Summary
Background and objectives
The Initiating Dialysis Early and Late study showed that planned early or late initiation of dialysis, based on the Cockcroft and Gault estimation of GFR, was associated with identical clinical outcomes. This study examined the association of all-cause mortality with estimated GFR at dialysis commencement, which was determined using multiple formulas.
Design, setting, participants, & measurements
Initiating Dialysis Early and Late trial participants were stratified into tertiles according to the estimated GFR measured by Cockcroft and Gault, Modification of Diet in Renal Disease, or Chronic Kidney Disease-Epidemiology Collaboration formula at dialysis commencement. Patient survival was determined using multivariable Cox proportional hazards model regression.
Results
Only Initiating Dialysis Early and Late trial participants who commenced on dialysis were included in this study (n=768). A total of 275 patients died during the study. After adjustment for age, sex, racial origin, body mass index, diabetes, and cardiovascular disease, no significant differences in survival were observed between estimated GFR tertiles determined by Cockcroft and Gault (lowest tertile adjusted hazard ratio, 1.11; 95% confidence interval, 0.82 to 1.49; middle tertile hazard ratio, 1.29; 95% confidence interval, 0.96 to 1.74; highest tertile reference), Modification of Diet in Renal Disease (lowest tertile hazard ratio, 0.88; 95% confidence interval, 0.63 to 1.24; middle tertile hazard ratio, 1.20; 95% confidence interval, 0.90 to 1.61; highest tertile reference), and Chronic Kidney Disease-Epidemiology Collaboration equations (lowest tertile hazard ratio, 0.93; 95% confidence interval, 0.67 to 1.27; middle tertile hazard ratio, 1.15; 95% confidence interval, 0.86 to 1.54; highest tertile reference).
Conclusion
Estimated GFR at dialysis commencement was not significantly associated with patient survival, regardless of the formula used. However, a clinically important association cannot be excluded, because observed confidence intervals were wide.
Introduction
Early observational cohort studies in the 1980s and 1990s suggested that commencing dialysis at a higher level of GFR was associated with improved morbidity and mortality (1–9). These studies led to promulgation of the so-called healthy start dialysis concept, where dialysis was instigated before the development of uremic signs and symptoms (10). Conversely, a number of more recently published observational studies, including a meta-analysis (11), has shown that dialysis initiation at higher estimated GFR (eGFR) levels is paradoxically associated with an increased risk of mortality compared with initiation at lower levels (12–22). These results may have been limited by selection, indication, survivor, and misclassification biases. Moreover, a study by the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD) Group found that the Modification of Diet in Renal Disease (MDRD) method of estimation of GFR was influenced by muscle mass to a greater extent than GFR estimated by creatinine clearance (21). Specifically, MDRD eGFR at dialysis commencement was inversely associated with muscle mass and survival, whereas mean urinary urea and creatinine clearances were not significantly associated with either muscle mass or survival (21).
In 2010, the Initiating Dialysis Early and Late (IDEAL) randomized controlled trial involving 828 patients with ESRD showed that planned early initiation of dialysis at eGFR values between 10 and 14 ml/min per 1.73 m2 based on the Cockcroft and Gault (CG) equation did not confer either a survival or quality of life benefit (23,24) compared with late dialysis commencement (GFR<7 ml/min per 1.73 m2 or when traditional clinical indicators of uremia supervened). The aim of this preplanned post hoc analysis of the IDEAL trial was to further explore the relationship between eGFR at dialysis commencement using three different equations to estimate GFR and subsequent survival.
Materials and Methods
The IDEAL study design, methodology, and primary results have been described previously (23). The IDEAL trial (Australian and New Zealand Clinical Trials Registry number 12609000266268) was conducted during the period before the abbreviated MDRD equation widely replaced the CG formula in estimation of GFR. This prespecified post hoc analysis used an as-treated analysis to study the association between the eGFR determined by different formulas at the start of dialysis on the IDEAL participants’ survival. This study included participants who commenced on dialysis from 2000 to 2009, with a median follow-up period of 4.4 years. The study protocol was approved by ethics committees at all participating centers in adherence to the Declaration of Helsinki. All patients provided written informed consent before trial participation.
Participants
The IDEAL trial included adult patients with stage 5 CKD and a CG eGFR value (25) corrected for body surface area (BSA) (26) between 10 and 15 ml/min per 1.73 m2. Participants from 32 centers in Australia and New Zealand were randomized 1:1 to either commence dialysis at a GFR of 10–14 ml/min per 1.73 m2 or continue routine medical care and commence dialysis at a GFR of 5–7 ml/min per 1.73 m2. The IDEAL trial participants who commenced dialysis during the study were divided into tertiles according to the CG GFR at dialysis commencement. Separate sensitivity analyses were also conducted, in which the GFR at dialysis commencement was alternatively estimated by either the MDRD equation or the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (27). Sensitivity analyses were also performed using eGFR uncorrected for BSA (i.e., by multiplying BSA-corrected GFR by actual BSA divided by 1.73) (26) and analyses by sex.
Study Outcomes
The primary outcome measure was all-cause mortality. The secondary outcomes are broadly classified as cardiovascular (CV) death and non-CV mortality.
Statistical Analyses
Results were expressed as frequencies (percentages) for categorical variables, mean ± SD for continuous normally distributed variables, and median (range) for continuous non-normally distributed variables. Group comparisons were performed by chi-squared test, ANOVA, or Kruskal–Wallis test as appropriate. For the primary outcome of all-cause mortality, survival estimates and curves were generated using the Kaplan–Meier method. The agreement between two eGFR formulas was shown by Bland and Altman plots. Survival time was defined as the period from dialysis commencement until death with censoring for transplantation, loss to follow-up, or end of study. A Cox proportional hazards model analysis was used to investigate the association of tertiles of eGFR at dialysis commencement for all-cause, CV, and non-CV mortality. Covariates used in the multivariable analyses were age, sex, racial origin, body mass index, presence or absence of diabetes mellitus, and presence or absence of CV disease (defined as presence of at least one of congestive heart failure, ischemic heart disease, peripheral vascular disease, and prior cerebrovascular event). Statistical analyses were performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and R version 2.15.1 (The R Foundation for Statistical Computing). P values less than 0.05 were considered statistically significant.
Results
Patient Characteristics
In total, 768 of 828 IDEAL (93%) participants in the IDEAL trial commenced dialysis during the study period (early start group, n=382; late start group, n=386). One patient from the early start group had no GFR recorded at the commencement of dialysis and was excluded from analysis. Patients were divided into tertiles according to CG eGFR at dialysis commencement (i.e., <9.5 ml/min per 1.73 m2 [n=248], 9.5–11.9 ml/min per 1.73 m2 [n=256], and ≥12.0 ml/min per 1.73 m2 [n=264]). Based on the CG equation, women were more likely to commence dialysis with a higher eGFR. However, the three groups had similar comorbidities at the start of dialysis (Table 1).
Table 1.
Baseline characteristics of the Initiating Dialysis Early and Late trial participants divided in tertiles of estimated GFR at initiation using the Cockcroft and Gault, Modification of Diet in Renal Disease, and Chronic Kidney Disease-Epidemiology Collaboration formulas
| Characteristics | CG (ml/min per 1.73 m2) | MDRD (ml/min per 1.73 m2) | CKD-EPI (ml/min per 1.73 m2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <9.5 | 9.5–11.9 | ≥12 | P Value | <6.5 | 6.5–8.9 | ≥9 | P Value | <6 | 6.0–7.9 | ≥8 | P Value | |
| N | 248 | 256 | 264 | 255 | 268 | 245 | 271 | 238 | 259 | |||
| Randomized group, n (%) | ||||||||||||
| Early | 57 (23.0) | 138 (53.9) | 187 (70.8) | 80 (31.4) | 137 (51.1) | 165 (67.4) | 84 (31.0) | 124 (52.1) | 174 (67.2) | |||
| Late | 191 (77.0) | 118 (46.1) | 77 (29.2) | 175 (68.6) | 131 (48.9) | 80 (32.7) | 187 (69.0) | 114 (47.9) | 85 (32.8) | |||
| Women, n (%) | 68 (27.4) | 97 (37.9) | 100 (37.9) | 0.02 | 101 (39.6) | 88 (32.8) | 76 (31.0) | 0.10 | 101 (37.3) | 75 (31.5) | 89 (34.4) | 0.39 |
| Age (yr)a | 60.1 (11.7) | 59.3 (12.6) | 59.5 (12.7) | 0.74 | 55.2 (12.0) | 58.2 (11.7) | 65.7 (10.8) | <0.001 | 56.3 (12.0) | 59.4 (11.7) | 63.3 (12.3) | <0.001 |
| Duration first seen by nephrologist (mo)a | 56.2 (71.6) | 74.2 (100.7) | 66.9 (91.6) | 0.08 | 59.9 (75.0) | 70.0 (95.5) | 67.6 (95.4) | 0.41 | 58.1 (75.2) | 69.3 (92.4) | 70.8 (99.0) | 0.21 |
| Race, n (%) | ||||||||||||
| Caucasian | 169 (68.2) | 178 (69.5) | 199 (75.4) | 0.16 | 162 (63.5) | 196 (73.1) | 188 (76.7) | <0.01 | 171 (63.1) | 176 (74.0) | 199 (76.8) | <0.01 |
| Non-Caucasian | 79 (31.8) | 78 (30.5) | 65 (24.6) | 93 (36.5) | 72 (26.9) | 57 (23.3) | 100 (36.9) | 62 (26.0) | 60 (23.2) | |||
| Comorbidities, n (%) | ||||||||||||
| Diabetes | 109 (44.0) | 110 (43.0) | 112 (42.4) | 0.94 | 120 (47.1) | 113 (42.2) | 98 (40.0) | 0.26 | 130 (48.0) | 100 (42.0) | 101 (39.0) | 0.10 |
| Congestive cardiac failure | 12 (4.8) | 16 (6.3) | 15 (5.9) | 0.79 | 14 (5.5) | 10 (3.7) | 19 (7.8) | 0.14 | 15 (5.5) | 10 (4.2) | 18 (6.9) | 0.41 |
| Peripheral vascular disease | 47 (19.0) | 43 (16.8) | 47 (17.8) | 0.82 | 44 (17.3) | 48 (17.9) | 45 (18.4) | 0.95 | 49 (18.1) | 39 (16.4) | 49 (18.9) | 0.76 |
| Ischemic heart disease | 68 (27.4) | 68 (26.6) | 78 (29.6) | 0.74 | 53 (20.8) | 76 (28.4) | 85 (34.7) | <0.01 | 60 (22.1) | 70 (29.4) | 84 (32.4) | <0.05 |
| Planned dialysis modality, n (%) | ||||||||||||
| Hemodialysis | 97 (39.1) | 123 (48.1) | 119 (45.1) | 0.12 | 125 (49.0) | 121 (45.1) | 93 (38.0) | 0.04 | 130 (48.0) | 110 (46.2) | 99 (38.2) | 0.06 |
| Peritoneal dialysis | 151 (60.9) | 133 (52.0) | 145 (54.9) | 130 (51.0) | 147 (54.9) | 152 (62.0) | 141 (52.0) | 128 (53.8) | 160 (61.8) | |||
| Clinical parametersa | ||||||||||||
| Body mass index | 28.2 (5.4) | 29.7 (6.2) | 29.2 (6.4) | 0.01 | 31.1 (6.8) | 29.4 (5.7) | 26.5 (4.6) | <0.001 | 31.1 (6.7) | 29.5 (5.7) | 26.5 (4.6) | <0.001 |
| Systolic BP | 143 (20) | 145 (21) | 141 (21) | 0.17 | 143 (20) | 143 (21) | 143 (22) | 0.96 | 143 (19) | 143 (21) | 143 (22) | 0.99 |
| Diastolic BP | 79 (11) | 80 (11) | 78 (12) | 0.37 | 80 (11) | 79 (11) | 78 (12) | <0.05 | 80 (10) | 79 (12) | 78 (12) | 0.23 |
CG, Cockcroft and Gault; MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration.
Mean (SD).
When eGFR at dialysis commencement was determined by the MDRD formula, the tertile cut points were <6.5 ml/min per 1.73 m2 (n=255), 6.5–8.9 ml/min per 1.73 m2 (n=268), and ≥9.0 ml/min per 1.73 m2 (n=245). When the CKD-EPI equation was used for GFR estimation, the tertile cut points were <6.0 ml/min per 1.73 m2 (n=271), 6.0–7.9 ml/min per 1.73 m2 (n=238), and ≥8.0 ml/min per 1.73 m2 (n=259). When these equations were used to estimate GFR, the patients who commenced on dialysis in the highest eGFR tertile were older, had a lower body mass index, were more likely to be Caucasian, and were more likely to have ischemic heart disease as a comorbidity. Patients with diabetes mellitus were equally distributed across the tertiles (Table 1). Patients who commenced renal replacement therapy with a higher GFR estimated by the MDRD formula were more likely to commence on perito-neal dialysis than hemodialysis (P<0.05), with a similar trend that did not reach statistical significance when the CKD-EPI formula was used.
In a subanalysis, measured GFR using mean urinary urea and creatinine clearances was obtained from 301 participants with a 24-hour urine collection at the point of dialysis commencement. The tertile cut points were <5.9 ml/min (n=99), 5.9–8.2 ml/min (n=99), and ≥8.2 ml/min (n=103). The baseline characteristics when using this method of GFR measurement are listed in Supplemental Table 1.
The baseline characteristics that were similar across all three formulas included hemoglobin, serum albumin, C-reactive protein, calcium, and medications, including angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and statins.
Agreement between eGFR Formulas
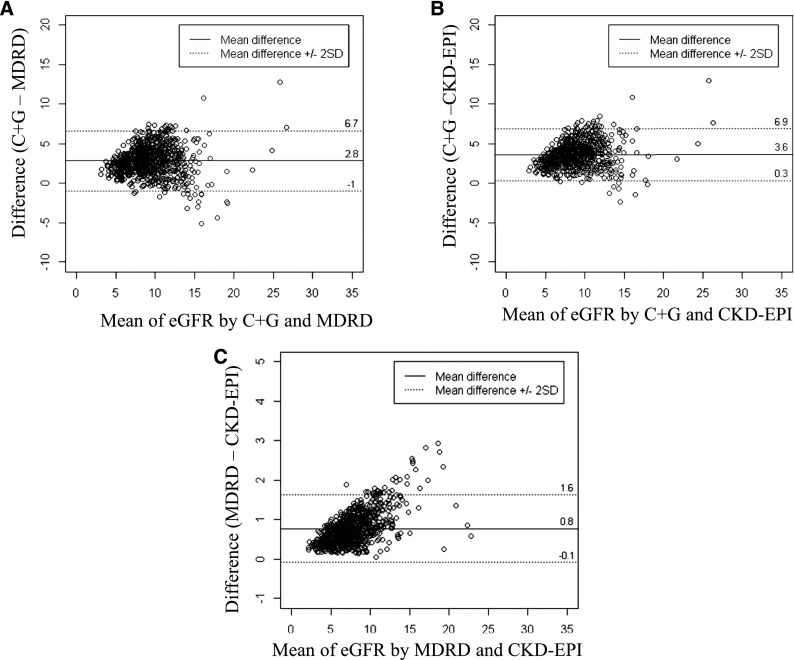
The CG estimation of GFR was a mean of 2.8 ml/min per 1.73 m2 greater than the GFR estimated by MDRD (limits of agreement=−1–6.7 ml/min per 1.73 m2). The CG estimation of GFR was a mean of 3.6 ml/min per 1.73 m2 greater than the GFR estimated by CKD-EPI (limits of agreement=0.3–6.9 ml/min per 1.73 m2). The closest agreement was observed between the eGFR values determined by the MDRD and CKD-EPI formulas, with the difference being 0.8 ml/min per 1.73 m2 (limits of agreement=−0.1–1.6 ml/min per 1.73 m2). Greater differences were observed at higher levels of eGFR (Figure 1).
Figure 1.
Bland and Altman plot comparing two different estimated GFR (eGFR) formulas in Initiating Dialysis Early and Late participants at dialysis commencement. (A) Comparison between Cockcroft and Gault (CG) and Modification of Diet in Renal Disease (MDRD) formulas. (B) Comparison between CG and Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) formulas. (C) Comparison between MDRD and CKD-EPI formulas. The continuous lines represent the mean difference between the two formulas; the dotted lines represent the upper and lower limits of agreement between the two formulas.
Patient Survival and eGFR Grouped in Tertiles
A total of 275 (35.8%) patients who commenced dialysis died during the 9-year study period, with a median follow-up period of 4.4 years. Overall mortality was comparable among the three tertiles when divided using the CG eGFR at dialysis commencement (hazard ratio [HR], 1.02; 95% confidence interval [95% CI], 0.76 to 1.37; P=0.38; lowest eGFR tertile versus highest eGFR tertile) (Figure 2A). When eGFR at dialysis initiation was calculated alternatively, using the MDRD formula, the group that had the lowest eGFR had the lowest mortality (HR, 0.56; 95% CI, 0.41 to 0.75; P<0.001) (Figure 2B). Similar results were observed using the CKD-EPI formula (HR, 0.64; 95% CI, 0.48 to 0.85; P=0.006) (Figure 2C). When GFR was measured by the mean of urinary urea and creatinine clearances, the results were similar to the results observed using the CG formula (HR, 1.05; 95% CI, 0.67 to 1.66; P=0.86) (Figure 2D).
Figure 2.
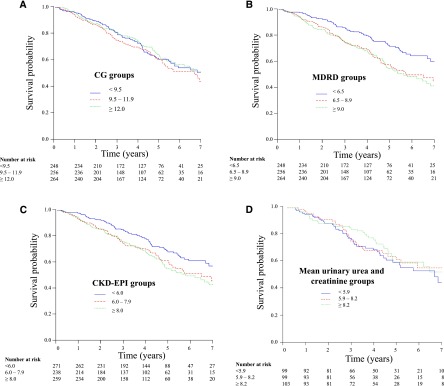
Kaplan–Meier plots of patient survival based on estimated GFR (eGFR) tertile groups by three different formulas or measured mean urinary urea and creatinine clearance at dialysis commencement in Initiating Dialysis Early and Late trial participants. (A) Cockcroft and Gault (CG) eGFR: the differences between the groups were not statistically significant (log-rank test: P=0.38). (B) Modification of Diet in Renal Disease (MDRD) eGFR: the differences between the groups were highly statistically significant (log-rank test: P<0.001). (C) Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) eGFR: the differences between the groups were highly statistically significant (log-rank test: P<0.001). (D) Mean urinary urea and creatinine clearance: the differences between the groups were not statistically significant (log-rank test: P=0.86).
Patient Survival and eGFR Uncorrected for BSA
In sensitivity analyses, there was no interaction between eGFR formulas and sex for any of the three formulas. When eGFR values were uncorrected for BSA, CG-determined eGFR at dialysis start was not associated with all-cause mortality, whereas the groups with the lower start eGFR determined by MDRD and CKD-EPI eGFR values were still significantly associated with lower mortality (Supplemental Figure 1). The Bland and Altman plots were similar to those plots of eGFR corrected for BSA, with the closest agreement observed between the MDRD and CKD-EPI estimates of GFR (data not shown).
Patient Survival Based on CV and Non-CV Mortality
In total, 121 patients died of CV causes, and 154 patients died from non-CV causes. The unadjusted HR of the lowest eGFR group estimated by all three formulas was not statistically significant for CV-related death (Table 2). When MDRD and CKD-EPI were used to determine eGFR, the lowest eGFR tertiles had 58% and 51% lower risks of dying because of non-CV–related death, respectively, compared with the highest eGFR tertile (reference group) (Table 2). When the causes of non-CV deaths were further analyzed, 42% were attributable to infection, 20% were because of patient initiated withdrawal from dialysis, and 16% were caused by malignancy. Of the infection-related deaths, peritonitis accounted for 14.7%, which was followed by lung infection (12.8%).
Table 2.
Unadjusted hazard ratios for cardiovascular and noncardiovascular mortality according to GFR tertile groups by each formula
| GFR Tertilea | Hazard Ratio | Lower CI | Upper CI | P Value |
|---|---|---|---|---|
| CV deaths | ||||
| CG<9.5 | 1.06 | 0.69 | 1.65 | 0.78 |
| CG=9.5–11.9 | 1.15 | 0.74 | 1.78 | 0.54 |
| MDRD<6.5 | 0.83 | 0.52 | 1.33 | 0.44 |
| MDRD=6.5–8.9 | 1.38 | 0.90 | 2.13 | 0.14 |
| CKD-EPI<6.0 | 0.90 | 0.58 | 1.41 | 0.65 |
| CKD-EPI=6.0–7.9 | 1.26 | 0.81 | 1.95 | 0.31 |
| Non-CV deaths | ||||
| CG<9.5 | 0.99 | 0.67 | 1.48 | 0.97 |
| CG=9.5–11.9 | 1.25 | 0.85 | 1.84 | 0.26 |
| MDRD<6.5 | 0.42 | 0.28 | 0.62 | <0.001 |
| MDRD=6.5–8.9 | 0.70 | 0.48 | 1.00 | >0.05 |
| CKD-EPI<6.0 | 0.49 | 0.33 | 0.73 | <0.001 |
| CKD-EPI=6.0–7.9 | 0.75 | 0.52 | 1.09 | 0.13 |
CI, confidence interval; CV, cardiovascular; CG, Cockcroft and Gault; MDRD, Modification of Diet in Renal Disease; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration.
Reference group is the tertile with the highest estimated GFR (CG≥12.0 ml/min per 1.73 m2; MDRD≥9.0 ml/min per 1.73 m2; CKD-EPI≥8.0 ml/min per 1.73 m2).
Patient Survival in Multivariate Adjusted Analysis
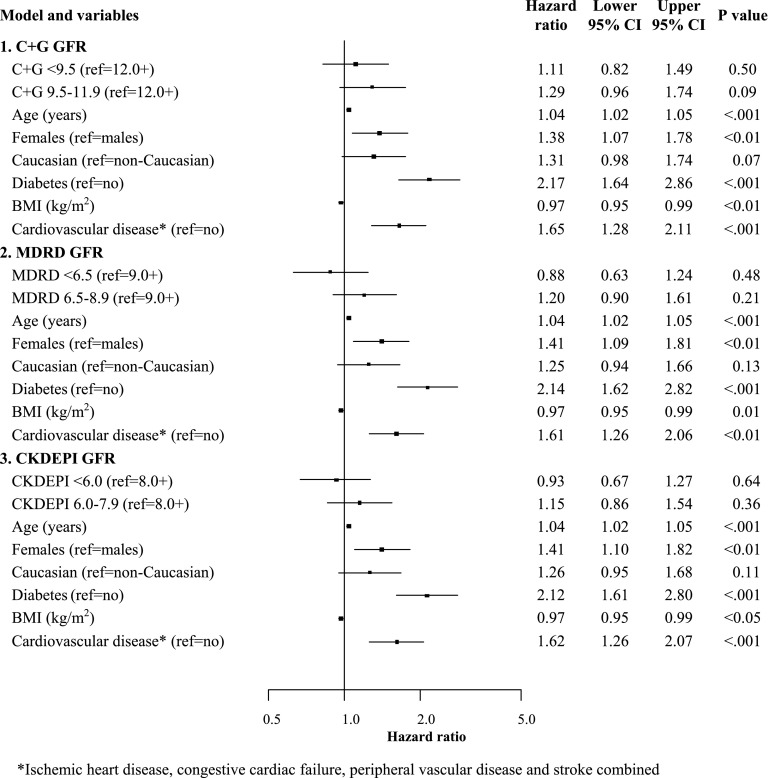
Using multivariable Cox proportional hazards model analysis, the observed apparent longer survival in the lowest MDRD or CKD-EPI eGFR tertiles compared with the highest tertiles of eGFR at dialysis commencement was no longer significant after adjustment for age, sex, racial origin, body mass index, and comorbidities (Figure 3). Being older, being a woman, and having diabetes or CV disease conferred a higher risk of death (Figure 3). At the time of dialysis commencement, having diabetes mellitus conferred a more than twofold increased risk of death in patients with stage 5 CKD.
Figure 3.
Forest plot of the association between estimated GFR (eGFR) tertiles at dialysis commencement and patient survival in Initiating Dialysis Early and Late trial participants. In multivariable Cox proportional hazards model analyses, patient survival was not significantly associated with eGFR at dialysis commencement, regardless of the formula used. Older age, women, diabetes mellitus, and cardiovascular disease at dialysis commencement were associated with a significantly increased hazard of death. Cardiovascular disease was defined as the presence of ischemic heart disease, congestive cardiac failure, peripheral vascular disease, or stroke. BMI, body mass index; C+G, Cockcroft and Gault; CI, confidence interval; CKDEPI, Chronic Kidney Disease-Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease.
Discussion
The IDEAL trial reported that planned early start dialysis did not result in better clinical outcomes or quality of life but incurred additional cost. The current as-treated analysis of the IDEAL trial using the MDRD or CKD-EPI formulas for GFR estimation at the time of commencement of dialysis showed that those patients who commenced dialysis with a lower eGFR experienced longer survival compared with those patients who started dialysis with higher levels of renal function. This result was not seen when GFR was estimated using the CG formula. Although the Kaplan–Meier plot clearly showed a longer survival for the lowest eGFR tertile when MDRD and CKD-EPI equations were used, this association was no longer statistically significant after adjustment for age, sex, race, body mass index, and comorbidities. This study also supports the observations of other cohort studies using the MDRD eGFR in suggesting that patients who commence dialysis with higher levels of renal function are more likely to be older, be women, and have diabetes or CV disease. Hence, these characteristics are more likely to result in a higher risk of dying compared with the characteristics of patients who remain clinically well and biochemically stable and hence, are able to defer the initiation of dialysis until later in the course of CKD.
The NECOSAD suggested that this finding may be primarily influenced by an increased muscle mass in healthy patients, resulting in a lower eGFR when determined by the MDRD study. Our study did not find that correction of eGFR for BSA modified the relationship between eGFR and mortality. Only one third of the IDEAL participants had timed urinary collection at the time of dialysis commencement. Despite this limitation, this study was able to replicate the data by the NECOSAD study group that showed a survival advantage in those patients with the lowest renal function measured by MDRD but not GFR was measured by mean urinary urea and creatinine clearances (Figure 2D). Although we could not definitively determine the impact of muscle mass in the estimation of GFR and hence, the association with outcome in this study, our findings reinforced that estimation of GFR by equations using plasma creatinine in the denominator is less likely to be useful in patients with end stage kidney disease, because the plasma creatinine is affected by muscle mass as well as glomerular filtration.
Our study also shows that there is a clear trend for patients who start dialysis early to commence on peritoneal dialysis. Thus, the observed initial mortality benefit shown in the peritoneal dialysis cohort (28) may well be influenced by an unmeasured lead time bias rather than a specific benefit of peritoneal dialysis.
Before the results of the IDEAL study, guidelines increasingly considered the eGFR as a determinant for initiation of dialysis. Hence, the difference in eGFR estimated by different formulas and associated clinical outcomes are likely to be of considerable clinical interest. Our results confirm significant reclassification of patients among the tertiles when the MDRD and CKD-EPI formulas were used instead of CG equation to estimate GFR at the commencement of dialysis. The Bland and Altman plots indicate strong agreement between MDRD and CKD-EPI eGFR values, but neither had strong agreement with the CG-determined eGFR. In the absence of a gold standard measurement GFR, this study is unable to provide information regarding which GFR formula most accurately determines GFR in stage 5 CKD.
Recently, many investigators had questioned the validity of using creatinine-based eGFR to estimate renal function in patients with stage 5 CKD (29), largely because of the fact that very few patients with stage 5 CKD were included in the original validation cohorts. For instance, the CG equation was derived from 249 patients ages 18–92 years who were all Caucasian men in an in-patient veterans hospital. The lowest GFR was reported in the 80- to 92-year-old group, with a mean of 37 ml/min per 1.73 m2 (2,25). The MDRD study included 1070 patients in the development sample and 558 patients in the validation sample, with a mean eGFR of 39.8 ml/min per 1.73 m2 (2,30). Similarly, less than 3% in the validation cohort using the CKD-EPI formula had a GFR<15 ml/min per 1.73 m2 (2,31). Hence, using creatinine-based eGFR to estimate renal function in patients with stage 5 CKD has questionable validity. Moreover, the results of the IDEAL study suggest that there is limited clinical relevance of eGFR in stage 5 CKD for guiding decisions regarding renal replacement therapy.
The strength of this study was its inclusive nature, which encompassed a variety of centers (metropolitan, regional, and rural as well as general and university) across two countries (Australia and New Zealand). The end points were independently adjudicated by an adjudication committee to reduce bias. However, there are several limitations. Despite randomization, there remains the possibility of misclassification bias, survivor bias, and indication bias with residual confounding (i.e., patients starting dialysis at lower GFR values may have had less comorbidity, leading to lower mortality that may not have been adequately adjusted for in multivariable analysis). Because this analysis is an on treatment analysis, lead time bias remains. However, in contrast to other observational studies, all patients in the IDEAL study were under the care of nephrologists before the start of dialysis.
This analysis is based on the premise that overall kidney function is a function of GFR calculated using various GFR formulas. It has not addressed other key physiologic kidney functions, which may not decline in parallel to GFR. Because there were only a few participants with timed urinary collection at the time of dialysis commencement, this study was unable to determine whether measurement of mean urinary urea and creatinine clearances provided additional useful clinical information in patients with stage 5 CKD. Our limited results suggest that this is not the case.
In conclusion, these data suggest that there are discrepancies between estimations of GFR using the available formulas in patients with stage 5 CKD independent of BSA. More importantly, the timing of dialysis, which was determined by eGFR, does not impact on survival after conventional risk factors, such as age, sex, diabetes, and CV risk factors, are taken into account. The results are consistent across most populations, including those groups historically considered likely to benefit most from early start dialysis, namely the elderly and diabetic cohorts. Moreover, patients who commenced dialysis at higher MDRD or CKD-EPI eGFR values are more likely to die because of non-CV–related mortality, which is mostly related to dialysis-related complications, such as peritonitis and withdrawal of dialysis. The latter is consistent with a recent report from Canadian registry data (32). These data support the conclusions of the IDEAL trial that the decision to initiate dialysis should be based on clinical judgment rather than solely eGFR. The appropriateness and applicability of measuring eGFR in stage 5 CKD should be reconsidered by the nephrological community.
Disclosures
C.P. chairs a clinical trial steering committee for Baxter and is a member of an advisory committee for Amgen. The other authors have nothing to disclose.
Supplementary Material
Acknowledgments
The Initiating Dialysis Early and Late (IDEAL) study was an investigator-initiated and -conducted study funded by National Health and Medical Research Council of Australia Grants 211146 and 465095, Australian Health Ministers Advisory Council Grant PDR 2001/10, the Royal Australasian College of Physicians/Australian and New Zealand Society of Nephrology (Don and Lorraine Jacquot Fellowship; 2001 and 2012), the National Heart Foundation (Australia; 2003), and the National Heart Foundation (New Zealand; 2003). The IDEAL trial was supported by unrestricted grants from Baxter Healthcare Corporation, the Health Funding Authority New Zealand (Te Mana Putea Hauora O Aotearoa), the International Society for Peritoneal Dialysis, Amgen Australia Pty Ltd, and Janssen Cilag Pty Ltd.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02310213/-/DCSupplemental.
References
- 1.Bonomini V, Baldrati L, Stefoni S: Comparative cost/benefit analysis in early and late dialysis. Nephron 33: 1–4, 1983 [DOI] [PubMed] [Google Scholar]
- 2.Bonomini V, Albertazzi A, Vangelista A, Bortolotti GC, Stefoni S, Scolari MP: Residual renal function and effective rehabilitation in chronic dialysis. Nephron 16: 89–102, 1976 [DOI] [PubMed] [Google Scholar]
- 3.Bonomini V, Feletti C, Scolari MP, Stefoni S: Benefits of early initiation of dialysis. Kidney Int Suppl 17: S57–S59, 1985 [PubMed] [Google Scholar]
- 4.Jungers P, Zingraff J, Albouze G, Chauveau P, Page B, Hannedouche T, Man NK: Late referral to maintenance dialysis: Detrimental consequences. Nephrol Dial Transplant 8: 1089–1093, 1993 [PubMed] [Google Scholar]
- 5.Jungers P, Zingraff J, Page B, Albouze G, Hannedouche T, Man NK: Detrimental effects of late referral in patients with chronic renal failure: A case-control study. Kidney Int Suppl 41: S170–S173, 1993 [PubMed] [Google Scholar]
- 6.McCusker FX, Teehan BP, Thorpe KE, Keshaviah PR, Churchill DN: How much peritoneal dialysis is required for the maintenance of a good nutritional state? Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Kidney Int Suppl 56: S56–S61, 1996 [PubMed] [Google Scholar]
- 7.Sesso R, Belasco AG: Late diagnosis of chronic renal failure and mortality on maintenance dialysis. Nephrol Dial Transplant 11: 2417–2420, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Tattersall J, Greenwood R, Farrington K: Urea kinetics and when to commence dialysis. Am J Nephrol 15: 283–289, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Churchill DN: An evidence-based approach to earlier initiation of dialysis. Am J Kidney Dis 30: 899–906, 1997 [DOI] [PubMed] [Google Scholar]
- 10.Golper TA: The rationale for Healthy Start dialysis. Blood Purif 17: 1–9, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Susantitaphong P, Altamimi S, Ashkar M, Balk EM, Stel VS, Wright S, Jaber BL: GFR at initiation of dialysis and mortality in CKD: A meta-analysis. Am J Kidney Dis 59: 829–840, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG: Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol 13: 2125–2132, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Clark WF, Na Y, Rosansky SJ, Sontrop JM, Macnab JJ, Glassock RJ, Eggers PW, Jackson K, Moist L: Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ 183: 47–53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazmi WH, Gilbertson DT, Obrador GT, Guo H, Pereira BJ, Collins AJ, Kausz AT: Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis 46: 887–896, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, Finne P, Ioannidis GA, Salomone M, Traynor JP, Zurriaga O, Verrina E, Jager KJ: Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant 24: 3175–3182, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Lassalle M, Labeeuw M, Frimat L, Villar E, Joyeux V, Couchoud C, Stengel B: Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int 77: 700–707, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Wright S, Klausner D, Baird B, Williams ME, Steinman T, Tang H, Ragasa R, Goldfarb-Rumyantzev AS: Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol 5: 1828–1835, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC, Taiwan Society of Nephrology : Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: A national cohort study in Taiwan. Nephrol Dial Transplant 25: 2616–2624, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Fink JC, Burdick RA, Kurth SJ, Blahut SA, Armistead NC, Turner MS, Shickle LM, Light PD: Significance of serum creatinine values in new end-stage renal disease patients. Am J Kidney Dis 34: 694–701, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A: Survival and dialysis initiation: Comparing British Columbia and Scotland registries. Nephrol Dial Transplant 24: 3186–3192, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Grootendorst DC, Michels WM, Richardson JD, Jager KJ, Boeschoten EW, Dekker FW, Krediet RT, NECOSAD Study Group : The MDRD formula does not reflect GFR in ESRD patients. Nephrol Dial Transplant 26: 1932–1937, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF: Early start of hemodialysis may be harmful. Arch Intern Med 171: 396–403, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA, IDEAL Study : A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med 363: 609–619, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Harris A, Cooper BA, Li JJ, Bulfone L, Branley P, Collins JF, Craig JC, Fraenkel MB, Johnson DW, Kesselhut J, Luxton G, Pilmore A, Rosevear M, Tiller DJ, Pollock CA, Harris DC: Cost-effectiveness of initiating dialysis early: A randomized controlled trial. Am J Kidney Dis 57: 707–715, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16: 31–41, 1976 [DOI] [PubMed] [Google Scholar]
- 26.Du Bois D, Du Bois EF: A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5: 303–311, 1989 [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA: Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: More accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis 55: 622–627, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM: Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol 6: 2642–2649, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White CA, Akbari A: The estimation, measurement, and relevance of the glomerular filtration rate in stage 5 chronic kidney disease. Semin Dial 24: 540–549, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellwood AD, Jassal SV, Suri RS, Clark WF, Na Y, Moist LM: Early dialysis initiation and rates and timing of withdrawal from dialysis in Canada. Clin J Am Soc Nephrol 8: 265–270, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.