Abstract
Purpose
For prostate cancer that is thought to be locally recurrent after prostatectomy, the optimal timing, dose and techniques for salvage radiotherapy (SRT) have not been established. Here we perform a systematic review of published reports including regression meta-analysis and radiobiologic modelling to identify predictors of biochemical disease control and late toxicity.
Methods
We performed a review of published series reporting treatment outcomes following SRT. Studies with at least 30 patients, median PSA before SRT of less than 2.0 ng/mL, and median follow-up of greater than 36 months were identified. Univariate and multivariate analyses were performed to test Gleason Score, SRT dose, SRT timing, pre-SRT PSA, whole pelvic irradiation and androgen deprivation therapy as predictors of 5-year biochemical progression-free survival (bPFS) and severe (grade ≥ 3) late GI and GU toxicity. bPFS and toxicity data were fit to tumour control probability and normal tissue complication probability models, respectively.
Results
Twenty-five articles met the inclusion criteria for this analysis. Five-year bPFS ranged from 25% to 70%. Severe late GI toxicity rates were 0% to 9%, and severe late GU toxicity rates were 1–11%. On multivariate analysis, bPFS increased with SRT dose by 2.5% per Gy and decreased with pre-SRT PSA by 18.3% per ng/mL (p < 0.001). Late GI and GU toxicity increased with SRT dose by 1.2% per Gy (p = 0.012) and 0.7% per Gy (p = 0.010), respectively. Radiobiological models demonstrate the interaction between pre-SRT PSA, SRT dose and bPFS. For example, an increase in pre-SRT PSA from 0.4 to 1.0 ng/mL increases the SRT dose required to achieve a 50% bPFS rate from 60 to 70 Gy. This could increase the rate of severe late toxicity by approximately 10%.
Conclusion
Biochemical control rates following SRT increase with SRT dose and decrease with pre-SRT PSA. Severe late GI and GU toxicity rates also increase with SRT dose. Radiobiological models suggest that the therapeutic ratio of SRT may be improved by initiating treatment at low PSA levels.
Keywords: Prostate cancer, Salvage radiotherapy, PSA, Dose, Late toxicity
1. Introduction
Following radical prostatectomy for localised prostate cancer, 15–28% of men will subsequently develop a detectable serum prostate specific antigen (PSA) level.1,2 In the setting of suspected local recurrence, salvage radiotherapy (SRT) offers the potential for cure and is the mainstay of treatment. The optimal timing, dose and techniques for SRT, however, have not been established.
Due to a lack of published randomized trials, SRT practices to this point are not yet based on high-level evidence. Retrospective analyses have identified factors that may affect disease free survival rates following SRT such as pre-SRT PSA, PSA doubling time, Gleason Score (GS), surgical margin status, SRT dose and utilisation of androgen deprivation therapy (ADT).3–10
The interaction between pre-SRT PSA, SRT dose and prostate cancer control is of particular interest, as patients with high PSA levels may have more significant disease burden and require higher doses of radiotherapy to achieve cure.3 SRT dose escalation, however, may be associated with increased toxicity.4,11 The relationship between radiotherapy dose and toxicity in the postoperative setting has not been established but is clearly of concern to both clinicians and patients.
Here we perform a systematic review of published SRT series to identify predictors of disease control and late toxicity. We utilise both regression analyses and straightforward radiobiological models to explore the relationships between SRT dose, pre-SRT PSA and treatment outcome.
2. Methods
2.1. Search strategy and selection criteria
We performed a PubMed literature search for published series describing outcomes following salvage radiotherapy (SRT) for prostate cancer. The search was restricted to English-language articles and included the key words ‘prostate cancer’, ‘salvage radiotherapy’, ‘PSA’, and ‘late toxicity’. Series that reported 5-year biochemical progression-free survival (bPFS) rates and/or late toxicity rates following SRT were included in this analysis. Studies with median PSA levels of greater than 2.0 ng/mL before SRT were excluded, as PSA values in that range may be associated with distant progression.12 Series were also excluded if they contained fewer than 30 patients or had a median follow-up time of less than 36 months. Care was taken not to include multiple reports of outcomes from a single patient cohort.
We tabulated patient characteristics (median age, PSA before SRT, Gleason Score), treatment techniques (SRT doses, SRT timing, SRT techniques and androgen deprivation therapy [ADT] use), and outcomes (5-year bPFS, grade ≥ 3 late gastrointestinal (GI) and genitourinary (GU) toxicity) for each series that met the selection criteria. For the few series where SRT fraction sizes other than 1.8–2.0 Gy were used,13–15 we converted nominal total dose to the bioequivalent dose in 2.0 Gy fractions using the Linear Quadratic Equation with α/β = 3 Gy.16
2.2. Data analysis – Biochemical progression-free survival (bPFS)
Simple linear regression was utilised to identify factors associated with 5-year bPFS rate. Variables tested included median patient age, median interval from prostatectomy to SRT, use of ADT, use of whole pelvic radiotherapy, proportion of patients with Gleason Score ≤ and ≤7, and SRT dose. All of these were treated as continuous variables. These factors were also tested in a forward stepwise multivariate linear regression to identify independent predictors of bPFS. Terms with p-values less than 0.05 were retained in the model.
We devised the following tumour control probability (TCP) model,17 in which bPFS is a function of both SRT dose and pre-SRT PSA (as a surrogate for tumour burden):
| (1) |
| (2) |
| (3) |
| (4) |
where S is the surviving fraction of tumour cells, α represents intrinsic cell radiosensitivity, D is total SRT dose, n represents the number of tumour cells present at the time of SRT, C is a scaling constant relating the number of tumour cells to the pre-SRT PSA and K is a constant (≤1) that represents the highest rate of disease control that can be achieved with SRT (e.g. to account for the risk of occult metastasis at the time of SRT). This model was fit to clinical data (bPFS, dose and PSA), using the least-squares approach to determine optimal values for α, C and K.
2.3. Data analysis – Late toxicity
Simple linear regression was used to identify factors associated with severe (grade ≥ 3) late GI and GU toxicity. Stepwise multivariate linear regression was also utilised to identify independent predictors of severe toxicity. Again, terms with p-values less than 0.05 were retained in each model.
We also utilised the normal tissue complication probability (NTCP) model18:
where D is SRT dose, TCD50 is the dose that would cause a 50% complication rate and k is a fitting parameter equal to 25 divided by the slope of the NTCP curve at the TCD50 dose point. We considered severe GI, GU and aggregate (GI + GU) toxicity. In each case, the model was fit to toxicity data using the least squares approach to determine optimal values for TCD50 and k.
3. Results
3.1. Series
We identified 25 articles that met the inclusion criteria for this analysis (Table 1), including outcomes for a total of 3828 patients. All but three papers provided 5-year bPFS data, but numerous definitions for biochemical progression were utilised. Common definitions included any detectable PSA,7,19–22 PSA rise to above 0.2–0.4 ng/mL23–30, and PSA rise to 0.1–0.2 ng/mL above the nadir PSA.5,13,14,31,32 Thirteen articles described the incidence of severe (grade ≥ 3) GI and/or GU toxicity. Eleven of those papers used the RTOG/EORTC Late Radiation Morbidity Scoring Schema to grade toxicity.
Table 1.
Study characteristics
| Author | Number of patients | Median follow-up (months) | Median age (years) | Pelvic RT (%) | ADT (%) | Median time to RT (months) | Median PSA before RT (ng/mL) | RT dose (Gy) | GS ≤ 6 (%) | GS ≤ 7 (%) | 5-year bPFS (%) | Grade 3 GI toxicit (%) | Grade 3 GU toxicity (%) | Toxicity scale |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anscher19 | 89 | 48 | 64 | 0 | 9 | 14 | 1.4 | 66.0 | 39 | 78 | 50 | 0.0 | 1.1 | RTOG |
| Brooks31 | 114 | 76 | 64 | 10 | 11 | 17 | 0.9 | 64.0 | 33 | 76 | 40 | 0.0 | 1.8 | RTOG |
| Chawla20 | 54 | 45 | 67 | 0 | 0 | NR | 1.3 | 64.8 | 24 | 65 | 35 | 0.0 | 3.7 | RTOG |
| Choo23 | 62 | 46 | 65 | 0 | 14 | 16a | 1.0 | 60.0 | NR | 71 | 38 | NR | NR | - |
| Cozzarini11 | 186 | 92 | 68 | 17 | 67 | 29 | 0.3 | 72.0 | 55 | 79 | NR | NR | 11.0 | CTCAE v. 3.0 |
| Cremers13 | 197 | 40 | 64 | 0 | 0 | 26 | 0.6 | 66.2c | NR | 82 | 59 | 0.6 | 5.8 | RTOG |
| Katz24 | 115 | 42 | 64 | 0 | 39 | 27 | 0.9 | 66.6 | 16 | 72 | 39 | 0.0 | 10.0 | RTOG |
| King7 | 38 | >60 | 62 | 59 | 56 | 14b | 0.9 | 60.0 | 28 | 74 | 25 | NR | NR | - |
| 84 | 0.5 | 70.0 | 58 | |||||||||||
| Kruser14 | 50d | 43 | 63 | 13 | 17 | NR | 0.4 | 71.5c | 36a | 77a | 67 | NR | NR | - |
| MacDonald26 | 60 | 50 | 66 | 0 | 0 | 17 | 0.7 | 64.0 | NR | 75 | 48 | 2.0 | 2.9 | RTOG |
| 42 | 52 | 71 | 52 | 1.8 | 68.4 | NR | 81 | 38 | ||||||
| Maier25 | 170 | 49 | 64 | 0 | 16 | 34 | 1.2 | 68.0 | 23 | 74 | 55 | 3.0 | 6.0 | RTOG |
| Moreira32 | 102 | 50 | 66 | NR | 0 | NR | 0.6 | 66.0 | 30 | 87 | 70 | NR | NR | - |
| Neuhof49,51 | 171 | 39 | 67 | 0 | 29 | 38 | 1.1 | 63.0 | 40 | 74 | 35 | 0.0 | 4.7 | RTOG |
| Pearse50,52 | 72 | 45 | 67 | 0 | 100 | 36 | 0.9 | 66.0 | 19 | 86 | NR | 1.4 | 5.6 | CTCAE v. 2.0 |
| Peterson51,53 | 308 | 60 | 68 | 0 | 19 | 18 | 0.7 | 64.8 | 39 | 81 | NR | 0.3 | 1.0 | RTOG/CTCAE v. 3.0 |
| Pisansky27 | 166 | 52 | 68 | 2 | 4 | 13 | 0.9 | 64.0 | 30 | 60 | 46 | NR | NR | - |
| Quero28 | 59 | 38 | 62 | 0 | 12 | 26 | 1.4 | 68.6c | 20 | 71 | 41 | 9.1 | 3.6 | RTOG |
| Stephenson5 | 328 | 53 | 64 | NR | 14 | NR | 0.3 | 64.8 | 26 | 78 | 58 | NR | NR | - |
| 414 | 0.8 | 50 | ||||||||||||
| 243 | 1.3 | 35 | ||||||||||||
| Symon52,54 | 50 | 40 | NR | 100 | 0 | 24 | 1.2 | 66.6 | 56 | 82 | 33 | NR | NR | - |
| Taylor21 | 71 | 39 | 62a | 0 | 49 | NR | 0.8 | 70.0 | 26 | 65 | 66 | NR | NR | - |
| Thompson29 | 57 | 127 | 64 | 0 | 0 | 4a | 0.6 | 62.0 | 57 | 91 | 34 | NR | NR | - |
| Trabulsi30 | 96 | 58 | 63 | NR | 0 | 30 | 0.7 | 64.8 | 23 | 85 | 50 | NR | NR | - |
| Tsien53,55 | 57 | 74 | 66 | 23 | 0 | 28 | 1.2 | 65.0 | 33 | 85 | 35 | NR | NR | - |
| Ward36 | 211 | 50 | 66a | 0 | 0 | 20 | 0.6 | 64.0 | NR | NR | 54 | NR | NR | - |
| Wiegel22 | 162 | 42 | 66 | 0 | 0 | 19 | 0.3 | 66.0 | 53 | NR | 53 | 0.0 | 2.4 | RTOG |
Abbreviations: RT = radiotherapy, GI = gastrointestinal/Bowel, GU = genitourinary/Bladder, ADT = androgen deprivation therapy, GS = Gleason Score at prostatectomy, NR = not reported.
Estimate.
Time to failure after prostatectomy.
Bioequivalent dose.
Subset of patients with adequate follow-up.
Median follow-up in these articles ranged from 38 to 127 months (median: 50). The proportion of patients with Gleason Scores of 8 or higher ranged from 9% to 40%. Median interval from prostatectomy to SRT generally ranged from 13 to 52 months. The one exception to this was a subset of patients who were treated as part of an adjuvant radiotherapy trial but in fact had an elevated postoperative PSA29; median interval from surgery to radiotherapy was estimated to be 4 months for that cohort. Median PSA at the time of SRT ranged from 0.3 to 1.8 ng/mL (median: 0.9 ng/mL). Median SRT dose ranged from 60 to 72 Gy (median: 65 Gy). 2- or 3-dimensional RT planning was used in all but one series where intensity-modulated radiotherapy (IMRT) was utilised.14 In seven series, a portion of patients (range: 2–100%) received pelvic nodal irradiation. A portion of patients received ADT in 15 series (range: 4–100%).
3.2. Biochemical progression-free survival (bPFS)
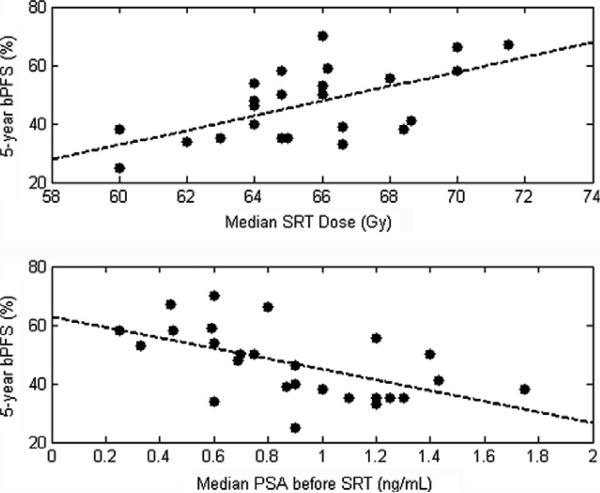
Five-year bPFS following SRT ranged from 25% to 70% (median: 47%). Scatter plots of bPFS against median SRT dose and PSA at the time of SRT are shown in Fig. 1.
Fig. 1.
Scatter plots of 5-year biochemical progression-free survival (bPFS) against median salvage radiotherapy (SRT) dose [top] and PSA prior to SRT [bottom]. (dotted lines represent results of simple linear regression).
Results of univariate analyses are shown in Table 2. Five-year bPFS was found to increase with median SRT dose (2.5% per Gy, 95% confidence interval: [1.0–4.0% per Gy]). bPFS also decreased significantly with increasing PSA before SRT (–18.1% per 1 ng/mL increase, 95% CI: [–29.2% to –7.0%]). bPFS was not significantly correlated with median patient age, timing of SRT, use of ADT, pelvic irradiation or Gleason Score. Stepwise multivariate linear regression yielded nearly identical results; 5-year bPFS increased with median SRT dose (2.5% per Gy, 95% CI: [1.5–3.6% per Gy]) and decreased with pre-SRT PSA (–18.3% per 1 ng/mL increase, 95% CI: [–26.3% to –10.4%]). No other variables were independently predictive of bPFS (Table 3).
Table 2.
Univariate analysis results. Each parameter was tested as a continuous variable. Shaded boxes indicate significant p-values (p< 0.05).
| Coefficient (95% CI, p-value) |
|||
|---|---|---|---|
| 5-year bPFS | Grade ≥ 3 late GI toxicity | Grade ≥ 3 late GU toxicity | |
| Time to SRT | 0.0% per month ([–0.5 to 0.6], p = 0.892) | 0.1% per month ([–0.2 to 0.3], p = 0.552) | 0.2% per month ([0.0–0.5], p = 0.045) |
| Median SRT dose | 2.5% per Gy ([1.0–4.0], p = 0.002) | 1.2% per Gy ([0.3–2.1], p = 0.012) | 0.8% per Gy ([0.1–1.6], p = 0.030) |
| Median PSA before SRT | –18.1% per ng/mL ([–29.2 to –7.0], p = 0.003) | 3.6% per ng/mL ([–1.7 to 8.8], p = 0.158) | –2.8% per ng/mL ([–8.3 to 2.7], p = 0.287) |
| ADTuse | 0.3% ([–28.0 to 28.8], p = 0.979) | –0.1% ([–7.3 to 7.1], p = 0.976) | 5.7% ([–0.6 to 12.0], p = 0.070) |
| Pelvic RT | –12.5% ([–32.8 to 7.8], p = 0.215) | 0.1% ([–0.2 to 0.3], p = 0.551) | 24.0% ([–14.5 to 62.4], p = 0.195) |
| Median age | –1.4% per year ([–3.9 to 1.0], p = 0.231) | –0.8% per year ([–1.7 to 0.1, p = 0.064) | 0.2% per year ([–1.0 to 1.3, p = 0.756) |
| Gleason Score ≤ 7 | 4.1% ([–68.9 to 77.1], p = 0.908) | –9.2% ([–44.2 to 25.8], p = 0.567) | –0.8% ([–41.4 to 39.8], p = 0.966) |
| Gleason Score ≤ 6 | –17.8% ([–66.9 to 31.3], p = 0.461) | –10.0% ([–26.4 to 6.4], p = 0.201) | –2.6% ([–19.9 to 14.8], p = 0.749) |
Abbreviations: bPFS = biochemical progression-free survival, SRT = salvage radiotherapy, RT = radiotherapy, GI = gastrointestinal/Bowel, GU = genitourinary/Bladder, ADT = androgen deprivation therapy.
Table 3.
Multivariate analysis results. In each case, forwards stepwise multilinear regression was used to arrive at the final model.
| Coefficient [95% CI] |
|||
|---|---|---|---|
| 5-year bPFS | Grade ≥ 3 late GI toxicity | Grade ≥ 3 late GU toxicity | |
| Time to SRT | - | - | 0.2% per month [0.0–0.4] |
| Median SRT dose | 2.5% per Gy [1.5–3.6] | 1.2% per Gy [0.3 to 2.1] | 0.7% per Gy [0.1–1.4] |
| Median PSA before SRT | –18.3% per ng/mL [–26.3 to –10.4] | - | - |
| p < 0.001 | P = 0.012 | p = 0.010 | |
Abbreviations: bPFS = biochemical progression-free survival, SRT = salvage radiotherapy, GI = gastrointestinal/Bowel, GU = genitourinary/Bladder.
3.3. Late toxicity
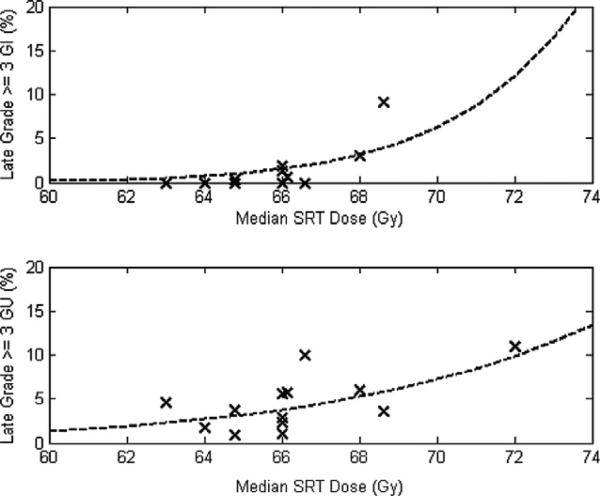
In the 13 series describing severe (grade ≥ 3) late GI toxicity, those complication rates ranged from 0% to 9%. Incidences of severe GU toxicity were between 1% and 11%. Scatter plots of late toxicity against median SRT dose are shown in Fig. 2.
Fig. 2.
Scatter plots of late grade ≥ 3 gastrointestinal [top] and genitourinary [bottom] toxicity against median salvage radiotherapy (SRT) dose. (dotted lines represent results of normal tissue complication probability modelling).
On univariate analysis, increasing median SRT dose was associated with increases in late grade ≥ 3 GI toxicity (1.2% per Gy, 95% CI: [0.3–2.1%], p = 0.012) as well as GU toxicity (0.8% per Gy, 95% CI: [0.1–1.6%], p = 0.030). GU toxicity rates also increased with increasing interval between prostatectomy and SRT (0.2% per month, 95% CI: [0.0–0.5%], p = 0.045). (Table 2) Stepwise multivariate linear regression yielded similar results. (Table 3) SRT dose was the only independent predictor of late GI toxicity. Late GU toxicity increased with both SRT dose (0.7% per Gy, 95% CI: [0.1–1.4%]) and median interval between prostatectomy and SRT (0.2% per month, 95% CI: [0.0–0.4%]).
3.4. Tumour control probability (TCP) and normal tissue complication probability (NTCP) modelling
Fitting the TCP model to bPFS data yielded an α of 0.12 Gy–1, C of 890 cells per ng/mL and K of 0.71. For late GI toxicity, optimal NTCP model parameters were a TCD50 of 74 Gy and k of 13 Gy. For late GU toxicity, these values were 83 Gy and 5 Gy, respectively. When GI and GU toxicity rates were added together, the optimal NTCP model parameters were a TCD50 of 74 Gy and k of 9 Gy.
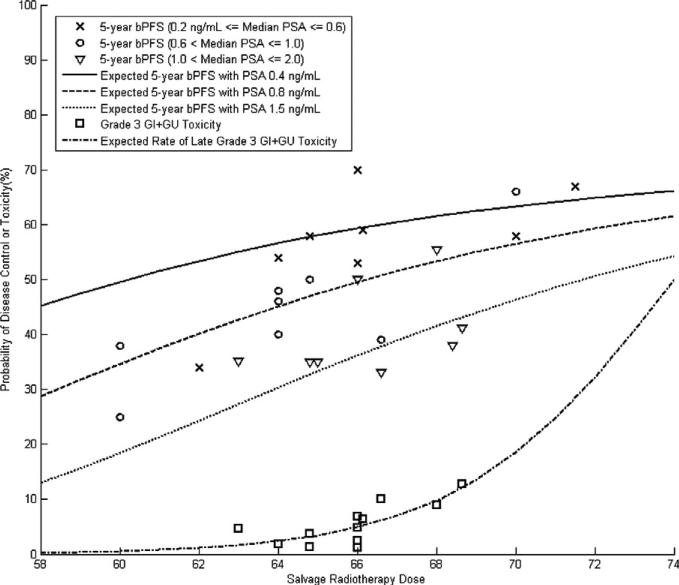
NTCP modelling results are shown graphically in Fig. 2. Scatter plots of disease control and combined GI and GU toxicity are displayed in Fig. 3. TCP and NTCP modelling results are also included in Fig. 3 to demonstrate the effects of SRT dose and pre-SRT PSA on the therapeutic ratio.
Fig. 3.
Scatter plots of biochemical progression free survival (bPFS) and combined grade ≥ 3 gastrointestinal (GI) and genitourinary (GU) toxicity. Curves represent the results of tumor control probability and normal tissue complication probability modelling.
4. Discussion
In this study, we have identified increased SRT dose and decreased pre-SRT PSA level as independent predictors of improved 5-year bPFS. SRT dose was also identified as an independent predictor of both late GI and late GU toxicity. Using data from published SRT series, we have generated TCP and NTCP models that can be used to predict rates of disease control and severe late toxicity. With a pre-SRT PSA level of 0.4 ng/mL, for e.g. an approximately 50% chance of 5-year bPFS can be achieved with an SRT dose of 60 Gy. Severe late toxicity rates with that SRT dose are on the order of 1%. If the PSA level before SRT is 1.0 ng/mL, on the other hand, a dose of approximately 70 Gy may be required to achieve the same probability of disease control. Severe late toxicity rates at that dose may reach 10%. This hypothesis-generating exercise demonstrates how the therapeutic ratio of SRT may be improved by initiation of treatment at low PSA levels.
The finding that bPFS increases with RT dose and decreases with pre-SRT PSA is consistent with existing data. Previous single-institution reports have suggested a dose-response for SRT after prostatectomy.7–10 ASTRO consensus guidelines released in 1999 recommend that ‘The highest dose of radiation therapy that can be given without morbidity is justifiable. . . the dose should be 64 Gy or slightly higher with standard fractionation.33 The relationship between PSA at salvage and bPFS has also been described in single-institution22,34–36 and multi-institutional5,29,37 reports. Common definitions for PSA failure following prostatectomy include PSA ≥ 0.4 ng/mL38–40 and PSA ≥ 0.2 ng/mL.41–43 Ultrasensitive PSA testing now allows for detection of PSA levels on the order of 0.01 ng/mL. This may allow earlier identification of patients destined to relapse after prostatectomy,44,45 but initiation of salvage therapy for patients with such low PSA levels may lead to overtreatment of men who would never develop clinically meaningful recurrence.45
Previous analyses by King et al. have separately described the importance of SRT dose and pre-SRT PSA.3,6 Our efforts build upon that work to investigate the interaction between these two factors as predictors of outcome. Because of the more complicated formulation of our TCP model, it is difficult to directly compare our results to previous reports. King et al. have reported that bPFS may increase by up to 3.8% per Gy6 and decrease by up to 4% with a 0.1 ng/mL increase in pre-SRT PSA.3 Our modelling results demonstrate the interaction of these variables; the bPFS gain from dose escalation is greatest when PSA is high, and the effect of PSA is greatest when SRT dose is low (Fig. 3).
Interestingly, in both King's recent editorial3 and our analysis, the maximum achievable 5-year bPFS following SRT appears to be between 70% and 80%. This suggests that a portion of patients who receive SRT with curative intent already have occult extrapelvic disease. Future efforts should focus on identifying such patients, who might benefit from systemic treatment rather than local therapy.
The incidence of grade ≥ 3 GU toxicity was found to increase slightly (0.2% per month) with median time from prostatectomy to initiation of SRT. This subtle effect may represent a chance finding. It also may be a result of clinician bias; physicians may be more likely to delay SRT when they perceive a high risk of treatment-related morbidity. Importantly, we did not find any evidence that late toxicity decreases when the interval from prostatectomy to SRT is increased. In light of the favourable toxicity profiles reported in adjuvant radiotherapy trials,46,47 this suggests that SRT should not be delayed for the sole purpose of limiting late toxicity.
It is important to note that all of the toxicity data used for this analysis came from series employing 2-D or 3-D treatment techniques. No published reports of toxicity following SRT delivered using more advanced techniques such as intensity-modulated radiotherapy (IMRT) had adequate follow-up for inclusion in this report. Existing reports with shorter follow-up, however, suggest that the late toxicity profile of SRT may be improved with the adoption of advanced treatment techniques. In one series with median follow-up of 30 months, SRT was delivered to a median dose of 75 Gy using IMRT, and grade ≥ 3 GI/GU toxicity was seen in only 6% of patients.48 In another IMRT series where patients received a median dose of 65 Gy in 26 fractions, no grade 3 toxicities have been reported after a median follow-up of 19 months.15 A third study, in which patients were treated with IMRT to a median dose of 68 Gy, reported a 2% incidence of late grade ≥ 3 toxicity after a median follow-up of 24 months.49 If modern treatment techniques are shown to reduce long-term morbidity following high-dose SRT, it would strengthen the case for dose escalation in the salvage setting. Interestingly, a 2010 survey suggests that the majority of radiation oncologists in the United States already use IMRT to deliver SRT doses of at least 70 Gy in their current practise.50
There are several limitations to this study that must be addressed. Our analysis utilised data from published reports; it would be more powerful if individual patient data were available. Though 5-year bPFS was reported in nearly every SRT study, its definition varied significantly. Events such as cause-specific death or development of distant metastasis are likely more meaningful endpoints than bPFS, but they were inconsistently reported and may require many years of follow-up. Several factors that may influence outcomes following SRT, such as PSA doubling time, were inconsistently reported and could not be incorporated into our analysis. While our modelling exercises were based on the assumption that PSA at the time of SRT is a surrogate for tumour burden, it is also possible that high PSA is simply an indicator of aggressive disease that is less likely to be cured by salvage therapy.
5. Conclusion
Our analysis indicates that biochemical control rates following SRT increase with SRT dose and decrease with pre-SRT PSA. Severe late GI and GU toxicity rates also increase with SRT dose. Modelling exercises demonstrate that the therapeutic ratio of SRT may be improved by initiating treatment at low PSA levels.
Footnotes
Conflict of interest statement
None declared.
REFERENCES
- 1.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 2.Boorjian SA, Karnes RJ, Crispen PL, et al. Radiation therapy after radical prostatectomy: impact on metastasis and survival. J Urol. 2009;182:2708–14. doi: 10.1016/j.juro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 3.King CR. Adjuvant Radiotherapy After Prostatectomy: Does Waiting for a Detectable Prostate-Specific Antigen Level Make Sense? Int J Radiat Oncol Biol Phys. 2011;80:1–3. doi: 10.1016/j.ijrobp.2010.10.073. [DOI] [PubMed] [Google Scholar]
- 4.Jereczek-Fossa BA, Zerini D, Fodor C, et al. Correlation between acute and late toxicity in 973 prostate cancer patients treated with three-dimensional conformal external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:26–34. doi: 10.1016/j.ijrobp.2009.07.1742. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson AJ, Scardino PT, Kattan MW, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol. 2007;25:2035–41. doi: 10.1200/JCO.2006.08.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King CR, Kapp DS. Radiotherapy after prostatectomy: is the evidence for dose escalation out there? Int J Radiat Oncol Biol Phys. 2008;71:346–50. doi: 10.1016/j.ijrobp.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 7.King CR, Spiotto MT. Improved outcomes with higher doses for salvage radiotherapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2008;71:23–7. doi: 10.1016/j.ijrobp.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 8.Bernard JR, Jr, Buskirk SJ, Heckman MG, et al. Salvage radiotherapy for rising prostate-specific antigen levels after radical prostatectomy for prostate cancer: dose-response analysis. Int J Radiat Oncol Biol Phys. 2010;76:735–40. doi: 10.1016/j.ijrobp.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 9.Tomita N, Kodaira T, Furutani K, et al. Early salvage radiotherapy for patients with PSA relapse after radical prostatectomy. J Cancer Res Clin Oncol. 2009;135:1561–7. doi: 10.1007/s00432-009-0603-7. [DOI] [PubMed] [Google Scholar]
- 10.Valicenti RK, Gomella LG, Ismail M, et al. Durable efficacy of early postoperative radiation therapy for high-risk pT3N0 prostate cancer: the importance of radiation dose. Urology. 1998;52:1034–40. doi: 10.1016/s0090-4295(98)00405-1. [DOI] [PubMed] [Google Scholar]
- 11.Cozzarini C, Fiorino C, Da Pozzo LF, et al. Clinical Factors Predicting Late Severe Urinary Toxicity After Postoperative Radiotherapy for Prostate Carcinoma. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.09.027. in press. [DOI] [PubMed] [Google Scholar]
- 12.Lee CT, Oesterling JE. Using prostate-specific antigen to eliminate the staging radionuclide bone scan. Urol Clin North Am. 1997;24:389–94. doi: 10.1016/s0094-0143(05)70385-2. [DOI] [PubMed] [Google Scholar]
- 13.Cremers RG, van Lin EN, Gerrits WL, et al. Efficacy and tolerance of salvage radiotherapy after radical prostatectomy, with emphasis on high-risk patients suited for adjuvant radiotherapy. Radiother Oncol. 2010;97:467–73. doi: 10.1016/j.radonc.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Kruser TJ, Jarrard DF, Graf AK, et al. Early hypofractionated salvage radiotherapy for postprostatectomy biochemical recurrence. Cancer. 2011;117:2629–36. doi: 10.1002/cncr.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong GW, Palazzi-Churas KL, Jarrard DF, et al. Salvage hypofractionated radiotherapy for biochemically recurrent prostate cancer after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2008;70:449–55. doi: 10.1016/j.ijrobp.2007.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–94. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 17.Suit H, Skates S, Taghian A, et al. Clinical implications of heterogeneity of tumor response to radiation therapy. Radiother Oncol. 1992;25:251–60. doi: 10.1016/0167-8140(92)90244-o. [DOI] [PubMed] [Google Scholar]
- 18.Niemierko A, Goitein M. Calculation of normal tissue complication probability and dose-volume histogram reduction schemes for tissues with a critical element architecture. Radiother Oncol. 1991;20:166–76. doi: 10.1016/0167-8140(91)90093-v. [DOI] [PubMed] [Google Scholar]
- 19.Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys. 2000;48:369–75. doi: 10.1016/s0360-3016(00)00645-3. [DOI] [PubMed] [Google Scholar]
- 20.Chawla AK, Thakral HK, Zietman AL, et al. Salvage radiotherapy after radical prostatectomy for prostate adenocarcinoma: analysis of efficacy and prognostic factors. Urology. 2002;59:726–31. doi: 10.1016/s0090-4295(02)01540-6. [DOI] [PubMed] [Google Scholar]
- 21.Taylor N, Kelly JF, Kuban DA, et al. Adjuvant and salvage radiotherapy after radical prostatectomy for prostate cancer. Int J Radiat Oncol Biol Phys. 2003;56:755–63. doi: 10.1016/s0360-3016(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 22.Wiegel T, Lohm G, Bottke D, et al. Achieving an undetectable PSA after radiotherapy for biochemical progression after radical prostatectomy is an independent predictor of biochemical outcome – results of a retrospective study. Int J Radiat Oncol Biol Phys. 2009;73:1009–16. doi: 10.1016/j.ijrobp.2008.06.1922. [DOI] [PubMed] [Google Scholar]
- 23.Choo R, Hruby G, Hong J, et al. Positive resection margin and/ or pathologic T3 adenocarcinoma of prostate with undetectable postoperative prostate-specific antigen after radical prostatectomy: to irradiate or not? Int J Radiat Oncol Biol Phys. 2002;52:674–80. doi: 10.1016/s0360-3016(01)02677-3. [DOI] [PubMed] [Google Scholar]
- 24.Katz MS, Zelefsky MJ, Venkatraman ES, et al. Predictors of biochemical outcome with salvage conformal radiotherapy after radical prostatectomy for prostate cancer. J Clin Oncol. 2003;21:483–9. doi: 10.1200/JCO.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 25.Maier J, Forman J, Tekyi-Mensah S, et al. Salvage radiation for a rising PSA following radical prostatectomy. Urol Oncol. 2004;22:50–6. doi: 10.1016/j.urolonc.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald OK, Schild SE, Vora S, et al. Salvage radiotherapy for men with isolated rising PSA or locally palpable recurrence after radical prostatectomy: do outcomes differ? Urology. 2004;64:760–4. doi: 10.1016/j.urology.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163:845–50. [PubMed] [Google Scholar]
- 28.Quero L, Mongiat-Artus P, Ravery V, et al. Salvage radiotherapy for patients with PSA relapse after radical prostatectomy: a single institution experience. BMC Cancer. 2008;8:26. doi: 10.1186/1471-2407-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson IM, Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296:2329–35. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 30.Trabulsi EJ, Valicenti RK, Hanlon AL, et al. A multi-institutional matched-control analysis of adjuvant and salvage postoperative radiation therapy for pT3–4N0 prostate cancer. Urology. 2008;72:1298–302. doi: 10.1016/j.urology.2008.05.057. [discussion 1302–1294] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks JP, Albert PS, Wilder RB, et al. Long-term salvage radiotherapy outcome after radical prostatectomy and relapse predictors. J Urol. 2005;174:2204–8. doi: 10.1097/01.ju.0000181223.99576.ff. [discussion 2208] [DOI] [PubMed] [Google Scholar]
- 32.Moreira DM, Jayachandran J, Presti JC, Jr, et al. Validation of a nomogram to predict disease progression following salvage radiotherapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2009;104:1452–6. doi: 10.1111/j.1464-410X.2009.08623.x. [DOI] [PubMed] [Google Scholar]
- 33.Cox JD, Gallagher MJ, Hammond EH, et al. Consensus statements on radiation therapy of prostate cancer: guidelines for prostate re-biopsy after radiation, for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. American Society for Therapeutic Radiology, Oncology Consensus Panel. J Clin Oncol. 1999;17:1155. doi: 10.1200/JCO.1999.17.4.1155. [DOI] [PubMed] [Google Scholar]
- 34.Pazona JF, Han M, Hawkins SA, et al. Salvage radiation therapy for prostate specific antigen progression following radical prostatectomy: 10-year outcome estimates. J Urol. 2005;174:1282–6. doi: 10.1097/01.ju.0000173911.82467.f9. [DOI] [PubMed] [Google Scholar]
- 35.Buskirk SJ, Pisansky TM, Schild SE, et al. Salvage radiotherapy for isolated prostate specific antigen increase after radical prostatectomy: evaluation of prognostic factors and creation of a prognostic scoring system. J Urol. 2006;176:985–90. doi: 10.1016/j.juro.2006.04.083. [DOI] [PubMed] [Google Scholar]
- 36.Ward JF, Zincke H, Bergstralh EJ, et al. Prostate specific antigen doubling time subsequent to radical prostatectomy as a prognosticator of outcome following salvage radiotherapy. J Urol. 2004;172:2244–8. doi: 10.1097/01.ju.0000145262.34748.2b. [DOI] [PubMed] [Google Scholar]
- 37.Macdonald OK, D'Amico AV, Sadetsky N, et al. Predicting PSA failure following salvage radiotherapy for a rising PSA post-prostatectomy: from the CaPSURE database. Urol Oncol. 2008;26:271–5. doi: 10.1016/j.urolonc.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 39.Amling CL, Bergstralh EJ, Blute ML, et al. Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol. 2001;165:1146–51. [PubMed] [Google Scholar]
- 40.Scher HI, Eisenberger M, D'Amico AV, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537–56. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 41.Cookson MS, Aus G, Burnett AL, et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: the American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J Urol. 2007;177:540–5. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 42.Heidenreich A, Aus G, Bolla M, et al. EAU guidelines on prostate cancer. Eur Urol. 2008;53:68–80. doi: 10.1016/j.eururo.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Boccon-Gibod L, Djavan WB, Hammerer P, et al. Management of prostate-specific antigen relapse in prostate cancer: a European Consensus. Int J Clin Pract. 2004;58:382–90. doi: 10.1111/j.1368-5031.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 44.Witherspoon LR, Lapeyrolerie T. Sensitive prostate specific antigen measurements identify men with long disease-free intervals and differentiate aggressive from indolent cancer recurrences within 2 years after radical prostatectomy. J Urol. 1997;157:1322–8. [PubMed] [Google Scholar]
- 45.Vassilikos EJ, Yu H, Trachtenberg J, et al. Relapse and cure rates of prostate cancer patients after radical prostatectomy and 5 years of follow-up. Clin Biochem. 2000;33:115–23. doi: 10.1016/s0009-9120(99)00099-5. [DOI] [PubMed] [Google Scholar]
- 46.Bolla M, van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366:572–8. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 47.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96–02/AUO AP 09/95. J Clin Oncol. 2009;27:2924–30. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 48.De Meerleer G, Fonteyne V, Meersschout S, et al. Salvage intensity-modulated radiotherapy for rising PSA after radical prostatectomy. Radiother Oncol. 2008;89:205–13. doi: 10.1016/j.radonc.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 49.Nath SK, Sandhu AP, Rose BS, et al. Toxicity analysis of postoperative image-guided intensity-modulated radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78:435–41. doi: 10.1016/j.ijrobp.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 50.Showalter TN, Ohri N, Teti KG, et al. Physician beliefs and practices for adjuvant and salvage radiation therapy after prostatectomy. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuhof D, et al. Long-term results and predictive factors of three-dimensional conformal salvage radiotherapy for biochemical relapse after prostatectomy. Int J Radiat Oncol Biol Phys. 2007;67:1411–7. doi: 10.1016/j.ijrobp.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 52.Pearse M, et al. Prospective assessment of gastrointestinal and genitourinary toxicity of salvage radiotherapy for patients with prostate-specific antigen relapse or local recurrence after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2008;72:792–8. doi: 10.1016/j.ijrobp.2008.05.063. [DOI] [PubMed] [Google Scholar]
- 53.Peterson JL, et al. Late toxicity after postprostatectomy salvage radiation therapy. Radiother Oncol. 2009;93:203–6. doi: 10.1016/j.radonc.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 54.Symon Z, et al. Radiation rescue for biochemical failure after surgery for prostate cancer: predictive parameters and an assessment of contemporary predictive models. Am J Clin Oncol. 2006;29:446–50. doi: 10.1097/01.coc.0000221237.58653.0e. [DOI] [PubMed] [Google Scholar]
- 55.Tsien C, et al. Long-term results of three-dimensional conformal adjuvant and salvage radiotherapy after radical prostatectomy. Urology. 2003;62:93–8. doi: 10.1016/s0090-4295(03)00127-4. [DOI] [PubMed] [Google Scholar]