Summary
Background and objectives
Tubulointerstitial nephritis and uveitis (TINU) syndrome is considered a rare cause of acute tubulointerstitial nephritis (ATIN) that is usually associated with renal recovery. This study sought to investigate the diagnosis, prognosis, and contributing factors of TINU syndrome using a large cohort of patients with prospective follow-up.
Design, setting, participants, & measurements
This study included patients with TINU syndrome from a prospective cohort of patients with ATIN from 2007 to 2012. Clinical-pathologic data were collected at biopsy and autoantibodies against modified C-reactive protein (mCRP-Ab) were measured. Serum levels and renal tissue expression of Kreb von den Lunge-6 were also detected. Independent risk factors for poor renal outcome at 12 months and late-onset uveitis were analyzed.
Results
Thirty-one patients (28%) with biopsy-proven ATIN were classified as having TINU syndrome. Of these patients, 18 (58%) developed late-onset uveitis and were misdiagnosed as having drug-induced ATIN at the time of biopsy. An abnormal level of mCRP-Ab was an independent risk factor for late-onset uveitis (odds ratio, 14.7; 95% confidence interval, 3.4 to 64.0). Patients with TINU syndrome and drug-induced ATIN had comparable levels of Kreb von den Lunge-6 in both serum and renal tissues. Ninety-two percent of patients developed stage 3–4 CKD and/or tubular dysfunction by 12 months postbiopsy. Age, serum creatine level, erythrocyte sedimentation rate, and the presence of concomitant thyroid disease or leukocyturia were related to poor renal outcome. Relapse was seen in 36% (11 of 31) of patients and potentiated poor renal outcome.
Conclusions
The diagnosis of TINU syndrome can be missed in a large fraction of patients with ATIN because uveitis can present well after the onset of tubulointerstitial nephritis. Elevated mCRP-Ab levels may be useful in predicting late-onset uveitis TINU syndrome. Unfortunately, patients with TINU tended to have frequent relapses and most patients had incomplete renal recovery. Long-term follow-up is needed to prevent misdiagnosis and properly manage TINU syndrome.
Introduction
Tubulointerstitial nephritis and uveitis (TINU) syndrome is regarded as a rare cause of acute tubulointerstitial nephritis (ATIN) (1). It is defined by tubulointerstitial nephritis associated with uveitis that can either occur concurrently or precede or follow the onset of renal dysfunction (2).
Since it was first documented in 1975 (3), >250 cases of TINU syndrome have been reported, with approximately 60% of cases occurring in children. The renal prognosis has been considered to be favorable, although this was largely based on case reports or retrospective studies of a small number of patients (4–16). The long-term outcome of TINU syndrome, especially in adults, has not been well studied. We investigated the diagnosis, prognosis, and factors affecting patients with TINU syndrome in a prospective cohort of patients with biopsy-proven ATIN.
Materials and Methods
Patients
This study was approved by the Committee on Research Ethics of the Peking University First Hospital. A prospective cohort of ATIN was established in 2007 and patients who had a biopsy were included in the study cohort and followed regularly for at least 12 months (n=112). Ophthalmologic consultations were performed at the time of biopsy and every 3–6 months thereafter for each patient. The diagnosis of TINU syndrome was made based on the criteria described by Mandeville et al. (2). Patients who were aged <16 years and those who had concurrent glomerular diseases, systemic autoimmune diseases, or infectious diseases were excluded from the study.
Sixty-one patients with drug-induced ATIN (DATIN) from the ATIN cohort were included for comparative analysis. The diagnosis of DATIN was made according to previously described criteria (17). Each patient was followed for at least 12 months to exclude the possibility of autoimmune diseases or TINU syndrome.
Evaluation of Clinical Parameters
Renal function was evaluated by serum creatinine (SCr) measurements and the estimated GFR (eGFR) was calculated using the Modification of Diet in Renal Disease study equation (18). Tubular dysfunction was identified by renal glycosuria, elevated levels of urinary N-acetyl-β-d-glucosaminidase (NAG) and α1-microglobulin (α1-MG), and decreased urine osmolality. Levels of urinary NAG and α1-MG were measured by immune transmission turbidity and spectrophotometric methods, respectively. Systemic inflammation was evaluated by erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
Histologic Examinations
Renal biopsy was performed in all patients. Kidney sections were processed for light microscopy, electron microscopy, and immunofluorescence examination. Semiquantitative scores were developed for classifying the interstitial inflammation, fibrosis, tubulitis, and tubular atrophy according to the Banff working classification (19,20). An activity index was calculated by adding the scores for interstitial inflammation and tubulitis. The chronicity index was the sum of the scores for interstitial fibrosis and tubular atrophy. Infiltrating cells were identified by immunofluorescence staining with antibodies against-CD3 (T lymphocytes), CD20 (B lymphocytes), CD38 (plasma cells), CD68 (monocytes/macrophages), and neutrophil elastase (neutrophils). Eosinophils were detected with hematoxylin and eosin staining.
Assay of mCRP-Ab
Autoantibodies against modified CRP (mCRP-Ab) were detected by ELISA as previously described (21). For each sample, the binding of the tested serum was expressed as the percentage of a known positive control serum. Sera from the normal controls were from 50 ethnically matched, voluntarily recruited, and selected Chinese blood donors. The normal range was defined as <19.2% (mean + 2 SD of normal controls, n=50).
Assay of Krebs von den Lunge-6
Serum Krebs von den Lunge-6 (KL-6) is a mucin-like high molecular weight glycoprotein. Its increased serum level has been reported to be an indicator for distinguishing patients with TINU syndrome from patients with uveitis (22). We used ELISA to detect KL-6 levels in the serum and renal tissue of our patients (23), and immunofluorescence (22) to investigate whether KL-6 can be a marker for identifying TINU syndrome from DATIN. Normal serum KL-6 levels were determined in the same 50 normal controls as the mCRP-Ab assay. Renal tissue adjacent to tumors was used as the normal control for the immunofluorescence assay (n=14). The number of tubules that were positively stained with KL-6 was counted under ×400 magnification and expressed as the number of positive tubules/×400.
Treatment
Prednisone (0.8 mg/kg per day) was prescribed for 6–8 weeks and tapered by 5 mg every 2 weeks until the patients were off prednisone. Four patients aged >60 years and/or who had diabetes mellitus started prednisone at 0.6 mg/kg per day. The entire prednisone treatment lasted for approximately 6 months. Ten patients (32%) received methylprednisolone (250–500 mg/d ×3 days) pulse therapy followed by prednisone treatment. Cyclophosphamide (1–2 mg/kg per day) was administered to 11 patients (36%). Three patients received cyclophosphamide after the initiation of prednisone for renal dysfunction requiring hemodialysis, which associated with a profound inflammatory response (ESR≥100 mm/h, CRP≥50 mg/L). An additional eight patients were administered cyclophosphamide during follow-up for relapses of acute increases in SCr. The cumulative dose of cyclophosphamide was 4–6 g for most patients.
Follow-Up and Prognosis
Patients were scheduled for follow-up at our renal clinic monthly for the first 6 months and then every 3 months until at least 1 year after biopsy. Clinic visits included event investigation, physical examination, laboratory testing, and treatment. We performed complete blood counts, blood tests for biochemistry, ESR, and CRP levels, urinalysis, and urinary NAG and α1-MG.
Short-term renal recovery was evaluated by the percent decrease in SCr at 1 month and 3 months after biopsy. Long-term renal recovery was evaluated by eGFR at 6 and 12 months after biopsy. Patients who had an eGFR of ≥60 ml/min per 1.73 m2 at 12 months were considered to have a good outcome. Those who had an eGFR<60 ml/min per 1.73 m2 were classified as having a poor outcome. Normal renal function was defined as an eGFR≥90 ml/min per 1.73 m2. Incomplete renal recovery was defined as an eGFR<60 ml/min per 1.73 m2 and/or elevated levels of urinary NAG and/or α1-MG.
During the recovery phase, any acute increase in SCr by >25% within 2–4 weeks was considered an episode of AKI. An episode of AKI was considered to be a relapse of the disease after excluding common etiologies for AKI such as infection, drug hypersensitivity or toxicity, and prerenal and postrenal causes. An increase in urinary NAG and α1-MG and active ocular inflammation reinforced the diagnosis of disease relapse.
Statistical Analyses
Statistical analysis was performed using SPSS 17.0 statistical software (SPSS, Chicago, IL). Normally distributed variables were expressed as the mean ± SD and were compared using the t test. Nonparametric variables were expressed as the median and range and were compared using the Mann–Whitney U test. Categorical variables were compared using the chi-squared or Fisher’s exact tests. Correlation analysis was performed to determine factors that related to poor renal outcome and late-onset uveitis. Univariate regression followed by multivariate logistic regression using a backward stepwise elimination was performed to determine independent risk factors for poor renal outcome and predictors for late-onset uveitis. Results of the regression analyses are reported as odds ratios with 95% confidence intervals. A receiver operating characteristic curve was used to determine the most discriminative threshold for mCRP-Ab in predicting late-onset uveitis. P<0.05 was considered statistically significant.
Results
TINU Syndrome Could Be Easily Missed without Proper Follow-Up
Of the entire ATIN cohort, 31 patients were diagnosed with TINU syndrome, which accounted for 27.7% of patients with biopsy-proven ATIN and 0.7% of all individuals who had a biopsy. The mean follow-up period was 35±22 months (median 37 months; range, 4–62 months).
Thirteen patients (42%) were diagnosed with TINU at the time of biopsy because uveitis occurred concurrently or before the onset of renal insufficiency. The other 18 patients (58%) developed late-onset uveitis, which occurred 2–14 months after biopsy; these patients were thus misdiagnosed as having DATIN at biopsy. The absence of longitudinal follow-up would thus have resulted in underdiagnosis of TINU by 58%.
Clinical Characteristics
The mean age of our cohort was 47.7±12.1 years, with a female predominance (5.2:1). Comorbidities included hypertension (32%), diabetes mellitus (13%), thyroid diseases (29%), and history of allergies (26%). Six patients had hypothyroidism and three patients had hyperthyroidism. Hashimoto’s thyroiditis was the major cause of the thyroid abnormalities.
The median time from onset of first symptoms to renal biopsy was 30 days (range, 20–150 days). The most commonly reported symptoms were fatigue, anorexia, nausea, vomiting, fever, polyuria, and nocturia. Eighteen patients—whose uveitis was bilateral, anterior, and took place between 2 months before and 12 months after biopsy—were identified as having typical uveitis. The remaining 13 patients were diagnosed as having atypical uveitis (2). Of 31 patients, 9 (29%) had ocular inflammation lasting for >6 weeks (chronic uveitis) and 8 patients (26%) presented with insidious uveitis.
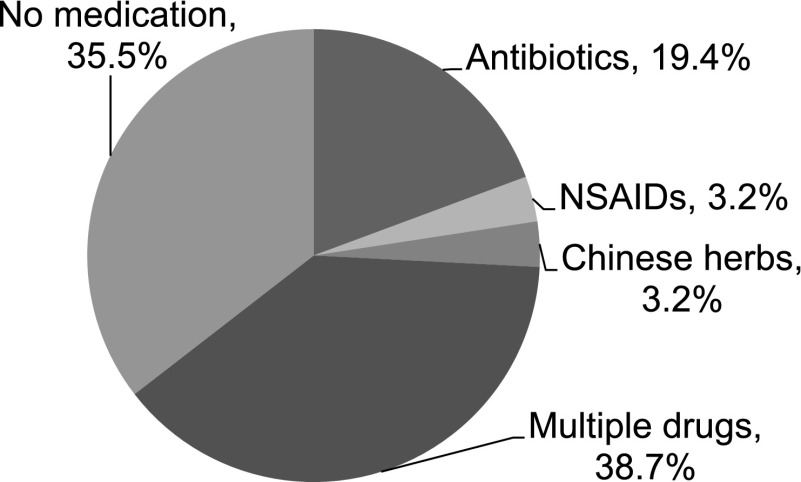
Twenty patients (65%) had been exposed to various medications within 20 days before the presentation of the disease (Figure 1). Manifestations of hypersensitivity, such as rash, fever, and joint pain, were seen in 7%, 58%, and 10% of the patients, respectively.
Figure 1.
Drugs applied in patients with TINU within 20 days before the onset of renal dysfunction (n=31). The related drugs included β-lactams (amoxicillin, cephalexin, cefoperazone, cefuroxime), meropenem, azithromycin, levofloxacin, ibuprofen, and omeprazole, which were also known to cause DATIN. DATIN, drug-induced acute tubulointerstitial nephritis; NSAID, nonsteroidal anti-inflammatory drug; TINU, tubulointerstitial nephritis and uveitis.
Laboratory Findings
The main laboratory findings are presented in Table 1. Increased SCr levels were seen in all patients and five patients (16%) needed hemodialysis. Increased urinary α1-MG excretion and decreased urinary osmolality were seen in all patients. Renal glycosuria was present in 94% of patients. Approximately 50% of patients had elevated urinary NAG excretion and leukocyturia. A systemic inflammatory response was apparent in each patient and was associated with significantly elevated ESR and CRP levels.
Table 1.
Laboratory findings of patients with TINU syndrome
| Measurement | Normal Range | Percent Change | Patients with TINU (n=31) |
|---|---|---|---|
| Serum creatinine (μmol/L) | 44–133 | ||
| Peak | 304.3 (142.0–1445.0) | ||
| Biopsy | 232.5 (103.0–825.0) | ||
| Hemoglobin (g/L) | 137–179 | 97 ↓ | 90.0 (74.0–137.0) |
| Erythrocyte sedimentation rate (mm/h) | 0–20 | 100 ↑ | 81.0 (25.0–140.0) |
| C-reactive protein (mg/L) | <8 | 68 ↑ | 13.1 (2.1–74.3) |
| C3 (g/L) | 0.6–1.5 | 0 ↓ | 1.2 (0.6–1.5) |
| Eosinophilia | 0.05–0.5×109/L | 0 (0) | |
| Autoantibodies | |||
| Antinuclear antibody (1:100)a | <1100 | 4 (13) | |
| ANCA (±)b | Negative | 2 (7) | |
| N-acetyl-β-d-glucosaminidase (U/L) | 0–21 | 58 ↑ | 30.0 (5.0–65.0) |
| α1-Microglobulin (mg/L) | 0–12 | 100 ↑ | 193.0 (16.9–392.0) |
| Urine osmotic pressure (mOsm/kg) | 600–1000 | 100 ↓ | 385.0 (113.0–596.0) |
| Renal glycosuria | Negative | 94 ↑ | 29 (94) |
| Leukocyturia (number of cells) | 0–5/H | 18 (58) | |
| Eosinophiluria (number of cells) | 0/HP | 0 (0) |
Data are presented as n (%) or median (interquartile range). TINU, tubulointerstitial nephritis and uveitis.
ANA was weakly positive (1:100) at biopsy in four patients and maintained the same level during follow-up. Double-stranded DNA and extractable nuclear antigen autoantibodies were all negative.
ANCA was weakly positive (±) at biopsy in two patients and turned negative during follow-up. Specific antibodies including anti-proteinase 3 and anti-myeloperoxidase were all negative.
Pathologic Characteristics
Diffuse interstitial inflammation and significant tubulitis were seen in 87% and 81% of patient biopsies, respectively. Seventy-four percent of patients had none or <25% fibrosis of the interstitial area. The infiltrates consisted of T lymphocytes (accounting for 48%±7% of all inflammatory cells), B lymphocytes (12%±4%), plasma cells (11%±5%), monocytes/macrophages (24%±6%), and neutrophils (6%±3%). Eosinophils were seen at 5.4±7.2 (median 4.3; interquartile range, 0.0–31.8) cells/×200.
Immunofluorescence staining was negative for Ig or complement in the kidney biopsies in all patients. Electron microscopy examinations helped to exclude glomerular disease.
Outcome and Affecting Factors
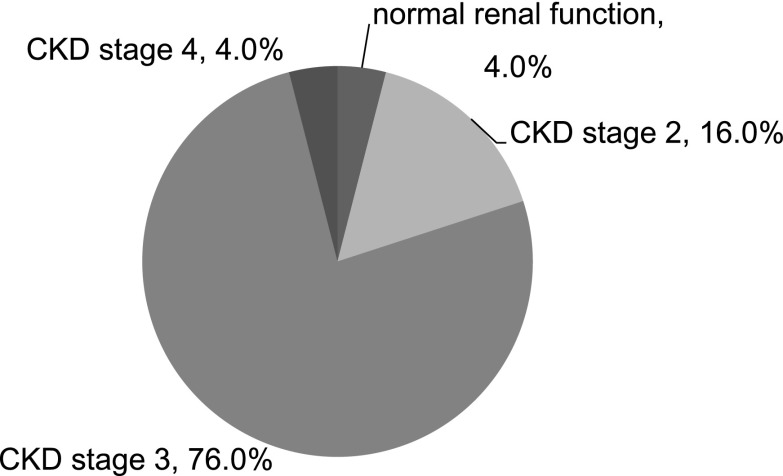
Patients who were followed for >1 year were included in the analysis for renal outcome (n=25). Short-term renal outcome was characterized by abrupt decreases in SCr in all patients within the first month after biopsy (Table 2). During months 1–3, 22 patients (88%) saw their rate of renal recovery decline. Only seven patients (28%) experienced sustained renal recovery during the entire year after biopsy. Eighty percent of patients developed stage 3–4 CKD at 12 months (Figure 2). Prolonged tubular dysfunction was seen in 88% of patients with elevated levels of urinary NAG and/or α1-MG. Altogether, 92% of patients developed CKD and/or tubular insufficiency, indicating incomplete recovery from the preceding AKI.
Table 2.
Renal function at every follow-up time point (n=25)
| Measurement | Follow-Up | Renal Function | Good Outcome (n=5) | Poor Outcome (n=20) | P Value |
|---|---|---|---|---|---|
| Serum creatinine (μmol/L) | At biopsy | 252.0 (103.0–825.0) | 191.0 (160.0–295.0) | 268.5 (103.0–825.0) | 0.03 |
| 1 wk | 193.5 (117.0–825.0) | 151.0 (118.0–206.0) | 195.5 (117.0–825.0) | 0.07 | |
| 2 wk | 164.5 (111.0–469.0) | 117.0 (114.0–171.0) | 164.8 (111.0–469.0) | 0.15 | |
| 1 mo | 120.0 (98.0–206.0) | 102.0 (100.0–103.5) | 121.5 (98.0–206.0) | 0.01 | |
| 3 mo | 110.0 (73.6–185.0) | 97.0 (95.0–125.0) | 110.3 (73.6–185.0) | 0.41 | |
| 6 mo | 112.0 (81.2–154.0) | 88.0 (81.2–92.0) | 113.0 (92.0–154.0) | 0.002 | |
| 12 mo | 108.0 (73.0–177.0) | 86.0 (73.0–102.0) | 112.0 (95.0–177.0) | 0.001 | |
| Estimated GFR (ml/min per 1.73 m2) | At biopsy | 17.7 (5.7–46.1) | 31.9 (15.4–38.0) | 17.1 (5.7–46.1) | 0.04 |
| 1 wk | 26.5 (5.8–52.9) | 45.4 (23.8–52.9) | 25.1 (5.8–41.8) | 0.02 | |
| 2 wk | 31.7 (11.4–55.2) | 50.9 (33.8–55.2) | 30.6 (11.4–50.2) | 0.02 | |
| 1 mo | 44.6 (24.6–95.2) | 62.0 (57.0–63.1) | 43.8 (24.6–95.2) | 0.02 | |
| 3 mo | 49.3 (28.2–104.0) | 60.6 (49.3–67.1) | 48.9 (28.2–104.0) | 0.19 | |
| 6 mo | 50.1 (32.4–83.1) | 71.5 (66.5–83.1) | 47.3 (32.4–81.0) | 0.01 | |
| 12 mo | 52.0 (29.4–92.4) | 80.5 (66.6–92.4) | 49.4 (29.4–59.3) | <0.001 |
Figure 2.
Renal outcome of patients with TINU syndrome at 12 months after biopsy (n=25). Renal function was determined by eGFR. There was only one patient that had normal renal function (eGFR≥90 ml/min per 1.73 m2). Patients in CKD stages 3 and 4 accounted for 80% of all patients. eGFR, estimated GFR; TINU, tubulointerstitial nephritis and uveitis.
Compared with patients who experienced a good outcome, those with a poor renal outcome were older, had higher levels of SCr and ESR, and more exhibited prominent leukocyturia (Tables 2 and 3). There was no significant difference in the pathologic activity or chronicity index between the two groups. Correlation analysis showed that age (r=0.54, P=0.01), accompanied thyroid diseases (r=0.44, P=0.03), levels of ESR (r=0.47, P=0.03), leukocyturia (r=0.45, P=0.03), and SCr concentrations at biopsy (r=0.44, P=0.03) were related to the eGFR levels at 12 months after biopsy. Unfortunately, multivariate regression analysis failed to identify any of the clinical, laboratory, or histologic factors that could predict poor renal outcome.
Table 3.
Comparison of clinical findings at biopsy between patients with good and poor renal outcome
| Characteristic | Good Outcome (n=5) | Poor Outcome (n=20) | P Value |
|---|---|---|---|
| Age (yr) | 37.4±7.5 | 49.4±12.3 | 0.05 |
| Male/female sex (% male) | 1/4 (20) | 2/18 (10) | 0.50 |
| Hemoglobin (g/L) | 99.0 (89.0–106.0) | 89.5 (74.0–137.0) | 0.13 |
| Erythrocyte sedimentation rate (mm/h) | 45.0 (32.0–100.0) | 87.5 (25.0–140.0) | 0.04 |
| C-reactive protein (mg/L) | 8.0 (2.1–33.7) | 17.7 (2.7–74.3) | 0.29 |
| mCRP-Ab (↑%)a | 22.6 (18.2–55.3); 80 | 25.3 (6.4–39.2); 75 | 0.57 |
| Serum creatinine at biopsy (μmol/L) | 191.0 (160.0–295.0) | 268.5 (103.0–825.0) | 0.03 |
| Estimated GFR at biopsy (ml/min per 1.73 m2) | 31.9 (15.4–38.0) | 17.1 (5.7–46.1) | 0.04 |
| N-acetyl-β-d-glucosaminidase (U/L) | 41.0 (13.0–65.0) | 28.0 (10.0–57.0) | 0.29 |
| α1-Microglobulin (mg/L) | 314.0 (177.0–328.0) | 174.5 (16.9–392.0) | 0.07 |
| Leukocyturia | 1 (20) | 15 (75) | 0.04 |
| Histologic activity index ≥4 | 5 (100) | 18 (90) | 0.46 |
| Histologic chronicity index ≤2 | 4 (80) | 12 (60) | 0.41 |
Data are presented as mean ± SD, n (%), or median (interquartile range) unless otherwise specified. mCRP-Ab, autoantibody against modified C-reactive protein.
Percentage of positive control.
Relapse in TINU Syndrome Might Potentiate Poor Long-Term Outcome
Patients with a good outcome had continuous renal recovery within 12 months postbiopsy, whereas those who had poor outcome failed to regain renal function after 3 months postbiopsy (Table 2). A high relapse rate was seen because 11 patients (44%) had an acute rise in SCr by 28%–89% on at least one occasion during the follow-up period. In addition, another seven patients (28%) had a less extensive SCr increase by 13%–23% during follow-up.
The relapse episodes of kidney injury were all accompanied by elevated urinary NAG and/or α1-MG as well as active ocular inflammation. The median time to relapse was 6 months (2–25 months) postbiopsy, when prednisone was discontinued or tapered down to 5–10 mg/d. The readministration of prednisone (15–30 mg/d) or the addition of cyclophosphamide (50 mg/d) decreased SCr levels. There was no significant difference in the clinical, laboratory, or histologic parameters when comparing patients with relapse and those without. Moreover, correlation analysis did not find any parameters that were related to disease relapse. Patients who relapsed seemed to have higher mCPR-Ab levels than those who had no relapse (29% [range, 6–55] versus 20% [range, 9–37] of positive control), but this did not reach the statistical significance (P=0.07).
Parameters that Could Predict Late-Onset Uveitis TINU Syndrome
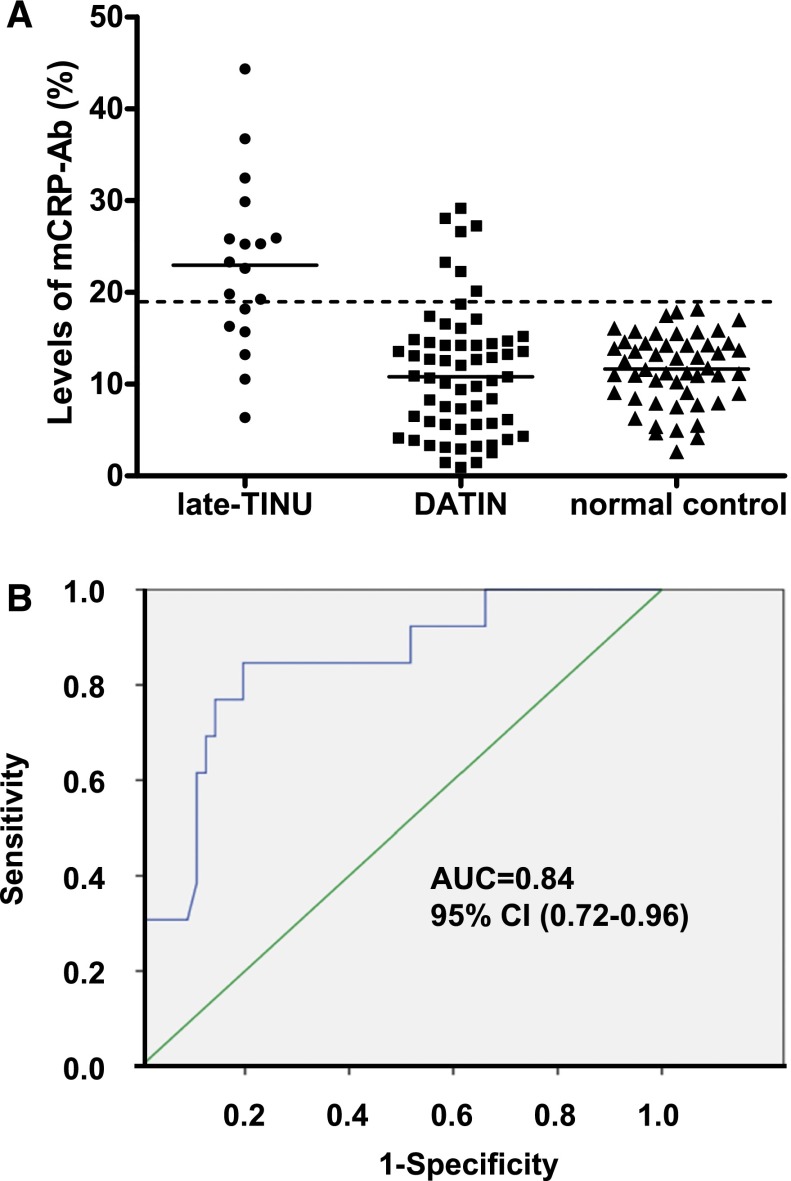
There were no significant differences in age, sex, medication history, renal dysfunction, or histologic parameters between the TINU syndrome group and the DATIN group (Table 4). Patients in the late-TINU group had a higher percentage of concomitant diseases and higher levels of ESR and CRP. Positive mCRP-Ab was detected not only in more patients but at higher levels in the late-TINU group (Table 4 and Figure 3A). By multivariate regression analysis, an elevated mCRP-Ab level at biopsy was found to be an independent risk factor for the development of uveitis during follow-up (odds ratio, 14.7; 95% confidence interval, 3.4 to 64.0; P<0.001). Analysis of the receiver operating characteristic curve yielded a mCRP-Ab level of 20.2% of positive controls to predict late-TINU, with 88% specificity and 64% sensitivity (Figure 3B). Five patients that were diagnosed and enrolled in 2011 had serial mCRP-Ab tested over time. An SCr increase was associated with a rise in mCRP-Ab levels in two patients who relapsed during follow-up. In the other three patients in whom renal function was stable, mCRP-Ab also remained stable and at lower levels during follow-up.
Table 4.
Comparison of clinical, laboratory, and pathologic findings between patients with late-onset uveitis TINU syndrome and DATIN
| Characteristic | Patients with Late-Onset Uveitis TINU (n=18) | Patients with DATIN (n=61) | P Value |
|---|---|---|---|
| Age (yr) | 46.2±10.9 | 47.0±12.7 | 0.82 |
| Male/female sex (% male) | 1/17 (6) | 16/45 (26) | 0.06 |
| Thyroid disease | 6 (33) | 7 (12) | 0.03 |
| Hemoglobin (g/L) | 90.0 (76.0–137.0) | 94.0 (72.0–150.0) | 0.28 |
| Erythrocyte sedimentation rate (mm/h) | 88.5 (45.0–140.0) | 60.0 (2.0–140.0) | 0.02 |
| C-reactive protein (mg/L) | 18.5 (3.9–74.3) | 7.2 (1.0–136.0) | 0.01 |
| Eosinophilia | 0 (0) | 5 (8) | 0.21 |
| mCRP-Ab (↑%)a | 21.8 (6.4–44.3); 67 | 10.9 (0.9–29.1); 12 | <0.001 |
| Serum creatinine at biopsy (μmol/L) | 257.5 (160.0–825.0) | 239.0 (98.0–988.0) | 0.56 |
| Estimated GFR at biopsy (ml/min per 1.73 m2) | 17.6 (5.8–36.6) | 24.3 (3.4–82.8) | 0.35 |
| N-acetyl-β-d-glucosaminidase (U/L) | 32.0 (10.0–57.0) | 21.0 (4.0–117.0) | 0.34 |
| α1-Microglobulin (mg/L) | 213.0 (36.2–362.0) | 184.0 (2.4–402.0) | 0.32 |
| Urine osmotic pressure (mOsm/kg) | 399.5 (292.0–596.0) | 383.0 (222.0–655.0) | 0.63 |
| Renal glycosuria (↑%) | 17 (94) | 51 (84) | 0.24 |
| Leukocyturia (↑%) | 9 (50) | 28 (46) | 0.76 |
| Eosinophiluria | 0 (0) | 0 (0) | 1.00 |
| Histologic activity index≥4 | 14 (78) | 47 (77) | 0.95 |
| Histologic chronicity index≤2 | 14 (78) | 48 (79) | 0.93 |
| Eosinophil on biopsy (×200) | 2.6 (0.4–31.8) | 1.9 (0.0–33.8) | 0.08 |
Data are presented as mean ± SD, n (%), or median (interquartile range) unless otherwise specified. mCRP-Ab, autoantibody against modified C-reactive protein; TINU, tubulointerstitial nephritis and uveitis; DATIN, drug-induced acute tubulointerstitial nephritis.
Percentage of positive control.
Figure 3.
Positive serum mCRP-Ab could predict late-onset TINU. (A) Levels of serum mCRP-Ab in patients with late-onset TINU (n=18), patients with DATIN (n=61), and normal controls (n=50). The horizontal solid lines indicate the median value of mCRP-Ab. The dashed line indicates the normal range (19.2%). (B) Receiver operator characteristic curve analysis of mCRP-Ab on diagnosing late-onset uveitis. The AUC was 0.84 (95% CI, 0.72 to 0.96; P<0.001). AUC, area under the curve; DATIN, druginduced acute tubulointerstitial nephritis; mCRP-ab, autoantibodies against modified C-reactive protein; TINU, tubulointerstitial nephritis and uveitis; 95% CI, 95% confidence interval.
KL-6 Levels in Serum and Expression in Renal Tissue Were Comparable between Patients with TINU and DATIN
Serum levels of KL-6 were higher in patients with ATIN compared with normal controls (441.9 U/ml [range, 30.3–3920.4] versus 285.8 U/ml [range, 137.6–404.1]; P=0.004). However, there was no significant difference in the serum KL-6 levels between patients with TINU syndrome and patients with DATIN (451.2 U/ml [range, 174.9–3726.0] versus 441.9 U/ml [range, 30.3–3920.4]; P=0.88). Furthermore, KL-6 expression by immunostaining was significantly higher among patients with acute tubulointerstitial injury compared with normal kidney (P<0.001), but there was no difference in KL-6 levels between patients with TINU and patients with DATIN (9.2%±1.2% versus 10.0%±1.7%, respectively; P=0.24) (Figure 4).
Figure 4.
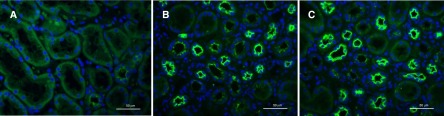
Immunofluorescence staining of KL-6 (green) on renal tissues from patients with ATIN. (A) Renal tissue adjacent to tumors presents weakly positive staining of KL-6 in a few tubules. (B and C) More positive tubules of KL-6 were seen in renal tissue of patients with DATIN (B) and patients with TINU (C). DATIN, drug-induced acute tubulointerstitial nephritis; KL-6, Kreb von den Lunge-6. Original magnification, ×400.
Discussion
Our study includes the highest number of adult patients with TINU syndrome from a single center reported to date in the literature. These patients were drawn from a prospective and longitudinally followed cohort of patients with ATIN, which represented 92% of all patients with biopsy-confirmed ATIN diagnosed over the past 6 years at the Peking University Institute of Nephrology. We found that TINU syndrome constituted 28% of the ATIN cohort, which was much higher than that reported in the literature (4.7%) (24). More than half of the patients (58%) had late-onset uveitis and would be clinically difficult to detect unless the patients were followed longitudinally. Furthermore, in 26% of our cohort, symptoms of uveitis were subtle and subclinical, which is similar to a recent prospective study in children with TINU (25). On the other hand, ophthalmologists have reported that up to 1%–2% of patients with uveitis have tubulointerstitial nephritis (26–28), which itself could be an underestimation because patients with TINU may have exhibited tubular dysfunction with normal SCr levels. Together with our findings, these data suggest that TINU syndrome is significantly underdiagnosed. Therefore, patients with ATIN require long-term monitoring by both nephrologists and ophthalmologists. Patients with uveitis also require screening for tubular function to minimize the false negative diagnosis of TINU syndrome.
We showed that late-uveitis TINU was clinically and pathologically indistinguishable from DATIN at biopsy. We previously reported that mCRP-Ab might be useful in distinguishing patients with TINU from patients with DATIN (21). In this study, of patients diagnosed with DATIN, those who went on to develop late-onset uveitis (i.e., TINU misclassified as DATIN), had higher levels of mCRP-Ab compared with those who had true DATIN. Further analysis revealed that an elevated mCRP-Ab level at biopsy would serve as an independent risk factor for late-onset uveitis. A mCRP-Ab level of >20.2% was deemed “elevated” and discriminated late-uveitis TINU from DATIN at biopsy. A limitation of this test is that mCRP-Ab can be detected in several other chronic diseases such as SLE (29,30) and chronic hepatitis C infection (31). We also identified some discrepancy between the normative value of mCRP-Ab in this study compared with our previous report (21). We speculate that this discrepancy was likely due to the instability of mCRP-Ab in the serum sample from the SLE patient (positive control) that had been stored for 2 years at −80°C. A recombinant antibody raised against the antigenic sequence of mCRP-Ab would detect mCRP-Ab directly and may be more useful in this respect.
The increased frequency of thyroid abnormalities and the higher levels of ESR and CRP in patients with TINU might suggest a more widespread immune disorder in these patients. This characteristic in patients with ATIN should prompt the clinician to suspect TINU. KL-6, a mucin-like high molecular weight glycoprotein secreted by type II alveolar cells found in interstitial lung disease, is another marker that is reported to be useful in distinguishing TINU from other causes of uveitis. Serum KL-6 levels have been reported to be significantly higher in patients with TINU syndrome compared with those with other causes of uveitis without nephritis. For this reason, the authors suggested that KL-6 might serve as a potential laboratory parameter for the diagnosis of TINU syndrome (23). In our study, however, KL-6 was found to be elevated in patients with TINU and patients with DATIN, both in the serum as well in the renal tubules. Another study found increased serum KL-6 levels in patients with diabetes and enhanced tubular KL-6 staining in biopsies of diabetic kidneys (32). Thus, KL-6 in the serum or kidneys may simply reflect tubulointerstitial injury and thus may not be helpful in differentiating TINU from ATIN of other causes.
Here, we reported that 92% of patients with TINU had incomplete renal recovery. In contrast, most reports thus far have suggested a good outcome (4–16), with only a few studies claiming prolonged renal insufficiency after TINU (2,27,33,34). This discrepancy might be due to three factors. First, approximately 60% of the reported patients were children, in whom better renal recovery is generally expected. Second, in most studies, renal outcome was assessed by SCr rather than eGFR or tubular function, which was a less accurate method for measuring renal function. Third, most of the abovementioned studies followed patients for a short time period and comprised small sample sizes.
There are several factors that may affect the long-term prognosis of TINU syndrome. We found that baseline characteristics such as older age, concurrent thyroid diseases, higher levels of SCr and ESR, and prominent leukocyturia were associated with prolonged renal dysfunction. Another important factor that could impede renal recovery was having a relapse during the repair phase after tubulointerstitial injury. Because relapses are often asymptomatic and untreated, the resulting repeated AKI would accelerate chronic progression. Few such patients with recurrent and progressive nephritis have been reported (35). Unfortunately, we did not identify any clinical parameters that could identify those patients with relapse during follow-up in this study.
There is no established standard treatment for TINU syndrome. Glucocorticoid therapy might have a beneficial effect on reducing interstitial inflammation and subsequent fibrosis (36). In this study, all patients that received glucocorticoid therapy experienced an abrupt decrease in SCr, a finding that is in accordance with that reported in the literature (2,6,13,37–39); however, the long-term outcome was still not favorable in these patients. Therefore, more studies aimed at identifying treatment strategies are urgently needed for this disease.
Although our study is the largest reported thus far, the sample size of our patients with TINU is still somewhat limited. Given the observational features of this study, we are not able to define the advantages of various treatments. A multicenter study would thus be better suited to identifying improved diagnosis, prognostication, and treatment of this rare syndrome.
The diagnosis of TINU syndrome can be missed in a large fraction of patients with ATIN because uveitis can present well after the onset of tubulointerstitial nephritis. Elevated mCRP-Ab levels may be useful in predicting late-onset uveitis in patients that will ultimately be diagnosed with TINU syndrome. Approximately one-third of patients with TINU syndrome had relapses during follow-up, and most had incomplete renal recovery. Thus, long-term follow-up is needed for proper diagnosis and management of TINU syndrome.
Disclosures
None.
Acknowledgments
The authors thank Dr. Gunaratnam (Western University, Canada) for editing the manuscript.
This work was supported by the National Natural Science Foundation of China (Grant 81070549 to L.Y.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kaynar K, Ersoz S, Akyol N, Ersoz O, Unlu O, Ozbay T, Ulusoy S: Adult onset tubulointerstitial nephritis and uveitis syndrome. Nephrology (Carlton) 10: 418–420, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Mandeville JT, Levinson RD, Holland GN: The tubulointerstitial nephritis and uveitis syndrome. Surv Ophthalmol 46: 195–208, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Dobrin RS, Vernier RL, Fish AL: Acute eosinophilic interstitial nephritis and renal failure with bone marrow-lymph node granulomas and anterior uveitis. A new syndrome. Am J Med 59: 325–333, 1975 [DOI] [PubMed] [Google Scholar]
- 4.Igarashi T, Kawato H, Kamoshita S, Nosaka K, Seiya K, Hayakawa H: Acute tubulointerstitial nephritis with uveitis syndrome presenting as multiple tubular dysfunction including Fanconi’s syndrome. Pediatr Nephrol 6: 547–549, 1992 [DOI] [PubMed] [Google Scholar]
- 5.Gianviti A, Greco M, Barsotti P, Rizzoni G: Acute tubulointerstitial nephritis occurring with 1-year lapse in identical twins. Pediatr Nephrol 8: 427–430, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Takemura T, Okada M, Hino S, Fukushima K, Yamamoto S, Miyazato H, Maruyama K, Yoshioka K: Course and outcome of tubulointerstitial nephritis and uveitis syndrome. Am J Kidney Dis 34: 1016–1021, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Manjón MT, Sánchez-Bursón J, Montero R, Pérez-Requena J, Alonso M, Marenco JL: Two cases of acute tubulointerstitial nephritis associated with panuveitis (TINU syndrome). J Rheumatol 26: 234–236, 1999 [PubMed] [Google Scholar]
- 8.Nikolić V, Bogdanović R, Ognjanović M, Stajić N: [Acute tubulointerstitial nephritis in children]. Srp Arh Celok Lek 129[Suppl 1]: 23–27, 2001 [PubMed] [Google Scholar]
- 9.Wakaki H, Sakamoto H, Awazu M: Tubulointerstitial nephritis and uveitis syndrome with autoantibody directed to renal tubular cells. Pediatrics 107: 1443–1446, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Suzuki K, Nakahata T, Tateyama T, Waga S, Ito E: Repeat renal biopsy in a girl with tubulointerstitial nephritis and uveitis syndrome. Pediatr Nephrol 16: 885–887, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Howarth L, Gilbert RD, Bass P, Deshpande PV: Tubulointerstitial nephritis and uveitis in monozygotic twin boys. Pediatr Nephrol 19: 917–919, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Lee JW, Kim HJ, Sung SH, Lee SJ: A case of tubulointerstitial nephritis and uveitis syndrome with severe immunologic dysregulation. Pediatr Nephrol 20: 1805–1808, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Liakopoulos V, Ioannidis I, Zengos N, Karabatsas CH, Karasavvidou F, Salmas M, Kanelaki E, Eleftheriadis T, Stefanidis I: Tubulointerstitial nephritis and uveitis (TINU) syndrome in a 52-year-old female: A case report and review of the literature. Ren Fail 28: 355–359, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Ebihara I, Hirayama K, Usui J, Seki M, Higuchi F, Oteki T, Kobayashi M, Yamagata K: Tubulointerstitial nephritis and uveitis syndrome associated with hyperthyroidism. Clin Exp Nephrol 10: 216–221, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Thomassen VH, Ring T, Thaarup J, Baggesen K: Tubulointerstitial nephritis and uveitis (TINU) syndrome: A case report and review of the literature. Acta Ophthalmol (Copenh) 87: 676–679, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Jahnukainen T, Ala-Houhala M, Karikoski R, Kataja J, Saarela V, Nuutinen M: Clinical outcome and occurrence of uveitis in children with idiopathic tubulointerstitial nephritis. Pediatr Nephrol 26: 291–299, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Yang L, Su T, Wang C, Liu G, Li XM: Pathological significance of a panel of urinary biomarkers in patients with drug-induced tubulointerstitial nephritis. Clin J Am Soc Nephrol 5: 1954–1959, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y, Mihatsch MJ, Nadasdy TR, Nickerson PR, Steenolsen T, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose SN, Rush DD, Madrigal LS, Salomon DR, Sund SE, Taskinen EO, Trpkov KL, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff 07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Tan Y, Yu F, Qu Z, Su T, Xing GQ, Wu LH, Wang FM, Liu G, Yang L, Zhao MH: Modified C-reactive protein might be a target autoantigen of TINU syndrome. Clin J Am Soc Nephrol 6: 93–100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kase S, Kitaichi N, Namba K, Miyazaki A, Yoshida K, Ishikura K, Ikeda M, Nakashima T, Ohno S: Elevation of serum Krebs von den Lunge-6 levels in patients with tubulointerstitial nephritis and uveitis syndrome. Am J Kidney Dis 48: 935–941, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Munakata M, Suzuki I, Kawakami Y: Serum and bronchoalveolar fluid KL-6 levels in patients with pulmonary alveolar proteinosis. Am J Respir Crit Care Med 158: 1294–1298, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Baker RJ, Pusey CD: The changing profile of acute tubulointerstitial nephritis. Nephrol Dial Transplant 19: 8–11, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Saarela V, Nuutinen M, Ala-Houhala M, Arikoski P, Rönnholm K, Jahnukainen T: Tubulointerstitial nephritis and uveitis syndrome in children: A prospective multicenter study. Ophthalmology 120: 1476–1481, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum JT: Bilateral anterior uveitis and interstitial nephritis. Am J Ophthalmol 105: 534–537, 1988 [DOI] [PubMed] [Google Scholar]
- 27.Mackensen F, Smith JR, Rosenbaum JT: Enhanced recognition, treatment, and prognosis of tubulointerstitial nephritis and uveitis syndrome. Ophthalmology 114: 995–999, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Levinson RD: Tubulointerstitial nephritis and uveitis syndrome. Int Ophthalmol Clin 48: 51–59, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Sjöwall C, Bengtsson AA, Sturfelt G, Skogh T: Serum levels of autoantibodies against monomeric C-reactive protein are correlated with disease activity in systemic lupus erythematosus. Arthritis Res Ther 6: R87–R94, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan Y, Yu F, Yang H, Chen M, Fang Q, Zhao MH: Autoantibodies against monomeric C-reactive protein in sera from patients with lupus nephritis are associated with disease activity and renal tubulointerstitial lesions. Hum Immunol 69: 840–844, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Kessel A, Elias G, Pavlotzky E, Zuckerman E, Rosner I, Toubi E: Anti-C-reactive protein antibodies in chronic hepatitis C infection: Correlation with severity and autoimmunity. Hum Immunol 68: 844–848, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Takahashi T, Takamura K, Sakaue S, Ishii J, Yokouchi H, Nasuhara Y: Elevated serum KL-6 concentrations in patients with diabetes mellitus. J Diabetes Complications 16: 352–358, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi Y, Honda M, Yoshikawa N, Ito H: Acute tubulointerstitial nephritis in 21 Japanese children. Clin Nephrol 54: 191–197, 2000 [PubMed] [Google Scholar]
- 34.Hamdan JM, Obeidat FN: Tubulo-interstitial nephritis and uveitis syndrome in a 6-year-old boy: Case report. Ann Trop Paediatr 26: 145–148, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Oymak O, Unal A, Sipahioglu MH, Akcakaya M, Tokgoz B, Patiroglu T, Utas C: Recurrent and progressive nephritis in a patient with acute tubulointerstitial nephritis and uveitis syndrome. Clin Nephrol 69: 64–66, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Cacoub P, Deray G, Le Hoang P, Baumelou A, Beaufils H, de Groc F, Rousselie F, Jouanneau C, Jacobs C: Idiopathic acute interstitial nephritis associated with anterior uveitis in adults. Clin Nephrol 31: 307–310, 1989 [PubMed] [Google Scholar]
- 37.Tareeva IE, Kravets TA, Proskurneva EP: [Acute tubulo-interstitial nephritis combined with uveitis (clinical case and review of the literature)]. Ter Arkh 58: 89–92, 1986 [PubMed] [Google Scholar]
- 38.Buysen JG, Houthoff HJ, Krediet RT, Arisz L: Acute interstitial nephritis: A clinical and morphological study in 27 patients. Nephrol Dial Transplant 5: 94–99, 1990 [DOI] [PubMed] [Google Scholar]
- 39.Cigni A, Soro G, Faedda R, Caucci F, Amadori F, Manca A, Tanda F, Satta AE: A case of adult-onset tubulointerstitial nephritis and uveitis (“TINU syndrome”) associated with sacroileitis and Epstein-Barr virus infection with good spontaneous outcome. Am J Kidney Dis 42: E4–E10, 2003 [DOI] [PubMed] [Google Scholar]