Abstract
We previously reported that inhibition of nitric oxide (NO) increases the rate of bacteremia and maternal mortality in pregnant rats with uterine infection by Escherichia coli expressing the Dr fimbria (Dr+). Epithelial binding and invasion by Dr+ E. coli has also been shown to be dependent upon the expression level of the cellular receptor decay-accelerating factor (DAF; CD55). Here, we hypothesize that NO-related severity of infection could be mediated by changes in DAF expression and in the rate of epithelial invasion. The cellular basis of NO effects on epithelial invasion with Dr+ E. coli was studied using Ishikawa endometrial carcinoma cells as an in vitro model of the human endometrial epithelium. Initially, we show that Ishikawa cells produce NO and express both NO synthase enzymes, NOS II and NOS III, and DAF protein. We next tested the abilities of both Dr+ E. coli and a Dr− E. coli mutant to invade Ishikawa cells, and invasion was seen only with Dr+ E. coli. Invasion by Dr+ E. coli was decreased by elevated NO production and increased by NO inhibition. Elevated NO production significantly decreased DAF protein and mRNA expression in Ishikawa cells in a time- and dose-dependent manner. Here, we propose that in vitro invasion of an epithelial cell line is directly related to NO-regulated expression of DAF. The significance of NO-regulated receptor-ligand invasion is that it may represent a novel unrecognized phenomenon of epithelial defense against infection.
Although urogenital microbial infection in pregnancy is an important cause of maternal and neonatal morbidity and mortality, the mechanisms of defense against gestational intrauterine infection are poorly understood (8, 14, 23). Evidence obtained in studies of both humans and rats suggests that the bacteriostatic actions of nitric oxide (NO) are an important component of defense against urogenital infection (16, 30, 33). Nitric oxide is synthesized in situ from an l-arginine substrate by one or more of three NO synthase isoforms (NOS I, NOS II, and NOS III), each of which has been identified in the mouse, rat, and human (1, 27, 40, 41).
Several lines of evidence have demonstrated the involvement of intracellular NO in the host defense mechanisms against bacterial infections (5, 27). Recently, NO production and three NOS isoforms were reported to be present in rat uterine tissues, and elevated NOS II expression was demonstrated to contribute to the increased NO production in the rat uterus and consequent uterine quiescence during gestation (3, 4, 40). However, the role of the NO system in uterine defense mechanisms is not well understood.
Three separate lines of evidence from our laboratories have suggested that increased NO production by the urogenital tract in pregnancy protects against Escherichia coli infection. First, inhibition of NO synthesis in pregnant rats with an intrauterine infection increases maternal death (30). Second, the sensitivity of the female rat or mouse urinary tract to E. coli infection was increased with inhibition of NO (30, 33). Third, a spontaneous, localized increase in NO production and the expression of inducible NO synthase (NOS II) was observed in response to intrauterine infection (6). Interestingly, an in vitro NO donor had no bactericidal or static effect on bacterial growth, suggesting an indirect inhibitory effect on infection, possibly by modification of epithelial cell function.
Uropathogenic E. coli strains, especially those of the O75 serotype, have been found to be associated with unique gestational virulence (13). These strains express a gene cluster encoding Dr adhesins that allows E. coli invasion and accounts for 40% of pyelonephritis cases in the third trimester (12). In addition, Dr+ E. coli can cause chronic diarrhea in children and has been associated with recurrent or chronic urinary tract infection (32). Recent studies have demonstrated that adherence and invasion by E. coli in human cervical epithelial cells, HeLa cells, depends very much upon the presence of Dr fimbriae (10). In the absence of fimbriae, E. coli had no significant invasion.
The epithelial cell entry of Dr+ E. coli is mediated by a cellular receptor, decay-accelerating factor (DAF; CD55) (31, 36). Binding of Dr+ E. coli to the short consensus repeat 3 domain of DAF, expressed in Chinese hamster ovary (CHO) cells was found to be critical for internalization to occur (36). Moreover, the extent of Dr+ E. coli binding and internalization in these cells was shown to be proportional to the level of DAF protein expression (36). The physiological function of DAF is to protect the host cell from the cytotoxic effects of complement activation (24, 25). DAF is expressed by the human endometrial epithelium, and its expression is dynamically regulated through the menstrual cycle (42). DAF is also highly expressed at the feto-maternal interface. These locations of DAF are important, as the semiallogenic fetus requires protection from the complement attack. This is further supported by the studies showing that 100% of fetal mice deficient in the closely related protein CRRY are lost during pregnancy in a complement C3-dependent process (39).
Increasing NO production clearly decreases the rates and severity of infection by Dr+ E. coli, and treatment with an NO synthesis inhibitor clearly increases the severity of infection (30, 33). However, NO may not be very effective at directly killing E. coli, and Dr+ E. coli is not readily killed by human leukocytes (19). We therefore hypothesized that the urogenital (uterine) NO system might participate in a unknown host defense mechanism(s) preventing bacterial invasion by suppressing DAF expression. To test this hypothesis at the cellular level, we used a urogenital epithelial cell model (Ishikawa cells) that fulfills three criteria: (i) expression of NO synthases, (ii) expression of DAF, and (iii) allowing invasion of E. coli that is associated with gestational infections. We evaluated the modulating effects of NO on DAF expression and internalization of recombinant Dr+ E. coli and the Dr− mutant. Here, we provide evidence for a novel cellular mechanism of NO-controlled bacterial virulence by means of a unique modulatory effect on DAF receptor expression and therefore alteration of the risk of epithelial cell invasion.
Portions of these results have been presented previously in abstract form at the 47th annual meeting of the Society for Gynecologic Investigation, 21 to 25 March 2000, in Chicago, Ill.
MATERIALS AND METHODS
Cell culture and evaluation of nitrite production by Ishikawa cells.
Ishikawa cells are a human endometrial cell line derived from a well-differentiated endometrial adenocarcinoma that has been shown to mimic endometrial epithelial cells (29). Ishikawa cells were routinely cultured in Eagle's minimum essential medium (MEM) containing 2 mM l-glutamine, 100 μg of penicillin-streptomycin/ml, and 10 mM HEPES supplemented with 10% fetal calf serum at 37°C in a humidified 5% CO2 atmosphere. The cells were passaged every 10 days and replated in a 75-cm2 flask at 106/flask. In the subsequent experiments, we investigated the influence of NO-regulating reagents in the MEM and immunohistochemically assessed the presence of NOS II and NOS III enzymes in Ishikawa cells. For NO synthesis analysis, Ishikawa cells were incubated for 24 h in l-arginine-free medium (Life Technologies, Gaithersburg, Md.) or in medium containing d-arginine (Sigma Co., St Louis, Mo.), l-arginine (Life Technologies), or l-arginine combined with a nonselective NOS inhibitor, NG-nitro-l-argine methyl ester (L-NAME; Sigma Co.), at concentrations of 10−7, 10−5, and 10−3 M, respectively. The medium was collected and stored at −70°C for measuring nitrite production (assayed as a stable end stage product of NO).
Nitrite concentrations in the medium were measured in triplicate by the high-performance liquid chromatography method as described previously (6). Briefly, an aliquot of medium was injected into a spectrophotometric nitrite (NO2) and nitrate (NO3) analyzer, which consists of high-performance liquid chromatography pumps, a cadmium-reducing column, a postcolumn reactor, and a UV detector (System Bold 126; Beckman Inc., Fullerton, Calif.). The samples were carried through the analyzer in a 0.3% aqueous ammonium chloride buffer containing 0.07% EDTA (pH 8.0). Nitrates were reduced to nitrites on a cadmium-reducing column. The UV-absorbing derivative was formed by postcolumn reaction with a solution containing 0.1% N-(1-naphthyl) ethylenediamine dihydrochloride and 1% HCl. The areas of the absorbance peak at 546 nm were determined with an integrator.
Immunohistochemistry for detecting NOS II and NOS III in Ishikawa cells.
Ishikawa cells were grown and assayed at 37°C with 5% CO2 in MEM supplemented with 5% fetal calf serum. The cells were fixed in 75% cold acetone for 30 min, and primary monoclonal antibodies directed against NOS-II or NOS-III (Transduction Laboratory) were added. Staining was detected using a biotinylated secondary anti-mouse immunoglobulin G (IgG) (raised in horses) in combination with the avidin-biotin-peroxidase method (Elite ABC Kit; Vector Laboratories, Burlingame, Calif.) with 3,3′-diaminobenzidine as a chromogen. As controls, slides were incubated with mouse IgG to substitute for the primary NOS antibodies.
E. coli internalization assay.
To assess the effects of NO on bacterial invasion and internalization in Ishikawa cells, we used a Dr+ fimbriated clinical isolate of E. coli (strain IH11128 [O75:K5:H−])or its Dr− mutant E. coli Dr 14 (9). In addition, we used a recombinant strain, BN406 (the laboratory E. coli K-12 strain carrying plasmid pBJN406), encoding the production of Dr fimbriae, and the recombinant E. coli K-12 strain BN17, containing a transposon insertion mutation in the draE gene (Dr− phenotype) (9, 10, 31). Because the cellular receptor of Dr+ fimbriated E. coli, DAF, was shown to be expressed in epithelial cells, we initially confirmed the expression of DAF in Ishikawa cells. In experiments involving treatment of Ishikawa cells to modulate NO, 10,000 cells per well were plated in 24-well plates and incubated in l-arginine-free MEM with or without added d-arginine, l-arginine, NO donor, DETA-NO ([Z]-1-[2-aminoethyl]-N-[2-ammonioethyl]amino diazen-1-ium-1,2-diolate), l-arginine and L-NAME, or l-arginine and aminoguanidine (inducible NOS inhibitor) at concentrations of 10−7, 10−5, and 10−3 M for 24 h. Following the incubations, the media were removed and the plates were rinsed with phosphate-buffered saline (PBS). Then, bacterial suspensions in 500 μl of PBS, prepared as described previously (9, 10) for the adherence assay, were added to monolayers of Ishikawa cells. The plates were then incubated at 37°C in a CO2 incubator. The bacterial suspensions were removed at 30-min intervals, and the Ishikawa cells were washed twice and incubated for 1 h with MEM containing 200 μg of gentamicin per ml to kill the remaining extracellular bacteria. Equal volumes of 200 μl were transferred to Eppendorf tubes and lysed with 200 μl of 2× lysing mixture containing 20 mM TRIS-HCl, pH 7.5, 2 mM EDTA, and 2% Triton X-100 for 5 min. Three wells were assigned to each time point, and the assays were repeated three times. About 25 μl of the lysate was plated on Luria agar plates and incubated overnight at 37°C under 5% CO2. The following day, the colonies were counted, and the results are expressed as bacterial CFU per well (9, 10).
Expression of cellular receptor (DAF) protein in Ishikawa cells.
To assess the time-dependent effects of NO on DAF expression, Ishikawa cells were plated in 24-well plates (10,000/well) and then incubated in MEM without l-arginine or MEM containing d-arginine, l-arginine, NO donor, DETA-NO, l-arginine and L-NAME, or l-arginine and aminoguanidine at 10−3 M concentration for 12, 24, or 48 h. Following the incubations, the cells were rinsed, collected, and lysed for Western immunoblot analysis. In addition, we assessed the dose dependence of NO on DAF expression in these cells. Ishikawa cells in 24-well plates were incubated in the presence of different doses (10−5, 10−4, and 10−3 M) of the above-mentioned reagents for 24 h, and the cells were lysed for detection of DAF by Western immunoblotting.
Equal amounts of total protein of Ishikawa cells were size fractionated under nonreducing conditions in linear 10 to 20% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred onto polyvinylidene difluoride membranes. The cell lysate of CHO cells transfected with the human DAF gene served as a positive control. The membranes were blocked in PBS with 5% dry milk- 0.05% Tween 20 for 1 h. Incubation with primary monoclonal antibody (clone IH4) to human DAF (courtesy of D. M. Lublin, Washington University School of Medicine, Seattle, Wash.) was carried out overnight at 4°C. The membranes were then washed three times for 30 min each time with PBS-Tween 20 wash buffer and incubated with horseradish peroxidase-conjugated goat-anti-mouse antibody. The immunoconjugates were visualized with an enhanced chemiluminescence kit (Amersham Life Sciences, Piscataway, N.J.) as described previously. The intensities of specific immunoreactive bands were quantified using densitometric scanning. Densitometric units of specific protein bands are expressed relative to the values from background units.
RT-PCR analysis of DAF mRNA expression by Ishikawa cells.
For reverse transcription (RT)-PCR analysis, total RNA from 5 × 106 Ishikawa cells was extracted with Trizol (Life Technologies) RNA isolation reagent. The RT-PCRs were conducted using 1.0 μg of the purified RNA per RT-PCR bead (Amersham-Pharmacia) in a 25-μl volume. The primers for human DAF were CTG CGA GGT GCC AAC AAG and AGT AAT ACG ACT CAC TAT AGG GCA TTC AGG TGG TG (product size, 580 bp). The RT reaction was 30 min at 42°C, followed by a 5-min denaturation of the reverse transcriptase and 33 cycles of the following: 95°C for 1 min, 64°C for 1 min, 72°C for 50 s, and finally 72°C for 7 min. We used the human β-actin primers GTG GGG CGC CCC AGG CAC CA and CTC CTT AAT GTC ACG CAC GAT TTC (product size, 548 bp) as a positive control (23). The RT-PCRs were analyzed by running 10 μl of the RT-PCR mixtures in 1.5% agarose- 1× TAE gels. The results are expressed as the ratio of the densitometric readings for DAF mRNA to those for β-actin mRNA. Therefore, the results indicate relative changes in DAF mRNA levels. Finally, the RT-PCR products, obtained with the DAF primers, were identical to the published sequence of human DAF (24, 26), as confirmed by direct double-strand sequencing analysis (data not shown). We assessed the effects of various doses of NO on DAF mRNA expression in Ishikawa cells by incubating the cells in the presence of different doses (10−5, 10−4, and 10−3 M) of l-arginine, d-arginine, and DETA-NO for 24 h.
Statistical analysis.
The data on bacterial internalization in Ishikawa cells were analyzed using analysis of variance followed by the Bonferroni test. The Mann-Whitney U test was used when the data were nonparametrically distributed (9, 33). One-way analysis of variance or Student's t test was used to evaluate differences in the intensities of bands from various treatments. For all statistical tests, a P value of <0.05 was considered to represent a significant difference between the compared values.
RESULTS
Characterization of NO system in Ishikawa cells. (i) Production of NO in Ishikawa cells.
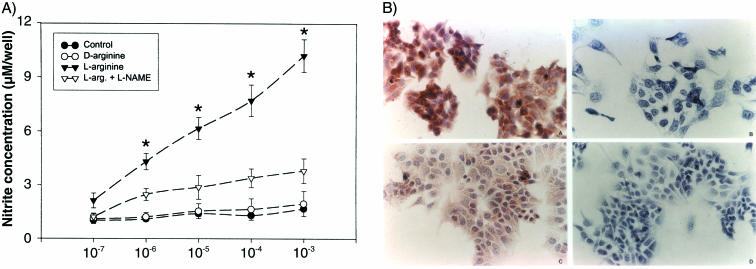
We examined NO synthesis by Ishikawa cells and determined the effects of treatment with the NOS substrate l-arginine, the nonsubstrate stereoisomer d-arginine, and the NOS inhibitor L-NAME. l-Arginine induced a dose-dependent and stereospecific increase in nitrite levels, which was inhibited by 0.1 mM L-NAME (Fig. 1A). These results suggest that Ishikawa cells express NOS and that the production of NO can be experimentally manipulated by NO substrate and inhibitors.
FIG. 1.
(A) Production of NO, measured as nitrites, by Ishikawa cells in culture. Equal numbers of cells (10,000/well) were incubated with various concentrations (10−7 to 10−3 M) of agents in l-arginine-free MEM or l-arginine-free medium alone (control). l-Arginine, but not d-arginine, is a substrate for NO synthesis, and L-NAME is an inhibitor of NO synthesis. The data are the mean ± standard error of the mean of six replicates. *, P < 0.05 compared with the controls. (B) Immunocytochemical staining for NOS II and NOS III in Ishikawa cells. Strong staining was apparent for both NOS II (upper right) and NOS III (lower right) in Ishikawa cells. The negative control (without NOS antibodies) shows an absence of staining either for NOS II (upper left) or for NOS III (lower left). Magnification, ×250.
(ii) Immunohistochemistry of NOS II and NOS III proteins in Ishikawa cells.
We assessed the presence of NOS II and NOS III enzyme proteins by immunohistochemistry. NOS II and NOS III proteins reactive with the respective monoclonal antibodies were detectable in the cytoplasm of Ishikawa cells (Fig. 1B). The specificity of staining was demonstrated in the presence of the respective antibodies (Fig. 1B, left) and was absent when the antibodies were replaced with nonspecific mouse IgG (Fig. 1B, right).
NO regulates E. coli internalization in Ishikawa cells.
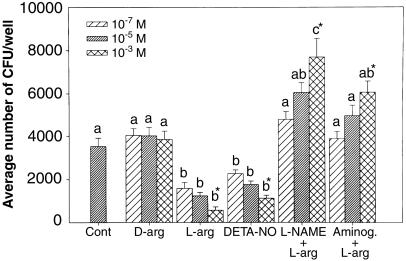
The Ishikawa model system was next utilized to study the effects of NO on internalization of Dr+ E. coli. Treatment with either NOS substrate, l-arginine, or a stable NO donor, DETA-NO, decreased invasion by Dr+ E. coli IH11128 in a dose-dependent manner (Fig. 2). The decreased invasion was blocked by the NO inhibitors L-NAME and aminoguanidine, also in a dose-dependent manner (Fig. 2). The effects of l-arginine were stereospecific, since the nonsubstrate stereoisomer, d-arginine, did not produce significant changes in bacterial internalization (Fig. 2). These data suggest that endogenous NO plays a role in modulating Dr+ E. coli invasion. In all of the experiments, invasion assays were done following the removal of NO-modifying agents. Therefore, the possible direct effect of NO on bacterial viability was eliminated.
FIG. 2.
Internalization of Dr+ E. coli IH11128 in Ishikawa cells following 24-h treatments with NO-modifying agents. The data represent means plus standard errors of the mean from six replicates. Prior to the bacterial internalization assay, Ishikawa cells were treated for 24 h in l-arginine (L-arg)-free medium (Cont) with or without various doses of d-arginine (10−5 to 10−3 M), l-arginine (10−5 to 10−3 M), DETA-NO (10−5 to 10−3 M), l-arginine (10−5 to 10−3 M) plus L-NAME (10−4 M), or l-arginine (10−5 to 10−3 M) plus aminoguanidine (Aminog.) (10−4 M). Groups with different letters above the bars vary significantly. Within each treatment group, the asterisk indicates statistical significance (P < 0.05) among the doses.
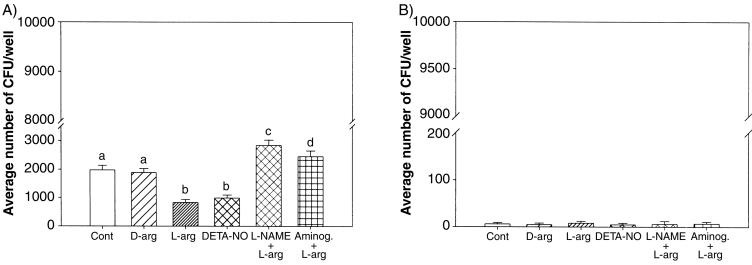
In order to determine whether the NO modulation of E. coli invasion is Dr specific, two laboratory E. coli strains, BN406 (Dr+) and BN17 (Dr−) were utilized. BN17 (draE mutant) is unable to express the structural protein of Dr fimbriae, while E. coli BN406 carries a Dr (draE)-expressing plasmid, pBJN406 (10). Dr+ E. coli BN406 displayed a level of invasion of Ishikawa cells similar to that of the clinical strain IH11128 (Fig. 2 and 3A). Suppression of NO production with different NOS inhibitors enhanced the invasiveness of BN406, and either an NO donor or NOS substrate reversed this effect (Fig. 3A). In contrast, Dr− BN17 E. coli did not invade Ishikawa cells (Fig. 3B), and reagents that alter NO synthesis in these cells had no effect. These results demonstrate that NO regulates E. coli invasion of Ishikawa cells in a Dr-specific manner.
FIG. 3.
Internalization of Dr+ E. coli (BN406 with expression of Dr fimbriae) (A) or its mutant Dr− E. coli (BN17, containing a transposon insertion mutation in the draE gene) (B) in Ishikawa cells following treatments with NO-modifying reagents as indicated in the legend to Fig. 2. Only a 10−3 M dose was used for all of the agents, except for L-NAME and aminoguanidine (Aminog.) (10−4 M). The data represent means ± standard errors of the mean from six replicates. Groups with different letters above the bars vary significantly (P < 0.05).
NO regulates the gene expression of DAF on Ishikawa cells.
Because the NO modulation of Ishikawa cells was specific for invasion by Dr+ E. coli, we hypothesized that NO regulation of bacterial invasion might be due to NO regulation of DAF expression. Therefore, we pharmacologically manipulated the NO system in Ishikawa cells and determined the effects on DAF protein and mRNA expression.
(i) Western analysis of DAF protein expression in Ishikawa cells:.
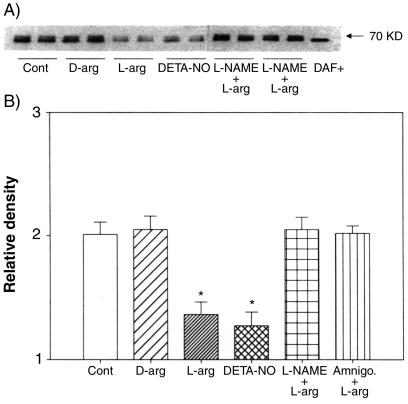
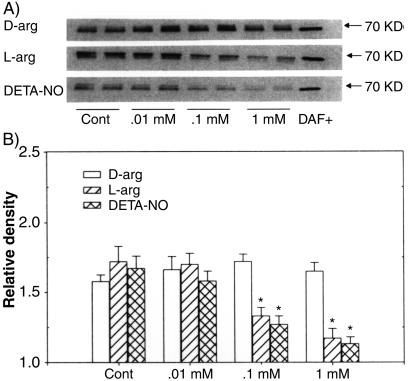
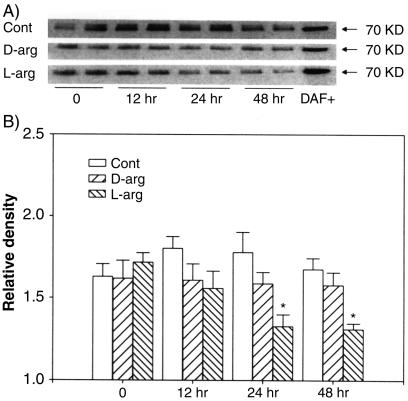
DAF protein isoforms were detected on Western blots clustered around the predicted mass of 70 kDa and almost identical in mass to the positive control protein from CHO cells overexpressing recombinant DAF (DAF+) (Fig. 4A). Densitometric analysis of the DAF bands showed that both l-arginine and DETA-NO inhibited, while L-NAME and aminoguamidine reversed, the inhibitory effects of l-arginine on the expression of DAF protein in Ishikawa cells (Fig. 4B). d-Arginine, the nonsubstrate stereoisomer of l-arginine, had no effect. The effects of l-arginine and DETA-NO were dose dependent (Fig. 5), with the effects of l-arginine showing pronounced time dependency (Fig. 6). Maximal inhibition by l-arginine was observed after 24 to 48 h of treatment (Fig. 6). These studies indicate that NO is capable of regulating DAF protein expression in Ishikawa cells in a time- and concentration-dependent manner.
FIG. 4.
Western blotting of DAF protein in Ishikawa cells treated for 24 h with 10−3 M l-arginine (L-arg), DETA-NO, d-arginine (D-arg), or l-arginine combined with 10−4 M L-NAME (L-NAME + L-arg) or aminoguanidine (Amnigo. + L-arg). (A) Cell lysates were size fractionated and probed with monoclonal antibodies to DAF. The bands at 70 kDa are representative of two replicates in each group. DAF+, positive control for DAF protein. (B) Relative densities of bands for DAF protein in cells treated with NO-modifying agents shown as the mean plus standard error of the mean of four replicates in each group. *, P < 0.05 compared with the densitometric units from the controls.
FIG. 5.
(A) Western blotting of DAF protein in Ishikawa cells treated for 24 h with various doses (0.01 to 1.0 mM) of l-arginine (L-arg), DETA-NO, or d-arginine (D-arg). The bands at 70 kDa represent two replicates from each treatment group. DAF+, positive control of DAF protein; Cont, control. (B) Relative densities of bands for DAF protein in treated cells; the data are expressed as the mean plus standard error of the mean of four replicates in each group. *, P < 0.05 compared with the densitometric units from the controls.
FIG. 6.
Western blotting of DAF protein in Ishikawa cells with 1 mM l-arginine (L-arg) or d-arginine (D-arg) for 12, 24, or 48 h. (A) The bands represent two replicates from each treatment group. DAF+, positive control of DAF protein; Cont, control. (B) Relative densities of bands for DAF protein in treated cells at various time; the data are the mean plus standard error of the mean from four replicates in each group. *, P < 0.05 compared with the densitometric units from the controls at time zero.
(ii) RT-PCR analysis of DAF mRNA in Ishikawa cells.
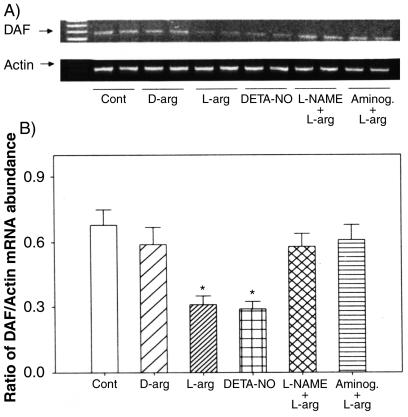
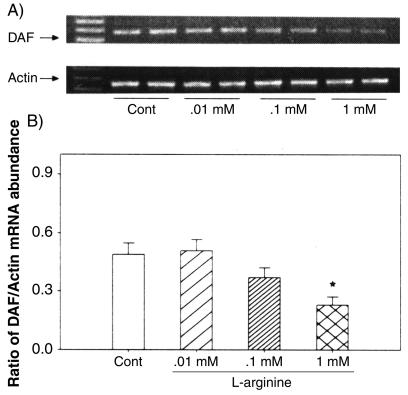
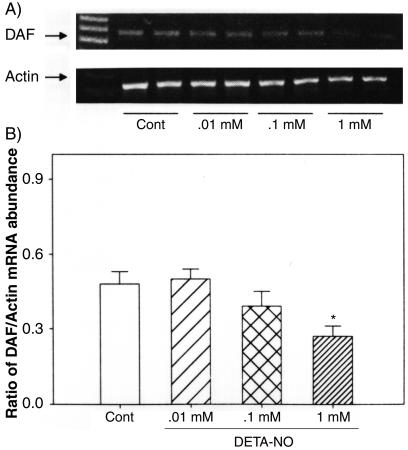
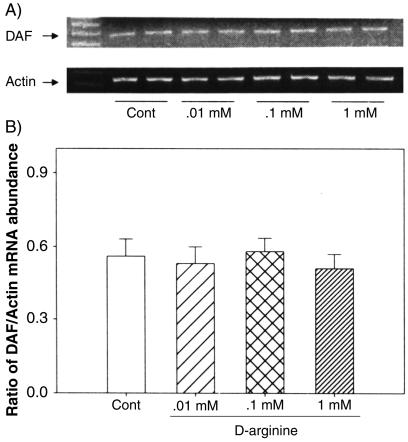
We examined the effects of NO manipulations on DAF gene expression at the mRNA level by RT-PCR analysis. The data on the expression of mRNA for DAF in Ishikawa cells following treatments with l-arginine, d-arginine, DETA-NO, L-NAME, and aminoguanidine are presented in Fig. 7. Treatment with either l-arginine or DETA-NO, but not d-arginine, decreased DAF mRNA expression, while treatment with either NO synthase inhibitor, L-NAME, or aminoguanidine reversed this effect (Fig. 7A). The densitometric analysis, relative to β-actin mRNA, indicated that the changes in DAF mRNA with these treatments were significant (Fig. 7B). The effects of l-arginine and DETA-NO were concentration dependent (Fig. 8 and 9), while d-arginine had no effect at the tested concentrations (Fig. 10) The results from the RT-PCR analysis are consistent with the data obtained by Western blotting of DAF protein in these cells, indicating that the effects of NO are at the mRNA level, likely secondary to changes in transcription.
FIG. 7.
RT-PCR analysis of DAF mRNA in Ishikawa cells treated for 24 h with 10−3 M l-arginine (L-arg), DETA-NO, d-arginine (D-arg), l-arginine combined with 10−4 M L-NAME (L-NAME + L-arg), or aminoguanidine (Aminog. + L-arg). Cont, control. (A) The bands show representative PCR products of DAF and the corresponding β-actin from two replicates in each group. (B) Densities of bands for DAF mRNA expressed as a ratio of β-actin mRNA in cells treated with NO-modifying agents expressed as the mean plus standard error of the mean of four replicates in each group. *, P < 0.05 compared with the densitometric units from the controls.
FIG. 8.
RT-PCR analysis of DAF mRNA in Ishikawa cells treated for 24 h with various doses (0.01 to 1.0 mM) of l-arginine. (A) The bands represent PCR products of DAF and the corresponding β-actin from two replicates in each treatment group. Cont, control. (B) Densities of bands for DAF mRNA expressed as a ratio of β-actin mRNA in treated cells; the data are expressed as the mean plus standard error of the mean of four replicates in each group. *, P < 0.05 compared with the densitometric units from the controls.
FIG. 9.
RT-PCR analysis of DAF mRNA in Ishikawa cells treated for 24 h with various doses (0.01 to 1.0 mM) of DETA-NO. (A) The bands represent PCR products of DAF and the corresponding β-actin from two replicates in each treatment group. Cont, control. (B) Densities of bands for DAF mRNA expressed as a ratio of β-actin mRNA in treated cells; the data are expressed as the mean plus standard error of the mean of four replicates in each group. *, P < 0.05 compared with the densitometric units from the controls.
FIG. 10.
RT-PCR analysis of DAF mRNA in Ishikawa cells treated for 24 h with various doses (0.01 to 1.0 mM) of d-arginine. (A) The bands represent PCR products of DAF and the corresponding β-actin from two replicates in each treatment group. (B) Densities of bands for DAF mRNA expressed as a ratio of β-actin mRNA in treated cells; the data are expressed as the mean plus standard error of the mean of four replicates in each group. *, P < 0.05 compared with the densitometric units from the controls.
DISCUSSION
In the present study, we utilized Ishikawa cells, a line derived from a well-differentiated endometrial adenocarcinoma, to investigate the role of NO in the regulation of bacterial invasion. We demonstrated that Ishikawa cells express both NOS II and NOS III isoforms and generate NO in the presence of l-arginine. The NO generated by Ishikawa cells substantially inhibited the invasion of Dr+ but not Dr− E. coli, an effect that was reversed by NOS inhibitors. The effects of NO modifiers on the bacterial invasion were both time and dose dependent. Furthermore, NO down-regulated expression of DAF protein and mRNA in a dose- and time-dependent manner, suggesting that the effects of NO on bacterial invasion may be mediated by regulation of DAF, the Dr− fimbria receptor. Therefore, we propose that NO generated in endometrial epithelial cells down-regulates DAF and thus reduces invasion of these cells, thereby playing a role in epithelial resistance to an invasive pathogen.
DAF (CD55) is a complement-regulatory protein that protects cells from autologous complement-mediated damage (15). DAF is widely expressed on blood cells, epithelial cells, endometrial cells, uterus, and kidney (15), and its levels of expression may play a role in modulating the pathophysiology of many disease processes. Low levels of DAF have been implicated in the destruction of red cells in patients with paroxysmal nocturnal hemoglobinuria and in luteal phase defect of the endometrium associated with infertility or pregnancy loss (37). Conversely, higher levels of expression of DAF were implicated in the escape of malignant cells of colon and endometrial carcinoma from complement-mediated lysis (11, 18, 34). In addition, DAF expression may have a protective role in renal transplant rejection (35). The altered expression of DAF has been associated with cytokine expression (interleukin-2 and interleukin-4) or the level of progesterone (17, 20, 22). Here, we report the first study to demonstrate the modulation of DAF levels by NO. Since DAF is a key immunoregulatory molecule protecting tissues from cytotoxic effects of complement, NO through the regulation of DAF, could also modulate the complement system and thus control the basic host defense mechanisms. Therefore, we speculate that several pathologies resulting in differential DAF expression could potentially benefit from the novel therapeutic approaches based upon NO regulation of DAF expression.
NO is known to be generated by different cell types in a variety of tissues. Our group and other researchers have demonstrated that NO is produced by a variety of uterine cell types, including endometrial epithelium, metrial glands, decidua, myometrial cells, NK cells, and macrophages (6, 7, 28, 38, 40). Previous work has also implicated NO as a modulator of uterine infection, including the finding that NO inhibition increased the lethality of experimental intrauterine infection in pregnant rats and that intrauterine infection results in a localized increase in NO synthesis (9, 30). These data, together with the observation that the uterine endometrium expresses both NOS II and NOS III (38), suggested that endogenous NO may be involved in the uterine defense against bacterial infection.
Using Ishikawa cells, we investigated a potential mechanism of NO regulation of epithelial infection. These studies demonstrated that the invasion by E. coli of Ishikawa cells was regulated by NO generation. The incubation of Ishikawa cells with l-arginine inhibited bacterial invasion in a time- and dose-dependent manner. This effect of l-arginine was specific, because d-arginine was ineffective and the effects of l-arginine were blunted in the presence of NOS inhibitors (L-NAME and aminoguanidine). In addition, provision of an NO donor, such as DETA-NO, also effectively inhibited the internalization of bacteria into the endometrial cells. Moreover, the inhibitory effects of NO on E. coli invasion were dependent upon the presence of Dr fimbriae, indicating the possible involvement of endometrial cellular receptors for Dr+ E. coli in these processes. It has been documented that clinical (IH11128) and laboratory (BN406) E. coli strains that express Dr fimbriae, but not BN17 (and the clinical isogenic mutantDR14 [data not shown]), which lacks Dr fimbria expression (9), could invade epithelial cells. In this study, using the same strains, we show that a NO donor decreases while NO inhibition increases invasion of fimbriated strains. The possible direct effect of NO on bacterial viability was eliminated in our studies, since the medium containing NO-modifying agents was removed prior to the invasion assay. Also, a prior study showed no direct effect of NO on E. coli viability in vitro (33). Previous studies of Dr fimbriae or other Dr family adhesins and their corresponding epithelial receptor, DAF, have provided evidence that adhesin ligand-mediated invasion is directly proportional to the receptor density. For example, an increase in DAF expression from 100 to 150% increases invasion by 50%. In this study, NO-induced decreases in DAF density are parallel to the reductions in bacterial invasion. Similarly, inverse effects were observed with NO inhibition. Although alternative explanations of this phenomenon may be considered, the most plausible is that NO regulates DAF expression, which in turn affects invasion (9, 10, 21).
The mechanisms by which NO alters the severity of infection in experimental rats is not well understood. Previously suggested mechanisms of antibacterial NO action include direct killing of bacteria through cytotoxic effects and DNA damage (2, 5). Several pathogens, including Dr+ E. coli, utilize DAF and structurally related complement regulators to attach and invade host cells. Intracellular pathogens have developed sophisticated mechanisms of intracellular survival, for example, by avoiding phagolysosomal fusion, and therefore escape NO-mediated intracellular killing (10, 36). We now postulate that in situations in which intracellular killing is limited, the host's best strategy to avoid protracted infection may be to reduce the expression of epithelial receptors and, as a result, decrease bacterial attachment and invasion. The significance of the NO-mediated decrease in invasion is that it may represent a novel unrecognized epithelial defense mechanism.
Acknowledgments
We gratefully acknowledge the grant support we received from NIH HD30273 HL-72650 and HL58144 to C. Yallampalli, NIDDK 42029 and 41687 to B. J. Nowicki, and NIH 5K12-HD-01269 to S. L. Young.
We thank W. D. Willis and his staff for help with high-pressure liquid chromatography experiments. For editorial and graphic assistance, we thank the Ob/Gyn Publications director and staff: R. G. McConnell, Kristi Barrett, John Helms, and Traci Smith.
Editor: V. J. DiRita
REFERENCES
- 1.Bredt, D. S., and S. H. Snyder. 1990. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc. Natl. Acad. Sci. USA 87:682-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Groote, M. A., and F. C. Fang. 1995. NO inhibitions: antimicrobial properties of nitric oxide. Clin. Infect. Dis. 21(Suppl. 2):S162-S165. [DOI] [PubMed] [Google Scholar]
- 3.Dong, Y.-L., B. S. Dai, P. Singh, and C. Yallampalli. 1997. Involvement of nitric oxide pathway in prostaglandin F2α-induced preterm labor in rats. Am. J. Obstet. Gynecol. 177:907-918. [DOI] [PubMed] [Google Scholar]
- 4.Dong, Y.-L., P. R. R. Gangula, and C. Yallampalli. 1996. Nitric oxide synthase isoforms in rat uterus: differential regulation during pregnancy and labor. J. Reprod. Fertil. 107:249-254. [DOI] [PubMed] [Google Scholar]
- 5.Fang, F. C. 1997. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 99:2818-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang, L., B. J. Nowicki, Y.-L. Dong, and C. Yallampalli. 1999. Localized increase in nitric oxide and the expression of nitric oxide synthase isoforms in rat uterus with experimental intra-uterine infection. Am. J. Obstet. Gynecol. 181:601-609. [DOI] [PubMed] [Google Scholar]
- 7.Gangula, P. R. R., Y.-L. Dong, and C. Yallampalli. 1997. Rat myometrial smooth muscle cells express endothelial nitric oxide synthase. Hum. Reprod. 12:561-568. [DOI] [PubMed] [Google Scholar]
- 8.Gibbs, R. S., R. Romero, S. L. Hillier, D. A. Eschenbach, and R. L. Sweet. 1992. A review of premature birth and subclinical infection. Am. J. Obstet. Gynecol. 166:1515-1528. [DOI] [PubMed] [Google Scholar]
- 9.Goluszko, P., S. L. Moseley, L. D. Truong, A. Kaul, J. R. Williford, R. Selvarangan, S. Nowicki, and B. Nowicki. 1997. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Investig. 99:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goluszko, P., V. Popov, R. Selvarangan, S. Nowicki, T. Pham, and B. J. Nowicki. 1997. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J. Infect. Dis. 176:158-167. [DOI] [PubMed] [Google Scholar]
- 11.Hara, T., M. Kojima, H. Fukuda, T. Masaoka, Y. Fukumori, and M. Matsumoto. 1992. Levels of complement regulatory proteins, CD35 (CR1), CD46 (MCP) and CD55 (DAF) in human haematological malignancies. Br. J. Haematol. 82:368-373. [DOI] [PubMed] [Google Scholar]
- 12.Hart, A., B. J. Nowicki, B. Reisner, E. Pawelczyk, P. Goluszko, P. Urvil, G. D. Anderson, and S. Nowicki. 2001. Ampicillin-resistant Escherichia coli in gestational pyelonephritis: increased occurrence and association with the colonization factor Dr adhesin. J. Infect. Dis. 183:1526-1529. [DOI] [PubMed] [Google Scholar]
- 13.Hart, A., T. Pham, S. Nowicki, E. B. Whorton, Jr., M. G. Martens, G. D. Anderson, and B. J. Nowicki. 1996. Gestational pyelonephritis-associated Escherichia coli isolates represent a nonrandom, closely related population. Am. J. Obstet. Gynecol. 174:983-989. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch, E., I. Saotome, and D. Hirsh. 1995. A model of intrauterine infection and preterm delivery in mice. Am. J. Obstet. Gynecol. 172:1598-1603. [DOI] [PubMed] [Google Scholar]
- 15.Hourcade, D., M. K. Liszewski, M. Krych-Goldberg, and J. P. Atkinson. 2000. Functional domains, structural variations and pathogen interactions of MCP, DAF and CR1. Immunopharmacology 49:103-116. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, C. D., E. Meaddough, K. Aversa, S. F. Hong, I. S. Lee, R. O. Bahodo-Singh, L. C. Lu, and J. A. Copel. 1998. Dual roles of amniotic fluid nitric oxide and prostaglandin E2 in preterm labor with intra-amniotic infection. Am. J. Physio. Heart Circ. Physiol. 15:683-687. [DOI] [PubMed] [Google Scholar]
- 17.Hyc, A., A. Osiecka-Iwan, R. Strzelczyk, and S. Moskalewski. 2003. Effect of IL-1β, TNF-α, and IL-4 on complement regulatory protein mRNA expression in human articular chondrocytes. Int. J. Mol. Med. 11:91-94. [PubMed] [Google Scholar]
- 18.Inoue, H., M. Mizuno, T. Uesu, and T. Tsuji. 1994. Distribution of complement regulatory proteins, decay-accelerating factor, CD59/homologous restriction factor 20 and membrane cofactor protein in human colorectal adenoma and cancer. Acta Med. Okayama 48:271-277. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, J. R., K. M. Skubitz, B. J. Nowicki, K. Jacques-Palaz, and R. M. Rakita. 1995. Nonlethal adherence to human neutrophils mediated by Dr antigen-specific adhesins of Escherichia coli. Infect. Immun. 63:309-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaul, A., M. Nagamani, and B. Nowicki. 1995. Decreased expression of endometrial decay accelerating factor (DAF), a complement regulatory protein, in patients with luteal phase defect. Am. J. Reprod. Immunol. 34:236-240. [DOI] [PubMed] [Google Scholar]
- 21.Kaul, A., B. J. Nowicki, M. G. Martens, P. Goluszko, A. Hart, M. Nagamani, T. Q. Pham, and S. Nowicki. 1994. Decay-accelerating factor is expressed in the human endometrium and may serve as the attachment ligand for Dr pili of Escherichia coli. Am. J. Reprod. Immunol. 32:194-199. [DOI] [PubMed] [Google Scholar]
- 22.Kaul, A. K., D. Kumar, M. Nagamani, P. Goluszko, S. Nowicki, and B. J. Nowicki. 1996. Rapid cyclic changes in density and accessibility of endometrial ligands for Escherichia coli Dr fimbriae. Infect. Immun. 64:611-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krohn, M. A., S. L. Hillier, R. P. Nugent, M. F. Cotch, J. C. Carey, R. S. Gibbs, D. A. Eschenbach, et al. 1995. The genital flora of women with intraamniotic infection. J. Infect. Dis. 171:1475-1480. [DOI] [PubMed] [Google Scholar]
- 24.Lublin, D. M., and J. P. Atkinson. 1989. Decay-accelerating factor: biochemistry, molecular biology, and function. Annu. Rev. Immunol. 7:35-58. [DOI] [PubMed] [Google Scholar]
- 25.Lublin, D. M., and K. E. Coyne. 1991. Phospholipid-anchored and transmembrane versions of either decay-accelerating factor or membrane cofactor protein show equal efficiency in protection from complement-mediated cell damage. J. Exp. Med. 174:35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medof, M. E., D. M. Lublin, V. M. Holers, D. J. Ayers, R. R. Getty, J. F. Leykam, J. P. Atkinson, and M. L. Tykocinski. 1987. Cloning and characterization of cDNAs encoding the complete sequence of decay-accelerating factor of human complement. Proc. Natl. Acad. Sci. USA 84:2007-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nathan, C., and Q. W. Xie. 1994. Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 269:13725-13728. [PubMed] [Google Scholar]
- 28.Natuzzi, E. S., P. C. Ursell, M. Harrison, C. Buscher, and R. K. Riemer. 1993. Nitric oxide synthase activity in the pregnant uterus decreases at parturition. Biochem. Res. Commun. 194:1-8. [DOI] [PubMed] [Google Scholar]
- 29.Nishida, M., K. Kasahara, M. Kaneko, and H. Iwasaki. 1985. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Acta Obst. Gynecol. Jpn. 37:1103-1111. [PubMed] [Google Scholar]
- 30.Nowicki, B., L. Fang, J. Singhal, and C. Yallampalli. 1997. Lethal outcome of uterine infection in pregnant but not in nonpregnant rats and increased rat death with inhibition of nitric oxide. Am. J. Reprod. Immunol. 38:309-312. [DOI] [PubMed] [Google Scholar]
- 31.Nowicki, B., A. Hart, K. E. Coyne, D. M. Lublin, and S. Nowicki. 1993. Short consensus repeat-3 domain of recombinant decay-accelerating factor is recognized by Escherichia coli recombinant Dr adhesin in a model of a cell-cell interaction. J. Exp. Med. 178:2115-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowicki, B., S. Nowicki, and R. Selvarangan. 2001. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 183:S24-S27. [DOI] [PubMed] [Google Scholar]
- 33.Nowicki, B. J., J. Singhal, L. Fang, S. Nowicki, and C. Yallampalli. 1999. Inverse relationship between severity of experimental pyelonephritis and nitric oxide production in C3H/Hej mice. Infect. Immun. 67:2421-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nowicki, S., B. Nowicki, T. Pham, R. Hasan, and M. Nagamani. 2001. Expression of decay accelerating factor in endometrial adenocarcinoma is inversely related to that stage of tumor. Am. J. Reprod. Immunol. 46:144-148. [DOI] [PubMed] [Google Scholar]
- 35.Schuurman, H. J., G. Pino-Chavez, M. J. Phillips, L. Thomas, D. J. White, and E. Cozzi. 2002. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF-transgenic donors. Transplantation 73:1146-1151. [DOI] [PubMed] [Google Scholar]
- 36.Selvarangan, R., P. Goluszko, V. Popov, J. Singhal, T. Pham, D. M. Lublin, S. Nowicki, and B. Nowicki. 2000. Role of decay-accelerating factor domains and anchorage in internalization of Dr-fimbriated Escherichia coli. Infect. Immun. 68:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tschudi, M. R., M. Barton, N. A. Bersinger, P. Moreau, F. G. Cosentino, G. Nell, T. Malinski, and T. F. Luscher. 1996. Effect of age on kinetics of nitric oxide release in rat aorta and pulmonary artery. J. Clin. Investig. 98:899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tschugguel, W., C. Schneeberger, G. Unfried, K. Czerwenka, W. Weninger, M. Mildner, J. R. Bishop, and J. Huber. 1998. Induction of inducible nitric oxide synthase expression in human secretory endometrium. Hum. Reprod. 13:436-444. [DOI] [PubMed] [Google Scholar]
- 39.Xu, C., D. Mao, V. M. Holers, B. Palanca, A. M. Cheng, and H. Molina. 2000. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 287:498-501. [DOI] [PubMed] [Google Scholar]
- 40.Yallampalli, C., Y. L. Dong, P. R. R. Gangula, and L. Fang. 1998. Role and regulation of nitric oxide in the uterus during pregnancy and parturition. J. Soc. Gynecol. Investig. 5:58-67. [DOI] [PubMed] [Google Scholar]
- 41.Yallampalli, C., H. Izumi, M. Byam-Smith, and R. E. Garfield. 1994. An l-arginine-nitric oxide-cyclic guanosine monophosphate system exists in the uterus and inhibits contractility during pregnancy. Am. J. Obstet. Gynecol. 170:175-185. [DOI] [PubMed] [Google Scholar]
- 42.Young, S. L., B. A. Lessey, M. A. Fritz, W. R. Meyer, M. J. Murray, P. L. Speckman, and B. J. Nowicki. 2002. In vivo and in vitro evidence suggest that HB-EGF regulates endometrial expression of human decay-accelerating factor. J. Clin. Endocrinol. Metab. 87:1368-1375. [DOI] [PubMed] [Google Scholar]