Abstract
We developed a live, fully attenuated Mycobacterium tuberculosis vaccine candidate strain with two independent attenuating auxotrophic mutations in leucine and pantothenate biosynthesis. The ΔleuD ΔpanCD double auxotroph is fully attenuated in the SCID mouse model and highly immunogenic and protective in the extremely sensitive guinea pig tuberculosis model, reducing both bacterial burden and disease pathology.
The rising worldwide incidence of tuberculosis (TB) caused by Mycobacterium tuberculosis infection, coupled with the concurrent human immunodeficiency virus (HIV)/AIDS epidemic, has created the need for improved TB therapies and a more effective preventative vaccine. The effectiveness of the currently available vaccine, Mycobacterium bovis bacillus Calmette-Guérin (BCG), derived by many passages from an M. bovis isolate, has varied enormously in different parts of the world. Furthermore, BCG vaccination has not provided adequate protection against pulmonary disease in adults (2). The reasons for the variability in efficacy of BCG remain unresolved (7). Exposure to environmental mycobacteria may provide some protection against M. tuberculosis, thereby masking the effects of BCG immunization, or alternatively interfere with the initial replication of the BCG vaccine strain (9, 14, 22). Another reason that BCG may not be optimally protective is that it is known to lack between 100 and 150 genes that are present in the M. tuberculosis genome (5), some of which encode immunologically important antigens (4, 5), including ESAT-6 (13), a protein that has been identified as a protective antigen in various animal immunization studies (3, 8, 24, 28). In considering vaccine development, it is important to bear in mind that the target population in the developing world will certainly include immunocompromised individuals infected with HIV, for whom even a live attenuated vaccine such as BCG presents a risk. Since the HIV-infected population is particularly vulnerable to mycobacterial disease (11), it is critical that any novel TB vaccine demonstrate safety in the setting of compromised immunity and previous M. tuberculosis exposure. Importantly, BCG immunization has been reported to be harmful in immunocompromised individuals, and a number of cases of disseminated BCG infection in HIV-positive children and adults have been documented (1, 6, 16, 20, 25, 30).
Of the several approaches being applied to the development of new TB vaccines (33), we have used strategies to create live, nonreplicating attenuated strains of M. tuberculosis. The advantage of this approach has been demonstrated by evidence that live vaccines are usually able to elicit a superior, longer-term memory immune response than nonliving vaccines (21, 29). In addition, vaccines derived from M. tuberculosis, which includes more than 100 genes deleted from BCG, express more M. tuberculosis-specific antigens. Previously, we demonstrated the feasibility of this approach by the creation of independent leucine and pantothenate auxotrophic mutants that elicited protective immunity in a murine model of M. tuberculosis infection (15, 27). Importantly, we showed that leucine auxotrophy alone rendered M. tuberculosis incapable of replicating in macrophages in vitro, and furthermore we observed no increase in bacterial numbers over time in the organs of immunocompetent and immunocompromised SCID mice challenged with the M. tuberculosis leucine auxotroph (15; unpublished data). The apparent lack of in vivo replication associated with leucine auxotrophy seemingly indicates that host leucine is inaccessible to the M. tuberculosis mutant, thus impairing its ability to replicate in the host. In this study, we describe the combination of two independent, attenuating, nonrevertible mutations, with deletions in the essential leuD (M. tuberculosis H37Rv gene number Rv2987c) and panCD (Rv3602c and Rv3601c) loci in M. tuberculosis, creating a strain that is highly attenuated in vivo and auxotrophic for both leucine and pantothenate. The rationale for combining two mutations was to ensure the safety of the strain in the unlikely occurrence of a second-site suppressor mutation or a recombination event leading to loss of auxotrophy at one or the other of the loci. In order to facilitate downstream development and testing of the double auxotroph as a potential vaccine candidate, additional in vitro and in vivo safety aspects were addressed. Finally, we chose to test the immunogenic and protective capacities of this strain in the highly susceptible guinea pig model, in which a very few bacilli represent a lethal dose (18).
Construction of a ΔleuD ΔpanCD double auxotroph of M. tuberculosis H37Rv.
For reasons of safety and in consideration of future clinical vaccine development, the ΔleuD ΔpanCD mutant was created using good laboratory practice for documentation of stepwise attenuation of the parent strain, M. tuberculosis H37Rv 102J23. This parent strain (obtained from Frank Collins, formerly of the Food and Drug Administration [FDA], Rockville, Md.) had not been previously cultured in medium containing bovine serum albumin. All subsequent culture steps were performed with documented lots of OADC (Becton Dickinson and Co., Sparks, Md.) so that the country of origin for the bovine-derived components could be established to exclude the possibility of contamination with bovine spongiform encephalopathy prions. The first step in the construction of the double auxotroph was to generate an unmarked leuD deletion mutant using a suicide vector approach similar to that which we previously described (23). Subsequently, the panCD locus in the ΔleuD strain was deleted and marked with a hygromycin resistance (Hygr) cassette by using specialized phage transduction (27). Southern blot and PCR analysis confirmed that the final strain had undergone allelic replacement at the leuD and panCD loci (see supplemental Fig. 1S at http://www.hsph.harvard.edu/bloomlab/), removing 359 bp of leuD and 1,298 bp of panCD.
FIG. 1.
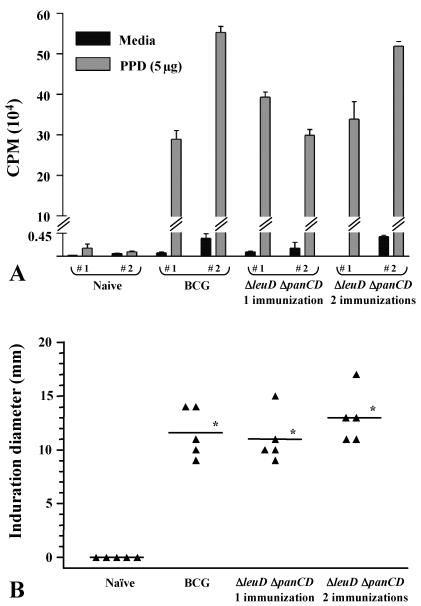
Immune response to PPD in guinea pigs immunized with the ΔleuD ΔpanCD strain. Outbred female Hartley guinea pigs were immunized i.d. with 5 × 105 CFU of the ΔleuD ΔpanCD strain, followed 6 weeks later by a second immunization with 3 × 106 CFU of the same strain. At the time that this group received the booster vaccination, a second group received a single immunization with 3 × 106 CFU of the ΔleuD ΔpanCD strain, while a third group received 3 × 106 CFU of BCG-P. A control group was unimmunized. Each experimental group consisted of five animals. (A) Five weeks after the last immunization, PBMCs were isolated from whole blood by Ficoll-Histopaque (Sigma, St. Louis, Mo.) density-gradient centrifugation. Two individual guinea pigs were tested per treatment group. PBMCs (2 × 105/well) were incubated at 37°C in 5% CO2 for 5 days with medium alone, phytohemagglutinin A (Sigma) (data not shown), or 5 μg of PPD (Mycos Research) per well. Proliferation was assessed on day 5 by [3H]thymidine (0.5 μCi/well) incorporation for 6 h. Cells were harvested with a Skatron cell harvester, and incorporated thymidine was measured using a beta-plate liquid scintillation counter. Values are expressed as mean counts per minute of triplicate samples. Error bars represent standard deviations from the mean. (B) DTH responses were determined 7 weeks after the last immunization. Four TU (data not shown) or 40 TU of PPD (FDA/Center for Biologics Evaluation and Research, Bethesda, Md.) was injected i.d. in the right flank, and the diameter of induration was measured at 24 h. Mean induration diameters (in millimeters) (indicated by horizontal lines) were 11.6 ± 2.3 for BCG-P-immunized animals, 11.0 ± 2.3 for animals immunized once with the ΔleuD ΔpanCD strain, and 13 ± 2.4 for animals immunized twice with the ΔleuD ΔpanCD strain. *, P < 0.001 as determined by one-way analysis of variance.
As predicted, the ΔleuD ΔpanCD strain failed to grow on minimal medium (7H9) (10% OADC, 0.2% glycerol, 0.05% Tween 80) or when the medium was singly supplemented with either leucine (50 μg/ml) or pantothenate (24 μg/ml). Growth could be restored only in the presence of both leucine and pantothenate (see supplemental Fig. 2S at the URL listed above), confirming that the strain was auxotrophic for these two compounds. The growth rate of the ΔleuD ΔpanCD strain in minimal medium supplemented with both leucine and pantothenate was similar to that of wild-type M. tuberculosis in minimal medium (see supplemental Fig. 2S). The ΔleuD ΔpanCD strain was complemented with both leuD and panCD (under the control of the mycobacterial hsp60 promoter) in a single copy integrated at the att site, eliminating the requirement for exogenous leucine and pantothenate and confirming that the observed auxotrophy was due solely to the mutation of these two loci.
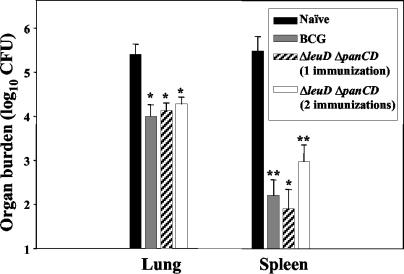
FIG. 2.
Protection of guinea pigs against aerosol challenge with M. tuberculosis H37Rv. Outbred female Hartley guinea pigs (n = 5 per group) were immunized i.d. once with M. bovis BCG-P, once with the ΔleuD ΔpanCD strain, or twice (6 weeks apart) with the ΔleuD ΔpanCD strain. A negative control group was unimmunized. Seven weeks subsequent to the last immunization, animals were challenged via the aerosol route with 20 CFU of M. tuberculosis H37Rv. Spleen and right caudal lung bacterial burdens were determined 5 weeks postchallenge. Error bars represent standard deviations from the mean. *, P < 0.001; **, P < 0.01; determined by one-way analysis of variance.
Because further development of the ΔleuD ΔpanCD strain as a potential vaccine candidate would require large-scale GMP production, involving serial passage in vitro, we conducted studies to determine the stability of the strain during in vitro growth. Reversion analysis was performed on three separate occasions. The ΔleuD ΔpanCD strain was cultured in broth medium supplemented with both leucine and pantothenate, but without hygromycin, and then plated onto 7H10 agar (with 10% OADC, 0.2% glycerol, and 100 μg of cycloheximide/ml) containing either pantothenate alone or leucine alone. No prototrophic clones (out of 2 × 109, 2 × 1010, and 2 × 1010 CFU plated for experiments 1, 2, and 3, respectively) were identified, establishing the reversion frequency as <10−11. Similarly, no reversion was observed after repeated in vitro subculturing of the strain. After six in vitro passages, representing approximately 41 doublings, no prototrophic clones arose out of a final 3 × 1010 CFU plated, further demonstrating that the auxotrophic phenotype of the strain is stable and does not revert to prototrophy during repeated in vitro passage.
To ensure that the inclusion of a Hygr marker in the strain did not influence susceptibility to commonly used antituberculous agents, the ΔleuD ΔpanCD strain was tested for sensitivity to the following antibiotics: ethambutol (2.5 μg/ml), isoniazid (0.1 and 0.4 μg/ml), pyrazinamide (100 μg/ml), rifampin (2.0 μg/ml), and streptomycin (2.0 μg/ml) (Focus Technologies Microbiology Laboratories, Cypress, Calif.). The strain was fully sensitive to all five antibiotics tested.
The ΔleuD ΔpanCD strain is attenuated in immunocompromised SCID mice.
A critical property of a live M. tuberculosis vaccine strain is that it be fully attenuated even in immunocompromised hosts. This is particularly relevant in consideration of the worldwide HIV epidemic, in which immunocompromised individuals represent an important potential target population for any new TB vaccine candidate (11). Previously, we found that the individual auxotrophic strains were highly attenuated in vivo (15, 27); therefore, the double auxotroph would also be expected to be fully attenuated. To confirm this, the in vivo attenuation of the ΔleuD ΔpanCD strain was assessed by infection of immunocompromised BALB/cJ SCID mice, which lack T and B cells. Mice were obtained from Jackson Laboratories (Bar Harbor, Maine) and housed according to institutional protocols. BALB/cJ SCID mice are highly susceptible to M. tuberculosis infection and succumb to intravenous challenge with 7 × 103 CFU of wild-type M. tuberculosis H37Rv in approximately 4 weeks (mean survival time, 31 days) (see supplemental Fig. 3S at the URL listed above). However, SCID mice challenged with the ΔleuD ΔpanCD strain at four times the wild-type challenge dose (3 × 104 CFU) were able to clear the infection and remained healthy for >41 weeks (Fig. 3S). This demonstrates that the ΔleuD ΔpanCD strain is attenuated even in a severely immunocompromised host which succumbs to BCG challenge (12). Furthermore, infection of SCID mice with a doubly complemented ΔleuD ΔpanCD strain (both the leuD and panCD loci) resulted in a rate of mortality similar to that of wild-type-infected animals (mean survival time, 40 days) (Fig. 3S), thereby confirming that the observed attenuation of the double auxotroph was due to the mutations in the leuD and panCD loci and not to polar effects on adjacent genes.
FIG. 3.
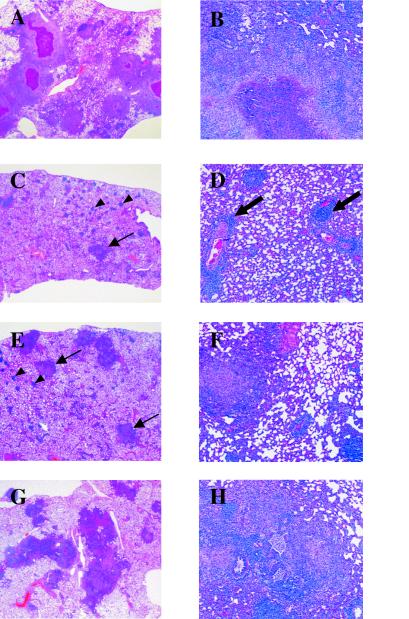
Histopathology of the lungs of vaccinated and unvaccinated guinea pigs 5 weeks after an aerosol challenge with M. tuberculosis. (A and B) Left caudal lung section from an unvaccinated guinea pig showing severe multifocal and spreading granulomatous pneumonia with central caseation necrosis. (B) Higher-power photomicrograph showing the spreading granulomatous reaction composed of epithelioid cells accompaniedby large numbers of macrophages and lymphocytes surrounding the central caseation necrosis. (C and D) Lung of a guinea pig vaccinated with BCG-P. The lower-power photomicrograph (C) shows multiple sites of focal perivascular and interstitial discrete accumulations of moderate to large numbers of lymphocytes (arrowheads). Also shown are scattered granulomatous foci composed of epithelioid cells and numerous lymphocytes (arrows). Very mild caseation necrosis was evident in a few of the granulomas. The higher-power photomicrograph (D) shows perivascular lymphoid accumulations (arrows). (E and F) Lung of a guinea pig vaccinated once with the ΔleuD ΔpanCD strain. (E) The lungs had lower numbers of discrete focal granulomas widely scattered in the parenchyma (arrows). Multifocal small, localized lymphocytic accumulations (arrowheads) were apparent, similar to those in the BCG-vaccinated guinea pigs. (F) Higher-power photomicrograph showing a discrete granuloma composed of predominantly epithelioid cells accompanied by low to moderate numbers of lymphocytes and focal lymphocytic accumulations in the parenchyma and perivascular regions. A few granulomas had mild central caseation necrosis. (G and H) Lung from a guinea pig vaccinated twice with the ΔleuD ΔpanCD strain, exhibiting extensive coalescing granulomatous pneumonia, particularly along airways (panel G). The spreading areas of inflammation were composed of epithelioid cells and moderate numbers of lymphocytes, accompanied by macrophages. Many of the granulomas showed mild to moderate central caseation necrosis, considerably less pronounced than in the unvaccinated guinea pigs, but more prominent than in BCG- and singly immunized animals.
Immunogenicity and protective efficacy in guinea pigs.
Previous investigations of the vaccine potential of the individual leucine and pantothenate auxotrophs suggested that these strains were able to elicit protection against challenge with virulent M. tuberculosis in the murine model (15, 27). Both strains reduced lung pathology and increased survival times of infected animals. However, the mouse is relatively resistant to M. tuberculosis infection, in contrast to the far more susceptible guinea pig (18, 26). Therefore, we chose to test the immunizing and protective capacity of the double auxotroph in the more rigorous and highly susceptible guinea pig aerosol TB challenge model.
Previous data had shown that the single ΔleuD auxotroph apparently does not replicate in vivo and is cleared from the lung, liver, and spleen of immunocompetent BALB/cJ mice within 13 weeks (15). As this relatively rapid clearance might limit the quantity of antigen available for immune priming, we also included a ΔleuD ΔpanCD strain-primed and -boosted group in the experimental design in an attempt to achieve more prolonged immunization. Female Hartley guinea pigs (300 to 400 g) (Elm Hill Breeders, Chelmsford, Mass.) were housed according to institutional protocols. One group of animals was immunized intradermally (i.d.) with the ΔleuD ΔpanCD strain, followed 6 weeks later by a second immunization with the ΔleuD ΔpanCD strain. At this time, a second group of animals received a single immunization with the ΔleuD ΔpanCD strain, and a third group received M. bovis BCG (Pasteur) (BCG-P) for comparison to the currently available M. tuberculosis vaccine. An unimmunized group was included as a negative control.
To confirm that the highly attenuated ΔleuD ΔpanCD strain was able to elicit a specific immune response to mycobacterial antigens, T-cell-proliferative responses of the vaccinated guinea pigs to purified protein derivative (PPD) were measured in vitro. Five weeks after the last immunization, peripheral blood mononuclear cells (PBMCs) were collected via cardiac puncture from two animals in each group and cultured with 5 μg of PPD/well (Mycos Research, LLC, Loveland, Colo.) and then pulsed with [3H]thymidine to assay specific proliferative responses to M. tuberculosis antigens. PBMCs from unimmunized guinea pigs did not proliferate in response to PPD, whereas those from animals immunized with the ΔleuD ΔpanCD strain showed proliferative responses comparable to BCG-P-immunized animals (Fig. 1A). Both singly immunized and boosted animals exhibited similar responses. Next, we assessed the cutaneous delayed-type hypersensitivity (DTH) response to PPD in all groups. At 7 weeks post-final immunization, guinea pigs were i.d. tested with 4 and 40 tuberculin units (TU) (Fig. 1B and supplementary Fig. 4S at the URL listed above) of PPD (FDA/Center for Biologics Evaluation and Research, Bethesda, Md.), followed by measurement of the diameter of induration 24 h later. As illustrated in Fig. 1B, immunization with the ΔleuD ΔpanCD strain induced statistically equivalent DTH responses when compared to immunization with viable BCG-P. These data demonstrate that despite its apparent inability to replicate, the double auxotroph is able to induce robust cell-mediated immune responses following immunization.
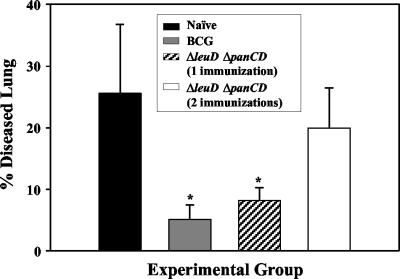
FIG. 4.
Morphometric analysis of lung pathology. The areas of lung lesions were measured on hematoxylin- and eosin-stained left caudal lobe sections by use of Scion Image software. These measurements were used to calculate the total area of the lung section occupied by diseased tissue. The percentage of diseased tissue was significantly reduced in animals immunized with BCG-P and the ΔleuD ΔpanCD strain relative to naive animals. *, P < 0.01 as determined by one-way analysis of variance.
Having established that the attenuated ΔleuD ΔpanCD auxotroph elicited strong cellular immune responses in vivo, we investigated whether this translated into protection against challenge with virulent M. tuberculosis. Therefore, the unimmunized control group and the groups vaccinated with the ΔleuD ΔpanCD strain and BCG-P were challenged at 7 weeks after the last immunization by aerosol with 20 CFU of M. tuberculosis H37Rv per animal. At 5 weeks postchallenge, right caudal lung and spleen bacterial burdens were determined by dilution plating of organ homogenates. Plates contained 2 μg of thiophene-2-carboxylic acid hydrazide (Sigma)/ml to prevent growth of any residual BCG-P. Guinea pigs immunized with the ΔleuD ΔpanCD strain (either once or twice) showed statistically significant reductions in both lung (1 log unit) and spleen (2 to 3 log units) bacterial burdens relative to naive controls. The protection afforded by single or double immunization with the ΔleuD ΔpanCD strain was statistically equivalent to that provided by BCG-P (Fig. 2). Splenic burden following aerosol challenge is an indication of hematogenous dissemination of the infection, and it is particularly noteworthy that a single immunization with the double auxotroph protected well against dissemination. In fact, in three of five animals receiving a single immunization with the ΔleuD ΔpanCD strain, no bacteria could be detected in the spleen (limit of detection, 50 CFU). Boosting with the ΔleuD ΔpanCD strain did not appear to enhance the protective effect.
Assessment of protective efficacy of vaccination with the ΔleuD ΔpanCD strain by lung histopathology evaluation.
Although guinea pig infection has many desirable features as a model for TB, the financial expense and space constraints associated with experiments using this species often preclude long-term survival studies. It has been suggested that bacillary burden at early time points may not necessarily correlate with survival; rather pulmonary pathology may be a better predictor of long-term disease outcome following M. tuberculosis infection (3). Histological evaluation of the lungs from guinea pigs in this study demonstrated that both BCG-P immunization and a single immunization with the ΔleuD ΔpanCD auxotroph caused significant reduction in the severity of lung damage following aerosol infection with a virulent strain of M. tuberculosis. Whereas the unvaccinated guinea pigs developed severe pneumonia with extensive widespread coalescing granulomas and severe caseation necrosis (Fig. 3A and B) , BCG-P-vaccinated animals developed milder, focal, localized granulomas and perivascular lymphocytic accumulations dispersed throughout the lung (Fig. 3C and D). The guinea pigs vaccinated once i.d. with the ΔleuD ΔpanCD double auxotrophic strain showed multiple scattered peribronchial, well-defined, fewer and smaller granulomas, reduced in severity compared to those of the unvaccinated group (Fig. 3E and F). However, guinea pigs boosted with the ΔleuD ΔpanCD double auxotroph developed more extensive granulomatous pneumonia compared to the guinea pigs given either BCG or a single dose of the ΔleuD ΔpanCD strain (Fig. 3G and H). The booster immunization offered no advantage, and the pulmonary pathology appeared to be more severe than after a single immunization. In summary, a single immunization with the live attenuated ΔleuD ΔpanCD M. tuberculosis auxotrophic vaccine strain induced protection in the susceptible guinea pig TB model comparable to that provided by BCG.
To semiquantitatively evaluate the extent of lung pathology, morphometric analysis was performed (Fig. 4) using Scion Image software (Scion Corporation, Frederick, Md.) (10) to measure the area of lung inflammation as a percentage of the total lung area. The groups immunized with BCG-P and singly immunized with the ΔleuD ΔpanCD strain showed statistically significant and equivalent reductions in the extent of lung damage compared to unvaccinated animals after challenge (Fig. 4). However, the lung pathology was exacerbated in the group that received a booster dose (Fig. 4). This observation is consistent with other recent studies (19, 31, 32). For example, it has been reported that existing M. tuberculosis infection in mice was worsened by the delivery of additional mycobacterial antigens administered either subcutaneously or by aerosol in the form of viable or heat-killed BCG and M. tuberculosis (19). The mechanism proposed to explain these findings is that, analogous to the well-known Koch phenomenon (17), the increased load of mycobacterial antigen induced a stronger inflammatory response and a worsening of lung pathology (19). This may explain the more severe lung pathology associated with the booster dose as reported here. These observations could have important implications for the design and implementation of any future TB vaccines. It will be important to know whether prior BCG immunization or subclinical infection would result in similar adverse inflammatory responses if such an individual was boosted with the new vaccine and whether adverse reactions could be avoided by adjusting the timing and/or dose of the prime-boost strategy.
In summary, our work describes the development of a live M. tuberculosis vaccine candidate with two attenuating auxotrophic mutations. This strain is fully attenuated and yet retains immunogenicity and protective capacity in a sensitive guinea pig TB aerosol challenge model. We have taken into consideration certain regulatory issues in the hope of facilitating subsequent development of the vaccine for human clinical trials. The ΔleuD ΔpanCD strain is safe even in severely immunocompromised (SCID) mice. Importantly, although the ΔleuD ΔpanCD strain is apparently nonreplicating and is fully attenuated in contrast with live BCG, the two vaccines elicited equivalent protection. In the case of the double auxotroph, since the protection achieved is independent of bacterial replication, this vaccine may have the advantage of greater safety in the context of target populations with a high prevalence of HIV infection, high exposure to environmental mycobacteria, or prior BCG vaccination. Importantly, recent data demonstrate that long-term protection in mice can be achieved by immunization with a related double auxotroph of M. tuberculosis (V. K. Sambandamurthy et al., unpublished data). We believe these data justify further development and testing of the ΔleuD ΔpanCD double auxotroph as a vaccine candidate against tuberculosis.
Acknowledgments
We thank Ilona Breiterene and Xiaowei Xiong for providing technical assistance.
This study was supported by the Aeras Global TB Vaccine Foundation.
Editor: A. D. O'Brien
REFERENCES
- 1.Armbruster, C., W. Junker, N. Vetter, and G. Jaksch. 1990. Disseminated bacille Calmette-Guerin infection in an AIDS patient 30 years after BCG vaccination. J. Infect. Dis. 162:1216. [DOI] [PubMed] [Google Scholar]
- 2.Baily, G. V. 1980. Tuberculosis prevention trial, Madras. Ind. J. Med. Res. 72(Suppl.):1-74. [PubMed] [Google Scholar]
- 3.Baldwin, S. L., C. D'Souza, A. D. Roberts, B. P. Kelly, A. A. Frank, M. A. Lui, J. B. Ulmer, K. Huygen, D. M. McMurray, and I. M. Orme. 1998. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect. Immun. 66:2951-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behr, M. A., and P. M. Small. 1997. Has BCG attenuated to impotence? Nature 389:133-134. [DOI] [PubMed] [Google Scholar]
- 5.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 6.Besnard, M., S. Sauvion, C. Offredo, J. Gaudelus, J. L. Gaillard, F. Veber, and S. Blanche. 1993. Bacillus Calmette-Guerin infection after vaccination of human immunodeficiency virus-infected children. Pediatr. Infect. Dis. J. 12:993-997. [DOI] [PubMed] [Google Scholar]
- 7.Bloom, B. R., and P. E. M. Fine. 1994. The BCG experience: implications for future vaccines against tuberculosis, p. 531-557. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection and control. ASM Press, Washington, D.C.
- 8.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect. Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandt, L., J. Feino Cunha, A. Weinreich Olsen, B. Chilima, P. Hirsch, R. Appelberg, and P. Andersen. 2002. Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis. Infect. Immun. 70:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dascher, C. C., K. Hiromatsu, X. Xiong, C. Morehouse, G. Watts, G. Liu, D. N. McMurray, K. P. LeClair, S. A. Porcelli, and M. B. Brenner. 2003. Immunization with a mycobacterial lipid vaccine improves pulmonary pathology in the guinea pig model of tuberculosis. Int. Immunol. 15:915-925. [DOI] [PubMed] [Google Scholar]
- 11.Dye, C., S. Scheele, S. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. W. H. O. Global Surveillance Monitoring Project. J. Am. Med Assoc. 282:677-686. [DOI] [PubMed] [Google Scholar]
- 12.Guleria, I., R. Teitelbaum, R. A. McAdam, G. Kalpana, W. R. Jacobs, Jr., and B. R. Bloom. 1996. Auxotrophic vaccines for tuberculosis. Nat. Med. 2:334-337. [DOI] [PubMed] [Google Scholar]
- 13.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez-Pando, R., L. Pavon, K. Arriaga, H. Orozco, V. Madrid-Marina, and G. Rook. 1997. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect. Immun. 65:3317-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hondalus, M. K., S. Bardarov, R. Russell, J. Chan, W. R. Jacobs, Jr., and B. R. Bloom. 2000. Attenuation of and protection induced by a leucine auxotroph of Mycobacterium tuberculosis. Infect. Immun 68:2888-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houde, C., and P. Dery. 1988. Mycobacterium bovis sepsis in an infant with human immunodeficiency virus infection. Pediatr. Infect. Dis. J. 7:810-812. [PubMed] [Google Scholar]
- 17.Koch, R. 1891. Fortsetzung uber ein Heilmittel gegen Tuberculose. Dutch Med. Wochenschr. 17:101-102. [Google Scholar]
- 18.McMurray, D. M. 1994. Guinea pig model of tuberculosis, p. 135-147. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection and control. ASM Press, Washington, D.C.
- 19.Moreira, A. L., L. Tsenova, M. H. Aman, L. G. Bekker, S. Freeman, B. Mangaliso, U. Schroder, J. Jagirdar, W. N. Rom, M. G. Tovey, V. H. Freedman, and G. Kaplan. 2002. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect. Immun. 70:2100-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien, K. L., A. J. Ruff, M. A. Louis, J. Desormeaux, D. J. Joseph, M. McBrien, J. Coberly, R. Boulos, and N. A. Halsey. 1995. Bacillus Calmette-Guerin complications in children born to HIV-1-infected women with a review of the literature. Pediatrics 95:414-418. [PubMed] [Google Scholar]
- 21.Opie, E. L., and J. Freund. 1937. An experimental study of protective inoculation with heat killed tubercle bacilli. J. Exp. Med. 66:761-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer, C. E., and M. W. Long. 1966. Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis. Am. Rev. Respir. Dis. 94:553-568. [DOI] [PubMed] [Google Scholar]
- 23.Pavelka, M. S., Jr., and W. R. Jacobs, Jr. 1999. Comparison of the construction of unmarked deletion mutations in Mycobacterium smegmatis,Mycobacterium bovis bacillus Calmette-Guérin, and Mycobacterium tuberculosis H37Rv by allelic exchange. J. Bacteriol. 181:4780-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and S. T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9:533-539. [DOI] [PubMed] [Google Scholar]
- 25.Reynes, J., C. Perez, I. Lamaury, F. Janbon, and A. Bertrand. 1989. Bacille Calmette-Guerin adenitis 30 years after immunization in a patient with AIDS. J. Infect. Dis. 160:727. [DOI] [PubMed] [Google Scholar]
- 26.Riley, R. L., C. C. Mills, W. Nyka, N. Weinstock, P. B. Storey, L. U. Sultan, M. C. Riley, and W. F. Wells. 1959. Aerial dissemination of pulmonary tuberculosis. A two year study of contagion in a tuberculosis ward. Am. J. Hyg. 70:185-196. [DOI] [PubMed] [Google Scholar]
- 27.Sambandamurthy, V. K., X. Wang, B. Chen, R. G. Russell, S. Derrick, F. M. Collins, S. L. Morris, and W. R. Jacobs. 2002. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat. Med. 9:9. [DOI] [PubMed] [Google Scholar]
- 28.Skinner, M. A., A. J. Ramsay, G. S. Buchan, D. L. Keen, C. Ranasinghe, L. Slobbe, D. M. Collins, G. W. de Lisle, and B. M. Buddle. 2003. A DNA prime-live vaccine boost strategy in mice can augment IFN-gamma responses to mycobacterial antigens but does not increase the protective efficacy of two attenuated strains of Mycobacterium bovis against bovine tuberculosis. Immunology 108:548-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith, T. 1917. Certain aspects of natural and acquired resistance to tuberculosis and their bearing on preventative measures. J. Am. Med. Assoc. 68:764-769. [Google Scholar]
- 30.Talbot, E. A., M. D. Perkins, S. F. Silva, and R. Frothingham. 1997. Disseminated bacille Calmette-Guerin disease after vaccination: case report and review. Clin. Infect. Dis. 24:1139-1146. [DOI] [PubMed] [Google Scholar]
- 31.Taylor, J. L., O. C. Turner, R. J. Basaraba, J. T. Belisle, K. Huygen, and I. M. Orme. 2003. Pulmonary necrosis resulting from DNA vaccination against tuberculosis. Infect. Immun. 71:2192-2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner, J., E. R. Rhoades, M. Keen, J. T. Belisle, A. A. Frank, and I. M. Orme. 2000. Effective preexposure tuberculosis vaccines fail to protect when they are given in an immunotherapeutic mode. Infect. Immun. 68:1706-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young, D. B. 2003. Tuberculosis, p. 279-289. In B. R. Bloom and P.-H. Lambert (ed.), The vaccine book. Academic Press, San Diego, Calif.