Summary
Background and objectives
Early detection of CKD is important for slowing progression to renal failure and preventing cardiovascular events. Automated laboratory reporting of estimated GFR (eGFR) has been introduced in many health systems to improve CKD recognition, but its effect in large, United States–based health systems remains unclear.
Design, setting, participants, & measurements
Using Veterans Affairs (VA) laboratory and administrative data, two nonoverlapping national cohorts of patients receiving care in VA medical centers before (n=66,323) and after (n=16,670) implementation of automated eGFR reporting between 2004 and 2010 were identified. Recognition was assessed by the presence of new CKD diagnostic codes, use of additional diagnostic testing, outpatient nephrology visits, or overall CKD recognition (receipt of at least one of these outcomes) for each patient during the 12-month period after their first eligible creatinine or eGFR laboratory result. Generalized estimating equations were used to assess change before and after automated eGFR reporting.
Results
Overall CKD recognition increased from 22.1% of veterans before eGFR reporting to 27.5% in the post-eGFR reporting period (odds ratio [OR], 1.19; 95% CI, 1.12 to 1.27; P<0.001). Higher overall CKD recognition was driven largely by increased documentation of CKD diagnosis codes in medical records (OR, 1.31; 95% CI, 1.21 to 1.41; P<0.001) and diagnostic testing for CKD (OR, 1.13; 95% CI, 1.03 to 1.24; P<0.01) rather than outpatient nephrology consultation. Automated eGFR reporting was not associated with greater CKD recognition among black or older patients (P=0.07).
Conclusions
Automated eGFR laboratory reporting improved documentation of CKD diagnoses but had no effect on nephrology consultation. These findings suggest that to advance CKD care, further strategies are needed to ensure appropriate follow-up evaluation to confirm and effectively evaluate CKD.
Introduction
Early detection of CKD is important for timely intervention and clinical management to slow progression to ESRD and to prevent cardiovascular events (1–3). Automated laboratory reporting of estimated GFR (eGFR) has been introduced in many health systems to improve CKD recognition. Despite clinical guidelines promoting the use of eGFR in practice and increased implementation of eGFR reporting (4), the effect of eGFR reporting on CKD management outcomes remains unclear.
Evaluations of automated eGFR reporting have assessed the effect in large, population-based samples of national, publicly funded health care systems (4). The few studies from the United States evaluated eGFR reporting in small clinical practices with limited sample sizes (4). The effect of eGFR reporting in large integrated health systems is unknown. Furthermore, outcomes of eGFR reporting have largely focused on CKD recognition, nephrology referrals, or pharmacologic management of CKD as the primary outcome of interest (4). A detailed examination of how CKD is recognized would have implications for treatment and may better inform the effectiveness of optimal eGFR reporting strategies and CKD prevention efforts.
The Veterans Health Administration (VHA) is the nation’s largest integrated health care system, and prevalence of CKD among veterans is 30% greater than among the general population (5). In 2004, the VHA National Pathology and Laboratory Service mandated all Veterans Affairs medical centers (VAMCs) to provide eGFR values as part of standardized laboratory reporting of blood panel results. We hypothesized that implementation of systematic reporting of eGFR would improve diagnosis, testing, and referral of patients with CKD. Results from this large national sample seek to inform efforts to improve screening and evaluation of CKD in large health systems (6).
Methods
Study Design and Data Sources
We used a retrospective cohort design to examine changes in CKD recognition among eligible patients before and after implementation of eGFR reporting by VAMC laboratories during a 5-year period. The population sample, patient outcomes, and covariates were drawn from the VA Decision Support System’s Laboratory Results National Data Extract, National Patient Care Database Patient Treatment File (PTF) for inpatient care, and Outpatient Care (OPC) file. The institutional review board of the Durham VAMC approved this study.
VA facility laboratories implemented eGFR reporting from 2.5 months to 4 years after the VHA mandate (7). We accounted for facility-specific initiation and eGFR reporting patterns in determining pre- and post-eGFR reporting periods. We defined the pre-eGFR reporting period as the 12 months leading up to the date that each VAMC laboratory began reporting eGFR for 1% of creatinine tests. The post-eGFR reporting period was the longer period of either 90 days after the end of a VAMC’s pre-eGFR period or the date it began reporting eGFR for 90% of their creatinine tests—the threshold that we expected to have the potential to inform changes in provider practice. Activation of eGFR reporting software varied across VAMCs, where the median time between initial and full implementation was 9.6 months (interquartile range, 5 months) (7); thus, our pre-eGFR and post-eGFR reporting intervals reflected a range of reporting patterns.
Study Population
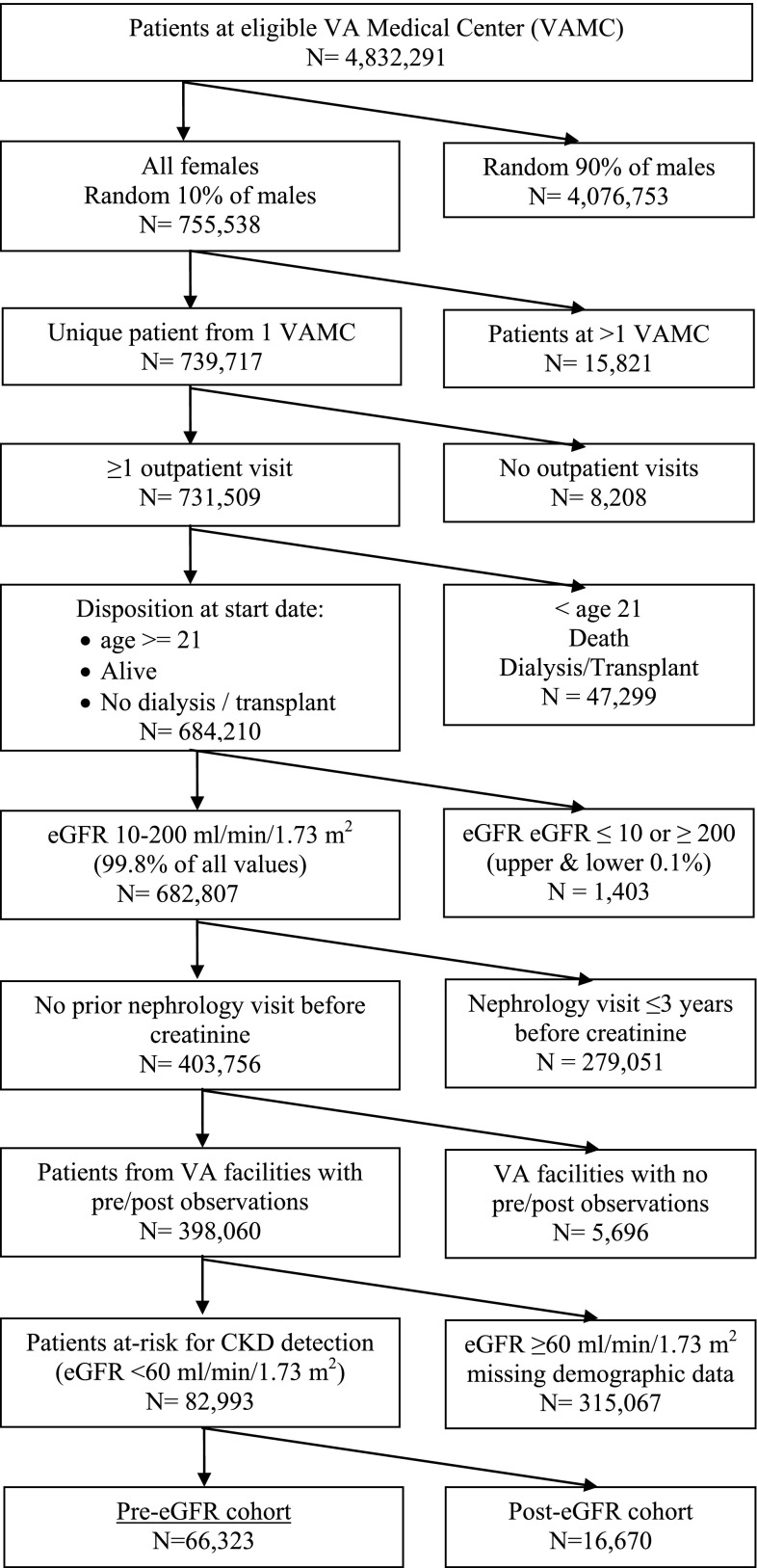
The initial sample consisted of >4.8 million patients from 148 VAMCs with available laboratory data between July 2004 and September 2010. We sampled all women and a random 10% sample of men age 21 years or older who had at least one outpatient visit in a VA clinic and had not previously received dialysis or transplant services (Figure 1). We identified a 10% sample of men to simplify data management and cleaning because 94%–98% of all VA users are men and the VA provides care for nearly 5 million veterans per year. We excluded patients with laboratory values for kidney function in the upper and lower 0.1 percentile distribution tails (eGFR ≤10 or ≥200 ml/min per 1.73 m2; n=1403). We then excluded patients seen in VA facilities that never implemented eGFR reporting or did not have their own clinical laboratories (44 facilities) and patients who had a prior nephrology clinic visit up to 3 years before their first study-eligible creatinine laboratory test (n=279,051). To examine the effect of eGFR reporting during the pre-eGFR and post-eGFR periods, we further limited the sample to patients in VAMCs with observations during both pre-eGFR and post-eGFR reporting periods (143 facilities; 398,060 patients). Finally, we retained patients with complete marital and VA copayment status information and patients with CKD detectable through laboratory testing, defined as eGFR <60 ml/min per 1.73 m2, by applying the Modification of Diet in Renal Disease equation (8) to creatinine values during the pre-eGFR reporting period. The final analytic sample included 82,993 patients with CKD.
Figure 1.
Study flow diagram per Consolidated Standards of Reporting Trials. eGFR, estimated GFR; VA, Veterans Affairs; VAMC, Veterans Affairs Medical Center.
To examine the extent of CKD recognition among patients at risk for CKD who had no indication of prior kidney disease-related utilization, we identified a cohort of patients based on the earliest date of an eligible creatinine value during the available laboratory data period (i.e., index date). Eligible patients’ first eGFR values ranged from 10 to 59 ml/min per 1.73 m2, where no other creatinine result was reported within the prior 180 days and no nephrology visit occurred in the prior 3 years. We then assigned patients to the pre-eGFR or post-eGFR cohort according to each patient’s creatinine index date and their VAMC activation of automated eGFR reporting, which eliminated potential overlap of patients between cohorts. Most patients were assigned to the pre-eGFR cohort (n=66,323), and the remainder were assigned to the post-eGFR cohort (n=16,670). We followed each patient for 12 months after the index date.
Measurements
Consistent with previous studies (4,6,9–13), our primary outcome was recognition of CKD within 1 year of the patient’s first creatinine test, defined as (1) a diagnosis of kidney disease in the VA electronic medical record (International Classification of Diseases, Ninth Revision, Clinical Modification codes 250.4, 250.40, 504.1, 250.42, 504.3, 582.×, 583.×, 586.×, 587.×, 403.×, 404.×, and 585.×); (2) use of urine microalbumin (VA laboratory codes 32, 56), kidney ultrasonography, or biopsy diagnostic procedures (codes 76705, 76770, 76776, 76778, 50200, and 50205) to test for kidney disease; or (3) an outpatient nephrology visit. We also pooled the three outcomes to generate a general outcome of CKD identification. These outcomes were chosen because they serve as proxies for disease identification (e.g., diagnosis) or represent an active response (e.g., testing to evaluate CKD or nephrology consultation) and therefore greater recognition of CKD in response to eGFR reporting. All outcomes were identified from procedural and diagnosis codes found in administrative records from Decision Support System’s Laboratory Results National Data Extract and OPC files.
To examine whether eGFR reporting led to changes in recognition of CKD, the explanatory variable of interest was a pre-post indicator to distinguish the pre-eGFR cohort from the post-eGFR cohort. The patient-level analysis controlled for several baseline demographic characteristics that may influence VA health care utilization. We obtained date of birth and sex from the VA Vital Status File and determined VA eligibility and copayment status (requirement to pay versus exemption from copayment) from the VA Enrollment file. We obtained marital status from encounters in the OPC data. Patient race and comorbid conditions were assessed from Patient Treatment File and OPC files. The total number of outpatient visits in the year before each patient’s creatinine index date was obtained from the OPC file.
Statistical Analyses
We compared unadjusted differences in patient characteristics and outcomes between pre-eGFR and post-eGFR reporting periods using chi-squared tests for categorical and two-tailed unpaired t tests for continuous variables. We examined unadjusted pre-post differences in CKD recognition rates in the overall sample and by subgroup of interest (black versus white, female versus male, eGFR 10–29 versus 30–44 versus 45–59 ml/min per 1.73 m2) using chi-squared tests.
To estimate the population average impact of eGFR reporting on CKD recognition (overall and by mode of identification), we used generalized estimating equations with eGFR report (i.e., pre-post eGFR period indicator) as the key independent variable of interest. The models assumed a binomial distribution, logit link, and exchangeable working covariance structure. All models were also clustered at VAMCs to account for local clinical practice patterns and clustering of data specific to VAMCs that would influence CKD recognition modalities. To examine differences in CKD recognition by patient subgroups, we conducted secondary analyses comparing outcomes by sex (female versus male), race (black versus white), and eGFR group (10–29 versus 30–44 versus 45–59 ml/min per 1.73 m2). All adjusted models controlled for patient age, marital status, status of exemption from VA copayments, number of prior outpatient visits, comorbid conditions, and the year of each patient’s VAMC facility eGFR activation date (to control for time effects).
Following VA convention (14), we performed sensitivity analyses to account for the possible misspecification of race from VA inpatient and outpatient encounters. To generate eGFR values for patients whose race was unknown (16.1% of the sample), we assigned veterans with unknown race to “white” to calculate eGFR and identify patients with CKD detectable by laboratory testing (eGFR <60 ml/min per 1.73 m2). To assess the sensitivity of this default assumption, we performed the analyses again after resetting unknown race to “black” for deriving eGFR.
Results
The final analytic sample consisted of 82,993 patients with eGFR <60 ml/min per 1.73 m2 who were at risk for CKD identification (Table 1). Patients in the post-eGFR reporting period had more comorbid conditions and were more likely to be women, nonwhite, unmarried, and exempt from VA copayments than patients in the pre-eGFR reporting period (P<0.001 for all comparisons). A majority of the patients in the sample obtained care in VAMCs that initiated eGFR reporting in 2005–2006, with the remainder initiating in 2007–2009.
Table 1.
Baseline characteristics of the study population by eGFR reporting cohort
| Characteristic | All Patients (n=82,993) | eGFR Reporting Cohort | ||
|---|---|---|---|---|
| Pre-Reporting (n=66,323) | Post-Reporting (n=16,670) | P Valuea | ||
| Mean age ± SD (yr) | 71.5 ± 12.3 | 71.5 ± 12.2 | 71.3 ± 12.8 | 0.15 |
| Age group, n (%) | <.001 | |||
| <50 y | 4979 (6.0) | 3964 (6.0) | 1015 (6.1) | |
| 50–59 y | 11,059 (13.3) | 8707 (13.1) | 2352 (14.1) | |
| 60–69 y | 13,293 (16.0) | 10,267 (15.5) | 3026 (18.2) | |
| 70–79 y | 27,598 (33.3) | 22,725 (34.3) | 4873 (29.2) | |
| ≥80 y | 26,064 (31.4) | 20,660 (31.2) | 5404 (32.4) | |
| Women, n (%) | 21,323 (25.7) | 16,604 (25.0) | 4719 (28.3) | <.001 |
| Race, n (%)b | <.001 | |||
| Black | 5290 (6.4) | 3977 (6.0) | 1313 (7.9) | |
| White | 76,378 (92.0) | 61,306 (92.4) | 15,072 (90.4) | |
| Other | 1325 (1.6) | 1040 (1.6) | 285 (1.7) | |
| Married, n (%) | 49,767 (60.0) | 40,300 (60.8) | 9467 (56.8) | <.001 |
| VA copayment exemption, n (%) | 55,950 (67.4) | 44,006 (66.4) | 11,944 (71.6) | <.001 |
| Mean prior outpatient visits ±SDc | 13.7 ± 20.6 | 12.9 ± 19.6 | 16.8 ± 23.8 | <.001 |
| Comorbid conditions, n (%) | ||||
| Cancer | 11,291 (13.6) | 8753 (13.2) | 2538 (15.2) | <.001 |
| Cerebrovascular disease | 8913 (10.7) | 6756 (10.2) | 2157 (12.9) | <.001 |
| Chronic lung disease | 17,649 (21.3) | 13,461 (20.3) | 4188 (25.1) | <.001 |
| Diabetes mellitus | 24,518 (29.5) | 18,754 (28.3) | 5764 (34.6) | <.001 |
| Heart failure | 8561 (10.3) | 6477 (9.8) | 2084 (12.5) | <.001 |
| Hypertension | 63,469 (76.5) | 50,185 (75.7) | 13,284 (79.7) | <.001 |
| Myocardial infarction | 4233 (5.1) | 3292 (5.0) | 941 (5.6) | <.001 |
| Peripheral vascular disease | 9887 (11.9) | 7595 (11.5) | 2292 (13.7) | <.001 |
| eGFR, n (%) | <.001 | |||
| 45–59 ml/min per 1.73 m2 | 60,688 (73.1) | 48,747 (73.5) | 11,941 (71.6) | |
| 30–44 ml/min per 1.73 m2 | 18,542 (22.3) | 14,641 (22.1) | 3901 (23.4) | |
| 10–29 ml/min per 1.73 m2 | 3763 (4.5) | 2935 (4.4) | 828 (5.0) | |
| Year eGFR reporting began, n (%) | <.001 | |||
| 2005 | 28,521 (34.4) | 22,482 (33.9) | 6039 (36.2) | |
| 2006 | 23,262 (28.0) | 18,512 (27.9) | 4750 (28.5) | |
| 2007 | 12,631 (15.2) | 10,353 (15.6) | 2278 (13.7) | |
| 2008 | 18,501 (22.3) | 14,925 (22.5) | 3576 (21.5) | |
| 2009 | 78 (0.1) | 51 (0.1) | 27 (0.2) | |
eGFR, estimated GFR; VA, Veterans Affairs.
Statistical significance between the pre-reporting and post-reporting groups was measured with t tests for continuous variables and chi-square tests for dichotomous variables.
To identify patients with CKD detectable through laboratory testing (i.e., eGFR <60 ml/min per 1.73 m2), patients of unknown race (n=13,396 [16.1%]) were assigned to the category “white” for calculations of eGFR.
Prior outpatient visits were defined as the patient’s total number of VA outpatient visits during the 12 months before the creatinine index date.
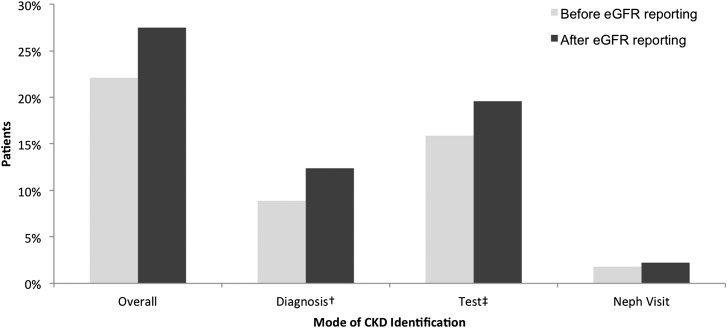
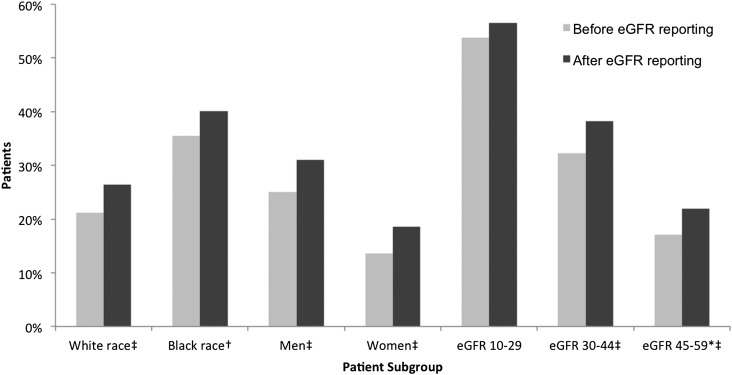
Overall recognition of CKD increased after implementation of laboratory eGFR reporting (Figure 2): Of patients with eGFR <60 ml/min per 1.73 m2, 22.1% were identified with CKD in the pre-eGFR period, which improved to 27.5% in the post-eGFR reporting period (P<0.001). The higher overall CKD recognition was driven largely by increased documentation of CKD diagnosis coding in medical records (8.9% in the pre-eGFR reporting period to 12.4% in the post-eGFR reporting period; P<0.001) and additional diagnostic procedures for CKD (15.9% pre-eGFR to 19.6% post-eGFR; P<0.001), rather than outpatient nephrology visits (1.8% pre-eGFR to 2.2% post-eGFR; P<0.001). Upon further inspection of CKD recognition by patient subgroups (Figure 3), we found that eGFR reporting was generally associated with a small increase in CKD identification among all subgroups.
Figure 2.
Unadjusted pre-post CKD identification rate by mode of identification. Pre-post differences were statistically significant at P<0.01. †Diagnosis codes for CKD included International Classification of Diseases, Ninth Revision, Clinical Modification codes 250.4, 250.40, 504.1, 250.42, 504.3, 582.×, 583.×, 586.×, 587.×, 403.×, 404.×, and 585. ×. ‡Tests for CKD included urine microalbumin, kidney ultrasonography, or kidney biopsy.
Figure 3.
Unadjusted pre-post CKD identification rate by patient subgroup. *Patients with estimated GFR (eGFR) >60 ml/min per 1.73 m2 were not at risk for identification of CKD and were, therefore, excluded from the analysis. †P<0.01. ‡P<0.001.
In adjusted analyses, eGFR laboratory reporting was positively associated with overall CKD recognition among patients with eGFR <60 ml/min per 1.73 m2 (odds ratio [OR], 1.19; 95% confidence interval [CI], 1.12 to 1.27; P<0.001) (Table 2). This significant pooled outcome was driven by a greater likelihood of CKD recognition through CKD diagnoses (OR, 1.31; 95% CI, 1.21 to 1.41; P<0.001) and additional diagnostic testing (OR, 1.13; 95% CI, 1.03 to 1.24; P<0.01).
Table 2.
Associations between eGFR reporting and identification of CKD after multivariable adjustment
| Group | CKD Identification Modality, OR (95% CI)a | |||
|---|---|---|---|---|
| Overall | Diagnosisb | Diagnostic Testc | Nephrology Visit | |
| All patients | 1.19 (1.12 to 1.27) | 1.31 (1.21 to 1.41) | 1.13 (1.03 to 1.24) | 1.04 (0.88 to 1.23) |
| Sex | ||||
| Female | 1.27 (1.15 to 1.40) | 1.46 (1.24 to 1.71) | 1.22 (1.07 to 1.39) | 1.19 (0.85 to 1.68) |
| Male | 1.17 (1.09 to 1.26) | 1.29 (1.18 to 1.41) | 1.11 (1.01 to 1.23) | 1.01 (0.84 to 1.21) |
| Raced | ||||
| Black | 1.13 (0.99 to 1.28) | 1.09 (0.93 to 1.27) | 1.11 (0.93 to 1.31) | 1.08 (0.83 to 1.40) |
| White | 1.20 (1.12 to 1.29) | 1.35 (1.25 to 1.46) | 1.13 (1.02 to 1.25) | 1.01 (0.85 to 1.20) |
| eGFR | ||||
| 45–59 ml/min per 1.73 m2 | 1.21 (1.11 to 1.31) | 1.46 (1.32 to 1.62) | 1.15 (1.03 to 1.28) | 0.94 (0.71 to 1.25) |
| 30–44 ml/min per 1.73 m2 | 1.22 (1.12 to 1.33) | 1.33 (1.20 to 1.48) | 1.16 (1.04 to 1.30) | 1.29 (1.03 to 1.63) |
| 10–29 ml/min per 1.73 m2 | 1.05 (0.89 to 1.23) | 1.14 (0.96 to 1.36) | 0.86 (0.72 to 1.04) | 0.83 (0.65 to 1.07) |
CI, confidence interval; eGFR, estimated GFR; OR, odds ratio.
Adjusted models also controlled for the following patient characteristics: age, sex, race, marital status, Veterans Affairs copayment exemption status, number of outpatient visits in prior year, comorbid conditions, and year of Veterans Affairs medical center facility eGFR activate date (not shown).
Diagnosis codes for kidney disease included International Classification of Diseases, Ninth Revision, Clinical Modification codes 250.4, 250.40, 504.1, 250.42, 504.3, 582.×, 583.×, 586.×, 587.×, 403.×, 404.×, and 585.×.
Tests for CKD included urine microalbumin, kidney ultrasonography, or kidney biopsy.
To identify patients with CKD detectable through laboratory testing (i.e., eGFR <60 ml/min per 1.73 m2), patients of unknown race (n=13,396 [16.1%]) were assigned to the category “white” for calculations of eGFR.
Systematic reporting of eGFR was positively associated with overall CKD recognition (OR, 1.20; 95% CI, 1.12 to 1.29; P<0.001) and diagnosis (OR, 1.35; 95% CI, 1.25 to 1.46; P<0.001) among white patients and with overall recognition, diagnosis, and diagnostic testing for those with eGFR 30–59 ml/min per 1.73 m2 (P≤0.01). Among men and women, eGFR reporting was associated with increased CKD recognition through documented diagnosis and diagnostic testing (P≤0.003). In overall and subgroup analyses, there was no significant effect of automated eGFR reporting on nephrology visits.
Sensitivity analyses that reassigned patients of unknown race to black race yielded similar adjusted results. An exception was the significance of eGFR reporting on overall CKD detection among black patients (OR, 1.15; 95% CI, 1.04 to 1.27; P<0.01), which was driven largely by increased documentation of CKD diagnosis alone (OR, 1.16; 95% CI, 1.03 to 1.32; P=0.02). Nonetheless, the results of the primary analyses were robust to misspecification of race in the VA data.
Discussion
To our knowledge, this is the first study to examine the effect of eGFR laboratory reporting on changes in CKD recognition in a national sample of patients in the VHA, the largest integrated health care system in the United States that serves patients particularly vulnerable to CKD. Although previous studies examined recognition of CKD through nephrology referrals and visits (4), we used a national electronic medical record of patients to examine an expanded list of non–mutually exclusive modalities of CKD recognition. Overall, we observed a 19% increase in the odds of CKD detection after eGFR reporting.
The increase in CKD recognition was driven largely by a 31% increase in CKD diagnosis codes in the medical record. The effect of adding eGFR values to standard laboratory reports may increase CKD recognition through increases in diagnostic coding—a surrogate for disease detection. However, this effect has not been commonly reported in population-level studies. Our findings are consistent with Wyatt and colleagues’ study (13), which showed a nearly 50% increase in documentation of CKD among patients with moderate CKD (eGFR 30–59 ml/min per 1.73 m2) after the introduction of eGFR reporting in a single urban VA outpatient setting. Despite differences in definitions for recognition of CKD and magnitude of effects, several other studies have also reported increases in identification of CKD (9,15). Still, the overall proportion of those with eGFR <60 ml/min per 1.73 m2 who received a CKD diagnosis remains low.
We also found an increase in the use of new diagnostic tests for CKD. With the exception of a large Canadian study that did not find a significant change in laboratory testing with serum creatinine (11), few studies have evaluated the effect of eGFR reporting on follow-up tests for CKD. The significant effect of eGFR reporting on diagnostic testing was largely driven by the use of urine microalbumin testing. In separate analyses (not shown), the unadjusted increase in kidney ultrasonography and biopsies was no longer significant after adjustment for patient characteristics, suggesting that the differences in pre-eGFR and post-eGFR reporting cohorts may have been associated with the observed increases. Compared with kidney biopsy or ultrasonography, it is also possible that urine microalbumin may not be a sensitive marker for CKD detection because it is a standard test for patients with diabetes and hypertension and is a preventive care quality metric routinely tracked for primary care provider performance. Moreover, our results may reflect the relative ease of documenting a new diagnosis of CKD in medical records and performing urine microalbumin tests while highlighting that recognition may not translate to further evaluation of CKD.
Surprisingly, we did not observe an increase in nephrology referrals after adjustment for patient-level characteristics. Although overall (unadjusted) CKD recognition improved to 54% among patients with eGFR 10–29 ml/min per 1.73 m2, only 2% of all patients with CKD saw a nephrologist in the 12 months after VA laboratories implemented automated eGFR reporting. Our results contrast with notable increases in nephrology referrals that occurred after eGFR reporting in several vertically integrated health care settings in Canada (10,11,16), the United Kingdom (17), and Australia (12). Several factors may explain these differences. First, the VHA provides varying amounts of care to a small segment of the entire United States population, whereas publicly funded health care systems in other countries typically provide the majority of care to large segments of their populations. Second, the VHA operates in a fixed resource environment that may leave local VAMCs with limited capacity to conduct additional diagnostic tests or schedule follow-up nephrology consultations. Although resource constraints have been noted for some aspects of VA care (18), its contribution to effective evaluation of CKD is not known. Finally, interventions to improve outpatient referrals to subspecialty care may be more effective when they include provider education (19,20), but the VA’s mandated eGFR reporting did not include an educational campaign, nor could the lack of such a campaign fully explain the divergent rates of diagnostic coding, nephrology tests, and referrals.
CKD is often under-recognized in women and black patients (21,22), so eGFR reporting has the potential to narrow disparities in CKD identification and treatment. Increased rates of referral among women were observed in other studies (10,11), but recognition of CKD was greater for both men and women. Although we found larger increases in diagnoses, testing, and referrals among white patients and those with mild CKD, CKD recognition was not greater for black patients or those with more advanced CKD. The contrast of our findings with those of other studies suggests tremendous opportunity to reduce disparities in the recognition and management of CKD. In addition to including eGFR values in laboratory reports, additional prompts within the patient electronic medical record could facilitate providers’ interpretations of low eGFR values to encourage appropriate follow-up testing, improve medication prescribing, and decrease late referrals before initiation of dialysis (4,23,24).
Our study also has some limitations. First, the study was limited to outpatient care financed by the VA. To the extent that patients had limited access to VA nephrologists or received diagnostic tests and nephrology consultation from non-VA providers, the results may underestimate the effect of eGFR reporting on CKD detection. Second, when evaluating nephrology visits, we were unable to distinguish between appropriate and inappropriate referrals (25), determine the effect of variation in reporting eGFR values (26), or evaluate wait times to assess whether VAMC resource constraints limited access to nephrologists.
In summary, although recent efforts to expand eGFR laboratory reporting have improved CKD detection in other settings, the effects of eGFR reporting on CKD recognition in the VHA were limited, without higher recognition overall and among at-risk subgroups. To advance CKD care beyond greater recognition, appropriate follow-up evaluations and consultation are needed to confirm the presence and severity of CKD (27). Future research should investigate the extent to which physicians act upon and prioritize laboratory-reported clinical information in their treatment decisions. In addition, further attention should be paid to the recognition of CKD among high-risk subgroups like women, black patients, and patients with advanced CKD. While newer, more precise estimating equations are likely to replace existing ones in the near future (28), reporting alone will have minimal effect on improving VHA’s rates of CKD recognition without additional changes in care delivery or provider education to facilitate appropriate and timely evaluation and to reduce the disease burden of CKD.
Disclosures
M.L.M. reported serving as a consultant for Daiichi Sankyo and Takeda Pharmaceuticals and owning stock in Amgen because of his spouse’s employment. U.D.P. reported serving as a consultant for Kai Pharmaceuticals and Abbott Laboratories and receiving research funding from Amgen, Eli Lilly & Co., and Daiichi Sankyo for clinical trial event adjudication activities (detailed listings available at https://www.dcri.org/about-us/conflict-of-interest/).
Acknowledgments
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health, the U.S. Department of Veterans Affairs, AHRQ, or the American Society of Nephrology.
Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the manuscript. Linda Sanders, Duke University, provided programming and statistical support.
This work was supported by grant K23DK075929 from the National Institute of Diabetes and Digestive and Kidney Diseases. V.W. was supported in part by a postdoctoral fellowship award from the Offices of Research and Development and Academic Affiliations, U.S. Department of Veterans Affairs, and by grant K12HS19479-01 from the Agency for Healthcare Research and Quality (AHRQ). M.L.M. was supported in part by Research Career Scientist Award 10-391 from the Health Services Research and Development Service, U.S. Department of Veterans Affairs. R.K.H. was supported in part by grant T32HS019490-01 from AHRQ. U.D.P. was supported in part by a Gottschalk Research Scholar grant from the American Society of Nephrology.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Huisman RM: The deadly risk of late referral. Nephrol Dial Transplant 19: 2175–2180, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG, African American Study of Kidney Disease and Hypertension Study Group : Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the AASK trial. JAMA 288: 2421–2431, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Kagoma YK, Weir MA, Iansavichus AV, Hemmelgarn BR, Akbari A, Patel UD, Garg AX, Jain AK: Impact of estimated GFR reporting on patients, clinicians, and health-care systems: a systematic review. Am J Kidney Dis 57: 592–601, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Arora P, Rajagopalan S, Patel N, Nainani N, Venuto RC, Lohr JW: The MDRD equation underestimates the prevalence of CKD among blacks and overestimates the prevalence of CKD among whites compared to the CKD-EPI equation: A retrospective cohort study. BMC Nephrol 13: 4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel TG, Pogach LM, Barth RH: CKD screening and management in the Veterans Health Administration: The impact of system organization and an innovative electronic record. Am J Kidney Dis 53[Suppl 3]: S78–S85, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Hall RK, Wang V, Jackson GL, Hammill BG, Maciejewski ML, Yano EM, Svetkey LP, Patel UD: Implementation of automated reporting of estimated glomerular filtration rate among Veterans Affairs laboratories: A retrospective study. BMC Med Inform Decis Mak 12: 69, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Greene T, Sarnak MJ, Wang X, Beck GJ, Kusek JW, Collins AJ, Kopple JD: Effect of dietary protein restriction on the progression of kidney disease: Long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis 48: 879–888, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Akbari A, Swedko PJ, Clark HD, Hogg W, Lemelin J, Magner P, Moore L, Ooi D: Detection of chronic kidney disease with laboratory reporting of estimated glomerular filtration rate and an educational program. Arch Intern Med 164: 1788–1792, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Hemmelgarn BR, Zhang J, Manns BJ, James MT, Quinn RR, Ravani P, Klarenbach SW, Culleton BF, Krause R, Thorlacius L, Jain AK, Tonelli M, Alberta Kidney Disease Network : Nephrology visits and health care resource use before and after reporting estimated glomerular filtration rate. JAMA 303: 1151–1158, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Jain AK, McLeod I, Huo C, Cuerden MS, Akbari A, Tonelli M, van Walraven C, Quinn RR, Hemmelgarn B, Oliver MJ, Li P, Garg AX: When laboratories report estimated glomerular filtration rates in addition to serum creatinines, nephrology consults increase. Kidney Int 76: 318–323, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Noble E, Johnson DW, Gray N, Hollett P, Hawley CM, Campbell SB, Mudge DW, Isbel NM: The impact of automated eGFR reporting and education on nephrology service referrals. Nephrol Dial Transplant 23: 3845–3850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyatt C, Konduri V, Eng J, Rohatgi R: Reporting of estimated GFR in the primary care clinic. Am J Kidney Dis 49: 634–641, 2007 [DOI] [PubMed] [Google Scholar]
- 14.VA Information Resource Center (VIReC): Data Quality Alert (June 2007) in Medical SAS Datasets. Available at: http://vaww.virec.research.va.gov/DataSourcesCategory/DataQuality/HSRD_Data_Quality_Alert.pdf Hines, IL, VA Information Resource Center, 2007
- 15.Quartarolo JM, Thoelke M, Schafers SJ: Reporting of estimated glomerular filtration rate: Effect on physician recognition of chronic kidney disease and prescribing practices for elderly hospitalized patients. J Hosp Med 2: 74–78, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Akbari A, Grimshaw J, Stacey D, Hogg W, Ramsay T, Cheng-Fitzpatrick M, Magner P, Bell R, Karpinski J: Change in appropriate referrals to nephrologists after the introduction of automatic reporting of the estimated glomerular filtration rate. CMAJ 184: E269–E276, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards N, Harris K, Whitfield M, O’Donoghue D, Lewis R, Mansell M, Thomas S, Townend J, Eames M, Marcelli D: The impact of population-based identification of chronic kidney disease using estimated glomerular filtration rate (eGFR) reporting. Nephrol Dial Transplant 23: 556–561, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Krein SL, Bernstein SJ, Fletcher CE, Makki F, Goldzweig CL, Watts B, Vijan S, Hayward RA: Improving eye care for veterans with diabetes: An example of using the QUERI steps to move from evidence to implementation: QUERI Series. Implement Sci 3: 18, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbari A, Mayhew A, Al-Alawi MA, Grimshaw J, Winkens R, Glidewell E, Pritchard C, Thomas R, Fraser C: Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev (4): CD005471, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens PE, O’Donoghue DJ: The UK model for system redesign and chronic kidney disease services. Semin Nephrol 29: 475–482, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Waterman AD, Browne T, Waterman BM, Gladstone EH, Hostetter T: Attitudes and behaviors of African Americans regarding early detection of kidney disease. Am J Kidney Dis 51: 554–562, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Zhang QL, Rothenbacher D: Prevalence of chronic kidney disease in population-based studies: Systematic review. BMC Public Health 8: 117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Navaneethan SD, Aloudat S, Singh S: A systematic review of patient and health system characteristics associated with late referral in chronic kidney disease. BMC Nephrol 9: 3, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Udayaraj UP, Haynes R, Winearls CG: Late presentation of patients with end-stage renal disease for renal replacement therapy—is it always avoidable? Nephrol Dial Transplant 26: 3646–3651, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Kader K, Fischer GS, Johnston JR, Gu C, Moore CG, Unruh ML: Characterizing pre-dialysis care in the era of eGFR reporting: A cohort study. BMC Nephrol 12: 12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kilpatrick ES, Verrill H, National Clinical Biochemistry Audit Group : A national audit of estimated glomerular filtration rate and proteinuria reporting in the UK. Ann Clin Biochem 48: 558–561, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Levin A, Stevens PE: Early detection of CKD: the benefits, limitations and effects on prognosis. Nat Rev Nephrol 7: 446–457, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]