Abstract
We tested the hypothesis that cathepsins and specifically toxopain-1, a cathepsin B, play a critical role in the pathogenesis of toxoplasmosis. We found that inhibiting the expression of toxopain-1-specific mRNA and protein by >60% significantly decreased the capacity of the parasites to multiply and invade in vitro. To relate these in vitro results to the role of toxopain-1 in pathogenesis in vivo, we developed a novel chicken embryo model of congenital toxoplasmosis. Inhibiting either toxopain-1 expression or specific cysteine proteinase activity significantly reduced congenital infection of chicken embryos, as determined by histopathology and by the number of parasites quantified by real-time PCR. Our new model provides key in vivo validation for the hypothesis that toxopain-1 is a potential drug target in Toxoplasma gondii and also provides a new animal model for rapid, inexpensive screening of antiparasitic compounds.
Toxoplasma gondii is a widespread, obligate, intracellular parasite capable of infecting virtually any nucleated cell. As an opportunistic human pathogen, T. gondii causes devastating disease in immunocompromised individuals, especially AIDS patients and congenitally infected neonates (9). Toxoplasma encephalitis is the most common cause of central nervous system infection in patients with AIDS and is uniformly fatal unless it is treated (9). Almost 1 in 1,000 infants are born in the United States with toxoplasmosis (10). Treatment is often limited by toxic side effects of drugs, making identification of new drug targets a critical goal.
Parasite-derived proteases, particularly cysteine proteinases, are critical for invasion and survival of both protozoans and helminths and are plausible targets for drug therapy (17). We found that the sole T. gondii cathepsin B (TgCPB), toxopain-1, localizes to the unique rhoptry organelle of T. gondii, which is required for parasite invasion (16). One crucial biological function of toxopain-1 appears to be the processing of rhoptry proteins (16). Toxopain-1 is also secreted in the parasitophorous vacuole, where it may be involved in the degradation of host cell peptides, a process extensively studied in the related apicomplexan Plasmodium falciparum (4, 6, 19). Specific cysteine proteinase inhibitors disrupt host cell invasion by toxoplasma tachyzoites in vitro (16). Therefore, we tested the hypothesis that toxopain-1 is a key virulence factor in in vivo infection.
In apicomplexan parasites, null mutants can be generated by gene knockout (3). However, this approach is not feasible for investigating the biological function of essential genes. To circumvent the difficulties, we developed an antisense strategy to inhibit toxopain-1 expression in T. gondii. Substitution of the normal polyadenylation signal with cis-acting ribozymes leads to nuclear retention of the product RNAs by generation of export-deficient transcripts (2), which, when stably transfected into T. gondii, lead to dramatic and specific reductions in endogenous gene expression (13-15). We exploited this system to inhibit expression of toxopain-1 and examine its role in pathogenesis.
To identify in vivo effects of inhibition of toxopain-1, we developed a novel chicken embryo model of congenital toxoplasmosis. This model allows easy screening of drugs or genetically altered parasites during a course of infection that is shorter than that in mice. We report that specific inhibition of toxopain-1 blocks infection both in vitro and in vivo, a finding which supports the hypothesis that toxopain-1 is important in the pathogenesis of toxoplasmosis and is a potential drug target.
MATERIALS AND METHODS
Host cells and parasite cultures.
T. gondii strain RH tachyzoites and RH(EP)/ΔHXGPRT knockout mutants (NIH AIDS Reference and Reagent Repository, Bethesda, Md.) were maintained by serial passage in primary human foreskin fibroblasts (HFF). The host cells (HFF) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (Irvine Scientific, Irvine, Calif.) in the presence of penicillin and streptomycin.
Antisense RNA construct and selection of transformants.
The antisense TgCPB (AS-TgCPB) RNA construct was derived from a ribozyme-histone cassette, pNTPRZ, which contains a 5′ NTP3 promoter (1.6 kb) and a 3′ hammerhead ribozyme (0.1 kb) with a histone stem-loop structure as a stabilizing element against 3′-to-5′ exonuclease degradation (15). The complete coding region of TgCPB cDNA was amplified by PCR by using primers 5′-ATA GAA TTC ATG GAG GGG CGA AAG TCT TTT CGCG-3′ and 5′-GAA CCT AGG CAT TTC TCT CTC CTC TTC TG-3′ and was digested with AvrII and EcoRI. The TgCPB cDNA (1.7 kb) was cloned in an antisense orientation into the AvrII and EcoRI sites of the pNTPRZ vector, generating plasmid pAS-TgCPB-RZ. The pAS-TgCPB-RZ antisense cassette (3.4 kb) was reamplified by PCR and subcloned into the SpeI site of the parental pminHXGPRT+ vector (Ogden Bioservices Corp.), generating pAS-TgCPB with the hypoxanthine-xanthine-guanine-phosphoribosyltransferase (HXGPRT) gene serving as a selectable marker in genetically null T. gondii strain RH(EP)ΔHXGPRT. Tachyzoites of RH(EP)ΔHXGPRT transfected with the pAS-TgCPB vector or the control vector pminHXGPRT+-NTPRZ without an AS-TgCPB insert were selected in culture media containing mycophenolic acid (25 μg/ml) and supplemented with 50 μg of xanthine per ml to ensure that there was sufficient substrate for the enzyme.
A total of 2 × 107 purified T. gondii tachyzoites [strain RH(EP)ΔHXGPRT] were resuspended in 0.4 ml of cytomix buffer supplemented with 2 mM ATP (pH 7.6) and 5 mM (final concentration) glutathione. The parasite suspension in a 0.2-cm-gap cuvette was mixed with 50 μg of purified pAS-TgCPB or control vector plasmid DNA and then electroporated by using a model 600 BTX set to a 1.5-keV pulse at a resistance of 24 Ω. Electroporated cells were allowed to recover for 15 min at room temperature and inoculated onto monolayers of HFF in T-25 flasks containing growth medium. Transformants were selected 24 h postinfection in medium containing 25 μg of mycophenolic acid per ml and 50 μg of xanthine per ml and then cloned in 96-well microtiter plates containing HFF by limiting dilution with drugs. Clonal parasite lines were isolated and evaluated for depletion of the targeted protein.
Southern analysis.
T. gondii genomic DNA was extracted from pAS-TgCPB- and control vector-transformed tachyzoites (5 × 107 parasites) by using DNA STAT-60 reagent (Tel-Test, Friendswood, Tex.). Total DNA was digested with restriction enzyme HindIII or SpeI, electrophoresed through a 1% agarose gel, and transferred in duplicate to GeneScreen Plus membranes (Perkin-Elmer Life Sciences, Boston, Mass.). The TgCPB cDNA probe was amplified by PCR by using TgCPB cDNA (GenBank accession no. AY071839) as a template (16). The PCR fragment was gel purified with a QIAquick gel extraction kit (Qiagen, Valencia, Calif.) and was labeled by random priming with [α-32P]dCTP (Amersham Pharmacia Biotech). Hybridization of duplicate blots was performed at 68°C for 16 h in PerfectHyb Plus hybridization buffer (Sigma) with the labeled TgCPB cDNA fragment. Following hybridization, the blots were washed once in low-stringency wash buffer (2× SSC, 0.1% sodium dodecyl sulfate [SDS] [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate]) at room temperature for 10 min and then twice (30 min each) in high-stringency wash buffer (0.5× SSC, 0.1% SDS) at 68°C. The blots were exposed to X-ray film for 24 h at −70°C.
Northern analysis.
Total RNA was isolated from pAS-TgCPB- and control vector-transformed tachyzoites by using an RNAeasy mini kit (Qiagen). Equal amounts of total RNA (5 μg) were loaded in duplicate, fractionated on a 1.2% (wt/vol) formaldehyde-agarose gel, and transferred to nylon membranes (GeneScreen Plus; Perkin-Elmer Life Sciences). To ensure that AS-TgCPB did not affect expression of other genes, expression of the SAG1 (P30) gene coding for the surface protein of T. gondii and expression of a cathepsin L gene (TgCPL) were also evaluated by Northern blot analysis. The SAG1 probe coding for a 282-bp region (positions 418 to 700) of the SAG1 gene of T. gondii (GenBank accession no. X14080) was amplified with primers 5′-ACT GAT GTC GTT CTT GCG ATG TGG C-3′ and 5′-CGT CCA CCA GCT ATC TTC TGC TTC A-3′. The cathepsin L probe contained the entire coding sequence (GenBank accession no. AF184984). Northern blot analyses were performed by using labeled TgCPB, SAG1, or cathepsin L cDNA probes. Hybridizations were performed at 68°C in PerfectHyb Plus buffer (Sigma). The filters were washed twice (30 min each) at 68°C in high-stringency wash buffer and then exposed for autoradiography. The SAG1 and cathepsin L probes were used to reprobe the blots after stripping of the previous TgCPB probe in boiling water. The blots were developed by autoradiography and scanned, and the intensities of the bands were determined with Image software from the National Institutes of Health.
Western analysis.
Tachyzoite lysates (5.25 μg) from pAS-TgCPB- and control vector-transfected parasites were separated on SDS—10% polyacrylamide electrophoresis gels, and the proteins were transferred to nitrocellulose membranes (Millipore). The filters were probed with monoclonal antibodies 1.7, 1.15, and 1.17, which react to TgCPB (16) at a 1:500 dilution or to anti-nucleoside triphosphate hydrolase rabbit antiserum at a 1:1,000 dilution (15). The bands were visualized with goat anti-mouse immunoglobulin G-horseradish peroxidase (ZyMed, South San Francisco, Calif.) by using an ECL kit (Amersham Pharmacia Biotech, Piscataway, N.Y.) or with mouse anti-rabbit alkaline phosphatase and were developed with a nitroblue tetrazolium-BCIP (5-bromo-4-chloro-3-indolylphosphate) buffer solution (ZyMed). The intensities of the bands were determined as described above.
Assay of cathepsin B activity.
Equal numbers of pAS-TgCPB- and control vector-transformed tachyzoites were suspended in 50 mM Tris-10 mM EDTA and lysed by three freeze-thaw cycles and 15 s of sonication. The proteinase activity was measured in the soluble fraction by cleavage of the synthetic peptide substrate Z-Arg-Arg-4-amino-7-methylcoumarin (4 μM), which was monitored by determining the increase in fluorescence with an automated microtiter plate spectrofluorometer (Labsystems Fluoroskan II). All synthetic 4-amino-7-methylcoumarin substrates were obtained from Enzyme System Products (Livermore, Calif.).
Effect of AS-TgCB1 on tachyzoite invasion.
A total of 5 × 105 control or pAS-TgCPB tachyzoites were added directly to triplicate wells of chamber slides (Lab Tek; Nunc) containing fibroblast monolayers and incubated for 2 h. Following washing, the cells were fixed in 3% paraformaldehyde for 15 min and stained with acridine orange (5 μg/ml), and the numbers of infected cells and the numbers of intracellular parasites per cell were determined by fluorescent microscopy. Only parasites inside a parasitophorous vacuole away from the fibroblast cell membrane were counted to ensure that they were intracellular.
Effect of AS-TgCPB on intracellular tachyzoite multiplication.
A total of 2 × 105 pAS-TgCPB- and control vector-transformed tachyzoites were added to fibroblast monolayers on eight-well chamber slides and allowed to invade for 2 h. Free parasites were then washed away, fresh medium containing mycophenolic acid and xanthine was added, and the monolayers were incubated for an additional 24 h. Following washing, fixation with 3% paraformaldehyde, and permeabilization with 0.25% Triton X-100, the monolayers were stained with acridine orange. The numbers of tachyzoites per parasitophorous vacuole were determined, and statistical significance was assessed by measures of variance and a two-tailed t test.
Chicken embryo model of congenital toxoplasmosis.
Fertilized COFAL (stands for complement fixation for avian leukosis)-negative eggs (SPAFAS, Storrs, Conn.) were incubated at 37°C in a humid incubator. To mimic human congenital infection, tachyzoites were injected directly into the chorioallantoic veins of 12-day-old chicks. A small window was cut with a hand drill directly over the blood vessel in each egg, and the vein was visualized with 1 drop of sterile mineral oil on the exposed membrane. Tachyzoites in Dulbecco's modified Eagle's medium or in the presence of the test inhibitor or RH tachyzoites expressing the AS-TgCPB or control vector were injected with a 28-gauge needle. The windows were sealed with tape, and the embryos were incubated in a 37°C incubator. The controls included eggs that did not receive injections and embryos that were injected with media and synthetic hydrazide inhibitor alone (ZLIII115; from James McKerrow, University of California at San Francisco). The eggs were candled daily to assess viability, and the embryos were sacrificed, weighed, and fixed in 10% formalin for histopathological examination. Sections were prepared for hematoxylin and eosin staining and for immunostaining for T. gondii with the DAKO EnVision system (DAKO, Carpenteria, Calif.). The brain and liver tissues of chicken embryos were also frozen at −70°C for quantitative PCR analysis.
Quantitation of T. gondii by real-time PCR.
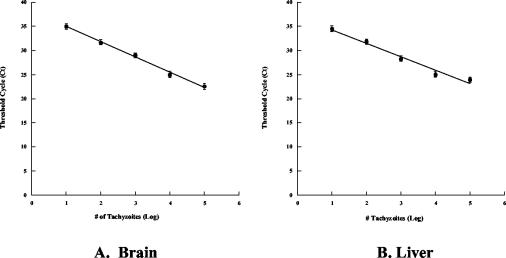
To quantitate tachyzoites in vivo, a standard curve was constructed by mixing 101 to 107 tachyzoites with brain or liver samples (100 mg) from 17-day-old chicken embryos and homogenizing the preparations with a Dounce homogenizer in cell lysis solution. Total genomic DNA was purified with a Puregene kit by following the instructions of the manufacturer (Gentra Systems, Minneapolis, Minn.). Purified DNA was rehydrated in 100 μl of DNA hydration solution, and aliquots (1 μl) of genomic DNA were used as templates for PCR amplification to determine the presence of a major T. gondii surface antigen (SAG1) gene (1). The relative amounts of Toxoplasma DNA were determined by real-time PCR. Primers 5′-GTT CTT GCG ATG TGG CGT T-3′ and 5′-GGC AGG TGA CAA CTT GAT TGG-3′ specific for part of the SAG1 gene sequence were designed with the ABI PRISM Primer Express software (PE Applied Biosystems, Foster City, Calif.) to obtain a 65-bp amplicon. The PCR was performed in triplicate by using the SYBR Green PCR core reagents (PE Applied Biosystems), and the cycling conditions were 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. The relative amount of product generated was measured by determining the threshold cycle when the level of specific PCR product increased exponentially and crossed the threshold of a passive reference dye in each sample. The melting curves of the amplicons were evaluated to ensure the specificity of the reaction, and the controls included uninfected embryo DNA. The standard curves were used to extrapolate the numbers of tachyzoites present in unknown samples (see Fig. 8).
FIG. 8.
Standard curves for quantitative real-time PCR. The standard curves were generated by amplification of the SAG1 sequence from DNA samples extracted from known numbers of infected tachyzoites added to normal chick liver or brain tissue. The curves are plots of the threshold cycle numbers versus log10 normalized numbers of tachyzoites for the brain (A) and liver (B).
RESULTS
Antisense toxopain-1 construct stably integrates and inhibits toxopain-1 expression.
To inhibit endogenous toxopain-1 gene expression in T. gondii, we generated a chimeric antisense toxopain-1 (AS-TgCPB) RNA-ribozyme construct in which the 3′ untranslated region was replaced by a hammerhead ribozyme (15). The antisense plasmid, pAS-TgCPB, or the control vector was electroporated into HXGPRT null tachyzoites by using the HXGPRT gene as a selectable marker. The majority of transiently transfected parasites did not grow, suggesting that a higher level of expression of the AS-TgCPB RNA-ribozyme construct was lethal to tachyzoites. However, a few viable mycophenolic acid-resistant parasites were cloned by dilution in 96-well microtiter plates with continued drug selection. Seven clonal parasite lines were isolated from the stable population of antisense transgenics by limiting dilution, and individual clones were analyzed.
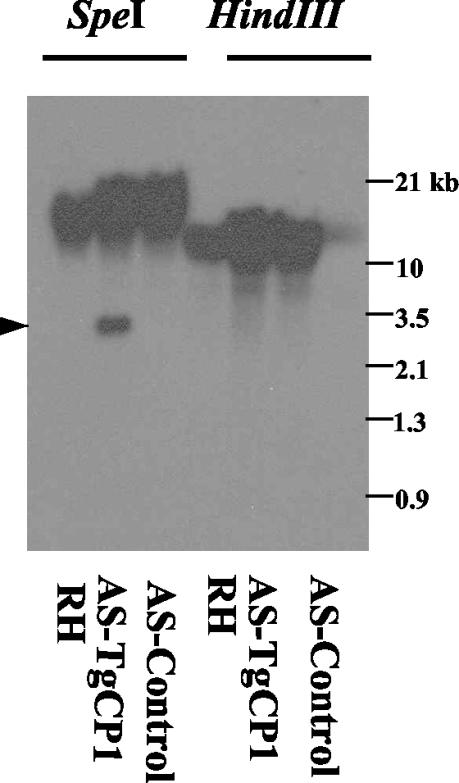
DNA was purified from independently cloned resistant parasites, digested with restriction enzymes (HindIII and SpeI), and examined by DNA hybridization to investigate the physical structure of stable transgenic clones. As the transfected TgCPB gene was derived from a cDNA sequence lacking introns, the presence of unaltered genomic bands in all clones indicated that no homologous recombination had occurred, perhaps because of insufficient continuous homology between the cDNA-derived transformation vector and the parasite genome. Southern blot analysis of T. gondii genomic DNA hybridized with a TgCPB cDNA probe indicated that TgCPB cDNA was present in transgenic tachyzoites (Fig. 1), suggesting that stable integration had occurred, most likely by nonhomologous recombination.
FIG. 1.
Southern blot analyses to determine the presence of episomal and integrated forms of AS-TgCPB. Genomic DNA from the RH wild type (RH), the AS-TgCPB strain (AS-TgCPB), and the antisense vector control (AS-Control) were digested with either SpeI or HindIII and probed for the TgCPB cDNA insert. The cDNA probe cross-reacted with the genomic DNA of all three cell lines. As expected, a 3.4-kb fragment of the AS-TgCPB-ribozyme antisense insert was detected in SpeI-digested AS-TgCPB clones but not in the RH wild type or the control. The intensity and size of the HindIII-digested genomic DNA indicates that plasmid pminHXGPRT-AsTgCP1-RZ (8.3 kb) was integrated into the parasite genome.
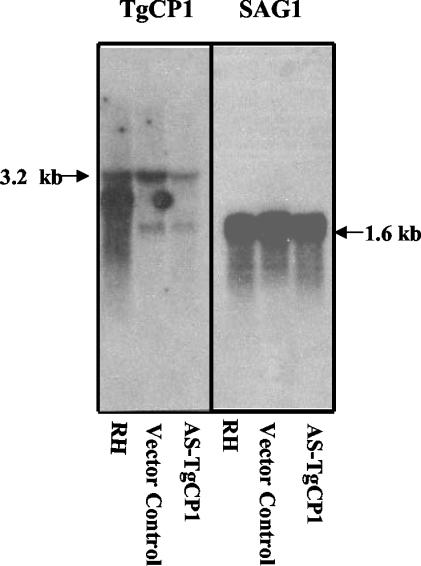
Northern blot analysis showed that there was a significant reduction in the steady-state levels of TgCPB mRNA in parasites that were stably transfected with the antisense RNA-ribozyme construct. Expression of TgCPB-specific mRNA was decreased 64% as determined by densitometric scanning of the blot, while control SAG1 (1) mRNA levels were unchanged (Fig. 2). AS-TgCPB had no effect on the levels of cathepsin L mRNA (data not shown).
FIG. 2.
Northern blot analysis of steady-state levels of RNA following transfection with the antisense plasmid. Total RNA (5 μg/lane) was extracted from T. gondii strain RH tachyzoites (wild type), an antisense vector control preparation, and an AS-TgCPB preparation. The Northern blots were hybridized with a toxopain-1 probe (left panel) and with the SAG1 probe (right panel).
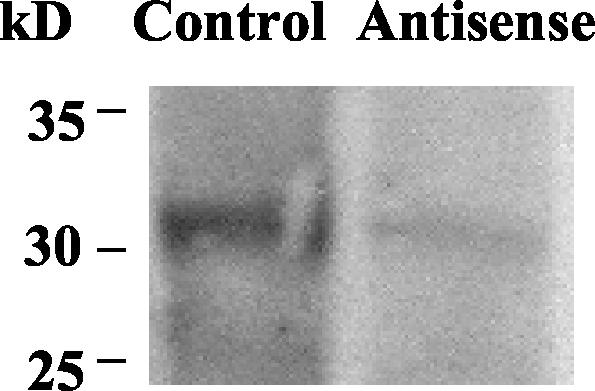
To compare the effects of antisense inhibition on TgCPB expression, Western blotting was performed with lysates of tachyzoites expressing the control or antisense vectors. TgCPB expression was decreased by 67%, which correlated with the decrease in the level of specific mRNA (Fig. 3). Equivalent levels of expression of nucleoside triphosphate hydrolase (63 kDa) (15) were observed in immunoblots of lysates from control vector- and pAS-TgCPB-transfected tachyzoites, indicating that AS-TgCPB did not affect expression of other proteins (data not shown).
FIG. 3.
Western blot analysis of TgCPB levels following antisense inhibition. Total soluble tachyzoite lysates (5.25 μg) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and probed with monoclonal antibodies 1.7, 1.15, and 1.17. Immunolabeled proteins were visualized with an ECL kit. The results for tachyzoites transfected with a control vector or pAS-TgCPB are shown.
Total cathepsin B activity was also measured in lysates of tachyzoites that were transfected with the control vector and the antisense vector. Enzyme activity in the AS-TgCPB-ribozyme-transfected tachyzoites was decreased by a maximum of 35% (P < 0.01, as determined by Student's paired t test). The disparity between the decrease in the amount of toxopain-1-specific mRNA and protein (>60%) and the decrease in the overall proteinase activity (35%) may reflect cleavage of the same substrate by cathepsin L, which has recently been cloned (K. Hirata and S. L. Reed, unpublished observations).
Antisense toxopain-1 inhibits host cell invasion and intracellular multiplication in vitro.
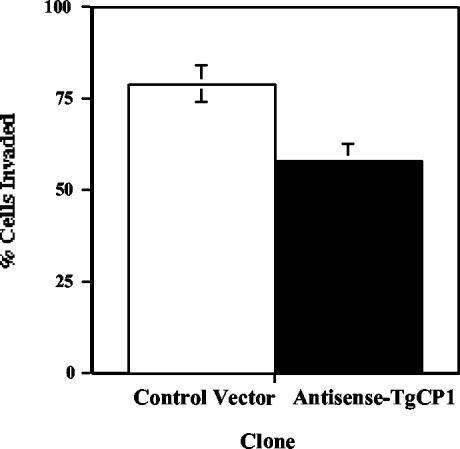
The effect of control vector- or pAS-TgCPB-transformed tachyzoites on in vitro invasion was evaluated by allowing tachyzoites to invade fibroblast monolayers on coverslips for 2 h. After washing, fixation in paraformaldehyde, and staining with acridine orange for counting, the number of infected cells was 26.4% lower in the presence of antisense inhibition (P < 0.006, as determined by Student's t test) (Fig. 4).
FIG. 4.
Antisense expression limits tachyzoite invasion. A total of 2.5 × 105 control or antisense toxopain-1 (AS-TgCP1) tachyzoites were allowed to invade fibroblast monolayers for 2 h, and the number of infected cells was counted after staining with acridine orange. The values are means ± standard errors.
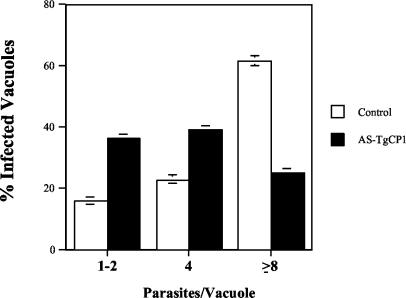
In contrast, antisense expression had much more dramatic effects on intracellular parasite multiplication than on invasion. The difference may have been due in part to the rapid invasion (∼30 s) compared with the 24-h intracellular multiplication assay, which allowed amplification of the effect of limited cathepsin B. Tachyzoites containing the control or AS-TgCPB vector were allowed to invade monolayers for 2 h, free tachyzoites were washed away, and the intracellular tachyzoites were allowed to multiply for 24 h in medium containing mycophenolic acid and xanthine. The number of antisense transfected tachyzoites per vacuole was compared with the number of control tachyzoites. Parasites treated with AS-TgCPB grew more slowly than the parasites in control cultures, and many abnormal parasite masses were observed. Infection with the control vector resulted in significantly more tachyzoites per vacuole. At 24 h, 60% of the parasitophorous vacuoles of the control parasites contained eight or more tachyzoites, whereas with the antisense clones, only 25% of the vacuoles contained eight or more cells (P < 0.001, as determined by Student's t test) (Fig. 5). Thus, the significant inhibition of parasite replication is closely linked to the specific reduction in toxopain-1 caused by antisense RNA.
FIG. 5.
Inhibition of intracellular multiplication by AS-TgCPB. HFF monolayers were infected with tachyzoites for 2 h, and all free parasites were then removed and replaced with fresh medium. Cultures were incubated for 24 h and fixed, and then the numbers of parasites per vacuole were determined. The values are means ± standard errors from at least three separate experiments. Expression of AS-TgCPB significantly reduced intracellular multiplication of tachyzoites (solid bars) compared to multiplication of the antisense vector control (open bars). The P value was <0.01 as determined by Student's t test.
Chicken embryo model mimics human congenital infection.
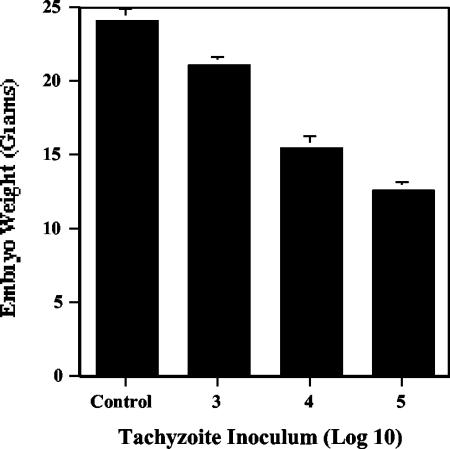
For in vivo studies, we needed a quantitative model of toxoplasmosis. Although mice are an excellent animal model, large amounts of inhibitors are required for daily treatment of the animals during the 7- to 10-day course of infection, and death is a crude endpoint that gives few insights into mechanisms of disease. Therefore, we modified a chicken embryo model that had been developed for the study of metastatic disease (8). Intravenous inoculation of RH strain tachyzoites into the chorioallantoic vein resulted in infections that could be measured by embryo size and histopathology and could be correlated with the number of invasive parasites quantified by real-time PCR. We found that there was a significant decrease in the size of the chicks at day 18 in a dose-response curve with the initial tachyzoite inoculum (P < 0.02, as determined by Student's paired t test), mimicking the intrauterine growth retardation seen in congenitally infected human babies (Fig. 6).
FIG. 6.
RH tachyzoite infection inhibits chicken embryo growth in a dose-dependent manner. Groups of day 12 eggs (n = 4) were injected intravenously with 103 (bar 3), 104 (bar 4), or 105 (bar 5) RH tachyzoites or with phosphate-buffered saline alone (Control). On day 18, the embryos were sacrificed and weighed. The values are means ± standard errors.
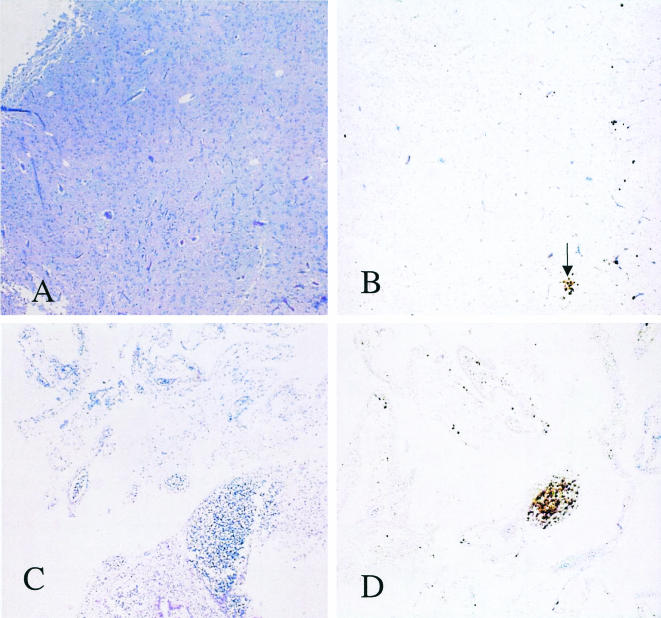
Histopathology revealed essentially normal brain morphology for chicks infected with 103 RH tachyzoites (Fig. 7A), and a few toxoplasma foci were revealed by immunohistochemistry (Fig. 7B). In contrast, chicks infected with 105 RH tachyzoites had almost complete necrosis of the brain (Fig. 7C) with extensive foci of infection with tachyzoites (Fig. 7D). As in human congenital infection, tachyzoites were present in virtually all organs (data not shown).
FIG. 7.
Histopathology of infected chick brains. Chicks were infected intravenously on day 12 with 103 or 105 RH tachyzoites, and their brains were formalin fixed on day 15. (A) Chicks infected with 103 RH tachyzoites. Hematoxylin and eosin stain. Magnification, ×40. (B) Immunohistochemical staining of the brain shown in panel A for T. gondii. The arrow points to a small focus of T. gondii infection. (C) Chick infected with 105 RH tachyzoites. Hematoxylin and eosin stain. (D) Immunostaining of the brain shown in panel C.
We constructed a standard curve to quantitate toxoplasma in vivo by mixing known numbers of T. gondii RH tachyzoites (10 to 107 tachyzoites) with chick tissue and using aliquots of total extracted genomic DNA as templates for PCR amplification of the T. gondii SAG1 sequence (1). The standard curve (Fig. 8) was used to extrapolate the numbers of toxoplasma cells present in unknown samples. Within a 1- to 6-log range, there was a direct relationship between the number of PCR threshold cycles and the number of tachyzoites present in the mixtures for either brains (Fig. 8A) or livers (Fig. 8B). We could detect as few as 10 tachyzoites in 100 mg of chick tissue, and the dose-response curve was highly reproducible.
Antisense toxopain-1 and specific cysteine proteinase inhibitors block in vivo infection.
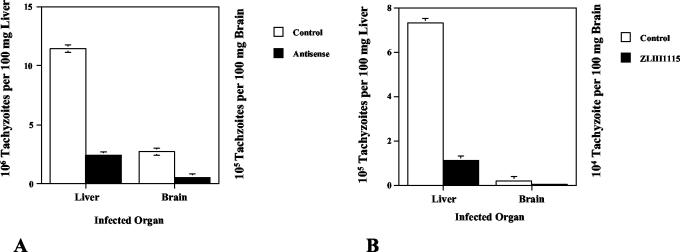
To definitively test the importance of toxopain-1 in vivo, we infected 12-day-old chicken embryos with 104 tachyzoites expressing pAS-TgCPB or the control vector. The chicks were sacrificed at day 15, and the DNA was extracted from their brains and livers. Infection with antisense clones resulted in 80% fewer tachyzoites in chick tissues (Fig. 9A).
FIG. 9.
Expression of antisense toxopain-1 and treatment with a hydrazide proteinase inhibitor limit toxoplasma infection in the chicken embryo. (A) Twelve-day-old chicken embryos were injected with 104 tachyzoites expressing the control vector or antisense toxopain-1, and the brains and livers were harvested on day 15. Total DNA was extracted, and the numbers of copies of SAG1 present in the organs were determined by real-time PCR and were extrapolated to the number of toxoplasma cells present based on the standard curve (Fig. 8). (B) RH tachyzoites (104 cells) were preincubated in medium alone or in medium containing the hydrazide inhibitor ZLIII115. The organs were harvested on day 18 and processed as described above. The results of a representative experiment (one of four experiments) are shown. The y axes of the two panels are different because of the higher number of tachyzoites in the liver.
To evaluate the utility of the chick model for drug testing, tachyzoites were preincubated with the cell-permeable, second-generation, hydrazide cysteine proteinase inhibitor ZLIII115 at a concentration of 50 μM (18) and injected intravenously into the chorioallantoic vein of a 12-day-old chick to obtain a level in serum of 10 μM. A second dose of inhibitor (final concentration, 10 μM) was administered intravenously at 24 h, the chicks were sacrificed at day 15, and the brains and livers were processed for pathological analysis and real-time PCR. Infection was inhibited by 85% in both the brains and livers (Fig. 9B). Importantly, no developmental differences were detected in uninfected embryos treated with inhibitor alone (data not shown).
DISCUSSION
Cysteine proteinases are key virulence factors in a number of parasites and provide attractive targets for rational drug design (17). Specific peptide inhibitors of Trypanosoma cruzi were concentrated only in the intracellular parasite and did not cause morphological changes in host cells (5, 7). Indeed, current clinical trials of a cysteine proteinase inhibitor may provide the first effective therapy against Chagas' disease (Jim McKerrow, personal communication). Treatment of toxoplasmosis is also problematic because of the high incidence of sulfa allergies, particularly in AIDS patients. Previously, a cathepsin B (TgCPB) was characterized, and the results showed that specific inhibitors blocked invasion of tachyzoites in vitro (16). To definitively prove that toxopain-1 is a key virulence factor and to evaluate its role in infection, we inhibited expression with an antisense ribozyme construct, as complete knockout was likely to be lethal. An antisense strategy generally does not result in complete inhibition of gene function and therefore is not suitable for creation of a null phenotype mutant. In fact, we never recovered clones with more than 35% inhibition of proteinase activity, supporting the idea that complete knockout would be lethal.
Consistent with our hypothesis, we found that decreased expression of toxopain-1 inhibited the growth of tachyzoites in cultured fibroblasts. Antisense expression decreased the level of toxopain-1-specific mRNA by 64% (Fig. 2) and the level of protein by 67% (Fig. 3), and there was no effect on control mRNA or protein. AS-TgCPB expression showed lower host cell invasion (Fig. 4) and intracellular multiplication of parasites (Fig. 5). Thus, in vitro studies support the hypothesis that toxopain-1 is a valid drug target.
For in vivo studies, we needed to develop a quantitative model of toxoplasmosis. Although mice are an excellent model for toxoplasmosis, large amounts of inhibitors are required for daily treatment of the animals during the 7- to 10-day course of infection, and death is a very crude endpoint. Animal models of congenital infection have been problematic. Therefore, we modified a chicken embryo model that had been developed for the study of metastatic disease (8). Following intravenous inoculation of tachyzoites, the infection was quantitated by determining the embryo size, histopathology, and number of invasive parasites. Like human congenital toxoplasmosis (12), intravenous infection of chicken embryos resulted in growth retardation (Fig. 6) and widespread necrotic infection (Fig. 7).
The new chicken model is sensitive and quantitative, as we could detect as few as 10 cells per 100 mg of tissue, and the pathology was proportional to the number of infecting parasites. Using this model, we showed that specific inhibition of toxopain-1 expression significantly lowered the level of infection and that there was a >80% decrease in the number of parasites in the brain and liver (Fig. 8A). In addition, this model proved to be very useful for drug testing. Tachyzoites were preincubated with the specific toxopain-1 inhibitor before intravenous injection, followed by a single injection 24 h later. Two doses of the drug resulted in >85% inhibition of infection in the brain and liver (Fig. 9B). The proteinase inhibitor had no evident effect on embryo development, which likely reflects the redundancy of cathepsins in higher eukaryotes and differences between the host and parasite cathepsins. To date, only one cathepsin B, one cathepsin L, one cathepsin D, and three cathepsin Cs have been detected in the toxoplasma genome project (http://www.tigr.org/tdb/e2k1/tga1/). In addition, intracellular parasites, including T. cruzii (11) and T. gondii (Que and Reed, unpublished observations), concentrate small peptide inhibitors. Our new model provides direct validation of the hypothesis that toxopain-1 is an important drug target due to the significant effect of inhibition on infection, and it is a valuable and physiologically relevant animal model for screening compounds by using a quantitative measure of infection.
Acknowledgments
We thank Charles Davis and Frances Gillin for their helpful comments, Andrew Zijlstra and James Quigley for their assistance with the chicken embryo model, and Jane Burns for assistance with real-time PCR.
This work was supported by funds from NIH grants AI41093 (S.L.R.), AI30060 (K.A.J.), and TWO1594 (K.A.J.) and by the Sandler Center for Basic Research in Parasitic Diseases (S.L.R.).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Burg, J. L., D. Perelman, L. H. Kasper, P. L. Ware, and J. C. Boothroyd. 1988. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J. Immunol. 141:3584-3591. [PubMed] [Google Scholar]
- 2.Dan, M., A. L. Wang, and C. C. Wang. 2000. Inhibition of pyruvate-ferredoxin oxidoreductase gene expression in Giardia lamblia by a virus-mediated hammerhead ribozyme. Mol. Microbiol. 36:447-456. [DOI] [PubMed] [Google Scholar]
- 3.Donald, R. G., and D. S. Roos. 1998. Gene knock-outs and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit-and-run mutagenesis. Mol. Biochem. Parasitol. 91:295-305. [DOI] [PubMed] [Google Scholar]
- 4.Eggleson, K. K., K. L. Duffin, and D. E. Goldberg. 1999. Identification and characterization of falcilysin, a metallopeptidase involved in hemoglobin catabolism within the malaria parasite Plasmodium falciparum. J. Biol. Chem. 274:32411-32419. [DOI] [PubMed] [Google Scholar]
- 5.Engel, J. C., P. S. Doyle, I. Hsieh, and J. H. McKerrow. 1998. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J. Exp. Med. 188:725-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenbaum, D. C., A. Baruch, M. Grainger, Z. Bozdech, K. F. Medzihradskzy, J. Engel, J. DeRisi, A. A. Holder, and M. Bogyo. 2002. A role for the protease falcipain-1 in host cell invasion by the human malaria parasite. Science 298:2002-2006. [DOI] [PubMed] [Google Scholar]
- 7.Harth, G., N. Andrews, A. A. Mills, J. C. Engel, R. Smith, and J. H. McKerrow. 1993. Peptide-fluoromethyl ketones arrest intracellular replication and intercellular transmission of Trypanosoma cruzi. Mol. Biochem. Parasitol. 58:17-24. [DOI] [PubMed] [Google Scholar]
- 8.Kim, J., W. Yu, K. Kovalski, and L. Ossowski. 1998. Requirement for specific proteases in cancer cell intravassation as revealed by a novel semiquantitative PCR-based assay. Cell 94:353-362. [DOI] [PubMed] [Google Scholar]
- 9.Luft, B. J., and J. S. Remington. 1992. Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15:211-222. [DOI] [PubMed] [Google Scholar]
- 10.McAuley, J., K. M. Boyer, D. Patel, M. Mets, C. Swisher, N. Roizen, C. Wolters, L. Stein, M. Stein, W. Schey, J. Remington, P. Meier, D. Johnson, P. Heydeman, E. Holfels, S. Withers, D. Mack, C. Brown, D. Patton, and R. McLeod. 1994. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin. Infect. Dis. 18:38-72. [DOI] [PubMed] [Google Scholar]
- 11.McGrath, M. E., A. E. Eakin, J. C. Engel, J. H. McKerrow, C. S. Craik, and R. J. Fletterick. 1995. The crystal structure of cruzain: a therapeutic target for Chagas' disease. J. Mol. Biol. 247:251-259. [DOI] [PubMed] [Google Scholar]
- 12.McLeod, R., K. Boyer, N. Roizen, L. Stein, C. Swisher, E. Holfels, J. Hopkins, D. Mack, T. Karrison, D. Patel, L. Pfiffner, J. Remington, S. Withers, S. Meyers, V. Aitchison, M. Mets, P. Rabiah, and P. Meier. 2000. The child with congenital toxoplasmosis. Curr. Clin. Top. Infect. Dis. 20:189-208. [PubMed] [Google Scholar]
- 13.Nakaar, V., E. O. Ngo, and K. A. Joiner. 2000. Selection based on the expression of antisense hypoxanthine-xanthine-guanine-phosphoribosyltransferase RNA in Toxoplasma gondii. Mol. Biochem. Parasitol. 110:43-51. [DOI] [PubMed] [Google Scholar]
- 14.Nakaar, V., H. M. Ngo, E. P. Aaronson, I. Coppens, T. T. Stedman, and K. A. Joiner. 2003. Pleiotrpic effect due to targeted depletion of secretory rhoptry protein ROP2 in Toxoplasma gondii. J. Cell Sci. 116:2311-2320. [DOI] [PubMed] [Google Scholar]
- 15.Nakaar, V., B. U. Samuel, E. O. Ngo, and K. A. Joiner. 1999. Targeted reduction of nucleoside triphosphate hydrolase by antisense RNA inhibits Toxoplasma gondii proliferation. J. Biol. Chem. 274:5083-5087. [DOI] [PubMed] [Google Scholar]
- 16.Que, X., H. Ngo, J. Lawton, M. Gray, Q. Liu, J. Engel, L. Brinen, P. Ghosh, K. A. Joiner, and S. L. Reed. 2002. The cathepsin B of Toxoplasma gondii, toxopain-1, is critical for parasite invasion and rhoptry protein processing. J. Biol. Chem. 277:25791-25797. [DOI] [PubMed] [Google Scholar]
- 17.Sajid, M., and J. H. McKerrow. 2002. Cysteine proteases of parasitic organisms. Mol. Biochem. Parasitol. 120:1-21. [DOI] [PubMed] [Google Scholar]
- 18.Selzer, P. M., X. Chen, V. J. Chan, M. Cheng, G. L. Kenyon, I. D. Kuntz, J. A. Sakanari, F. E. Cohen, and J. H. McKerrow. 1997. Leishmania major: molecular modeling of cysteine proteases and prediction of new nonpeptide inhibitors. Exp. Parasitol. 87:212-221. [DOI] [PubMed] [Google Scholar]
- 19.Sijwali, P. S., B. R. Shenai, J. Gut, A. Singh, and P. J. Rosenthal. 2001. Expression and characterization of the Plasmodium falciparum haemoglobinase falcipain-3. Biochem. J. 360:481-489. [DOI] [PMC free article] [PubMed] [Google Scholar]