Summary
Background and objectives
At least four definitions of AKI have recently been proposed. This study sought to characterize the epidemiology of AKI according to the most recent consensus definition proposed by the Kidney Disease Improving Global Outcomes (KDIGO) Work Group, and to compare it with three other definitions.
Design, setting, participants, & measurements
This was a retrospective cohort study of 31,970 hospitalizations at an academic medical center in 2010. AKI was defined and staged according to KDIGO criteria, the Acute Dialysis Quality Initiative’s RIFLE criteria, the Acute Kidney Injury Network (AKIN) criteria, and a definition based on a model of creatinine kinetics (CK). Outcomes of interest were incidence, in-hospital mortality, length of stay, costs, readmission rates, and posthospitalization disposition.
Results
AKI incidence was highest according to the KDIGO definition (18.3%) followed by the AKIN (16.6%), RIFLE (16.1%), and CK (7.0%) definitions. AKI incidence appeared markedly higher in those with low baseline serum creatinine according to the KDIGO, AKIN, and RIFLE definitions, in which AKI may be defined by a 50% increase over baseline. AKI according to all definitions was associated with a significantly higher risk of death and higher resource utilization. The adjusted odds ratios for in-hospital mortality in those with AKI were highest with the CK definition (5.2; 95% confidence interval [95% CI], 4.1 to 6.6), followed by the RIFLE (2.9; 95% CI, 2.2 to 3.6), KDIGO (2.8; 95% CI, 2.2 to 3.6), and AKIN (2.6; 95% CI, 2.0 to 3.3) definitions. Concordance in diagnosis and staging was high among the KDIGO, AKIN, and RIFLE definitions.
Conclusions
The incidence of AKI in hospitalized individuals varies depending on the definition used. AKI according to all definitions is associated with higher in-hospital mortality and resource utilization. AKI may be inappropriately diagnosed in those with low baseline serum creatinine using definitions that incorporate percentage increases over baseline.
Introduction
AKI is recognized as one of the most serious complications of hospitalized individuals. AKI is strongly associated with increased resource utilization (1–3), higher short- and long-term mortality (1,4–9), and a higher risk for the development of CKD (10–13). Since 2004, at least four proposals have been put forth to define and stage AKI. The RIFLE (Risk, Injury, Failure, Loss, and ESRD) criteria (14), the first consensus definition, have been studied in a number of settings and validated by showing that a stepwise relationship exists between AKI severity and mortality (15). The Acute Kidney Injury Network (AKIN) criteria (16) modified RIFLE by incorporating an absolute increase in creatinine after the finding that small increases in serum creatinine (SCr) were of prognostic significance (1). In 2009, Waikar and Bonventre proposed a creatinine kinetics (CK)–based definition of AKI using absolute changes in SCr over 24 hours or 48 hours (17). Finally, in 2012, the Kidney Disease Improving Global Outcomes (KDIGO) Work Group proposed another definition that builds upon the AKIN definition (18). The KDIGO definition has been adopted by the Renal Association of the United Kingdom (19) and has been the subject of commentary by other professional societies from the United States (20), Europe (21), and Canada (22).
The purposes of this study were to provide detailed estimates of the epidemiology of AKI using the KDIGO definition—including incidence, in-hospital mortality, costs, readmission rates, length of stay (LOS), and posthospital disposition—and to investigate how the epidemiology differs according to the definition used.
Materials and Methods
Study Cohort
This study was conducted at Brigham and Women’s Hospital, a 777-bed urban academic medical center in Boston, Massachusetts. We included all hospitalizations of adult patients (aged ≥18 years) admitted between January 1, 2010 and December 31, 2010. We excluded patients with ESRD and those who received kidney transplantation (553 patients with 946 hospitalizations; Supplemental Table 1). To minimize misclassification we manually reviewed electronic discharge summaries of those with codes for renal replacement therapy (RRT) (Supplemental Tables 1 and 2) to verify that all ESRD patients were excluded and that RRT was provided for AKI (23).
Data Collection
We obtained data through the Partners Healthcare Research Patient Data Registry, a central clinical data warehouse for >1.8 million inpatients and outpatients designed for research and quality improvement (23–25). Approval for this study was granted by the Institutional Review Board at Brigham and Women’s Hospital and the need for informed consent was waived.
We obtained information on patient demographics, LOS, vital status at hospital discharge, discharge disposition, International Classification of Diseases (Ninth Revision, Clinical Modification) (ICD-9-CM) codes and Current Procedural Terminology (CPT) codes, diagnosis-related group codes, and inpatient costs. In addition, we obtained SCr measurements during and before hospitalization (performed on Roche Cobas autoanalyzers using a modified Jaffe reaction and an isotope dilution mass spectroscopy traceable standard) as well as the following initial laboratory values during hospitalization: hemoglobin, platelet count, white blood cell count, bicarbonate, BUN, total bilirubin, albumin, and serum sodium. Data regarding costs (excluding professional fees) were obtained from the hospital’s TSI database (Transition Systems, Boston, MA), an activity-based costing system that evaluates costs based on actual resources utilized in performing a specific activity. From this database, we obtained charges, actual variable costs, actual fixed costs, actual direct variable costs, and actual direct fixed costs. For the purposes of these analyses we used total actual costs, which were obtained by summing actual variable costs and actual fixed costs (26). We identified concurrent diseases, comorbid conditions (27), and procedures using ICD-9-CM and CPT codes (Supplemental Tables 2 and 3).
Definition and Staging of AKI
We defined and staged AKI according to SCr-based criteria per the KDIGO, RIFLE, AKIN, and CK criteria; urine output data were not available. We used all available SCr measurements along with date- and time-stamps to adhere specifically to the four definitions and staging systems (Table 1 and Supplemental Table 4). Patients with no SCr measurements during hospitalization were classified as not having AKI. Baseline SCr for KDIGO, AKIN, and RIFLE criteria was defined as the lowest SCr measurement during hospitalization (18) or, if available, the arithmetic mean of all outpatient SCr measurements 7–365 days before the index admission (28). In sensitivity analyses, we also explored the effects on incidence of alternate methods of assessing baseline SCr when missing (18), including multiple imputation (29). Baseline estimated GFR (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration study equation (30).
Table 1.
Diagnosis and staging criteria for AKI of RIFLE, AKIN, KDIGO, and CK definitions based on serum creatinine
| Classification | Definition for AKI | Stage | Serum Creatinine Criteria for AKI Staginga |
|---|---|---|---|
| RIFLE | Increase in SCr ≥50% within 7 d | Risk | To ≥1.5 times baseline |
| Injury | To ≥2 times baseline | ||
| Failure | To ≥3 times baseline or ≥0.5 mg/dl increase to at least 4.0 mg/dl | ||
| AKIN | Increase in SCr ≥0.3 mg/dl or ≥50% within 48 h | 1 | Increase of ≥0.3 mg/dl or to 1.5–1.9 times baseline |
| 2 | To 2–2.9 times baseline | ||
| 3 | To ≥3 times baseline or ≥0.5 mg/dl increase to at least 4.0 mg/dl or initiation of RRT | ||
| KDIGO | Increase in SCr ≥0.3 mg/dl within 48 h or ≥50% within 7 d | 1 | Increase in SCr ≥0.3 mg/dl within 48 h or to 1.5–1.9 times baseline |
| 2 | To 2.0–2.9 times baseline | ||
| 3 | To 3.0 times baseline or to at least 4.0 mg/dl or initiation of RRT | ||
| CK | Increase in SCr ≥0.3 mg/dl within 24 h or ≥0.5 mg/dl within 48 h | 1 | Increase in SCr ≥0.3 mg/dl within 24 h or ≥0.5 mg/dl within 48 h |
| 2 | Increase in SCr ≥0.5 mg/dl within 24 h or ≥1.0 mg/dl within 48 h | ||
| 3 | Increase in SCr ≥1.0 mg/dl within 24 h or ≥1.5 mg/dl within 48 h |
For patients meeting diagnosis criteria for AKI according to RIFLE, AKIN, or KDIGO, the stages based on percentage increase were determined by the ratio of peak SCr value obtained during hospitalization to baseline. RIFLE, Risk Injury Failure Loss ESRD; AKIN, Acute Kidney Injury Network; KDIGO, Kidney Disease Improving Global Outcomes; CK, creatinine kinetics; SCr, serum creatinine; RRT, renal replacement therapy.
Urine output was not used, because records of hourly urine output were not available in the majority of patients.
Statistical Analyses
Analyses were performed with SAS software (version 9.2; SAS Institute, Cary, NC). Continuous variables were expressed as means ± SDs or medians with interquartile ranges (IQRs), and were tested by the t test, Wilcoxon rank sum test, ANOVA, or Kruskal–Wallis test, as appropriate. Categorical variables were described as proportions and were compared using the chi-squared test. We calculated the observed proportional agreement and Cohen’s weighted κ statistic, a statistic of inter-rater agreement (31), to assess agreement between AKI according to KDIGO and the three other definitions. For analyses of in-hospital mortality, LOS, and costs, we randomly selected one hospitalization per patient if the patient was admitted multiple times; all other analyses used hospitalizations as the unit of analysis.
Odds ratios (ORs) for in-hospital mortality across stages of AKI were estimated by fitting logistic regression models. To explore the extent of confounding, we fitted increasingly adjusted models, first adjusting for demographic variables and then additionally for the following a priori selected covariates: concurrent diseases, major procedures, diagnosis-related group weights, baseline eGFR, and initial laboratory results. Laboratory data were modeled as being above or below the median result in the study population, with a missing indicator variable. LOS and costs were modeled using multivariable quantile regression analyses, a statistical technique that permits estimation of how the conditional median, or other quantiles (e.g., 10th percentile, 90th percentile), of a dependent variable y changes with an independent variable x. Unlike ordinary least-squares regression, quantile regression does not assume normality or homoscedasticity (32,33). Two-tailed P values <0.05 were considered significant.
Results
The study cohort included 25,859 patients with a total of 31,970 hospitalizations between January 1, 2010 and December 31, 2010. The median age was 54 years (IQR, 36–68); 64.7% were women, and 72.9% were white (Table 2). The median LOS among the 31,970 hospitalizations was 4 days (IQR, 2–7). SCr was measured more than once in 63.2% of hospitalizations, only once in 13.0%, and not at all in 23.8%. Those with one or no SCr measurements during hospitalization were younger (median 36 years versus 62 years; P<0.001), had shorter LOS (median 2 days versus 5 days; P<0.001), and were more likely to be admitted for childbirth (52.8% versus 2.8%; P<0.001). Among those with two or more SCr measurements during hospitalization, the median frequency of SCr measurements was 1.3 per day. A total of 167 patients were treated with RRT for AKI during hospitalization.
Table 2.
Demographic and clinical characteristics according to the development of AKI by the KDIGO definition during hospitalization
| Characteristic | No AKI | All AKI Stagesa | Stage 1 | Stage 2 | Stage 3 |
|---|---|---|---|---|---|
| Participants, n | 26,122 | 5848 | 4148 | 1001 | 699 |
| Demographic data | |||||
| Age, yr | 50 (35–66) | 64 (52–75) | 64 (53–75) | 62 (52–72) | 64 (52–74) |
| Men, % | 31.9 | 50.4 | 50.3 | 46.0 | 57.1 |
| Race, % | |||||
| White | 71.5 | 79.4 | 79.6 | 79.6 | 78.0 |
| Black | 11.1 | 8.6 | 8.5 | 8.3 | 10.2 |
| Hispanic | 7.8 | 5.2 | 5.5 | 4.5 | 4.7 |
| Other | 9.7 | 6.8 | 6.5 | 7.6 | 7.2 |
| Laboratory values on admissionb | |||||
| Serum creatinine, mg/dl | 0.8 (0.7–1.0) | 1.0 (0.8–1.5) | 1.0 (0.7–1.3) | 1.1 (0.8–1.5) | 1.9 (1.0–3.6) |
| Hemoglobin, g/dl | 11.8 (10.4–13.0) | 11.1 (9.7–12.7) | 11.2 (9.9–12.7) | 11.1 (9.7–12.8) | 10.3 (8.9–11.8) |
| White blood cell count, 1000/μl | 8.7 (6.5–11.6) | 8.9 (6.3–12.8) | 8.7 (6.3–12.4) | 9.6 (6.5–14.1) | 9.3 (5.9–14.3) |
| Platelet count, 1000/μl | 245 (193–311) | 234 (167–315) | 236 (172–315) | 233 (158–319) | 220 (117–310) |
| BUN, mg/dl | 13 (10–18) | 19 (13–31) | 18 (12–27) | 20 (14–33) | 35 (19–63) |
| Sodium, mmol/L | 137 (135–139) | 137 (134–139) | 137 (134–139) | 137 (133–139) | 136 (133–139) |
| CO2, mmol/L | 25 (23–27) | 24 (22–27) | 25 (22–27) | 24 (21–27) | 22 (19–26) |
| Albumin, g/dl | 3.7 (3.3–4.0) | 3.5 (3.0–3.9) | 3.6 (3.1–4.0) | 3.4 (2.9–3.9) | 3.2 (2.8–3.7) |
| Total bilirubin, mg/dl | 0.4 (0.3–0.6) | 0.5 (0.3–0.8) | 0.5 (0.3–0.7) | 0.5 (0.3–0.8) | 0.5 (0.3–1.0) |
Results are presented as medians with interquartile ranges or column percentages. KDIGO, Kidney Disease Improving Global Outcomes.
P<0.001 for all comparisons between patients with AKI and without AKI.
The following indicate the laboratory values that were missing in patients without AKI and with AKI, respectively: hemoglobin, 31.1% and 1.4%; white blood cell count, 31.1% and 1.4%; platelet count, 31.1% and 1.4%; BUN, 30.5% and 1.1%; sodium, 33.7% and 1.5%; CO2, 33.7% and 1.5%; albumin, 62.4% and 26.1%; total bilirubin, 62.8% and 26.9%; and serum creatinine, 28.5% and 0.0%.
Incidence and Clinical Settings
AKI according to the KDIGO definition complicated 18.3% of all hospitalizations (5848 of 31,970 hospitalizations, 70.9% of which were stage 1, 17.1% stage 2, and 12.0% stage 3). Among those with two or more SCr measurements (n=20,209), the incidence of AKI was 28.8%; for those with available prehospitalization outpatient SCr measurements (n=10,552; median baseline SCr 0.9 mg/dl), the incidence of AKI was 24.6% compared with 33.4% (P<0.001) in those without outpatient SCr values, in whom the nadir SCr was used to define baseline (n=9657; median nadir SCr during hospitalization 0.7 mg/dl). Alternative methods to define baseline, such as back-calculation of SCr based on eGFR 75 ml/min per 1.73 m2 and multiple imputation (29), led to slightly lower estimates of incidence (16.7% and 17.0%, respectively, versus 18.3%).
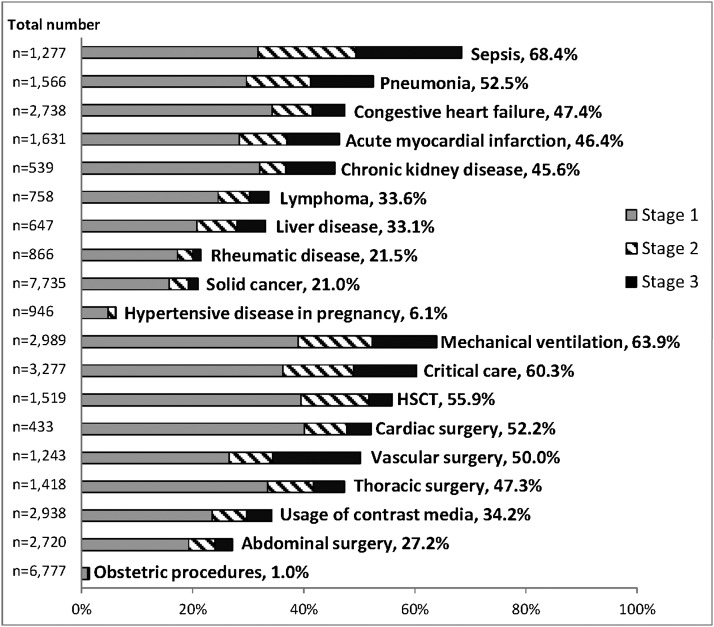
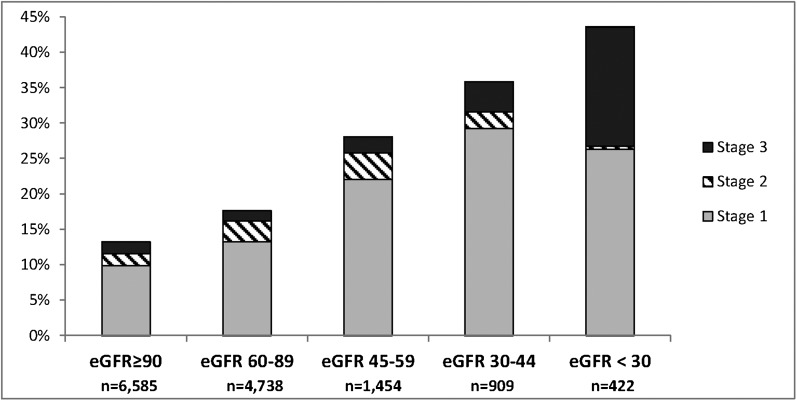
Compared with patients without AKI, those with AKI were older and more likely to be men (Table 2). The clinical settings with the highest incidence of AKI were sepsis (68.4%), mechanical ventilation (63.9%), critical care (60.3%), hematopoietic stem cell transplantation (55.9%), cardiac surgery (52.2%), and vascular surgery (50.2%) (Figure 1). In hospitalizations of patients with a known prehospitalization outpatient SCr baseline, the incidence of AKI differed substantially across baseline eGFR, from 13.2% in those with eGFR ≥90 ml/min per 1.73 m2 to 43.6% in those with eGFR <30 ml/min per 1.73 m2 (P<0.001) (Figure 2).
Figure 1.
Incidence of AKI according to the KDIGO definition across clinical settings. The percentage of hospitalizations complicated by AKI is shown for a number of clinical diagnoses and procedures, as identified by administrative codes. The total number of hospitalizations for each setting is shown on the left of the bar graph. Procedures and diagnoses were not mutually exclusive. HSCT, hematopoietic stem cell transplantation; KDIGO, Kidney Disease Improving Global Outcomes.
Figure 2.
Incidence of AKI by baseline eGFR according to the KDIGO definition. Baseline eGFR (in ml/min per 1.73 m2) was determined in 14,108 hospitalizations of 10,384 patients who had outpatient SCr measurements before admission. eGFR, estimated GFR; KDIGO, Kidney Disease Improving Global Outcomes.
In-Hospital Mortality
The overall in-hospital mortality rate (n=25,859, with random selection of one hospitalization from patients admitted more than once) was 2.2%, and varied substantially according to the presence and severity of AKI (0.6% in those without AKI, 5.3% in stage 1 AKI, 13.4% in stage 2 AKI, and 35.4% in stage 3 AKI; P<0.001). In-hospital mortality in AKI did not differ between patients who did and did not have prehospitalization outpatient SCr to define baseline (10.6% versus 10.1%; P=0.54). The highest in-hospital mortality rate in AKI was observed in sepsis (30.2% versus 6.4% without AKI), followed by pneumonia (27.2% versus 6.2% without AKI), acute myocardial infarction (21.7% versus 4.9% without AKI), and mechanical ventilation (20.3% versus 6.9% without AKI).
The association between AKI and in-hospital mortality persisted but was attenuated after additional adjustment in multivariable models for a number of demographic characteristics, clinical variables, baseline eGFR, and laboratory parameters (Table 3). In the fully adjusted model, AKI stages 1, 2, and 3 were associated with 2.0-, 3.4-, and 10.1-fold higher odds of death, respectively, compared with those without AKI.
Table 3.
Odds ratios for death in patients with and without AKI according to KDIGO
| Model | No AKI | All AKI Stages | Stage 1 | Stage 2 | Stage 3 |
|---|---|---|---|---|---|
| Unadjusted | 1.0 (ref) | 18.1 (14.9 to 22.0) | 8.9 (7.0 to 11.2) | 24.4 (18.6 to 32.1) | 86.4 (67.4 to 110.6) |
| 1 | 1.0 (ref) | 12.9 (10.6 to 15.8) | 6.0 (4.7 to 7.6) | 18.0 (13.7 to 23.8) | 65.6 (50.9 to 84.6) |
| 2 | 1.0 (ref) | 3.4 (2.6 to 4.3) | 2.4 (1.8 to 3.1) | 4.0 (2.8 to 5.6) | 13.3 (9.4 to 18.7) |
| 3 | 1.0 (ref) | 2.8 (2.2 to 3.6) | 2.0 (1.5 to 2.7) | 3.4 (2.4 to 4.9) | 10.1 (7.1 to 14.4) |
Results show odds ratios with 95% confidence intervals from logistic regression models. Model 1 is adjusted for age, sex, and race; model 2 for age, sex, race, procedures, diagnoses, and DRG weights; model 3 for age, sex, race, procedures, diagnoses, DRG weights, and laboratory values including estimated glomerular filtration rate. KDIGO, Kidney Disease Improving Global Outcomes; DRG, diagnosis-related group.
LOS and Cost
Hospital LOS was significantly higher in patients with AKI (3 days [IQR, 2–5] versus 10 days [IQR, 6–16] versus 3 days [IQR, 2–5]; P<0.001), and increased with increasing severity of AKI (n=25,859 patients). In multivariable quantile regression analysis, AKI was associated with a 2.8-day higher LOS and a $7082 increase in costs (P<0.001). Table 4 shows adjusted differences in LOS and cost modeled at the median, 10th, and 90th percentiles for all stages of AKI.
Table 4.
Excess lengths of stay and costs associated with AKI by the KDIGO definition according to quantile regression analyses
| Percentile | All AKI Stages | Stage 1 | Stage 2 | Stage 3 |
|---|---|---|---|---|
| Length of stay, d | ||||
| 10th | 1.5 (1.4–1.6) | 1.3 (1.2–1.5) | 2.0 (1.7–2.3) | 2.0 (1.5–2.5) |
| 50th | 2.8 (2.6–2.9) | 2.5 (2.3–2.7) | 4.2 (3.5–4.9) | 6.4 (5.3–7.5) |
| 90th | 6.2 (5.4–7.0) | 4.7 (4.1–5.4) | 10.5 (8.6–12.4) | 17.6 (14.3–21.0) |
| Costs, $1000 USD | ||||
| 10th | 3.5 (3.1–3.8) | 3.1 (2.8–3.5) | 4.2 (3.2–5.3) | 5.2 (3.8–6.5) |
| 50th | 7.1 (6.4–7.8) | 5.4 (4.7–6.1) | 15.2 (13.3–17.2) | 27.3 (22.6–32.0) |
| 90th | 18.7 (16.4–21.0) | 13.1 (10.9–15.4) | 35.4 (26.0–44.9) | 88.8 (72.6–105.0) |
Results are presented as the increase in length of stay and costs in patients with AKI versus without AKI according to the KDIGO definition, using multivariable adjusted quantile regression models adjusting for age, sex, race, procedures, diagnoses, laboratory values including estimated GFR, and diagnosis-related group weights. Values represent the increase in days or $1000 USD in those with AKI versus without AKI. KDIGO, Kidney Disease Improving Global Outcomes.
Discharge Disposition and AKI
Patients hospitalized with AKI were more likely to be discharged to rehabilitation facilities or other medical institutions than those without AKI (37.0% versus 12.3%; P<0.001). Discharge to hospice, skilled nursing facilities, and other hospitals was more common in successively higher stages of AKI (data not shown). A total of 5.4% of those with stage 3 AKI were discharged to hospice compared with 0.7% of those without AKI. Excluding those who died in the hospital, patients with AKI were significantly more like to be readmitted within 30 days of discharge (16.2% versus 8.7%; P<0.001).
Comparisons across Four Definitions of AKI
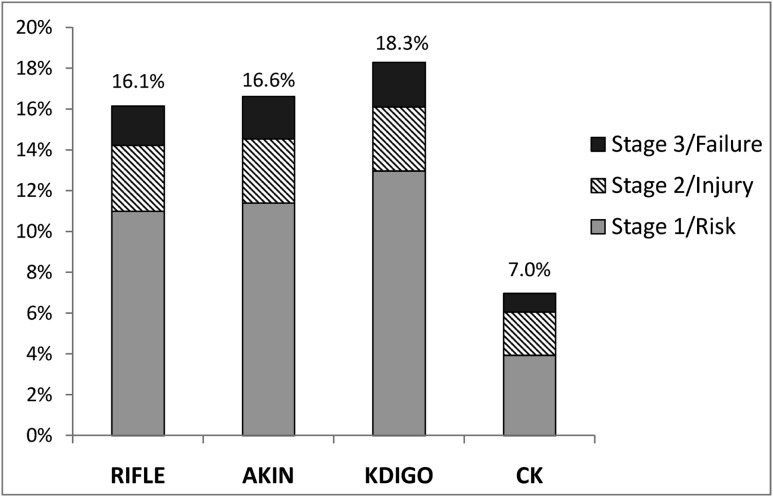
Figure 3 illustrates the comparative incidence of AKI according to the KDIGO, RIFLE, AKIN, and CK definitions and stages (n=31,970 hospitalizations). AKI incidence was highest according to the KDIGO definition (18.3%) followed by the AKIN (16.6%), RIFLE (16.1%), and CK (7.0%) definitions. Using patient-level analysis (n=25,859), we cross-tabulated the diagnosis, staging, and in-hospital mortality according to the KDIGO definition compared with the RIFLE, AKIN, and CK definitions (Table 5). All patients classified by the KDIGO definition as not having AKI were also classified as such by the RIFLE and AKIN definitions; concordance was 97.9% between the KDIGO and RIFLE definitions (weighted κ=0.96; 95% confidence interval [95% CI], 0.95 to 0.96) and 98.5% between the KDIGO and AKIN definitions (weighted κ=0.97; 95% CI, 0.97 to 0.98), with discordance due only to more frequent diagnosis or higher staging of AKI by the KDIGO definition than by the RIFLE or AKIN definitions. Concordance between the KDIGO and CK definitions was 87.4% (weighted κ=0.61; 95% CI, 0.59 to 0.62), with the large majority due to lower staging by the CK than KDIGO definition. Incidence, in-hospital mortality, LOS, and costs are shown in Supplemental Table 5 for all four definitions.
Figure 3.
Incidence and stages of AKI according to the RIFLE, AKIN, KDIGO, and CK definitions. The percentage of hospitalizations complicated by AKI is shown according to the four definitions and staging systems. AKIN, Acute Kidney Injury Network; KDIGO, Kidney Disease Improving Global Outcomes; CK, creatinine kinetics; RIFLE, Risk Injury Failure Loss ESRD.
Table 5.
Concordance of AKI designation
| Definition | Comparison | No AKI by RIFLE, AKIN, or CK | AKI Stage by RIFLE, AKIN, or CK | ||
|---|---|---|---|---|---|
| Risk/Stage 1 | Injury/Stage 2 | Failure/Stage 3 | |||
| KDIGO | RIFLE | ||||
| No AKI | 21,561 (0.6) | 0 (N/A) | 0 (N/A) | 0 (N/A) | |
| Stage 1 | 488 (3.9) | 2573 (5.6) | 0 (N/A) | 0 (N/A) | |
| Stage 2 | 1 (0) | 0 (N/A) | 722 (13.4) | 0 (N/A) | |
| Stage 3 | 20 (15.0) | 19 (42.1) | 20 (50.0) | 455 (35.4) | |
| KDIGO | AKIN | ||||
| No AKI | 21,561 (0.6) | 0 (N/A) | 0 (N/A) | 0 (N/A) | |
| Stage 1 | 365 (4.9) | 2696 (5.4) | 0 (N/A) | 0 (N/A) | |
| Stage 2 | 9 (11.1) | 0 (N/A) | 714 (13.4) | 0 (N/A) | |
| Stage 3 | 2 (0) | 12 (8.3) | 9 (0) | 491 (36.9) | |
| KDIGO | CK | ||||
| No AKI | 21,561 (0.6) | 0 (N/A) | 0 (N/A) | 0 (N/A) | |
| Stage 1 | 2114 (3.6) | 721 (8.0) | 217 (12.0) | 9 (33.3) | |
| Stage 2 | 390 (8.0) | 139 (18.0) | 147 (21.1) | 47 (21.3) | |
| Stage 3 | 126 (6.4) | 69 (34.8) | 150 (51.3) | 169 (43.2) | |
Shown are a cross-tabulation of patients with AKI according to KDIGO, RIFLE, and AKIN. Values in parentheses represent percent in-hospital mortality. KDIGO, Kidney Disease Improving Global Outcomes; AKIN, Acute Kidney Injury Network; CK, creatinine kinetics; RIFLE, Risk Injury Failure Loss ESRD; N/A, not applicable.
AKI with Low Baseline SCr
Among 5170 hospitalizations with baseline or nadir SCr <0.6 mg/dl and at least one inpatient SCr measurement, AKI incidence was 32.2% by the KDIGO and RIFLE definitions, 28.0% by the AKIN definition, and 4.2% by the CK definition. According to the KDIGO definition, 82.1% of the AKI cases were diagnosed based on a 50% increase in SCr over 7 days without meeting the 0.3 mg/dl absolute increase criterion. The incidence of AKI based on the KDIGO definition was even higher in 654 hospitalizations with baseline or nadir SCr <0.4 mg/dl (65.4%); in these patients with extremely low SCr, the risk of death did not differ between those with and without AKI (OR, 1.5; 95% CI, 0.5 to 4.9).
Discussion
In this large, single-center study of 31,970 hospitalizations during 2010, we found that approximately one of every six hospitalizations was complicated by AKI as defined by recent consensus definitions of AKI. The primary findings were that AKI is common in hospitalized individuals, particularly in sepsis, critical illness, and cardiovascular surgery—in which the incidence according to KDIGO exceeds 50%—and that higher stages of AKI are associated with graded increases for the risk of in-hospital mortality, excess LOS, higher costs, readmission, and discharge to skilled nursing facilities, hospice, or other medical institutions. The majority of cases of AKI according to KDIGO, the most recent consensus definition, were stage 1 (70.9%), with a smaller fraction of stages 2 and 3 (17.1% and 12.0%, respectively). Our estimates of incidence are comparable to those identified by other investigators using previous consensus definitions (5).
A major strength of our study is its internal validity, particularly with respect to the definition and staging of AKI. We used outpatient SCr values when available to establish a baseline (28), and all inpatient SCr values with corresponding date- and time-stamps in order to adhere specifically to the definitions according to KDIGO, RIFLE, AKIN, and CK. We excluded patients with ESRD and kidney transplants by careful review of electronic discharge summaries. Another key strength is the availability of additional laboratory values on admission and the analyses of LOS, costs, and postdischarge disposition and rehospitalization, which permitted a detailed examination of AKI in hospitalized individuals. Several of our findings deserve to be highlighted and placed into the context of previous studies.
Our finding that higher degrees of AKI severity are associated with increasing risks of in-hospital mortality is consistent with previous studies (1,4,5,15,34), and extends them in several ways. It should be noted that the association between AKI and in-hospital mortality is strongly confounded by a number of other variables. Previous studies have either not adjusted for comorbid conditions (4,5) or adjusted only for clinical conditions identified by ICD-9 codes (1). In our analyses, we found attenuation of the effect size when introducing additional variables in multivariable models of in-hospital mortality and other outcomes. Although AKI was still associated with mortality in the fully adjusted model, we found that diagnoses, procedures, and laboratory values were all confounders, and should be considered in future epidemiologic studies addressing the relationship between AKI and outcomes (35).
Our results showed substantial differences in the prognostic significance of AKI according to KDIGO in patients with low baseline SCr. We believe that the ability to define AKI by a 50% increase over baseline SCr may be driving the lower relative odds for death with AKI in patients with low SCr. Patients with low baseline SCr concentrations can be labeled as having AKI with very small, clinically insignificant absolute increases in SCr (e.g., 0.2 mg/dl in those with baseline SCr of 0.4 mg/dl). Indeed, we found that the incidence of AKI based on percentage increase criteria was markedly higher in those with SCr <0.6 mg/dl, and that mortality rates were not different in those with and without AKI with baseline SCr <0.4 mg/dl. Our findings suggest that a percentage increase of 50% to define AKI may be inappropriate at low baseline SCr.
Another important finding from our study is the association of AKI with resource utilization in hospitalized individuals. We found that all measures of resource utilization—including LOS, costs, readmission rates, and discharge to outside facilities—were higher in patients with AKI. Other investigators have found that costs and LOS are higher in AKI (1–3). Our findings provide an estimate of the actual magnitude of the effect of AKI. For example, adjusting for a number of other potential confounders, patients with the most severe stage of AKI according to the KDIGO definition have a median 6.4-day longer hospital stay and nearly $27,300 USD excess in costs, estimates that are comparable with the excess resource utilization observed in patients with hospital-acquired infections (36). The effect on the 90th percentile is even more striking, with 18.7-day longer hospital stay and approximately $88,800 USD excess in costs.
We compared KDIGO against three other definitions, including two previous consensus definitions, and found that the majority of hospitalized patients have concordant diagnoses, particularly between KDIGO versus AKIN and RIFLE. The incidence of AKI according to the KDIGO definition is the highest due to the addition of an absolute increase criterion (≥0.3 mg/dl over 48 hours) to the RIFLE definition and expansion of the time limit for percentage increase (≥50%) in the AKIN definition from 48 hours to 7 days. As a result, the higher incidence according to the KDIGO definition results primarily from more frequent identification of patients with mild (stage 1) AKI. The CK definition of AKI, which requires a 24-hour rather than 48-hour limit for an increase in SCr of ≥0.3 mg/dl, had the lowest observed incidence of AKI but identified a group of patients at higher risk of death than those with AKI according to the other three definitions. With respect to the comparative performance of the four definitions, we do not believe that comparisons of ORs, C statistics, or regression analyses provide insight into the clinical utility of AKI definitions and staging systems, which are not intended to serve as prognostic tools (37). We purposefully did not compare definitions by indices such as the net risk reclassification index, because this tool is used to compare nested models and is not appropriate for comparisons such as the ones here (38). We do believe the analyses are informative because they show that the KDIGO definition has the highest estimated incidence of AKI due to the identification of patients with stage 1 AKI. Whether increasing the frequency of diagnosis of stage 1 AKI will improve clinical care—due to increased attention to volume status, optimization of hemodynamics, and avoidance of nephrotoxins—or lead to unnecessary overdiagnosis of a condition of marginal clinical significance is a question that deserves further attention. Potential modifications to KDIGO could include caution in diagnosis of patients with low baseline SCr and a requirement of 24 hours for the increase in SCr of 0.3 mg/dl, as suggested in the CK definition.
Several limitations of our study deserve to be considered. As a single-center study from an academic hospital in the northeast United States, generalizability is limited. Incidence estimates, mortality rates, and concomitant diagnoses and procedures vary across hospital types and regions, although our estimates for AKI are in keeping with others from geographically disparate regions. We used administrative codes to identify medical conditions and procedures, which have limited accuracy, particularly for identification of diagnoses (23,39). Long-term mortality, CKD development, and CKD progression were not evaluated in this study but are important areas for future research (40).
Irrespective of the definition used, AKI is common in hospitalized patients and is associated with higher in-hospital mortality, longer LOS, and substantially higher resource utilization during and after hospitalization. The KDIGO definition of AKI provides an objective, graded system for the definition and staging of AKI, but the clinical significance of some cases of stage 1 AKI may be questionable, particularly in patients with low baseline SCr concentration.
Disclosures
S.S.W. reported serving as a consultant to CVS Caremark, BioTrends Research Group, Harvard Clinical Research Institute, and Takeda; providing expert testimony for GE Healthcare, Northstar Rx, and Salix; and receiving investigator-initiated grants from Otsuka, Merck, Genzyme, and Satellite Healthcare. Since completing work on this study, S.M.B. has become a full-time employee of DaVita Clinical Research.
Supplementary Material
Acknowledgments
X.Z. is supported by the China Scholarship Council. S.M.B. and S.S.W. were supported by the National Institutes of Health National Institute for Diabetes and Digestive and Kidney Diseases (DK079056 to S.M.B.; DK075941, DK093574, and U01DK085660 to S.S.W.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02730313/-/DCSupplemental.
References
- 1.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Parikh A, Shaw A: The economics of renal failure and kidney disease in critically ill patients. Crit Care Clin 28: 99–111, vii, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Lahoti A, Nates JL, Wakefield CD, Price KJ, Salahudeen AK: Costs and outcomes of acute kidney injury in critically ill patients with cancer. J Support Oncol 9: 149–155, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Wang HE, Muntner P, Chertow GM, Warnock DG: Acute kidney injury and mortality in hospitalized patients. Am J Nephrol 35: 349–355, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C: An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Mandelbaum T, Scott DJ, Lee J, Mark RG, Malhotra A, Waikar SS, Howell MD, Talmor D: Outcome of critically ill patients with acute kidney injury using the Acute Kidney Injury Network criteria. Crit Care Med 39: 2659–2664, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nin N, Lombardi R, Frutos-Vivar F, Esteban A, Lorente JA, Ferguson ND, Hurtado J, Apezteguia C, Brochard L, Schortgen F, Raymondos K, Tomicic V, Soto L, González M, Nightingale P, Abroug F, Pelosi P, Arabi Y, Moreno R, Anzueto A, VENTILA Group : Early and small changes in serum creatinine concentrations are associated with mortality in mechanically ventilated patients. Shock 34: 109–116, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Li SY, Chen JY, Yang WC, Chuang CL: Acute Kidney Injury Network classification predicts in-hospital and long-term mortality in patients undergoing elective coronary artery bypass grafting surgery. Eur J Cardiothorac Surg 39: 323–328, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Bouzas-Mosquera A, Vázquez-Rodríguez JM, Peteiro J, Alvarez-García N: Acute kidney injury and long-term prognosis after acute myocardial infarction. Arch Intern Med 169: 87, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, 2nd, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Wu VC, Huang TM, Lai CF, Shiao CC, Lin YF, Chu TS, Wu PC, Chao CT, Wang JY, Kao TW, Young GH, Tsai PR, Tsai HB, Wang CL, Wu MS, Chiang WC, Tsai IJ, Hu FC, Lin SL, Chen YM, Tsai TJ, Ko WJ, Wu KD: Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 80: 1222–1230, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafrance JP, Djurdjev O, Levin A: Incidence and outcomes of acute kidney injury in a referred chronic kidney disease cohort. Nephrol Dial Transplant 25: 2203–2209, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Workgroup : Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 8: R204–R212, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ricci Z, Cruz D, Ronco C: The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int 73: 538–546, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waikar SS, Bonventre JV: Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 20: 672–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidney Disease. Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 19.Lewington A, Kanagasundaram S: Renal Association Clinical Practice Guidelines on acute kidney injury. Nephron Clin Pract 118(Suppl 1):c349–c390, 2011 [DOI] [PubMed]
- 20.Palevsky PM, Liu KD, Brophy PD, Chawla LS, Parikh CR, Thakar CV, Tolwani AJ, Waikar SS, Weisbord SD: KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61: 649–672, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, Van Biesen W; Ad-Hoc Working Group of ERBP: A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: Part 1: Definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant 27: 4263–4272, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James M, Bouchard J, Ho J, Klarenbach S, LaFrance JP, Rigatto C, Wald R, Zappitelli M, Pannu N: Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis 61: 673–685, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa MA, Jaber BL: Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Fang MC, Chang Y, Hylek EM, Rosand J, Greenberg SM, Go AS, Singer DE: Advanced age, anticoagulation intensity, and risk for intracranial hemorrhage among patients taking warfarin for atrial fibrillation. Ann Intern Med 141: 745–752, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Rhee CM, Bhan I, Alexander EK, Brunelli SM: Association between iodinated contrast media exposure and incident hyperthyroidism and hypothyroidism. Arch Intern Med 172: 153–159, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Kaushal R, Bates DW, Franz C, Soukup JR, Rothschild JM: Costs of adverse events in intensive care units. Crit Care Med 35: 2479–2483, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Elixhauser A, Steiner C, Harris DR, Coffey RM: Comorbidity measures for use with administrative data. Med Care 36: 8–27, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Siew ED, Ikizler TA, Matheny ME, Shi Y, Schildcrout JS, Danciu I, Dwyer JP, Srichai M, Hung AM, Smith JP, Peterson JF: Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 7: 712–719, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siew ED, Peterson JF, Eden SK, Moons KG, Ikizler TA, Matheny ME: Use of multiple imputation method to improve estimation of missing baseline serum creatinine in acute kidney injury research. Clin J Am Soc Nephrol 8: 10–18, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleiss JL, Cohen J, Everitt BS: Large sample standard errors of kappa and weighted kappa. Psychol Bull 72: 323–327, 1969 [Google Scholar]
- 32.Koenker R, Hallock KF: Quantile regression. J Econ Perspect 15: 143–156, 2001 [Google Scholar]
- 33.Marrie RA, Dawson NV, Garland A: Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol 62: 511–, e1., 2009 [DOI] [PubMed] [Google Scholar]
- 34.Bagshaw SM, George C, Bellomo R, ANZICS Database Management Committe : A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 23: 1569–1574, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Katz MH: Multivariable Analysis: A Practical Guide for Clinicians and Public Health Researchers, New York, Cambridge University Press, 2011 [Google Scholar]
- 36.Roberts RR, Scott RD, 2nd, Hota B, Kampe LM, Abbasi F, Schabowski S, Ahmad I, Ciavarella GG, Cordell R, Solomon SL, Hagtvedt R, Weinstein RA: Costs attributable to healthcare-acquired infection in hospitalized adults and a comparison of economic methods. Med Care 48: 1026–1035, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Bellomo R, Kellum JA, Ronco C: Comment on “RIFLE classification in patients with acute kidney injury in need of renal replacement therapy” by Maccariello et al. Intensive Care Med 33: 1850–, author reply 1851–1852., 2007 [DOI] [PubMed] [Google Scholar]
- 38.Janes H, Pepe MS, Gu W: Assessing the value of risk predictions by using risk stratification tables. Ann Intern Med 149: 751–760, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quan H, Parsons GA, Ghali WA: Validity of information on comorbidity derived rom ICD-9-CCM administrative data. Med Care 40: 675–685, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Go AS, Parikh CR, Ikizler TA, Coca S, Siew ED, Chinchilli VM, Hsu CY, Garg AX, Zappitelli M, Liu KD, Reeves WB, Ghahramani N, Devarajan P, Faulkner GB, Tan TC, Kimmel PL, Eggers P, Stokes JB, Assessment Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury Study Investigators : The assessment, serial evaluation, and subsequent sequelae of acute kidney injury (ASSESS-AKI) study: Design and methods. BMC Nephrol 11: 22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.