Abstract
Background
Abnormalities of cyclic-AMP (cAMP) response element binding protein (CREB) function has been suggested in bipolar (BP) illness and schizophrenia (SZ), based on both indirect and direct evidence. To further elucidate the role of CREB in these disorders, we studied CREB expression and function in two brain areas implicated in these disorders, i.e., dorsolateral prefrontal cortex (DLPFC) and cingulate gyrus (CG).
Methods
We determined CREB protein expression using Western blot technique, CRE-DNA binding using gel shift assay, and mRNA expression using real-time RT-polymerase chain reaction (qPCR) in DLPFC and CG of the postmortem brain of BP (n = 19), SZ (n = 20), and normal control (NC, n = 20) subjects.
Results
We observed that CREB protein and mRNA expression and CRE-DNA binding activity were significantly decreased in the nuclear fraction of DLPFC and CG obtained from BP subjects compared with NC subjects. However, the protein and mRNA expression and CRE-DNA binding in SZ subjects was significantly decreased in CG, but not in DLPFC, compared with NC.
Conclusion
These studies thus indicate region-specific abnormalities of CREB expression and function in both BP and SZ. They suggest that abnormalities of CREB in CG may be associated with both BP and SZ, but its abnormality in DLPFC is specific to BP illness.
Keywords: Human DLPFC, Cingulate gyrus, CREB, CRE-DNA binding activity, Bipolar disorder, Schizophrenia
1. Introduction
Bipolar (BP) disorder and schizophrenia (SZ) are devastating illnesses that affect large numbers of individuals. BP is characterized by recurrent episodes of mania and depression, and it affects about 1.5% of the US population. It is a common, severe, chronic, and life-threatening illness (Goodwin and Jamison, 2007; Hunsberger et al., 2009) with poor recovery between episodes and a high relapse rate (Geller et al., 2004). About 1% to 2% of the total population is at risk for BP disorder in the United States (Judd and Akiskal, 2003). Although the BP disorder is a personal and social burden, the pathophysiology is poorly understood. Magnetic resonance imaging studies reported structural alterations in brain areas of BP and SZ patients (Beyer and Krishnan, 2002; Hajek et al., 2005; Haldane and Frangou, 2004; Savitz and Drevets, 2009). Accumulated evidence indicates decreased volume of neurons and glial cells in the brain of BP subjects (Cotter et al., 2002a; Cotter et al., 2002b; Rajkowska et al., 2001; Selemon and Rajkowska, 2003). Also, postmortem brain studies indicate a decreased density of neurons in the prefrontal cortex and cingulate cortex of schizophrenic subjects (Benes et al., 1986) and cell loss and cell atrophy in the PFC of subjects with depression and bipolar illness (Rajkowska, 2000). These studies suggest impaired neuroplasticity and resilience, and therefore, much attention has been paid to the imbalance of intracellular signaling systems in the pathophysiology of BP.
Several studies indicate that abnormalities of phosphoinositide (PI) and the adenylyl cyclase-cyclic AMP signaling system (AC), as well as several of their components may be associated with the pathophysiology of BP (Bezchlibnyk and Young, 2002; Du et al., 2003; Tanis and Duman, 2007) disorders and SZ (Muly, 2002). Activation of transcription factors is the final step in the signaling pathway that is mediated by the binding of the cell surface receptor with an agonist. One of the mechanisms by which these transcription factors are activated is by their phosphorylation and de-phosphorylation (Nestler and Greengard, 1994). The activation of protein kinase A (PKA), a component of the AC signaling system, and protein kinase C (PKC), a component of the PI signaling system, causes the phosphorylation of several transcription factors including the cAMP response element binding protein (CREB) (Nichols et al., 1992; Xie and Rothstein, 1995). There are some studies that suggest the abnormalities of PKA in the platelets of BP subjects (Tardito et al., 2003). Also, some studies indicate that the protein expression of some of the PKC isozymes may be abnormally expressed in the platelets of BP subjects (Pandey et al., 2002). These observations may suggest an abnormality of CREB that is a target for phosphorylation by these two enzymes, in addition to other signaling cascades. It is, therefore, possible that abnormalities of CREB may be associated with the pathophysiology of BP disorders.
CREB is a member of the basic leucine zipper family of transcription factors (Borrelli et al., 1992). CREB could be phosphorylated at ser-133 by many protein kinases, such as PKA and PKC (Akin et al., 2005; Hagiwara et al., 1993; Xie and Rothstein, 1995). The phosphorylation of CREB at serine-133 leads to its dimerization and activation by binding to the cAMP response element (CRE) at the consensus motif 5’-TGACGTCA, which is found in many neuronally expressed genes (Lee and Masson, 1993). On the other hand, CREB could also be phosphorylated at ser-129 residue by GSK-3β and inactivated (Bullock and Habener, 1998; Grimes and Jope, 2001). This inactivation is blocked by lithium treatment (Bullock and Habener, 1998; Fiol et al., 1994; Grimes and Jope, 2001). In its active form, the phosphorylated form of CREB regulates the transcription of many genes that are involved in several aspects of neuronal function, such as brain-derived neurotrophic factor (BDNF) (Moore et al., 1996; Walton and Dragunow, 2000). CREB plays a crucial role in regulating gene expression, participating in development of the nervous system, learning, memory, plasticity, adaptation, and cell survival (Carlezon et al., 2005; Hardingham et al., 2001; Lonze and Ginty, 2002; Shaywitz and Greenberg, 1999).
There is both direct and indirect evidence to suggest that abnormalities of CREB may be associated with the pathophysiology of BP disorders. Hammonds and Shim (2009) found that chronic treatment with lithium decreased CREB phosphorylation in rats’ cerebral cortex and hippocampus. On the other hand, Boer et al. (2008) observed that chronic lithium treatment significantly reduced CRE/CREB directed gene expression in the hippocampus, cortex, hypothalamus, and stratum in transgenic reporter gene mice. Since lithium is efficacious in treatment of BP disorder, this may suggest the importance of CREB in BP illness. CREB involvement in the pathophysiology of BP is also substantiated by a report by Young et al. (2004) who observed that the pCREB stem cells were significantly increased in several amygdala nuclei in subjects who died by suicide. On the other hand, those BP subjects who were treated with lithium at the time of death had significantly lower pCREB levels in the same region. There is some evidence to suggest abnormalities of CREB in BP illness. The role of CREB in SZ is less clear.
In order to further clarify the role of CREB in the pathophysiology of BP disorders and SZ, we determined the protein and mRNA expression of CREB and CRE-DNA binding in the dorsolateral prefrontal cortex (DLPFC) and cingulate gyrus (CG) obtained from BP, SZ, and normal control (NC) subjects. As mentioned before, since abnormalities of the DLPFC and CG have been implicated in BP disorders and SZ (Benes et al., 1986; Bouras et al., 2001; Haldane and Frangou, 2004), we have selected these two particular brain areas for our study.
2. Methods and Materials
2.1. Acquisition of Human Postmortem Brain Samples and Clinical Assessment
The frozen postmortem brain samples from DLPFC (Brodmann area 9 [BA9]), and CG (BA24) were obtained from the Harvard Brain Tissue Resource Center (HBTRC) at McLean Hospital, Belmont, Massachusetts, known as the McLean “66” cohort, and consisting of BP, SZ, and NC subjects. All diagnoses for the subjects in the collection were established by two psychiatrists at the HBTRC via retrospective review of all available medical records and extensive questionnaires about social and medical history completed by family members of the donors. The criteria of Feighner et al. (1972) for the diagnosis of SZ and DSM-III-R10 for the diagnosis of schizoaffective disorder and BP disorder were applied. Individuals with a documented history of substance dependence or neurological illnesses were excluded from the collection. The BP and SZ group also included subjects who died by suicide. This study was approved by the Institutional Review Boards of McLean Hospital and the University of Illinois at Chicago.
The demographics associated with these subjects are listed in Table 1. The sample included 19 BP subjects, 20 SZ subjects, and 20 NC subjects. Mean age, postmortem interval (PMI), and pH of the frozen brain samples did not differ significantly between the three groups: age (years, NC: 60.35 ± 16.94; BP: 62.68 ± 17.91; SZ: 56.25 ± 17.90); PMI (hours, NC: 21.19 ± 6.18; BP: 20.64 ± 9.70; SZ: 20.76 ± 5.35); and brain pH (NC: 6.37 ± 0.26; BP: 6.45 ± 0.23; SZ: 6.43 ± 0.25). The BD and SZ subjects had been exposed to various psychotropic medications (Table 1).
Table 1.
Summary of Demographic and Clinical Data Available on Subjects and Tissue Samples Used in the Present Study
| Group and Subject |
Age (year s) |
Race | Gend er |
PMI (hours) |
Brai n pH |
Cause of Death |
Psychiat ric Diagnosi s |
Medication at the time of death |
|---|---|---|---|---|---|---|---|---|
| CONTROLS | ||||||||
| 1. | 49 | White | M | 24.60 | 6.76 | Myocardial Infarction | Control | |
| 2. | 53 | Unknown | F | 24.00 | 5.8 | Cancer | Control | |
| 3. | 74 | White | F | 12.50 | 6.33 | Unknown | Control | |
| 4. | 54 | White | M | 24.20 | 6.53 | Congestive Heart Failure | Control | |
| 5. | 70 | White | F | 22.50 | 6.26 | Cancer | Control | |
| 6. | 67 | White | M | 22.33 | 6.42 | Heart Attack/Disease | Control | |
| 7. | 37 | White | M | 18.75 | 6.68 | Accidental | Control | |
| 8. | 73 | Unknown | M | 20.53 | 6.05 | Unknown | Control | |
| 9. | 35 | Unkno wn | M | 25.67 | 6.33 | Unknown | Control | |
| 10. | 89 | White | M | 7.42 | 6.39 | Cancer | Control | |
| 11. | 79 | White | M | 20.92 | 6.28 | Cancer | Control | |
| 12. | 78 | White | F | 23.91 | 6.67 | Cancer | Control | |
| 13. | 38 | White | M | 28.83 | 6.53 | Myocardial Infarction | Control | |
| 14. | 65 | White | F | 24.25 | 6.4 | Unknown | Control | |
| 15. | 66 | White | F | 7.42 | 6.03 | Cancer | Control | |
| 16. | 50 | Unkno wn | M | 24.13 | 6.01 | Unknown | Control | |
| 17. | 84 | White | M | 28.58 | 6.42 | Pneumonia | Control | |
| 18. | 40 | White | M | 16.60 | 6.24 | Myocardial Infarction | Control | |
| 19. | 66 | White | M | 18.70 | 6.76 | Heart Attack/Disease | Control | |
| 20. | 40 | White | M | 28.00 | 6.5 | Ski Accident | Control | |
| m mean: | 60.35 | 21.19 | 6.37 | |||||
| S.D.: | 16.94 | 6.18 | 0.26. | |||||
| BIPOLAR | ||||||||
| 1. | 80 | Unknown | F | 11.60 | 6.38 | Unknown | BD | Unknown |
| 2. | 74 | White | M | 7.18 | 6.7 | Pneumonia | BD | Gabapentin, zolpidem, olanzapine, lorazepam |
| 3. | 73 | White | F | 20.83 | 6.3 | Sepsis | BD | Carbamazepine, risperidone, diltiazem |
| 4. | 74 | White | M | 14.25 | 6.27 | Pneumonia | BD | Lithium,olanzapine, divalproex, zolpidem, lorazepam |
| 5. | 73 | White | F | 17.00 | 6.4 | Renal Failure | BD | Divalproex, risperidone, sertraline, donepezil |
| 6. | 40 | White | M | 30.75 | 6.03 | Suicide - hanging | BD | Risperidone, gabapentin, nefazodone, topiramate, ziprasidone |
| 7. | 38 | White | M | 22.00 | 6.24 | Suicide – CO poisoning | BD | Divalproex, Paroxetine, clonazepam, olanzapine, metoclopramide |
| 8. | 83 | Unknown | M | 17.50 | 6.6 | Cardiopulmonary arrest | BD | Divalproex, Paroxetine |
| 9. | 72 | White | M | 27.66 | 6.24 | Sepsis | BD | Lithium, Trazadone, |
| 10. | 82 | White | M | 5.02 | 6.37 | Cardiopulmonary arrest | BD | Unknown |
| 11. | 78 | White | M | 30.20 | 6.3 | Cardio-respiratory arrest | BD | Thorazine, clonazepam, divalproex, lithium, levodopa-carbidopa |
| 12. | 42 | White | F | 15.80 | 6.26 | Medication overdose | BD | Divalproex, lithium, perphenazine, zolpidem |
| 13. | 29 | White | F | 10.70 | 6.7 | Suicide - hanging | BD | Valproic acid, lithium, clonozepam, phenelzine, olanzapine, propranolol |
| 14. | 64 | White | F | 11.00 | 6.69 | Emphysema | BD | Divalproex, carbamazepine, trifluoperazine, Doxepin, trihexyphenidil, clonazepam |
| 15. | 38 | White | M | 41.50 | 6.52 | Suicide – Gunshot Wound | BD | Unknown (? Not taking meds at time of death) |
| 16. | 51 | White | M | 31.00 | 7.02 | Unknown | BD | Unknown |
| 17. | 76 | White | F | 22.80 | 6.6 | Heart Attack/Disease | BD | Lithium, lorazepam |
| 18. | 50 | White | M | 30.50 | 6.35 | Heart Attack/Disease | BD | Lithium |
| 19. | 74 | White | M | 24.80 | 6.53 | Pneumonia | BD | Divalproex, quetiapine |
| Mean: | 62.68 | 20.64 | 6.45 | |||||
| S.D.: | 17.91 | 9.70 | 0.23 | |||||
| SCHIZO. | ||||||||
| 1 | 55 | Unknown | F | 18.00 | 6.48 | Unknown | SA | Unknown |
| 2. | 66 | White | M | 22.1 | 6.43 | Pneumonia | SZ | Haloperidol |
| 3. | 61 | White | M | 19.9 | 6.68 | Renal failure | SZ | Haloperidol, lorazepm |
| 4. | 73 | White | F | 24.00 | 6.08 | Cancer | SZ | Risperidone, fluoxetine, clorazepate, fentanyl |
| 5. | 63 | White | M | 22.35 | 6.55 | Cardiac arrest | SZ | Clozapine, haloperidol, trazadone, lorazepam |
| 6. | 44 | White | M | 19.0 | 6.05 | Pneumonia | SZ | Clozapine |
| 7. | 35 | White | M | 28.00 | 6.25 | Heart Attack/Disease | SZ | None |
| 8. | 42 | White | M | 18.1 | 6.26 | Suicide –CO poisoning | SZ | Trazadone |
| 9. | 78 | White | F | 13.40 | 6.81 | Sick sinus syndrome | SA | Unknown |
| 10. | 46 | White | M | 18.5 | 6.31 | Cancer - sepsis | SZ | Olanzapine,divalp roex |
| 11. | 26 | White | M | 16.00 | 6.75 | Suicide - hanging | SZ | Prolixin decanoate |
| 12. | 42 | White | M | 27.1 | 6.64 | Cancer | SZ | None |
| 13. | 47 | White | M | 19.25 | 6.57 | Cancer | SZ | Clonazepam, hydroxyzine |
| 14. | 83 | White | F | 23.25 | 5.91 | GI bleed | SZ | Haloperidol decanoate |
| 15. | 84 | White | F | 25.75 | 6.14 | Congestive Heart Failure | SA | Risperidone, divalproex, temazepam |
| 16. | 31 | White | M | 15.0 | 6.46 | Unknown | SZ | Risperidone, olanzapine, bupropion |
| 17. | 72 | White | F | 21.75 | 6.65 | Cancer | SZ | Risperidone, paroxetine, clonidine |
| 18. | 48 | White | F | 33.78 | 6.63 | Heart Attack/Disease | SA | Risperidone, divalproex |
| 19. | 80 | White | M | 10.97 | 6.44 | Heart Attack/Disease | SZ | Thioridazine, mirtazapine |
| 20. | 49 | White | M | 19.08 | 6.6 | Suicide - hanging | SZ | Haloperidol decanoate, lorazepam |
| Mean: | 56.25 | 20.76 | 6.43 | |||||
| S.D.: | 17.90 | 5.35 | 0.25 | |||||
PMI, postmortem interval, SA, schizo-affective
2.2. Determination of Protein Expression and CRE-DNA Binding Activity in the Nuclear Fraction of BP, SZ, and NC Subjects
2.2.1. Preparation of Nuclear Fractions
The preparation of nuclear fraction followed the protocol from Pierce Biotechnology Inc. (Rockford, IL, USA). Briefly, tissue was homogenized in ice-cold cytoplasmic extraction reagent 1 (CER I) containing 0.5 mg/ml benzamidine, 2 ug/ml aprotinin, 2 ug/ml leupeptin, and 0.75 mM phenylmethylsulfonyl fluoride (PMSF). The homogenate was added to cytoplasmic extraction reagentII (CERII) and then centrifuged at 16000g for 5 min. The resulting pellet was suspended in ice-cold nuclear extraction reagent (NER) containing 0.5 mg/ml benazmide, 2 ug/ml aprotinin, 2 ug/ml leupeptin, and 2mM PMSF and incubated for 40 min on ice with frequent agitation. The nuclear extracts were separated by centrifugation at 16000g for 10 min. The protein content of the nuclear fraction was determined by the method of Lowry et al. (1951). This nuclear fraction was used to determine the protein expression of CREB and CRE-DNA binding activity.
2.2.2. Immunolabeling of CREB
The procedure for Western blotting has been described in detail (Dwivedi et al., 2003). Protein samples (30 ug protein) were loaded onto 10% (w/v) sodium dodecyl sulphate (SDS)-polyacrylaminde gel. The gels were run and transferred electrophoretically to an enhanced chemiluminescence (ECL) nitrocellulose membrane (Amersham, Arlington Heights, IL, USA). The membranes were washed with TBST buffer (10 mM Tris-base, 0.15 M NaCl, and 0.05% (w/v) Tween 20) for 10 min. The blots were blocked by incubating with 5% (w/v) powdered non-fat milk in TBST, 0.02% nonidet P-40, and 0.02% (w/v) SDS (pH 8.0). Then the bolts were incubated overnight at 4c with primary polyclonal anti-CREB antibody (Santa Cruz Biotechnilogy Inc., Santa Cruz, CA, USA) with a dilution of 1:3000. The membranes were washed with TBST and incubated with horseradish-peroxidase-linked secondary antibody (anti-rabbit immunoglobulin G(IgG);1:3000) for 5 h at room temperature. The membranes were extensively washed with TBST and exposed to ECL autoradiography film. The same nitrocellulose membrane was stripped and re-probed with β-actin antibody (Sigma Chemical Co., St. Louis, MO, USA). The bands on the autoradiogram were quantified using the Loats Image Analysis system (Westminster, MD, USA), and the optical density of each sample was corrected by the optical density of the corresponding β-actin band. The values are represented as a percent of control.
2.2.3. Determination of CRE-DNA Binding Activity by Gel Mobility Shift Assay
Preparation of DNA probe
Commercially available (Stratagene, La Jolla, CA, USA) oligonucleotides incorporating regulatory elements of the CREB sequence (5’-GATTGGCTGACGTCAGAGAGCT) were used. The probes are end-labeled with [y-32P) ATP using T4 polynucleotide kinase according to the manufacturer’s methods.
Gel mobility DNA binding assay
Binding reactions were carried out by incubating 10 µg of nuclear extract with 1 µg of poly(DI-DC) and BSA (6µg) in a reaction mixture of 20mM Hepes (pH7.9); 1mM DTT; 0.3 mM EDTA; 0.2 mM EGTA; 80 mM NaCl; 10% glycerol; and 0.2 mM PMSF for 15 min at room temperature. Approximately 5000 CPM of 32P-labelled CREB oligonucleotide were added and incubated for another 30 min. DNA-protein complexes were resolved on a 6.0% non-denaruring polyacrylamide gel in a buffer containing 25 mM Tris-borat (pH8.2) and 0.5 mM EDTA. The gel is dried and autoradiographed with intensifying screens on film (Kodak, Rochester, NY, USA) at −80°C. The bands of the DNA-protein complex are estimated quantitatively on the autoradiogram using the Loats Image Analysis system (Westminster, MD, USA).
2.3 Determination of mRNA Levels
2.3.1 RNA Isolation
Total RNA was extracted from 100 mg of tissue using the TRIZOL reagent according to the manufacturer's instructions and treated with DNAse 1 (Invitrogen, USA). The RNA yield was determined by absorbance at 260 nm using NanoDrop®ND-1000 (NanoDrop Technologies, Montchanin, DE, USA). RNA quality was assessed using Agilent Bioanalyzer 2100 and only samples with 28S/18S ratios >1.2 and RIN above 6.6 were included, 7.2 ± 0.6.
2.3.2 mRNA Quantitation
Expression levels of mRNA were determined using a two-step, real-time RT-PCR (qPCR) method, which we have previously published (Pandey et al., 2012). Briefly, 1 µg of total RNA was reverse transcribed using 50 ng random hexamers, 2mM dNTP mix, 10 units ribonuclease inhibitor, and 200 units MMLV-reverse transcriptase enzyme in a final reaction volume of 20 µl. qRT-PCR was performed with MX3005p sequence detection system (Agilent) using pre-designed Taqman gene expression assays (Applied Biosystems, Foster City, CA) targeting CREB1, Hs00231713_m1 two housekeeping genes β-actin (ACTB), Hs99999903_m1, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and Hs99999905_m1. The stability and optimal number of housekeeping genes was determined using geNORM version 3.4 (PrimerDesign Ltd, UK) according to the manufacturer's instructions (Vandesompele et al., 2002). This comparison identified ACTB and GAPDH as the most stable housekeeping genes for this cohort. PCR efficiency after 5-log dilution series of pooled cDNA was similar for all housekeeping and target genes. For each primer/probe set, qPCR reaction was carried out using 10 µl of cDNA (diluted 1:10) in 1X TaqMan Universal PCR Master Mix (Applied Biosystems) per manufacturer’s instructions. Each qPCR plate included a “no reverse transcriptase” and “no template” control to eliminate non-specific amplification and each sample was assayed in triplicate.
For qPCR gene expression analysis, raw expression data (Ct) were normalized to the geometric mean of the two housekeeping genes. Outliers were excluded if the normalized (delta Ct) values were greater than 2 standard deviations from the group mean. Relative expression levels, reported as fold change, were determined by 2−(ΔΔCt) method, as described in Applied Biosystems User Bulletin No. 2 (P/N 4303859), and ΔCt values were used for further statistical analysis.
2.4. Statistical Analysis and Effect of Confounding Variables
The data analyses were performed using the SAS 9.2 statistical software package. One-way ANCOVA was performed to compare the effects of three groups — BP, SZ, and NC — on CREB expression using covariates. In addition, for multiple comparisons we used t-test with Bonferroni Correction to adjust the type I error rates. We also performed a post-hoc t-test for each paired comparison separately.
To examine whether CREB protein and mRNA expression was affected by PMI, age, gender, or brain pH, we determined their dependencies by using a linear regression model.
3. Results
3.1. Effect of Age, Gender, and Brain pH on CREB Protein and Gene Expression and CRE-DNA Binding Activity
We determined CREB protein and mRNA expression and CRE-DNA binding activity in 19 BP, 20 SZ, and 20 NC subjects. The demographic and clinical characteristics of the study subjects are presented in Table 1. There were no significant differences in age between NC, BP, and SZ subjects. There was no significant correlation between CREB protein, CREB mRNA levels or CRE-DNA binding and age or gender. Our results from regression analyses showed that age has a significant effect (in positive direction) on CREB mRNA expression only in DLPFC. Brain pH has a significant effect (in positive direction) on both CREB protein expression and CRE-DNA binding only in DLPFC.
3.2. CRE-DNA Binding Activity in DLPFC and CG of BP, SZ, and NC Subjects
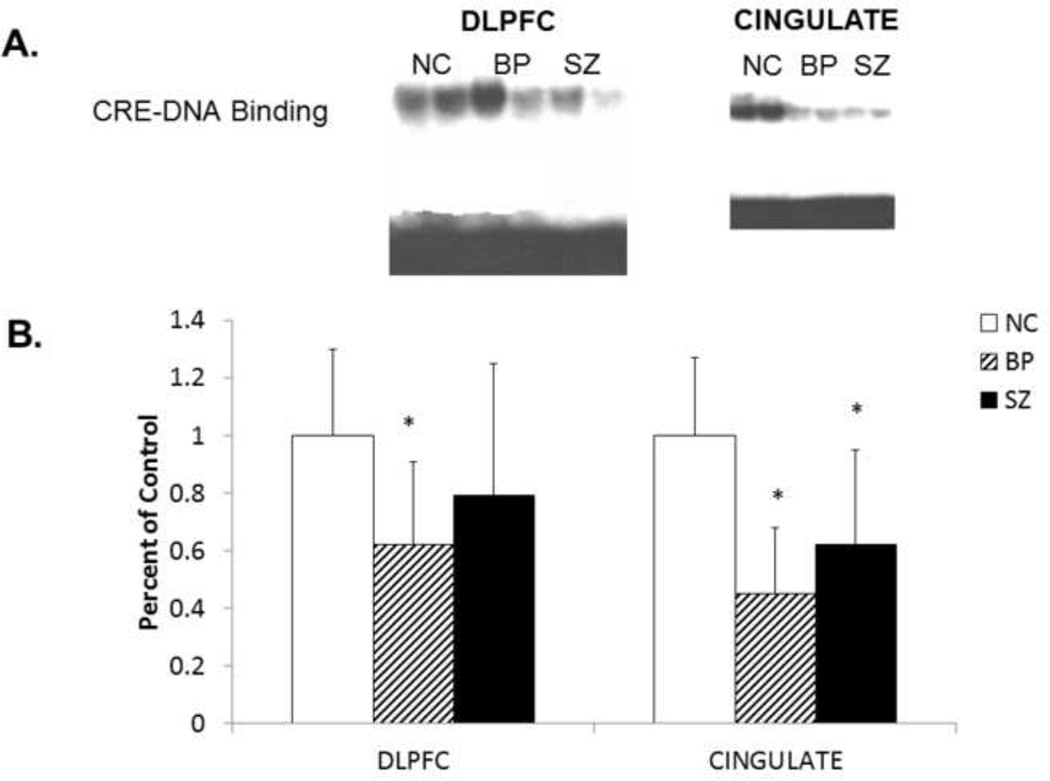
We determined the functional status of CREB by determining the CRE-DNA binding activity using a gel mobility shift assay in nuclear fraction of postmortem brain samples (DLPFC and CG) obtained from BP, SZ, and NC subjects. A representative autoradiogram showing CRE-DNA binding activity in the postmortem brain (DLPFC and CG) obtained from two BP, SZ, and NC subjects is shown in Figure 1A.
Figure 1.
A. Representative radiograms of the gel mobility shift assay showing CRE-DNA binding activity in nuclear fraction in dorsolateral prefrontal cortex (DLPFC) and cingulate gyrus (CG) of two normal controls (NC), two schizophrenic (SZ), and two bipolar (BP) subjects.
B. The mean CRE-DNA binding activity in the DLPFC nuclear fractions of NC (n = 20), SZ (n = 20), and BP (n = 19) subjects and in the CG nuclear fractions of NC (n = 15), SZ (n = 14), and BP (n = 13) subjects. The results are expressed as optical density (O.D.). Values are mean ± SD.
*p < .05
We performed ANCOVA to compare CRE-DNA binding activity between the groups. F-tests of ANCOVA showed that the three groups differ significantly in CRE-DNA binding in DLPFC (p =.007) and CG (p<.0001). For multiple comparisons, t-test with Bonferroni Correction showed that there is a significant difference in CRE-DNA binding between NC and BP in both DLPFC and CG. In the SZ group, the CRE-DNA binding activity was significantly different from NC only in the CG, and not the DLPFC.
Post-hoc t-test results showed that the mean CRE-DNA binding activity was significantly decreased in the nuclear fraction of DLPFC obtained from BP subjects (p = .0003), but not in the DLPFC of SZ subjects (p = .089), compared with NC subjects, as shown in Figure 1B. However, when we compared the CRE-DNA binding in the CG, we observed that it was significantly decreased in both BP (p<.0001) and SZ (p = 0.0023) subjects compared with NC, as shown in Figure 1B.
3.3. Immunolabeling of CREB in Nuclear Fraction of DLPFC and CG Obtained from BP, SZ, and NC Subjects
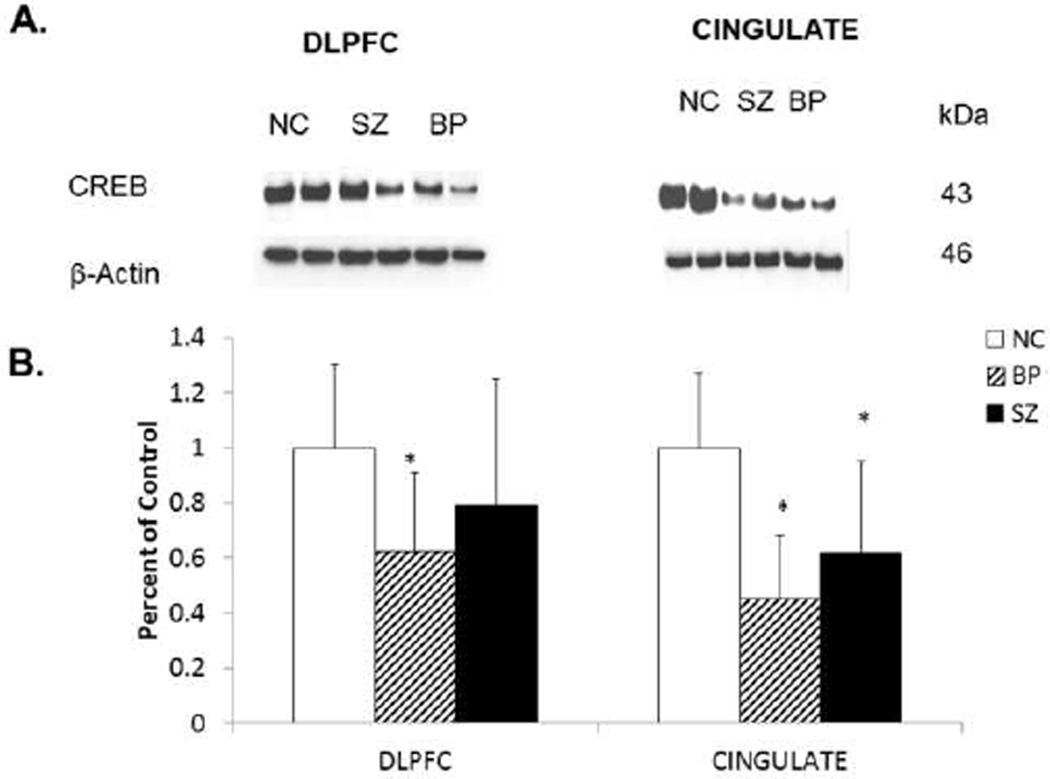
Because we observed a decrease in CRE-DNA binding activity in nuclear fraction of postmortem brain obtained from BP (in DLPFC and CG), and SZ subjects (in CG only) compared with NC subjects, we examined if this decrease in CRE-DNA binding activity in the nuclear fraction of DLPFC and CG obtained from BP was related to altered protein expression of CREB. We, therefore, determined the immunolabeling of CREB in the nuclear fraction of DLPFC and CG obtained from BP, SZ, and NC subjects. Representative Western blots showing immunolabeling of CREB protein in nuclear fraction of DLPFC and CG obtained from two BP subjects, two SZ subjects, and two NC subjects are presented in Figure 2A. As can be seen, the protein expression levels of CREB in these two BP subjects, but not SZ subjects, appeared to be lower in DLPFC than those in the NC subjects.
Figure 2.
A. Representative Western blots showing the immunolabeling of CREB and β-actin in the membrane fraction of DLPFC and CG of two normal controls (NC), two schizophrenic (SZ), and two bipolar (BP) subjects. kDa indicates kilo Daltons.
B. Mean protein expression levels of CREB in the DLPFC membrane fractions of NC (n = 20), SZ (n = 20), and BP (n = 19) subjects and in in the CG membrane fractions of NC (n = 15), SZ (n = 14), and BP (n = 13) subjects. The results are expressed as optical density (O.D.). Values are mean ± SD.
*p < .05
F-tests of ANCOVA show that the three groups differ significantly in CREB protein expression in the DLPFC (p = .001) and CG (p =.001). For multiple comparisons, t-test with Bonferroni correction showed that there is significant difference in CREB protein expression in both the DLPFC and CG between BP and NC subjects.
When we compared the mean protein expression levels of CREB in the nuclear fraction of DLPFC obtained from BP, SZ, and NC subjects, we found that the CREB protein levels were significantly decreased in the DLPFC of BP (p <.0001), but not in SZ subjects, as shown in Figure 2B.
The protein expression of CREB appeared to be lower in the CG of both BP and SZ subjects, as shown in the representative Western blots (Fig. 2A). When we compared the mean protein expression levels of CREB in the CG, we observed that they were significantly decreased in both BP (p<0.001) and SZ subjects (p<0.05) compared with NC subjects, as shown in Figure 2 B.
3.4. mRNA Expression Levels of CREB in the DLPFC and CG of BP, SZ, and NC Subjects
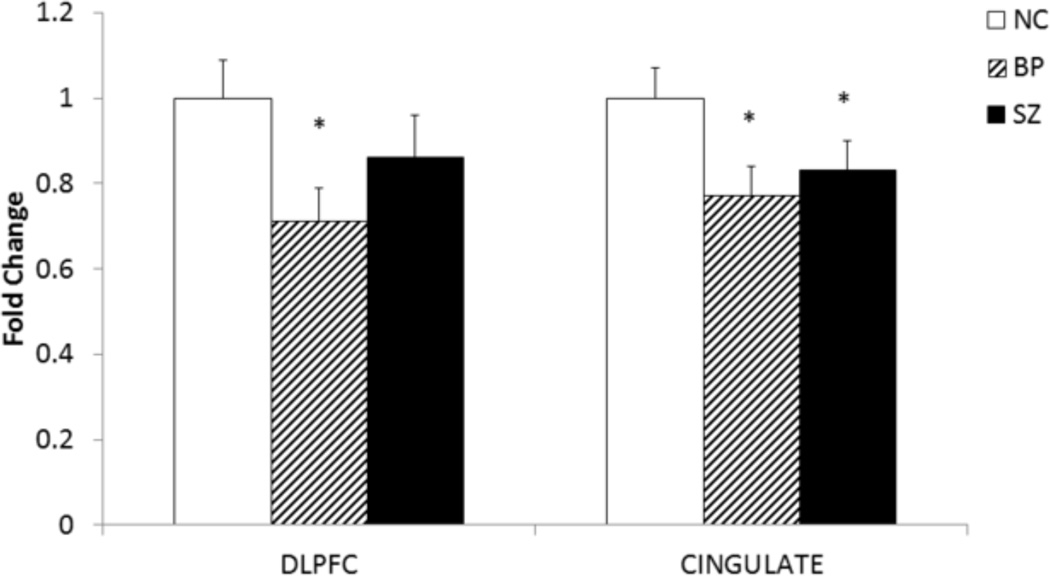
In order to examine if the abnormal protein expression of CREB in BP and SZ subjects is related to altered transcription of CREB, we determined the mRNA levels of CREB in the DLPFC and CG of BP, SZ, and NC subjects, and the results are shown in Figure 3.
Figure 3.
The mean mRNA expression levels of CREB in the DLPFC of NC 1.00 ± 0.09 (n = 20), SZ 0.86 ± 0.10 (n = 20), and BP 0.71 ± 0.08 (n = 19) subjects.
The mean mRNA expression levels of CREB in the CG of NC 1.00 ± 0.07 (n = 15), SZ 0.83 ± 0.07 (n = 13), and BP 0.77 ± 0.07 (n = 12) subjects.
The data are shown as fold change in mRNA levels. Values are fold change ± S.E.M.
*p< .05
F-tests of ANCOVA show that the CREB mRNA expression was significantly different in DLPFC (p = .0008) and CG (p = .0015) of the three groups studied.
For multiple comparisons, t-test with Bonferroni Correction showed that CREB mRNA expression was significantly decreased in the DLPFC and CG of BP subjects compared with NC. However, CREB mRNA expressions was significantly decreased in only in the CG of SZ subjects compared with NC
When we compared the mean mRNA expression levels of CREB in the nuclear fraction of DLPFC obtained from BP, SZ, and NC subjects, we found that the CREB mRNA levels were significantly decreased in the DLPFC of BP (p <.0004) subjects but not in the DLPFC of SZ subjects, as shown in Figure 3. However, when we compared the mean mRNA expression levels of CREB in the CG, we observed that they were significantly decreased in both BP (p =.0011) and SZ (p = 0.13) subjects compared with NC subjects, as shown in Figure 3.
4. Discussion
We determined the CREB protein and mRNA expression and CRE-DNA binding activity in the nuclear fraction of DLPFC and CG obtained from BP, SZ, and matched NC subjects. We found a significant decrease in the CRE-DNA binding, CREB protein, and mRNA expression in DLPFC obtained from BP subjects, but not SZ subjects, compared with NC subjects. On the other hand, the CRE-DNA binding, CREB protein, and mRNA expression levels were significantly decreased in the CG of both BP and SZ subjects compared with NC subjects.
Our observation that abnormality of CREB expression was found in the CG of both BP and SZ subjects, but was observed in the DLPFC of only BP subjects was intriguing. Structural and cellular abnormalities have been observed in the DLPFC and CG of both SZ and BP subjects. However, the patterns of the cellular abnormality in those two areas may differ in these disorders. For example, Bouras et al. (2001) observed decreased neuronal densities in the CG of BP subjects. On the other hand, they observed decreased cortical thickness without a decrease in neuronal density in the CG of SZ subjects. Decreased CG metabolic rate has been observed in SZ subjects by Haznedar et al. (1997). Carter et al. (1997) have reported anterior cingulate dysfunction and attention deficit in SZ subjects using a PET study. Selemon and Rajkowska (2003) reported cellular abnormalities in the postmortem brain of both BD and SZ subjects using morphometric studies. However, they found increased neuronal density in SZ subjects but decreased neuronal density in BP subjects.
Our studies suggest region-specific abnormalities of CREB in BP and SZ. Both disorders share CREB abnormality in the CG, but only BP subjects show CREB abnormality in the DLPFC.
There is both direct and indirect evidence suggesting that CREB abnormality may be associated with the pathophysiology of BP disorders. One of the major lines of evidence suggesting the involvement of CREB in the pathophysiology of BP is derived from the observation that lithium, used for treatment of BP illness, causes changes in the expression of CREB or the phosphorylation of CREB, both in vivo (Laifenfeld et al., 2005; Sairanen et al., 2007; Tiraboschi et al., 2004) and in vitro (Koch et al., 2003; Manier et al., 2002) conditions, thus suggesting that CREB alterations may be involved in the pathophysiology of BP disorder. In fact, mood stabilizers produce different effects on CREB activity and protein expression. For example, CREB protein expression and its activity are upregulated by mood stabilizers (Chen et al., 1999; Mai et al., 2002; Nibuya et al., 1996; Ozaki and Chuang, 1997; Thome et al., 2000; Yasuda et al., 2009). On the other hand, in an animal model of mania induced by d-AMPH, d-AMPH significantly increased GSK-3, PKC, PKA, CREB, and BDNF protein levels, and lithium could prevent and reverse these changes induced by d-AMPH (Cechinel-Recco et al., 2012). Boer et al. (2008) reported that chronic lithium salt treatment reduces CRE/CREB-directed gene transcription and reverses its upregulation by chronic psychosocial stress in transgenic reporter gene mice (Boer et al., 2008).
In a postmortem study, Young et al. (2004) found that the number of pCREB stained cells was significantly increased in the several amygdala nuclei in subjects who died by suicide. In contrast, patients treated with lithium at the time of death have significantly lower pCREB levels in the same region.
Mamdani et al. (2008) have studied lithium response and genetic variation in the CREB family of genes in a sample of 118 lithium responders and 69 non-responders, and 127 control subjects using single nucleotide polymorphisms (SNP) SNaPshot multiplex reaction. After correcting for multiple testing, they found that the CREB-1H SNP and CREB-1H7 SNP may be associated with BP disorders and/or lithium response, thus suggesting that genetic variations in CREB are associated with BP disorders and also that CREB may be involved in lithium response in BP patients.
Yuan et al. (2010) determined extracellular signal regulated kinase (ERK) signaling proteins, including CREB, in the cortex of subjects with BP, MDD, and SZ subjects. Whereas they found a significant decrease of CREB protein levels in MDD and SZ subjects, they did not observe any difference in CREB protein expression between BP and control subjects.
It is hard to compare our study with that of Young et al. (2004), as the latter was performed in amygdala. Nevertheless, our observation that CREB levels were lower in DLPFC is similar to their observation. In contrast to the observation of Yuan et al. (2010), who found no change in CREB protein levels between BP and control subjects, we observed a significant decrease in BP subjects and no change in SZ subjects.
While there are some studies of CREB in BP disorder, the role of CREB in SZ appears to be less clear. The only other study of CREB in SZ was reported by Kyosseva et al. (2000) in the cerebellar vermis of SZ subjects. They found increased levels of CREB protein in the cerebellar vermis of SZ subjects compared with controls, as opposed to our findings of decreased CREB in CG. The reasons for this discrepancy may be due to a different brain area used in their study.
The reasons or mechanisms that could alter CREB expression levels in BP or SZ subjects are unclear. One possibility is that it may be related to an altered signaling cascade that affects CREB expression and its translational activity. Several signaling systems converge at the level of CREB. For example, CREB is activated by PKA, a component of the AC signaling system, PKC, a component of the PI signaling system, or GSK-3β, a component of the Wnt signaling system (Grimes and Jope, 2001; Hagiwara et al., 1993; Xie and Rothstein, 1995). Since alteration of PKA, PKC, and GSK-3β has been suggested in BP illness (Pandey et al., 2003; Pandey et al., 2010), it is quite possible that altered expression and function of CREB may be related to abnormalities of these signaling cascades in BP disorder and schizophrenia. Decreased CREB function, as evidenced by decreased CRE-DNA binding activity, may be related to decreased CREB protein expression in the DLPFC and CG of BP subjects and the CG of SZ subjects.
Since abnormalities of CREB expression have been reported in teenage suicide (Pandey et al., 2007) and adult suicide (Dwivedi et al., 2003), we examined if CREB abnormalities in BP and SZ subjects were due to suicide. However, we did not find significant difference in CREB expression between total suicide and total non-suicide subjects, or BP suicide versus BP non-suicide, or SZ suicide versus SZ non-suicide subjects.
One limitation of this study is that the BP subjects, as well as the SZ subjects, were treated with lithium and other psychoactive drugs at the time of death. Specifically, it has been shown that the treatment with lithium, but not with other mood stabilizing drugs, causes changes in CREB levels (Wang et al., 1999). Thus, it is quite possible that the observed changes may be related to the effect of lithium treatment. However, when we compared the CREB in those BP patients who were treated with lithium or those who were not treated by lithium, or who were treated with other psychoactive drugs, we did not find any significant differences between the groups. Therefore, it appears unlikely that observed CREB changes in BP or SZ subjects are related to previous exposure to psychoactive drugs.
In conclusion, this study shows abnormalities of CREB functions and its protein and gene expression may be associated with the pathophysiology of BP illness and SZ. However, abnormalities of CREB in SZ may be specific to certain brain regions, such as CG, which has been shown to be associated with SZ. On the other hand, abnormalities of both DLPFC and CG have been observed in BP disorders and our observation does suggest CREB abnormality in these two areas of BP subjects. It will be of interest to examine if abnormal CREB expression also results in the abnormality of its target genes, such as BDNF, in the brain.
Acknowledgements
We thank the Harvard Brain Tissue Resource Center, Boston MA for providing the postmortem brain samples under PHS grant number R24 MH068855.
Role of funding source
This study was supported by a grant RO1 MH077254 to Dr. Pandey from the National Institute of Mental Health, Rockville, MD. This agency had no role in study design, acquisition and interpretation of data or writing of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that they have no financial interests or potential conflicts of interest related directly or indirectly to this work.
Contributors
XR performed CRE-DNA binding and CREB protein analyses in brain samples and participated in data interpretation. He also wrote the first draft of the paper and participated in critical revision of the manuscript.
HSR performed mRNA analyses of CREB in the brain samples.
MAK performed mRNA analyses of CREB in the brain samples.
RB Performed statistical analyses of the results.
YD performed literature search, participated in data interpretation and in critical revision of the manuscript.
GNP had access to all data (reported and unreported) from the study and had complete freedom to direct its analysis and its reporting, without influence from the sponsors. He further affirms that there was no editorial direction or censorship from the sponsor. He was responsible for the conception of the study, had the lead in the analysis and interpretation of the data, and wrote the manuscript.
All authors have contributed to and have approved the final version of the manuscript and approve the submission of the manuscript.
References
- Akin D, Manier DH, Sanders-Bush E, Shelton RC. Signal transduction abnormalities in melancholic depression. Int J Neuropsychopharmacol. 2005;8:5–16. doi: 10.1017/S146114570400478X. [DOI] [PubMed] [Google Scholar]
- Benes FM, Davidson J, Bird ED. Quantitative cytoarchitectural studies of the cerebral cortex of schizophrenics. Arch Gen Psychiatry. 1986;43:31–35. doi: 10.1001/archpsyc.1986.01800010033004. [DOI] [PubMed] [Google Scholar]
- Beyer JL, Krishnan KR. Volumetric brain imaging findings in mood disorders. Bipolar Disord. 2002;4:89–104. doi: 10.1034/j.1399-5618.2002.01157.x. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk Y, Young LT. The neurobiology of bipolar disorder: focus on signal transduction pathways and the regulation of gene expression. Can J Psychiatry. 2002;47:135–148. doi: 10.1177/070674370204700203. [DOI] [PubMed] [Google Scholar]
- Boer U, Cierny I, Krause D, Heinrich A, Lin H, Mayr G, Hiemke C, Knepel W. Chronic lithium salt treatment reduces CRE/CREB-directed gene transcription and reverses its upregulation by chronic psychosocial stress in transgenic reporter gene mice. Neuropsychopharmacology. 2008;33:2407–2415. doi: 10.1038/sj.npp.1301640. [DOI] [PubMed] [Google Scholar]
- Borrelli E, Montmayeur JP, Foulkes NS, Sassone-Corsi P. Signal transduction and gene control: the cAMP pathway. Critical Reviews in Oncology/Hematology. 1992;3:321–338. [PubMed] [Google Scholar]
- Bouras C, Kovari E, Hof PR, Riederer BM, Giannakopoulos P. Anterior cingulate cortex pathology in schizophrenia and bipolar disorder. Acta Neuropathol. 2001;102:373–379. doi: 10.1007/s004010100392. [DOI] [PubMed] [Google Scholar]
- Bullock BP, Habener JF. Phosphorylation of the cAMP response element binding protein CREB by cAMP-dependent protein kinase A and glycogen synthase kinase-3 alters DNA-binding affinity, conformation, and increases net charge. Biochemistry. 1998;37:3795–3809. doi: 10.1021/bi970982t. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends in Neurosciences. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154:1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- Cechinel-Recco K, Valvassori SS, Varela RB, Resende WR, Arent CO, Vitto MF, Luz G, de Souza CT, Quevedo J. Lithium and tamoxifen modulate cellular plasticity cascades in animal model of mania. J Psychopharmacol. 2012;26:1594–1604. doi: 10.1177/0269881112463124. [DOI] [PubMed] [Google Scholar]
- Chen G, Zeng WZ, Yuan PX, Huang LD, Jiang YM, Zhao ZH, Manji HK. The mood-stabilizing agents lithium and valproate robustly increase the levels of the neuroprotective protein bcl-2 in the CNS. J Neurochem. 1999;72:879–882. doi: 10.1046/j.1471-4159.1999.720879.x. [DOI] [PubMed] [Google Scholar]
- Cotter D, Landau S, Beasley C, Stevenson R, Chana G, MacMillan L, Everall I. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002a;51:377–386. doi: 10.1016/s0006-3223(01)01243-4. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002b;12:386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Du J, Gould TD, Manji Hk. Neurotrophic Signaling in Mood Disorders. In: Finkel T, Gutkind JS, editors. Signal Transduction and Human Disease. John Wiley & SOns, Inc.; 2003. pp. 411–445. Published online. [Google Scholar]
- Dwivedi Y, Rao JS, Rizavi HS, Kotowski J, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60:273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Robins E, Guze SB, Woodruff RA, Jr., Winokur G, Munoz R. Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry. 1972;26:57–63. doi: 10.1001/archpsyc.1972.01750190059011. [DOI] [PubMed] [Google Scholar]
- Fiol CJ, Williams JS, Chou CH, Wang QM, Roach PJ, Andrisani OM. A secondary phosphorylation of CREB341 at Ser129 is required for the cAMP-mediated control of gene expression. A role for glycogen synthase kinase-3 in the control of gene expression. J Biol Chem. 1994;269:32187–32193. [PubMed] [Google Scholar]
- Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Arch Gen Psychiatry. 2004;61:459–467. doi: 10.1001/archpsyc.61.5.459. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. 2nd Edition. New York: Oxford University Press; 2007. [Google Scholar]
- Grimes CA, Jope RS. CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J Neurochem. 2001;78:1219–1232. doi: 10.1046/j.1471-4159.2001.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara M, Brindle P, Harootunian A, Armstrong R, Rivier J, Vale W, Tsien R, Montminy MR. Coupling of hormonal stimulation and transcription via the cyclic AMP-responsive factor CREB is rate limited by nuclear entry of protein kinase A. Mol Cell Biol. 1993;13:4852–4859. doi: 10.1128/mcb.13.8.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek T, Carrey N, Alda M. Neuroanatomical abnormalities as risk factors for bipolar disorder. Bipolar Disord. 2005;7:393–403. doi: 10.1111/j.1399-5618.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- Haldane M, Frangou S. New insights help define the pathophysiology of bipolar affective disorder: neuroimaging and neuropathology findings. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:943–960. doi: 10.1016/j.pnpbp.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Hammonds MD, Shim SS. Effects of 4-week treatment with lithium and olanzapine on levels of brain-derived neurotrophic factor, B-cell CLL/lymphoma 2 and phosphorylated cyclic adenosine monophosphate response element-binding protein in the sub-regions of the hippocampus. Basic Clin Pharmacol Toxicol. 2009;105:113–119. doi: 10.1111/j.1742-7843.2009.00416.x. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nature Neuroscience. 2001;4:261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Luu C, Hazlett EA, Siegel BV, Jr., Lohr J, Wu J, Haier RJ, Bunney WE., Jr. Decreased anterior cingulate gyrus metabolic rate in schizophrenia. Am J Psychiatry. 1997;154:682–684. doi: 10.1176/ajp.154.5.682. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Austin DR, Chen G, Manji HK. Cellular mechanisms underlying affective resiliency: the role of glucocorticoid receptor- and mitochondrially-mediated plasticity. Brain Res. 2009;1293:76–84. doi: 10.1016/j.brainres.2009.06.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS. The prevalence and disability of bipolar spectrum disorders in the US population: re-analysis of the ECA database taking into account subthreshold cases. J Affect Disord. 2003;73:123–131. doi: 10.1016/s0165-0327(02)00332-4. [DOI] [PubMed] [Google Scholar]
- Koch JM, Kell S, Aldenhoff JB. Differential effects of fluoxetine and imipramine on the phosphorylation of the transcription factor CREB and cell-viability. Journal of Psychiatric Research. 2003;37:53–59. doi: 10.1016/s0022-3956(02)00061-4. [DOI] [PubMed] [Google Scholar]
- Kyosseva SV, Elbein AD, Hutton TL, Griffin ST, Mrak RE, Sturner WQ, Karson CN. Increased levels of transcription factors Elk-1, cyclic adenosine monophosphate response element-binding protein, and activating transcription factor 2 in the cerebellar vermis of schizophrenic patients. Arch Gen Psychiatry. 2000;57:685–691. doi: 10.1001/archpsyc.57.7.685. [DOI] [PubMed] [Google Scholar]
- Laifenfeld D, Karry R, Klein E, Ben-Shachar D. Alterations in cell adhesion molecule L1 and functionally related genes in major depression: a postmortem study. Biological Psychiatry. 2005;57:716–725. doi: 10.1016/j.biopsych.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Lee KA, Masson N. Transcriptional regulation by CREB and its relatives. Biochimica et Biophysica Acta. 1993;1174:221–233. doi: 10.1016/0167-4781(93)90191-f. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Ginty DD. Function and regulation of CREB family transcription factors in the nervous system. Neuron. 2002;35:605–623. doi: 10.1016/s0896-6273(02)00828-0. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Mai L, Jope RS, Li X. BDNF-mediated signal transduction is modulated by GSK3beta and mood stabilizing agents. J Neurochem. 2002;82:75–83. doi: 10.1046/j.1471-4159.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- Mamdani F, Alda M, Grof P, Young LT, Rouleau G, Turecki G. Lithium response and genetic variation in the CREB family of genes. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:500–504. doi: 10.1002/ajmg.b.30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manier DH, Shelton RC, Sulser F. Noradrenergic antidepressants: does chronic treatment increase or decrease nuclear CREB-P? Journal of Neural Transmission. 2002;109:91–99. doi: 10.1007/s702-002-8239-6. [DOI] [PubMed] [Google Scholar]
- Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. The Journal of Biological Chemistry. 1996;271:14214–14220. doi: 10.1074/jbc.271.24.14214. [DOI] [PubMed] [Google Scholar]
- Muly C. Signal transduction abnormalities in schizophrenia: the cAMP system. Psychopharmacol Bull. 2002;36:92–105. [PubMed] [Google Scholar]
- Nestler EJ, Greengard P. Protein phosphorylation and regulation of neuronal function. Little, Brown,, Boston. 1994 [Google Scholar]
- Nibuya M, Nestler EJ, Duman RS. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols M, Weih F, Schmid W, DeVack C, Kowenz-Leutz E, Luckow B, Boshart M, Schutz G. Phosphorylation of CREB affects its binding to high and low affinity sites: implications for cAMP induced gene transcription. The EMBO Journal. 1992;11:3337–3346. doi: 10.1002/j.1460-2075.1992.tb05412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Chuang DM. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J Neurochem. 1997;69:2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR. Cyclic AMP response element-binding protein in post-mortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. Int J Neuropsychopharmacol. 2007;10:621–629. doi: 10.1017/S1461145706007231. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR, Tamminga C. Altered expression and phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS) in postmortem brain of suicide victims with or without depression. J Psychiatr Res. 2003;37:421–432. doi: 10.1016/s0022-3956(03)00047-5. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, SridharaRao J, Ren X, Janicak PG, Sharma R. Protein kinase C and phospholipase C activity and expression of their specific isozymes is decreased and expression of MARCKS is increased in platelets of bipolar but not in unipolar patients. Neuropsychopharmacology. 2002;26:216–228. doi: 10.1016/S0893-133X(01)00327-X. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Ren X, Rizavi HS, Dwivedi Y. Glycogen synthase kinase-3beta in the platelets of patients with mood disorders: effect of treatment. J Psychiatr Res. 2010;44:143–148. doi: 10.1016/j.jpsychires.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiatry. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- Sairanen M, O'Leary OF, Knuuttila JE, Castren E. Chronic antidepressant treatment selectively increases expression of plasticity-related proteins in the hippocampus and medial prefrontal cortex of the rat. Neuroscience. 2007;144:368–374. doi: 10.1016/j.neuroscience.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G. Cellular pathology in the dorsolateral prefrontal cortex distinguishes schizophrenia from bipolar disorder. Curr Mol Med. 2003;3:427–436. doi: 10.2174/1566524033479663. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annual Review of Biochemistry. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Tanis KQ, Duman RS. Intracellular signaling pathways pave roads to recovery for mood disorders. Ann Med. 2007;39:531–544. doi: 10.1080/07853890701483270. [DOI] [PubMed] [Google Scholar]
- Tardito D, Mori S, Racagni G, Smeraldi E, Zanardi R, Perez J. Protein kinase A activity in platelets from patients with bipolar disorder. J Affect Disord. 2003;76:249–253. doi: 10.1016/s0165-0327(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Thome J, Sakai N, Shin K, Steffen C, Zhang YJ, Impey S, Storm D, Duman RS. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiraboschi E, Tardito D, Kasahara J, Moraschi S, Pruneri P, Gennarelli M, Racagni G, Popoli M. Selective phosphorylation of nuclear CREB by fluoxetine is linked to activation of CaM kinase IV and MAP kinase cascades. Neuropsychopharmacology. 2004;29:1831–1840. doi: 10.1038/sj.npp.1300488. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton MR, Dragunow I. Is CREB a key to neuronal survival? Trends in Neurosciences. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- Wang JF, Asghari V, Rockel C, Young LT. Cyclic AMP responsive element binding protein phosphorylation and DNA binding is decreased by chronic lithium but not valproate treatment of SH-SY5Y neuroblastoma cells. Neuroscience. 1999;91:771–776. doi: 10.1016/s0306-4522(98)00627-7. [DOI] [PubMed] [Google Scholar]
- Xie H, Rothstein TL. Protein kinase C mediates activation of nuclear cAMP response element-binding protein (CREB) in B lymphocytes stimulated through surface Ig. J Immunol. 1995;154:1717–1723. [PubMed] [Google Scholar]
- Yasuda S, Liang MH, Marinova Z, Yahyavi A, Chuang DM. The mood stabilizers lithium and valproate selectively activate the promoter IV of brain-derived neurotrophic factor in neurons. Mol Psychiatry. 2009;14:51–59. doi: 10.1038/sj.mp.4002099. [DOI] [PubMed] [Google Scholar]
- Young LT, Bezchlibnyk YB, Chen B, Wang JF, MacQueen GM. Amygdala cyclic adenosine monophosphate response element binding protein phosphorylation in patients with mood disorders: effects of diagnosis, suicide, and drug treatment. Biological Psychiatry. 2004;55:570–577. doi: 10.1016/j.biopsych.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Yuan P, Zhou R, Wang Y, Li X, Li J, Chen G, Guitart X, Manji HK. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord. 2010;124:164–169. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]