Summary
Calciphylaxis, also referred to as calcific uremic arteriolopathy, is a relatively rare but well described syndrome that occurs most commonly in patients with late stage CKD. It is characterized by very painful placques or subcutaneous nodules and violaceous, mottled skin lesions that may progress to nonhealing ulcers, tissue necrosis, and gangrene with a 1-year mortality rate >50%. The pathogenesis of calciphylaxis is poorly understood. Risk factors include female sex, obesity, hyperphosphatemia, hypercalcemia, hyperparathyroidism, longer dialysis vintage, hypercoagulable states, and use of calcium-containing phosphate binders and warfarin. Treatment strategies for calciphylaxis are limited by inadequate understanding of its pathophysiology. Therapy is generally focused on correcting disturbances of calcium, phosphorus, and parathyroid hormone metabolism. Additional therapy focuses on decreasing inflammation and on dissolution of tissue calcium deposits with sodium thiosulfate and/or bisphosphonates. Successful treatment generally results in improvement of pain and healing of the lesions within 2–4 weeks, but the disorder generally takes many months to completely resolve.
Case Description
Abhijit Naik, MD (Renal Fellow).
A 54-year-old white man was referred from an outside dialysis clinic for evaluation of necrotic skin lesions. He had a 10-year history of diabetes and hypertension, with ESRD secondary to diabetes. His history was also significant for coronary artery disease, with two prior myocardial infarctions and atrial fibrillation. He had been undergoing thrice-weekly hemodialysis for 2 years with a Kt/V between 1.3–1.4. Three months before presentation he noticed several small firm and very painful nodules on both anterior thighs. He stated that after several weeks the lesions became much larger, black, and spread to the lateral thighs and buttocks. He was treated with mupirocin ointment and a vascular evaluation revealed normal blood flow in his legs. Aside from these painful lesions, he stated that he generally felt “ok.” Dialysis had been proceeding without problems. He denied chest pain, dyspnea, abdominal pain, or any gastrointestinal complaints. Review of systems was otherwise unremarkable. He never smoked and drank alcohol very infrequently. Medications included aspirin, amiodarone, simvastatin, famotidine, glipizide, clopidogrel, sevelamer carbonate, hydrocodone, and gabapentin. He had no known allergies.
On examination his vital signs were as follows: temperature, 98.8°F; heart rate, 80 beats per minute; BP, 94/60 mmHg, with no orthostatic changes; and respiratory rate, 20 breaths per minute. His BP was generally low, with systolic BP averaging between 90 and 100 mmHg. He was an ill appearing male in mild distress from extremity pain. His lungs were clear and a cardiovascular examination revealed a regular rate and rhythm with a 2/6 holosystolic murmur. His abdomen had normal bowel sounds and was mildly distended with some ascites, but was otherwise nontender and without appreciable masses or organomegaly. He had a normal appearing dialysis graft in his left arm. The patient’s lower extremities revealed multiple necrotic lesions of both thighs with smaller erythematous areas of the lower legs (Figure 1). The surrounding erythematous areas were extremely tender with subcutaneous firmness to palpation. He had lower extremity edema (2+) and palpable pulses in both feet. There were no other skin lesions. Pertinent laboratory data included the following: serum calcium, 8.8 mg/dl; phosphorus, 5.1 mg/dl; albumin, 3.2 g/dl; parathyroid hormone (PTH), 560 pg/ml; and alkaline phosphatase, 354 IU/L.
Figure 1.
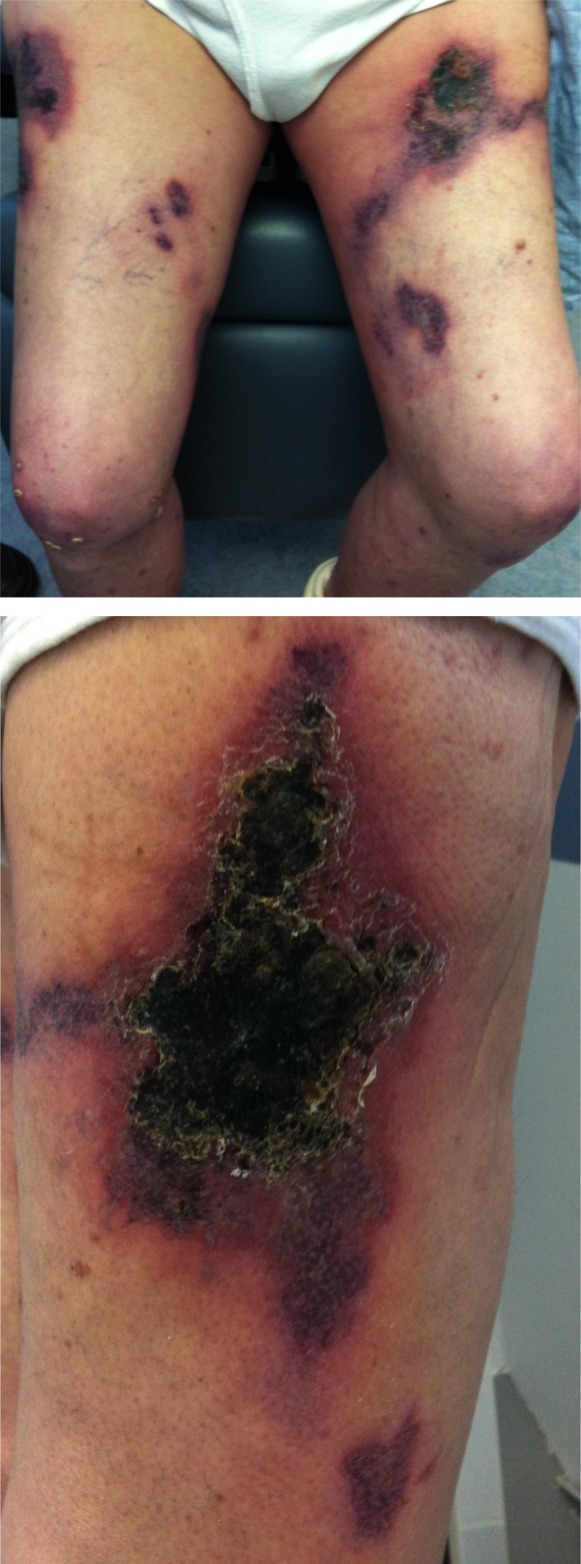
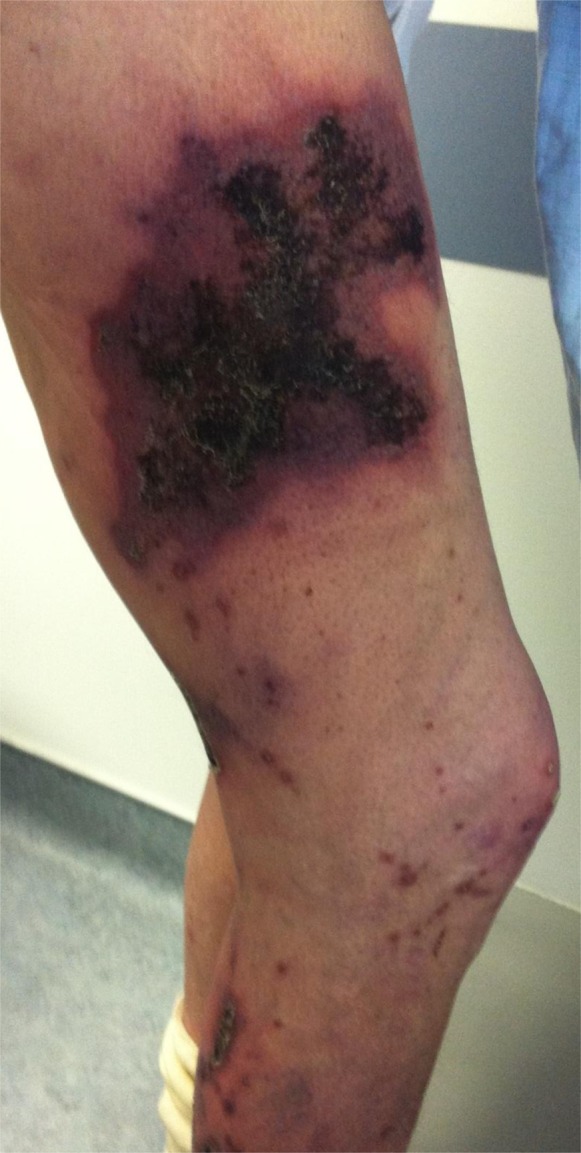
Calciphylactic lesions on the patient’s legs.
Discussion
In summary, this patient presented with a several month course of skin lesions progressing from small painful nodules to large necrotic lesions on both anterior thighs. The differential diagnosis of his presentation includes warfarin skin necrosis, peripheral vascular disease, vasculitis, cellulitis, and atheroembolic disease (1). However, this case represents a rather classic presentation of calciphylaxis, otherwise known as calcific uremic arteriolopathy (CUA). CUA is a relatively rare but well described entity that occurs most commonly in patients with late stage CKD, ESRD, or after transplantation. Although the initial clinical description was likely in 1898 (2), it was not until 1961 when Selye and colleagues coined the term calciphylaxis after inducing an anaphylactic-like hypersensitivity response in rats that resulted in soft tissue calcification and cutaneous necrosis (3). Clinically, CUA is characterized by very painful placques or subcutaneous nodules, and violaceous, mottled skin lesions that may progress to nonhealing ulcers, tissue necrosis, and gangrene. The clinical course may be complicated by surgical resections and amputations with a 1-year mortality rate >50%, with most deaths due to sepsis (4,5). Its pathology is significant for small vessel involvement and distal calcifications with intimal proliferation often accompanied by microthrombi. Although the pathogenesis of calciphylaxis is poorly understood, several factors appear to increase risk such as female sex, hyperphosphatemia, hypercalcemia, and hyperparathyroidism (5). Other factors associated with the development of calciphylaxis include obesity, longer dialysis vintage, hypercoagulable states, and the use of calcium-containing phosphate binders and warfarin (5–7). Risk factors in this patient included hyperparathyroidism and his long history of diabetes.
Diagnosis
CUA may develop in up to 4% of chronic dialysis patients (8,9). CUA can be very hard to diagnose clinically due to similar presentation of many other comorbid illnesses in CKD and dialysis patients. As previously mentioned, the differential diagnosis includes warfarin-induced skin necrosis, peripheral vascular disease, vasculitis, cellulitis, and atheroemboli (1). The absence of recent warfarin use rules out warfarin-induced skin necrosis. Peripheral vascular disease generally presents with nonhealing distal painful ulcers in the legs and feet that are associated with decreased blood flow to the extremities with diminished pulses. Atheroembolic disease is part of a multisystem disorder with systemic manifestations. The cutaneous manifestations are the result of occlusion of small arterioles and present as blue toe syndrome or livedo reticularis (10). Cutaneous vasculitis frequently presents with petechiae, palpable purpura, hemorrhagic bullae, digital ulceration, or livedo reticularis, which can be easily differentiated from the classic firm subcutaneous nodules of CUA. Finally, cellulitis generally has an area of erythema and warmth surrounding the necrotic area. In CUA, the lower extremities appear to be the most common site for lesions followed by the trunk, buttocks, upper extremities, and genitalia (4,11). It is good practice to classify lesions as proximal (above knee and elbow) and distal (below knee or elbow); proximal lesions are more likely to be due to calciphylaxis while ulcers from peripheral vascular disease tend to be distal. This is especially important because there is a high burden of peripheral vascular disease in dialysis patients.
Histopathologic features include circumferential wall calcification involving both the medial and intimal layers of small and medium size vessels. There is intimal hyperplasia with partial obliteration of the vessel lumen. Fibrin thrombi are often noted and are in close proximity to epidermal and dermal necrosis with necrosis frequently extending into the subcutaneous tissue (12).
A thorough clinical examination is the cornerstone of identifying these lesions early, especially when they are still nonulcerative (plaques), because they may represent potentially treatable disease (9). Tissue diagnosis is discouraged, first because it can initially be negative, but more importantly because performing biopsies may be associated with increased risk of progression of nonulcerative disease to ulcerative disease, infection, and mortality (9). Radiographic studies, including plain radiographs, high-resolution computed tomography scans, and mammographic techniques, can help distinguish calcium from surrounding noncalcium-containing radiolucent tissues but may be positive in <30% of patients with confirmed disease (9,13). Mammographic techniques are superior to computed tomography or plain films, although a significant limitation of mammographic imaging is that it can be quite painful, because it involves compressing the affected extremity between two plates (13). Bone scans may reveal increased technetium in affected areas in up to 97% of affected patients (8,9). The uptake is almost always subcutaneous. Given its noninvasive nature and high sensitivity, the bone scan could be the modality of choice to confirm a clinical suspicion of CUA. However, patients with florid and deep ulceration may have a negative scan due to complete denudation of subcutaneous tissue (9).
Risk Factors
Most information regarding risk factors for CUA is based on very small series and case reports, and thus should be interpreted with some caution. CUA rarely occurs in predialysis CKD patients and in the absence of renal failure (4,14) but most patients are undergoing dialysis. Peritoneal dialysis patients appear to have a slightly greater risk than hemodialysis patients (9,14,15). Most of the risk factors described point overwhelmingly to an altered calcium and phosphate milieu (Table 1), although CUA may occur in patients with serum calcium, phosphate and PTH concentrations in target ranges. Major risk factors for CUA include white race, younger age, elevated serum calcium or phosphate concentration, calcium×phosphate product and PTH concentrations (5), lower serum albumin, elevated serum alkaline phosphatase, and protein C and/or S deficiency (16–21). Local trauma, obesity (body mass index>30), liver disease, and use of systemic corticosteroids and warfarin have also been found to be independent risk factors (4,5,16–18). Warfarin likely promotes the development of CUA by reducing functional protein C levels and blocking vitamin K–dependent carboxylation of the matrix GLA protein, which is a local inhibitor of vascular calcification (22,23). Use of calcium supplements has been associated with increased risk of calciphylaxis in some (19,24), but not, all studies (7,17). Recent studies have suggested that parenteral iron may also be associated with development of CUA (25,26).
Table 1.
Risk factors for calciphylaxis
| Factor Type | Risk Factor |
| Demographic | Younger age |
| White | |
| Female sex | |
| Dialysis; peritoneal may be greater than hemodialysis | |
| Obesity | |
| Diabetes | |
| Liver disease | |
| Connective tissue disease | |
| Medication | Calcium-containing supplements or binders |
| Warfarin | |
| Corticosteroids | |
| Parenteral iron | |
| Biochemical | Hyperphosphatemia |
| Hypercalcemia | |
| Hyperparathyroidism | |
| Hypoalbuminemia | |
| Elevated alkaline phosphatase | |
| Protein C or S deficiency |
Risk factors in this patient included his being relatively young and white with a long history of diabetes and associated vascular disease. His laboratory data demonstrated mild hyperparathyroidism, although his PTH concentration was not especially elevated, nor were his serum phosphorus or calcium concentrations. He did have an elevated alkaline phosphatase and he was hypoalbuminemic. His dialysis vintage was not particularly long and he had not been exposed to warfarin.
Treatment and Outcome
Abhijit Naik, MD (Renal Fellow).
A diagnosis of CUA was made and the patient was treated with daily dialysis with 25 g of sodium thiosulfate infused over the last hour of dialysis. His phosphate binder was switched from sevelamer carbonate to 2000 mg of lanthanum carbonate with meals. Daily wound care consisted of dressing changes, leaving the wounds dry with no debridement. After about 2 weeks, his pain was much improved and dialysis and sodium thiosulfate was decreased to thrice weekly. The dialysate calcium concentration was maintained at 2.5 mmol/L. He did not receive vitamin D receptor activator (VDRA) agents or cinacalcet for his hyperparathyroidism. Serum phosphate remained between 3.0 and 4.2 mg/dl and PTH between 350 and 500 pg/ml. He remained in the hospital for 3 weeks and was then transferred to a skilled nursing facility. He continued to improve over the next 6 weeks, but approximately 2 months after presentation he suffered a myocardial infarction and died.
Management
General Measures.
Effective treatment options for CUA remain elusive. Treatment requires a multidisciplinary approach that may involve vascular and plastic surgeons, wound care experts, dieticians, and nephrologists. Early recognition is imperative to correct potential modifiable risk factors and initiate therapy that could prevent disease progression.
There is some evidence supporting the use of surgical debridement (4). However, most agree that debridement should be kept to a minimum because of poor wound healing with secondary infections and extension of the lesions (27). It is important to maintain a sterile environment with local wound care that should include gentle wound debridement while avoiding deep or wide surgical debridement and skin grafting. Appropriate sterile dressings should provide a moist environment while removing excessive exudates and should be easy to apply and remove in order to reduce surrounding skin trauma (4,27,28).
Warfarin should be discontinued, if possible (29). Alternatives such as heparin could be used for the short term if anticoagulation is necessary. The use of alternative anticoagulants, such as thrombin inhibitors, may have a role for patients needing long-term anticoagulation, although these agents have not been studied in calciphylaxis.
Tissues of patients with calciphylaxis have been shown to have low oxygen tension and hence hyperbaric oxygen is thought to have some utility (29–32). Hyperbaric oxygen therapy offers the ability to increase tissue oxygenation, improve angiogenesis, and enhance phagocytic activity and bactericidal action. Retrospective series have suggested that hyperbaric oxygen therapy reversed ulcerations in 8 of 11 (31) and 2 of 5 (32) patients with relatively well controlled hyperparathyroidism. Of note, among patients in whom tissue oxygenation was measured, those who failed hyperbaric therapy did not demonstrate an increase in tissue oxygenation (32). Favorable prognosis was associated with distal lesions and undergoing >20 sessions (31,32) Potential side effects include barotrauma, reversible myopia, and neurologic complications from oxygen toxicity (33).
Mineral Metabolism.
The primary focus of therapy should be to optimize calcium and phosphate homeostasis. Avoidance of a positive calcium balance and preventing the development of hypercalcemia should be a high priority (Table 2). Limited clinical data are available suggesting that lowering calcium intake improves CUA (27,34). Thus, discontinuation of calcium-based phosphate binders, adjustment of hemodialysis or peritoneal dialysis dialysate, and minimizing the use of VDRAs should be considered as initial therapeutic steps. Depending on the serum calcium concentration, hemodialysate calcium concentration can be reduced, with appropriate monitoring of blood chemistries. Dialysate calcium concentrations as low as 0–1.0 mEq/L have been used effectively (34–37). I generally do not recommend using dialysate concentrations lower than 1.25 mEq/L, however, because of risk of hemodynamic instability or cardiac arrhythmias from transient hypocalcemia.
Table 2.
Treatment options for calciphylaxis
| Intervention | Rationale |
| Reducing dietary phosphate | May prevent further worsening of disease due to elevated calcium×phosphate product |
| Phosphate binders | Will decrease calcium×phosphate product |
| Low calcium dialysate | May help lower calcium×phosphate product |
| Decreasing calcium intake, including use of noncalcium-containing phosphate binders | May help lower calcium×phosphate product |
| Increasing Kt/V | Help with better phosphate control |
| Hyperbaric oxygen | Increases oxygen tension in the tissues, may enhance healing and reduce pain |
| Sodium thiosulfate | Causes calcium to dissolve out of tissue and form soluble complexes, which can be more easily removed by dialysis; anti-inflammatory role |
| Bisphosphonates | Decrease bone turnover, anti-inflammatory action, reduces extraskeletal calcification |
| Cinacalcet | Decreases PTH levels and reduces bone turnover |
| Parathyroidectomy | Reduces bone turnover and decreases calcium and phosphate efflux from bone |
PTH, parathyroid hormone.
Hyperphosphatemia, with an associated increase in calcium×phosphate product, appears to be more important than calcium concentration alone in the pathophysiology of CUA. Thus, aggressive control of hyperphosphatemia with noncalcium-based binders (e.g., sevelamer carbonate, lanthanum carbonate, or even aluminum) and dietary phosphate restriction should be initiated. Intensifying dialysis or changing from peritoneal to hemodialysis has been suggested to be beneficial in some studies (24). Decreasing serum phosphorous levels by increasing the duration and frequency of hemodialysis has been reported to be beneficial in the resolution of CUA by several investigators (27,34,36,38–41). Although there are no studies demonstrating the optimal duration of intensified dialysis, I strongly recommend a course of dialysis 5–7 days per week for the first couple weeks of treatment, with the duration of this intensified dialysis therapy depending on clinical response.
The relationship of calciphylaxis with hyperparathyroidism is best seen in nonuremic patients (4,14,21,42). There are reports that parathyroidectomy may benefit wound healing (43–45) and increase survival (5,39,45), whereas other studies demonstrated no benefit to parathyroidectomy (4,46). In fact, Budisavljevic and colleagues reviewed 47 patients with CUA, 31of whom underwent parathyroidectomy; 50% of these patients died within 9 weeks of surgery (47). Thus, the role of parathyroidectomy remains controversial (5,8,42,48–50). Parathyroidectomy is unlikely to be useful when PTH concentrations are not markedly elevated (1,5,41). I generally would not consider parathyroidectomy with PTH concentrations of <800–1000 pg/ml.
Medical management of hyperparathyroidism remains important and relies on intensified dialysis, as well as the use of noncalcium-based phosphate binders and cinacalcet. Cinacalcet has been successfully used in several patients with CUA (22,51–55). VDRAs increase the risk of developing hypercalcemia or hyperphosphatemia; however, they have been used successfully in combination with cinacalcet (54,56,57).
Newer Therapies.
Several adjunctive therapies have recently been shown to have some success in the management of CUA (Table 2). Reports of sodium thiosulfate used to treat CUA first appeared in 2004 (51). There has since been numerous case reports and small series demonstrating effective treatment of CUA with the addition of sodium thiosulfate to the treatment regimen (22,38,52–55,58–61). Sodium thiosulfate is a chelating agent that has antioxidant activity and increases activity of endothelial nitric oxide synthetase (22); it has been used to treat cyanide toxicity, to reduce toxicity of cisplatin chemotherapy, and to chelate calcium in the treatment of nephrolithiasis (62–64). In CUA, sodium thiosulfate is believed to act by dissolving insoluble tissue calcium salts to form calcium thiosulfate, which is many thousand times more soluble than many other calcium salts (62).
There is no consensus as to the best dosing schedule for sodium thiosulfate (22,38,51–55,58–61). Intravenous doses have varied from 5 to 75 g after or during the last 30–60 minutes of hemodialysis. Sodium thiosulfate is predominantly metabolized by nonrenal mechanisms and is readily dialyzed. Serum concentrations drop quickly whether given before, during, or after dialysis, and more data are required to determine the optimal dosing schedule (65). The most commonly reported dose has been 25 g thrice weekly at the end of dialysis. In patients with more frequent dialysis, it is generally given after each dialysis session. Clinical improvement usually is noted within 2–3 weeks with a decrease in pain and stabilization of lesions. However, complete wound healing generally takes many months, and thus therapy may last for 6–12 months. Although it is generally well tolerated, adverse effects include nausea, emesis, and the development of a high anion gap metabolic acidosis. The acidosis is thought to occur through the generation of thiosulfuric acid when sodium thiosulfate is dissolved in aqueous solution (52) which can easily be managed by treating the patient with supplemental oral sodium bicarbonate or by altering the bicarbonate level of the dialysate. There are reports of a few patients undergoing successful intraperitoneal treatment with sodium thiosulfate (15,58), although it was thought to cause recurrent peritonitis in one patient (15). There is also a report of using oral sodium thiosulfate for prevention of recurrence in one patient (66) and as long-term maintenance therapy in four patients who had persistent skin lesions after 6 months of intravenous therapy (40). However, one of the four patients developed progression of disease, which was thought to be due to noncompliance.
Although there are no clear data as to the best treatment regimen, my standard protocol is to administer 25 g of sodium thiosulfate during the last hour of each dialysis session. Once there is a decrease in pain and stabilization of lesions, I generally decrease dialysis frequency and sodium thiosulfate dosage from 5–7 days weekly to thrice weekly, which generally occurs after 10–14 days. Once the patient becomes totally pain free and the lesions are clearly resolving, I then decrease the dose of sodium thiosulfate to 12.5 mg thrice weekly at the end of dialysis. This is generally 3–6 months into therapy. I also believe that it is easier to maintain more aggressive control of calcium and phosphate balance in patients with CUA with hemodialysis compared with peritoneal dialysis. Thus, I recommend that peritoneal dialysis patients be switched to hemodialysis and obtain intravenous sodium thiosulfate treatment.
Bisphosphonates are a class of antiresorptive drugs that inhibit osteoclastic activity and decrease bone turnover (67). There are several reports on the efficacy of bisphosphonate therapy in calciphylaxis (55,68–70). The potential mechanisms of bisphosphonate therapy likely include control of high bone resorption, an anti-inflammatory action (68), and an inhibition of calcification via inhibition of an NF-κB pathway (71). Unfortunately, bisphosphonates can have long-term adverse effects (and are typically contraindicated in patients with advanced CKD); thus, their role in treating calciphylaxis is unclear. Few data are available on the role of and the appropriate use of bisphosphonates in CUA. I suggest this therapy for those who have evidence of high bone turnover who are not responding to other therapies after 2–4 weeks.
Questions
Rebecca Seshasai, MD (Renal Fellow).
Are dialysis patients who are successfully treated for calciphylaxis at increased risk for recurrence? If so, does the disease tend to recur in the same location? Do you have any recommendations for monitoring and treatment of recurrences?
The overall occurrence of CUA is relatively rare and sporadic with a relatively poor response to therapy. There are many patients who do not have complete recovery and require long-term management. I would consider any patient who has developed CUA as being at increased risk of recurrence; however, there are no data available to assess risk of recurrence. In a review of 172 chronic hemodialysis patients who were treated with sodium thiosulfate, the long-term outcomes of 53 patients were obtained by a survey sent to the attending nephrologist. None of the patients were reported to have recurrence (61). As far as monitoring patients, I would recommend aggressive phosphate control, avoidance of hypercalcemia, avoidance of calcium-containing phosphate binders, as well as avoidance of agents that increase risk, such as warfarin, parenteral iron, and corticosteroids.
Suzanne Boyle, MD (Renal Fellow).
Given that there are reports of calciphylaxis after transplant, can patients with a previous history of calciphylaxis receive a kidney transplant? Are they at increased risk for developing calciphylaxis again after transplantation?
Unfortunately, there are no data to assess the risk of calciphylaxis after transplantation, nor are there data reporting the outcome of patients who have had CUA and were subsequently transplanted. In my opinion, if a patient had CUA and has totally recovered, I would recommend that patient be evaluated and transplanted based upon the same criteria used for any other potential transplant candidate.
Matt Denker, MD (Renal Fellow).
In an effort to prevent CUA, do you think we should have different target recommendations for serum calcium, phosphate, PTH, and choice of phosphate binder for patients who have multiple risk factors for CUA?
In terms of CKD-mineral bone disorder targets for patients at risk of CUA, I do not believe we should have different therapeutic targets. The primary focus should be on calcium and phosphate and we should aim to achieve normal values, as recommended by Kidney Disease Improving Global Outcomes guidelines (72). In regard to phosphate binders, it would be best to limit or avoid calcium-containing binders in those who are at increased risk for CUA.
In summary, calciphylaxis or CUA, is a relatively rare complication of CKD that results from disordered mineral metabolism, chronic inflammation, microvascular disease, and possibly a disordered coagulation system. It is critically important to correctly identify calciphylaxis due to its very high mortality rate of close to 50%. Therapy is multifactorial and includes aggressive management of calcium, phosphate, and PTH abnormalities, removal of potential inciting agents such as warfarin or calcium-containing drugs, and appropriate wound care. Most patients will require combined therapy that may include intensified dialysis, the early administration of sodium thiosulfate or possibly bisphosphonates, and the addition of cinacalcet or parathyroidectomy if severe hyperparathyroidism cannot be controlled.
Disclosures
S.M.S. has consulted or received grants from Abbott, Amgen, Cytochroma, Shire, and Fresenius.
Acknowledgments
The author thanks Dr. Abhijit Naik, Renal Fellow at the University of Chicago, for preparing the case presentation and assisting in the initial draft of the discussion, and Dr. Jeffrey Berns for his extensive review of the manuscript and helpful editorial comments.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Wilmer WA, Magro CM: Calciphylaxis: Emerging concepts in prevention, diagnosis, and treatment. Semin Dial 15: 172–186, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Bryant JH, White WH: A case of calcification of the arteries and obliterative endarterities associated with hydronephrosis in a child age six months. Guys Hosp Rep 55: 1719, 1898 [Google Scholar]
- 3.Selye H, Gentile G, Prioreschi P: Cutaneous molt induced by calciphylaxis in the rat. Science 134: 1876–1877, 1961 [DOI] [PubMed] [Google Scholar]
- 4.Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR: Calciphylaxis: Natural history, risk factor analysis, and outcome. J Am Acad Dermatol 56: 569–579, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Hafner J, Keusch G, Wahl C, Sauter B, Hürlimann A, von Weizsäcker F, Krayenbühl M, Biedermann K, Brunner U, Helfenstein U: Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): A complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol 33: 954–962, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Landray MJ, Baigent C: Renal function: An emerging risk factor for cardiovascular disease? Evid Based Cardiovasc Med 5: 32–33, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Bleyer AJ, Choi M, Igwemezie B, de la Torre E, White WL: A case control study of proximal calciphylaxis. Am J Kidney Dis 32: 376–383, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Angelis M, Wong LL, Myers SA, Wong LM: Calciphylaxis in patients on hemodialysis: A prevalence study. Surgery 122: 1083–1089, discussion 1089–1090, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Fine A, Zacharias J: Calciphylaxis is usually non-ulcerating: Risk factors, outcome and therapy. Kidney Int 61: 2210–2217, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Scolari F, Ravani P: Atheroembolic renal disease. Lancet 375: 1650–1660, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Rizvi T, Al-Nakshabandi NA: Penile necrosis due to calciphylaxis in a patient of end stage renal disease. Saudi Med J 30: 143–145, 2009 [PubMed] [Google Scholar]
- 12.Ng AT, Peng DH: Calciphylaxis. Dermatol Ther 24: 256–262, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Bleibel W, Hazar B, Herman R: A case report comparing various radiological tests in the diagnosis of calcific uremic arteriolopathy. Am J Kidney Dis 48: 659–661, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Nigwekar SU, Wolf M, Sterns RH, Hix JK: Calciphylaxis from nonuremic causes: A systematic review. Clin J Am Soc Nephrol 3: 1139–1143, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.New N, Mohandas J, John GT, Ratanjee S, Healy H, Francis L, Ranganathan D: Calcific uremic arteriolopathy in peritoneal dialysis populations. Int J Nephrol 2011: 982854, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Török LKL, Középessy L: Cutaneous gangrene due to hyperparathyroidism secondary to chronic renal failure (uraemic gangrene syndrome). Clin Exp Dermatol 21: 75–77, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, Davis CL, Stehman-Breen CO: Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int 60: 324–332, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Ahmed S, O’Neill KD, Hood AF, Evan AP, Moe SM: Calciphylaxis is associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis 37: 1267–1276, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Campistol JM, Almirall J, Martin E, Torras A, Revert L: Calcium-carbonate-induced calciphylaxis. Nephron 51: 549–550, 1989 [DOI] [PubMed] [Google Scholar]
- 20.Perez-Mijares R, Guzman-Zamudio JL, Payan-Lopez J, Rodriguez-Fernandez A, Gomez-Fernandez P, Almaraz-Jimenez M: Calciphylaxis in a haemodialysis patient: Functional protein S deficiency? Nephrol Dial Transplant 11: 1856–1859, 1996 [PubMed] [Google Scholar]
- 21.Mehta RL, Scott G, Sloand JA, Francis CW: Skin necrosis associated with acquired protein C deficiency in patients with renal failure and calciphylaxis. Am J Med 88: 252–257, 1990 [DOI] [PubMed] [Google Scholar]
- 22.Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R: Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: The emerging role of sodium thiosulfate. Cardiovasc Diabetol 4: 4, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howe AM, Webster WS: Warfarin exposure and calcification of the arterial system in the rat. Int J Exp Pathol 81: 51–56, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zacharias JM, Fontaine B, Fine A: Calcium use increases risk of calciphylaxis: A case-control study. Perit Dial Int 19: 248–252, 1999 [PubMed] [Google Scholar]
- 25.Farah M, Crawford RI, Levin A, Chan Yan C: Calciphylaxis in the current era: Emerging ‘ironic’ features? Nephrol Dial Transplant 26: 191–195, 2011 [DOI] [PubMed] [Google Scholar]
- 26.Goodkin DA, Larkina M, Robinson BM: Iron and calciphylaxis. Nephrol Dial Transplant 26: 3063, author reply 3063–3064, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Don BR, Chin AI: A strategy for the treatment of calcific uremic arteriolopathy (calciphylaxis) employing a combination of therapies. Clin Nephrol 59: 463–470, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Milas M, Bush RL, Lin P, Brown K, Mackay G, Lumsden A, Weber C, Dodson TF: Calciphylaxis and nonhealing wounds: The role of the vascular surgeon in a multidisciplinary treatment. J Vasc Surg 37: 501–507, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Banerjee C, Woller SC, Holm JR, Stevens SM, Lahey MJ: Atypical calciphylaxis in a patient receiving warfarin then resolving with cessation of warfarin and application of hyperbaric oxygen therapy. Clin Appl Thromb Hemost 16: 345–350, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Wilmer WA, Voroshilova O, Singh I, Middendorf DF, Cosio FG: Transcutaneous oxygen tension in patients with calciphylaxis. Am J Kidney Dis 37: 797–806, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Basile C, Montanaro A, Masi M, Pati G, De Maio P, Gismondi A: Hyperbaric oxygen therapy for calcific uremic arteriolopathy: A case series. J Nephrol 15: 676–680, 2002 [PubMed] [Google Scholar]
- 32.Podymow T, Wherrett C, Burns KD: Hyperbaric oxygen in the treatment of calciphylaxis: A case series. Nephrol Dial Transplant 16: 2176–2180, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Leach RM, Rees PJ, Wilmshurst P: Hyperbaric oxygen therapy. BMJ 317: 1140–1143, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Russell R, Brookshire MA, Zekonis M, Moe SM: Distal calcific uremic arteriolopathy in a hemodialysis patient responds to lowering of Ca x P product and aggressive wound care. Clin Nephrol 58: 238–243, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Wang HY, Yu CC, Huang CC: Successful treatment of severe calciphylaxis in a hemodialysis patient using low-calcium dialysate and medical parathyroidectomy: Case report and literature review. Ren Fail 26: 77–82, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Fernández E, Montoliu J: Successful treatment of massive uraemic tumoral calcinosis with daily haemodialysis and very low calcium dialysate. Nephrol Dial Transplant 9: 1207–1209, 1994 [DOI] [PubMed] [Google Scholar]
- 37.Lipsker D, Chosidow O, Martinez F, Challier E, Francès C: Low-calcium dialysis in calciphylaxis. Arch Dermatol 133: 798–799, 1997 [PubMed] [Google Scholar]
- 38.Baldwin C, Farah M, Leung M, Taylor P, Werb R, Kiaii M, Levin A: Multi-intervention management of calciphylaxis: A report of 7 cases. Am J Kidney Dis 58: 988–991, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Llach F: The evolving pattern of calciphylaxis: Therapeutic considerations. Nephrol Dial Transplant 16: 448–451, 2001 [DOI] [PubMed] [Google Scholar]
- 40.AlBugami MM, Wilson JA, Clarke JR, Soroka SD: Oral sodium thiosulfate as maintenance therapy for calcific uremic arteriolopathy: A case series. Am J Nephrol 37: 104–109, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Kang AS, McCarthy JT, Rowland C, Farley DR, van Heerden JA: Is calciphylaxis best treated surgically or medically? Surgery, 128: 967–971; discussion 971-962, 2000. [DOI] [PubMed]
- 42.Bishop J, Brown E, Podesta A, Troy C, Dong XE: Surgical management of calciphylaxis associated with primary hyperparathyroidism: A case report and review of the literature. Int J Endocrinol 2010: 823210, 4 pp, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adrogué HJ, Frazier MR, Zeluff B, Suki WN: Systemic calciphylaxis revisited. Am J Nephrol 1: 177–183, 1981 [DOI] [PubMed] [Google Scholar]
- 44.Kane WJ, Petty PM, Sterioff S, McCarthy JT, Crotty TB: The uremic gangrene syndrome: Improved healing in spontaneously forming wounds following subtotal parathyroidectomy. Plast Reconstr Surg 98: 671–678, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Duffy A, Schurr M, Warner T, Chen H: Long-term outcomes in patients with calciphylaxis from hyperparathyroidism. Ann Surg Oncol 13: 96–102, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Chan YL, Mahony JF, Turner JJ, Posen S: The vascular lesions associated with skin necrosis in renal disease. Br J Dermatol 109: 85–95, 1983 [DOI] [PubMed] [Google Scholar]
- 47.Budisavljevic MN, Cheek D, Ploth DW: Calciphylaxis in chronic renal failure. J Am Soc Nephrol 7: 978–982, 1996 [DOI] [PubMed] [Google Scholar]
- 48.Duh QY, Lim RC, Clark OH: Calciphylaxis in secondary hyperparathyroidism. Diagnosis and parathyroidectomy. Arch Surg 126: 1213–1218, discussion 1218–1219, 1991 [DOI] [PubMed] [Google Scholar]
- 49.Maeda H, Tokumoto M, Yotsueda H, Taniguchi M, Tsuruya K, Hirakata H, Iida M: Two cases of calciphylaxis treated by parathyroidectomy: Importance of increased bone formation. Clin Nephrol 67: 397–402, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Bahar G, Mimouni D, Feinmesser M, David M, Popovzer A, Feinmesser R: Subtotal parathyroidectomy: A possible treatment for calciphylaxis. Ear Nose Throat J 82: 390–393, 2003 [PubMed] [Google Scholar]
- 51.Cicone JS, Petronis JB, Embert CD, Spector DA: Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis 43: 1104–1108, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Brucculeri M, Cheigh J, Bauer G, Serur D: Long-term intravenous sodium thiosulfate in the treatment of a patient with calciphylaxis. Semin Dial 18: 431–434, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Araya CE, Fennell RS, Neiberger RE, Dharnidharka VR: Sodium thiosulfate treatment for calcific uremic arteriolopathy in children and young adults. Clin J Am Soc Nephrol 1: 1161–1166, 2006 [DOI] [PubMed] [Google Scholar]
- 54.Kyritsis I, Gombou A, Griveas I, Agroyannis I, Retsa K, Agroyannis B: Combination of sodium thiosulphate, cinacalcet, and paricalcitol in the treatment of calciphylaxis with hyperparathyroidism. Int J Artif Organs 31: 742–744, 2008 [DOI] [PubMed] [Google Scholar]
- 55.Raymond CB, Wazny LD: Sodium thiosulfate, bisphosphonates, and cinacalcet for treatment of calciphylaxis. Am J Health Syst Pharm 65: 1419–1429, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Vargemezis V, Liakopoulos V, Kriki P, Panagoutsos S, Leontsini M, Passadakis P, Thodis E: Pivotal role of paricalcitol in the treatment of calcific uremic arteriolopathy in the presence of a parathyroid adenoma. Am J Kidney Dis 55: 144–147, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Kakagia D, Kriki P, Thodis E, Roumeliotis A, Vargemezis V: Calcific uremic arteriolopathy treated with cinacalcet, paricalcitol, and autologous growth factors. J Cutan Med Surg 15: 121–124, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Mataic D, Bastani B: Intraperitoneal sodium thiosulfate for the treatment of calciphylaxis. Ren Fail 28: 361–363, 2006 [DOI] [PubMed] [Google Scholar]
- 59.Zitt E, König M, Vychytil A, Auinger M, Wallner M, Lingenhel G, Schilcher G, Rudnicki M, Salmhofer H, Lhotta K: Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant 28: 1232–1240, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Hackett BC, McAleer MA, Sheehan G, Powell FC, O’Donnell BF: Calciphylaxis in a patient with normal renal function: Response to treatment with sodium thiosulfate. Clin Exp Dermatol 34: 39–42, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Nigwekar SU, Brunelli SM, Meade D, Wang W, Hymes J, Lacson E, Jr: Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol 8: 1162–1170, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yatzidis H: Successful sodium thiosulphate treatment for recurrent calcium urolithiasis. Clin Nephrol 23: 63–67, 1985 [PubMed] [Google Scholar]
- 63.Baskin SI, Horowitz AM, Nealley EW: The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol 32: 368–375, 1992 [DOI] [PubMed] [Google Scholar]
- 64.Howell SB, Pfeifle CL, Wung WE, Olshen RA, Lucas WE, Yon JL, Green M: Intraperitoneal cisplatin with systemic thiosulfate protection. Ann Intern Med 97: 845–851, 1982 [DOI] [PubMed] [Google Scholar]
- 65.Farese S, Stauffer E, Kalicki R, Hildebrandt T, Frey BM, Frey FJ, Uehlinger DE, Pasch A: Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin J Am Soc Nephrol 6: 1447–1455, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Musso CG, Enz P, Vidal F, Gelman R, Di Giuseppe L, Bevione P, Garfi L, Galimberti R, Algranati L: Oral sodium thiosulfate solution as a secondary preventive treatment for calciphylaxis in dialysis patients. Saudi J Kidney Dis Transpl 19: 820–821, 2008 [PubMed] [Google Scholar]
- 67.Russell RG: Bisphosphonates: The first 40 years. Bone 49: 2–19, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Monney P, Nguyen QV, Perroud H, Descombes E: Rapid improvement of calciphylaxis after intravenous pamidronate therapy in a patient with chronic renal failure. Nephrol Dial Transplant 19: 2130–2132, 2004 [DOI] [PubMed] [Google Scholar]
- 69.Shiraishi N, Kitamura K, Miyoshi T, Adachi M, Kohda Y, Nonoguchi H, Misumi S, Maekawa Y, Murayama T, Tomita M, Tomita K: Successful treatment of a patient with severe calcific uremic arteriolopathy (calciphylaxis) by etidronate disodium. Am J Kidney Dis 48: 151–154, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Hanafusa T, Yamaguchi Y, Tani M, Umegaki N, Nishimura Y, Katayama I: Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol 57: 1021–1025, 2007 [DOI] [PubMed] [Google Scholar]
- 71.Weenig RH: Pathogenesis of calciphylaxis: Hans Selye to nuclear factor kappa-B. J Am Acad Dermatol 58: 458–471, 2008 [DOI] [PubMed] [Google Scholar]
- 72.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G; Kidney Disease: Improving Global Outcomes (KDIGO): Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]


