modality of ADT instead of the other is probably influenced by a host of other factors, such as personal preference, physician recommendations, or possibly, a combination of these factors characterizing differential access, among other factors. Although this study did not investigate factors associated with the use of a particular type of ADT, a previous study reported that patients primarily selected orchiectomy for convenience and LHRH agonists to avoid surgery.30 These findings suggest that selection of a particular type of ADT was probably not influenced by possible side effects, because the side effects for both therapies are comparable;31 however, the side effects may have been a factor for more than a quarter of our study population who did not receive any form of ADT and possibly among those with more comorbidities, for whom comprehensive medical management may have been more challenging.
Another potential limitation of our study is that the receipt of ADT was determined solely by Medicare claims data. This probably did not affect our finding of a racial difference in ADT receipt, because this would suggest that claim errors were disproportionately distributed by the race of the patient, which is unlikely. There is a possibility that some claims may not have been filed or may have been misfiled, although this is also unlikely to be a major source of error, because a claim is necessary to receive reimbursement for medical services rendered.32 In addition, the use of diethylstilbesterol (DES), an oral synthetic estrogen, could not be evaluated in this study, because it is not reimbursed by Medicare. The use of DES has declined over the years because of cardiovascular-related complications, but there is a possibility that some men may have elected to pay the out-of-pocket costs associated with receiving this alternative treatment and were incorrectly identified as not receiving hormonal therapy for metastatic prostate cancer in our study.
Policy changes in recent years may also have yielded differential incentives to choose specific therapies. Notably, the decline in Medicare reimbursement for medical castration, which resulted from the Medicare Modernization Act, may be influential in men’s treatment decisions.32 As new data become available, future research should extend the investigation of the trends presented in this study into the more current period while addressing the limitations presented earlier. Future research may also investigate whether the differences in therapies or timing of their initiation are associated with differences in survival, although such an investigation will be challenging, given the need for a large study population with a richly qualitative characterization of the disease at diagnosis, the specific indication associated with ADT initiation, and other health and clinical care factors arising through the post-diagnostic period.
The treatment and management of prostate cancer has improved over the past decade. Despite these advances, the disproportionate number of African-American men who did not receive ADT for metastatic prostate cancer mirrors the racial differences in the treatment of clinically localized prostate cancer, where African-American men disproportionately receive nonaggressive treatment.33–37 The reasons for not treating some patients with localized disease, such as patient preference for nonaggressive management of low-risk prostate cancer or limited patient survival because of illness or advanced age, are not applicable to palliative treatment of metastatic prostate cancer with ADT. Therefore, because African-American men tend to present with more clinically aggressive prostate cancer,2,3 it would be expected that they would be treated with ADT more, not less, than white men, and earlier, not later. These findings suggest racial differences in treatment persist throughout the stages of prostate cancer, and further research is warranted to identify and address those factors that contribute to racial disparities in ADT use and time to receipt of therapy for metastatic prostate cancer.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare Linked Database.
This work was funded by Department of Defense (PC040907; W81XWH-05-1-0208 to D.L.H.; PC06 0224; W81XWH-07-1-0350 to D.L.H.; PC060911 to P.A.G.); National Center on Minority Health and Health Disparities (P60MD000244 to P.A.G. and D.L.H.); and National Cancer Institute (2R25CA 057726 to W.R.C.; 1U01CA114629 to P.A.G.).
References
- 1.American Cancer Society . Cancer facts and figures. American Cancer Society; Atlanta, GA: 2005. [Google Scholar]
- 2.Stanford JL, Stephenson RA, Coyle LM, et al. Prostate cancer trends 1973-1995, SEER Program. National Cancer Institute; Bethesda, MD: 1999. NIH Pub. No. 99-4543. [Google Scholar]
- 3.Hoffman RM, Gilliland FD, Eley JW, et al. Racial and ethnic differences in advanced-stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001;93:388–395. doi: 10.1093/jnci/93.5.388. [DOI] [PubMed] [Google Scholar]
- 4.Ries LAG, Eisner MP, Kosary CL, et al. SEER cancer statistics review, 1975-2002. National Cancer Institute; Bethesda, MD: [Accessed March 18, 2007]. 2005. Available from http://seer.cancer.gov/csr/1975_2002/ [Google Scholar]
- 5.SEER Program . SEER*Stat Database: Incidence—SEER 9 registries public-use (1973-2002). Surveillance Research Program. Cancer Statistics Branch, National Cancer Institute; Bethesda, MD: 2005. [Google Scholar]
- 6.Huggins C, Hodges CV. Studies on prostatic cancer, I: the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 7.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006;98:839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolis G, Ackman D, Stellos A, et al. Tumor growth inhibition in patients with prostatic carcinoma treated with luteinizing hormone-releasing hormone agonists. Proc Natl Acad Sci USA. 1982;79:1658–1662. doi: 10.1073/pnas.79.5.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demark-Wahnefried W, Schildkraut JM, Iselin CE, et al. Treatment options, selection, and satisfaction among African American and white men with prostate carcinoma in North Carolina. Cancer. 1998;83:320–330. doi: 10.1002/(sici)1097-0142(19980715)83:2<320::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 13.Lu-Yao G, Moore DF, Oleynick J, DiPaola RS, Yao S-L. Use of hormonal therapy in men with metastatic prostate cancer. J Urol. 2006;176:526–531. doi: 10.1016/j.juro.2006.03.098. [DOI] [PubMed] [Google Scholar]
- 14.Zeliadt SB, Potosky AL, Penson DF, Etzioni R. Survival benefit associated with adjuvant androgen deprivation therapy combined with radiotherapy for high- and low-risk patients with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2006;66:395–402. doi: 10.1016/j.ijrobp.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 15.Shavers VL, Brown M, Klabunde CN, et al. Race/ethnicity and the intensity of medical monitoring under “watchful waiting” for prostate cancer. Med Care. 2004;42:239–250. doi: 10.1097/01.mlr.0000117361.61444.71. [DOI] [PubMed] [Google Scholar]
- 16.Messing E. The timing of hormone therapy for men with asymptomatic advanced prostate cancer. Urol Oncol. 2003;21:245–254. doi: 10.1016/s1078-1439(03)00016-4. [DOI] [PubMed] [Google Scholar]
- 17.Medical Research Council Prostate Cancer Working Party Investigators Group Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. Br J Urol. 1997;79:235–246. doi: 10.1046/j.1464-410x.1997.d01-6840.x. [DOI] [PubMed] [Google Scholar]
- 18.Nair B, Wilt T, MacDonald R, Rutks I. Early versus deferred androgen suppression in the treatment of advanced prostatic cancer. Cochrane Database Syst Rev. 2002;1:CD003506. doi: 10.1002/14651858.CD003506. [DOI] [PubMed] [Google Scholar]
- 19.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl IV):3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 20.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 21.Orbe J, Ferreira E, Nunez-Anton V. Comparing proportional hazards and accelerated failure time models for survival analysis. Stat Med. 2002;21:3493–3510. doi: 10.1002/sim.1251. [DOI] [PubMed] [Google Scholar]
- 22.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 23.Rogers EM. Diffusion of innovations. Free Press; New York: 2003. [Google Scholar]
- 24.National Comprehensive Cancer Network [Accessed March 18, 2007];NCCN clinical practice guidelines in oncology, prostate cancer, 2007. Available from http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf.
- 25.Byar DP. Treatment of prostatic cancer: studies by the Veterans Administration cooperative urological research group. Bull N Y Acad Med. 1972;48:751–766. [PMC free article] [PubMed] [Google Scholar]
- 26.Byar DP. Proceedings: The Veterans Administration Cooperative Urological Research Group’s studies of cancer of the prostate. Cancer. 1973;32:1126–1130. doi: 10.1002/1097-0142(197311)32:5<1126::aid-cncr2820320518>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 27.Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. doi: 10.1200/JCO.2006.10.1949. [DOI] [PubMed] [Google Scholar]
- 28.Kaisary AV, Tyrrell CJ, Peeling WB, Griffiths K. Comparison of LHRH analogue (Zoladex) with orchiectomy in patients with metastatic prostatic carcinoma. Br J Urol. 1991;67:502–508. doi: 10.1111/j.1464-410x.1991.tb15195.x. [DOI] [PubMed] [Google Scholar]
- 29.Peeling WB. Phase III studies to compare goserelin (Zoladex) with orchiectomy and with diethylstilbestrol in treatment of prostatic carcinoma. Urology. 1989;33(Suppl 5):45–52. doi: 10.1016/0090-4295(89)90106-4. [DOI] [PubMed] [Google Scholar]
- 30.Cassileth BR, Soloway MS, Vogelzang NJ, et al. Patients’ choice of treatment in stage D prostate cancer. Urology. 1989;33(Suppl 5):57–62. doi: 10.1016/0090-4295(89)90108-8. [DOI] [PubMed] [Google Scholar]
- 31.Potosky AL, Knopf K, Clegg LX, et al. Quality-of-life outcomes after primary androgen deprivation therapy: results from the Prostate Cancer Outcomes Study. J Clin Oncol. 2001;19:3750–3757. doi: 10.1200/JCO.2001.19.17.3750. [DOI] [PubMed] [Google Scholar]
- 32.Mariani AJ, Glover M, Arita S. Medical versus surgical androgen suppression therapy for prostate cancer: a 10-year longitudinal cost study. J Urol. 2001;165:104–107. doi: 10.1097/00005392-200101000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Godley PA, Schenck AP, Amamoo MA, et al. Racial differences in mortality among Medicare recipients after treatment for localized prostate cancer. J Natl Cancer Inst. 2003;95:1702–1710. doi: 10.1093/jnci/djg094. [DOI] [PubMed] [Google Scholar]
- 34.Klabunde CN, Potosky AL, Harlan LC, Kramer BS. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36:1337–1348. doi: 10.1097/00005650-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. doi: 10.1093/jnci/94.5.334. [DOI] [PubMed] [Google Scholar]
- 36.Shavers VL, Brown ML, Klabunde CN, et al. Race/ethnicity and the receipt of watchful waiting for the initial management of prostate cancer. J Gen Intern Med. 2004;19:146–155. doi: 10.1111/j.1525-1497.2004.30209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Underwood W, 3rd, Jackson J, Wei JT, et al. Racial treatment trends in localized/regional prostate carcinoma: 1992-1999. Cancer. 2005;103:538–545. doi: 10.1002/cncr.20796. [DOI] [PubMed] [Google Scholar]


