Summary
Background and objectives
Pregnancy in ESRD is rare and poses substantial risk for mother and baby. This study describes a large series of pregnancies in women undergoing long-term dialysis treatment and reviews maternal and fetal outcomes. Specifically, women who had conceived before and after starting long-term dialysis are compared.
Design, setting, participants, & measurement
All pregnancies reported to the Australian and New Zealand Dialysis and Transplantation Registry from 2001 to 2011 (n=77), following the introduction of specific parenthood data collection, were analyzed.
Results
Between 2001 and 2011, there were 77 pregnancies among 73 women. Of these, 53 pregnancies were in women who conceived after long-term dialysis was established and 24 pregnancies occurred before dialysis began. The overall live birth rate (after exclusion of elective terminations) was 73%. In pregnancies reaching 20 weeks gestation, the live birth rate was 82%. Women who conceived before dialysis commenced had significantly higher live birth rates (91% versus 63%; P=0.03), but infants had similar birthweight and gestational age. This difference in live birth rate was primarily due to higher rates of early pregnancy loss before 20 weeks in women who conceived after dialysis was established. In pregnancies that reached 20 weeks or more, the live birth rate was higher in women with conception before dialysis commenced (91% versus 76%; P=0.28). Overall, the median gestational age was 33.8 weeks (interquartile range, 30.6–37.6 weeks) and median birthweight was 1750 g (interquartile range, 1130–2417 g). More than 40% of pregnancies reached >34 weeks’ gestation; prematurity at <28 weeks was 11.4% and 28-day neonatal survival rate was 98%.
Conclusions
Women with kidney disease who start long-term dialysis after conception have superior live birth rates compared with those already established on dialysis at the time of conception, although these pregnancies remain high risk.
Introduction
Pregnancy in women with advanced CKD or ESRD is a rare, challenging clinical scenario with significant maternal and fetal morbidity (1–3). Conception rates in women undergoing long-term dialysis have been estimated at 0.3 per 100 patient-years (4,5) or ranging from <1% to 7% of women (3). Recently, the relationship between dialysis intensity and outcomes has become clearer (6–15), with increased dialysis delivery becoming standard practice and nocturnal hemodialysis potentially providing superior fertility and outcomes (16–18). Recent reports suggest that results of these pregnancies are superior to those reported prior to the 1990s, with fetal survival rates of up to 70%–90% (3–7,9,10,16,19–21).
This high-risk group can be separated into two subsets: women established on long-term dialysis at the time of conception and women who progress to ESRD and require dialysis after conception. This differentiation is clinically relevant because of the important effect of residual renal function. Women who conceive before starting dialysis may have better outcomes (4,19), be managed with less intensive dialysis, and maintain a superior live birth rate (6).
We reviewed pregnancies in women receiving dialysis who were reported to the Australian and New Zealand Dialysis and Transplantation Registry (ANZDATA), with a focus on pregnancies reported after the introduction of a specific parenthood data collection form in 2001 that expanded the perinatal information available. Detailed analysis of pregnancy rates and brief analysis of outcomes from this registry up to 2008 have been previously published (5) but excluded women who were not receiving maintenance dialysis before pregnancy. This current study involves a larger cohort, specifically comparing pregnancy outcomes in women who conceived while established on long-term dialysis versus women who conceived before dialysis commenced.
Materials and Methods
Patients
The ANZDATA registry receives incident data on patients with ESRD from 100% of renal units in Australia and New Zealand, collected annually. We retrospectively reviewed data for women who received dialysis during pregnancy and required ongoing renal replacement therapy after delivery. Data from women requiring <90 days of therapy were not captured.
Data
Pregnancies were categorized as occurring in women established on dialysis at conception (conception on dialysis [COD]) or in women with CKD who conceived before commencing dialysis (conception before dialysis [CBD]). After September 2001, a specific parenthood data collection form was introduced. Demographic data, duration and modality of dialysis, estimated conception date, fetal birth date, birthweight, pre-eclampsia and gestational diabetes rates, pregnancy outcome, and survival at 28 days were collected. For multiple pregnancies, maternal data were taken at first pregnancy; fetal data were included for each pregnancy. Ethnicity categories were Caucasian, Indigenous Australian (aboriginal or Torres Strait Islander), and other. Primary renal disease categories were condensed to chronic GN, reflux nephropathy, or other (including diabetic nephropathy, inherited renal diseases, cortical necrosis, and unknown causes).
Outcomes
Pregnancy outcomes were live birth, surgical (elective) termination of pregnancy, spontaneous abortion at <20 weeks’ gestation, or stillbirth at >20 weeks’ gestation. Gestational age (GA) was calculated in weeks from the reported date of conception to fetal birth date. Premature delivery was defined as GA <37 weeks, with early preterm delivery defined as <34 weeks gestation. Fetal birthweight (BW) was missing for six live births, and GA was missing for four pregnancies. Data related to maternal complications of diabetes and pre-eclampsia were not available for 10 pregnancies. The registry does not collect data on obstetric history, residual renal function, estimated GFR (eGFR) at pregnancy diagnosis, BP, medication, fetal monitoring, or obstetric/delivery details.
Statistical Analyses
Continuous data were expressed as median with interquartile range (IQR) and were compared using Wilcoxon rank-sum tests. Categorical variables were compared using the Fisher exact test. Proportions of each pregnancy outcome were compared between groups using logistic regression. Rates per 1000 patient-years or years at risk were calculated from the cohort of women on dialysis aged 15–45 years during each era. BW was regressed against GA, with COD or CBD as an interaction term. BW and GA were compared with published national Australian centiles (22). P<0.05 was considered to represent statistically significant differences. Data were analyzed using Stata IC software, 12.1 (Stata Corp., College Station, TX).
Results
From the commencement of the registry in 1963–2011, 115 pregnancies were reported in women receiving dialysis any time during pregnancy. The number of pregnancies/year over the last decade was higher than previously, with 38 pregnancies in 12,143 patient-years (3.1 pregnancies/1000 patient-years at risk) from 1963 to 2000 and 77 pregnancies in 9125 patient-years (8.4 pregnancies/1000 patient-years at risk) from 2001 to 2011. Detailed analysis from this registry of increasing pregnancy rates in women with COD from 1966 to 2008 have been reported previously (5).
During the period 2001–2011, 77 singleton pregnancies occurred in 73 women. Two women had two pregnancies each and one woman had three pregnancies. The cohort consisted of 53 pregnancies in 49 women with COD and 24 pregnancies in 24 women with CBD. Two women had a transplant that failed during pregnancy.
Demographic Data
The characteristics of the cohort are outlined in Table 1. The median age at time of first conception was 30.1 years (IQR, 26.1–32.3 years), and women with COD had a significantly older age distribution than those with CBD. The median eGFR at commencement of dialysis in the CBD group was higher than the COD group and the general dialysis population (23). There were no other demographic differences between the CBD and COD groups. As expected in this younger patient cohort, GN and reflux nephropathy were the most common causes of renal failure. Only six women had diabetes mellitus; four had diabetic nephropathy as their primary renal disease. Other comorbid conditions included chronic lung disease (n=6), peripheral vascular disease (n=2), and coronary artery disease (n=1).
Table 1.
Demographic data for women receiving dialysis during pregnancy 2001–2011.
| Variable | All Women | CBD Group | COD Group | P Value |
|---|---|---|---|---|
| Patients (n) | 73 | 24 | 49 | |
| Median maternal age at conception (IQR) (yr) | 30.1 (26.1–32.3) | 29.9 (23.1–31.9) | 30.6 (26.2–34.4) | 0.30 |
| Maternal age at conception, n (%) | ||||
| <20 yr | 4 (6) | 4 (17) | 0 | 0.01 |
| 20–34 yr | 53 (76) | 18 (75) | 35 (76) | |
| ≥35 yr | 13 (19) | 2 (8) | 11 (24) | |
| Dialysis modality at conception, n (%) | ||||
| HD | 41 (56) | 41 (84) | ||
| PD | 8 (11) | 8 (16) | ||
| Dialysis modality at end of pregnancy, n (%) | ||||
| HD | 64 (88) | 23 (96) | 41 (84) | 0.26 |
| PD | 9 (12) | 1 (4) | 8 (16) | |
| Median time from commencing dialysis to conception (IQR) (mo) | — | — | 12 (6–33) | |
| Median time from conception to commencing dialysis (IQR) (mo) | — | 3.2 (5.1–2.1) | — | |
| Race, n (%) | 0.24 | |||
| Caucasian | 40 (55) | 14 (58) | 26 (53) | |
| Indigenous | 7 (10) | 4 (17) | 3 (6) | |
| Other | 26 (36) | 6 (25) | 20 (41) | |
| Primary kidney disease, n (%) | 0.75 | |||
| Chronic GN | 41 (56) | 12 (50) | 29 (59) | |
| Other | 15 (21) | 6 (25) | 9 (18) | |
| Reflux nephropathy | 17 (23) | 6 (25) | 11 (22) | |
| Median weight (IQR) (kg) | 62.1 (54–85) | 65.2 (59–87) | 60 (53–83) | 0.15 |
| BMI category, n (%) | 0.28 | |||
| <18.5 kg/m2 | 6 (8) | 0 (0) | 6 (13) | |
| 18.5–24.9 kg/m2 | 33 (46) | 10 (42) | 23 (49) | |
| 25–29.9 kg/m2 | 10 (14) | 5 (21) | 5 (11) | |
| 30–34.9 kg/m2 | 11 (15) | 5 (21) | 6 (13) | |
| ≥35 kg/m2 | 11 (15) | 4 (17) | 7 (15) | |
| Current or former smokers, n (%) | 29 (40) | 10 (42) | 19 (39) | 0.90 |
| Diabetes, n (%) | 6 (8) | 2 (8) | 4 (8) | 0.90 |
| Median eGFR at commencement of dialysis (IQR) (ml/min per 1.73 m2) | 12.4 (6–20) | 4.9 (4–6) | <0.01 |
Continuous data were analyzed using Wilcoxon rank-sum tests, and categorical variables were analyzed using Fisher exact test. In women with multiple pregnancies, data were taken from the first pregnancy.
CBD, conception before dialysis; COD, conception on dialysis; IQR, interquartile range; HD, hemodialysis; PD, peritoneal dialysis; BMI, body mass index; eGFR, estimated GFR.
Dialysis Modality
In women with COD, dialysis modality was rarely changed during pregnancy, and the reason for change was not recorded. Most women with COD received hemodialysis therapy at conception (45 pregnancies in 41 women), and only 1 woman changed to peritoneal dialysis during pregnancy. Eight women received peritoneal dialysis at conception, and only 1 changed to hemodialysis. The median time from commencing dialysis to the first conception was 11.6 months (IQR, 5.8–33.1 months). However, several pregnancies occurred after many years on dialysis. In 1 woman pregnancy occurred after 13 years of hemodialysis treatment.
All women who commenced dialysis during pregnancy (CBD) remained on renal replacement therapy over the long term, hence their inclusion in this study. Hemodialysis was the predominant modality for women starting renal replacement therapy during pregnancy. Of 24 women with CBD, 22 commenced hemodialysis and 2 commenced peritoneal dialysis (however, 1 was switched to hemodialysis later in pregnancy). The median time from conception to commencing dialysis was 3.2 months (IQR, 5.1–2.1 months).
Pregnancy Outcomes
From 2001 to 2011, the crude live birth rate was 60% (46 live births from 77 pregnancies). After exclusion of surgical terminations, the live birth rate in women proceeding with pregnancy was 73%. In pregnancies reaching ≥20 weeks, the live birth rate was 82%. The live birth rate in the CBD and COD groups is shown in Table 2. The surgical termination rate and natural early pregnancy loss rate were higher in women with COD. The proportion of live births was significantly higher in the CBD group than in the COD group. There were no significant differences in maternal characteristics, including age at conception, dialysis modality, comorbidity, body mass index, or primary renal disease between the CBD and COD pregnancies that had a live birth (data not shown).
Table 2.
Outcome of pregnancy according to timing of conception before dialysis or conception on dialysis, after exclusion of surgical terminations
| Outcome | CBD Group | COD Group | P Value |
|---|---|---|---|
| All pregnancies | 24 | 53 | |
| Surgical termination | 2 (8) | 12 (23) | 0.20 |
| Pregnancies (termination excluded) | 22 | 41 | |
| LBR (all) | 20 (91) | 26 (63) | 0.03 |
| LBR > 14 wk | 20 (91) | 26/38 (68) | 0.06 |
| LBR > 20 wk | 20 (91) | 26/34 (76) | 0.28 |
| Spontaneous abortion < 20 wk | 0 (0) | 10 (24) | 0.01 |
| Stillbirth > 20 wk | 2 (9) | 5 (12) | 0.53 |
| Median GA for live births (IQR) (wk) | 33 (29.3–38) | 33.9 (31.4–37.1) | 0.90 |
| Median BW for live births (IQR) (g) | 1650 (1013–2370) | 1750 (1284–2595) | 0.36 |
Unless otherwise noted, data are expressed as number (percentage). P values were calculated by stepwise comparisons of the proportions between CBD and COD using Fisher exact test. GA and BW were compared using Wilcoxon rank-sum tests. CBD, conception before dialysis; COD, conception on dialysis; LBR, live birth rate; LBR > 14 wk, live birth rate in pregnancies proceeding beyond 14 weeks’ gestation; LBR > 20 wk, live birth rate in pregnancies proceeding beyond 20 weeks’ gestation; GA, gestational age (weeks); IQR, interquartile range; BW, fetal birth weight.
Fourteen women had a surgical termination, 13 in the first trimester (median GA, 9.4 weeks [IQR, 8–10.4 weeks]). The surgical termination rate fell from 26% during 2001 to 6%–8% during 2007–2011. Ten women had a spontaneous abortion (miscarriage) at <20 weeks, with a median GA of 14 weeks (IQR, 7–15.3 weeks). There were seven stillbirths >20 weeks, with a median GA of 22.7 weeks (IQR, 21.3–24.9 weeks). There were no significant associations between maternal demographic variables and pregnancy outcome (data not shown). Dialysis modality at conception was not associated with outcomes in women with COD (Table 3).
Table 3.
Pregnancy outcomes in women with conception on dialysis according to dialysis modality at conception
| Outcome | PD | HD | P Valuea |
|---|---|---|---|
| Patients (n) | 8 | 45 | |
| Surgical termination n (%) | 1 (13) | 11 (24) | |
| After exclusion of terminations | |||
| Patients (n) | 7 | 34 | |
| Live birth n (%) | 5 (71) | 21 (62) | 0.70 |
| Spontaneous abortion n (%) | 2 (29) | 8 (24) | |
| Stillbirth n (%) | 0 (0) | 5 (15) |
PD, peritoneal dialysis; HD, hemodialysis.
Comparing distribution of all pregnancy outcomes between PD and HD groups.
GA of Live Births
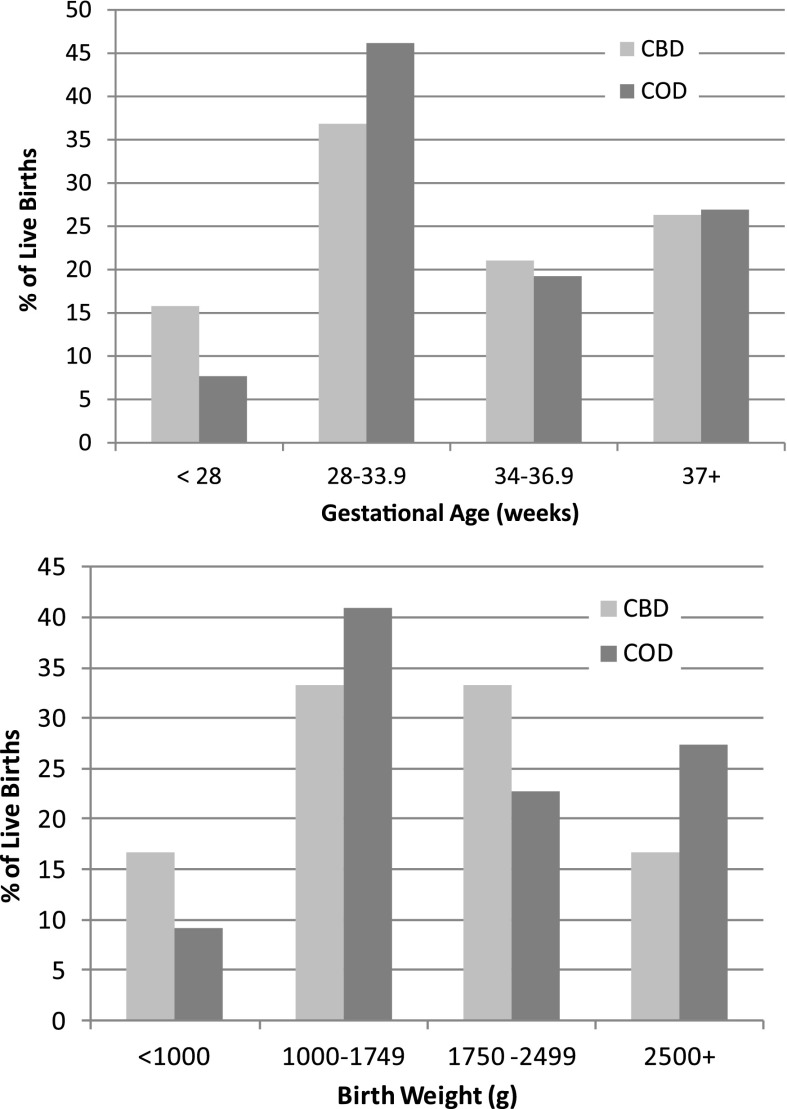
The distribution of GA is shown in Figure 1. The median GA for all live births was 33.8 weeks (IQR, 30.6–37.6 weeks). Of note, 66% of babies were born after 32 weeks’ gestation, 41% were born after 35 weeks’ gestation, and 25% were born after 38 weeks’ gestation. Extreme prematurity (GA < 28 weeks) occurred in 11% of pregnancies. GA was similar for CBD and COD groups (Table 2).
Figure 1.
Distribution of gestational age and birth weight of live births according to timing of conception before dialysis (CBD) or conception on dialysis (COD). The distribution of gestational ages and birth weights did not significantly differ between the CBD and COD cohorts in any age or weight category. CBD, conception before dialysis; COD, conception on dialysis.
BW of Live Births
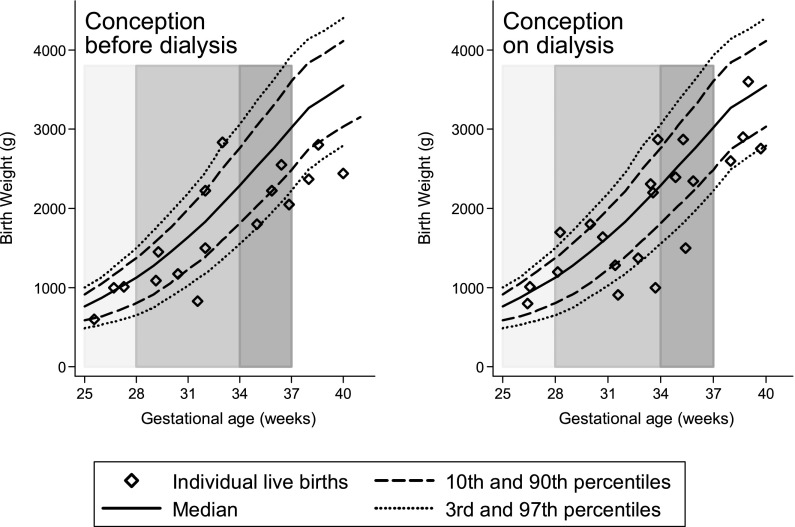
The BW distribution is shown in Figure 1. The median BW for all live births was 1750 g (IQR, 1130–2417 g), with no significant difference in BW between COD and CBD pregnancies (Table 2). As expected, BW increased significantly with increasing GA (P<0.01), but the relationship was not influenced by COD or CBD (P=0.58). BW and GA of live births were compared with average national Australian centiles, demonstrating that nearly half of the babies were born near or below the 10th centile (Figure 2).
Figure 2.
Fetal birth weight according to gestational age, compared with national Australian centiles (22). Gray bars show cutoffs at 28 weeks’, 34 weeks’, and 37 weeks’ gestation.
Deaths and Maternal Complications
Three neonatal deaths within 28 days were reported, but the cause of death was not available. Gestational diabetes was reported in only 1 pregnancy. Pre-eclampsia was reported in 15% of pregnancies; 8 of these 10 pregnancies resulted in a live birth.
At the latest survey, 28 women had received transplants, with 78% from deceased donors. No maternal deaths were related to pregnancy, but 4 women died during follow-up, with time from parenthood event to death ranging from 2.2 to 8.2 years. The causes of death were lung infection, withdrawal of dialysis due to comorbid conditions, cardiac arrest, and cerebrovascular accident.
Discussion
Pregnancy in women with CKD or ESRD remains a high-risk scenario. However, the once-dismal prognosis has improved substantially and more optimistic outcomes can be expected (2,3,6,11). Here we present one of the largest registry series of pregnancies in women requiring dialysis, with focus on the cohort from 2001 to 2011, providing previously unreported data on women with conception before dialysis who remained on dialysis after pregnancy.
The crude live birth rate for the 2001–2011 cohort was 60% for all pregnancies and 73% after exclusion of terminations. Women with CBD had superior live birth rates compared with those already established on dialysis at conception (91% versus 63%), even though both groups required ongoing renal replacement therapy after pregnancy. Our findings reflect other large studies demonstrating live birth rates or infant survival rates of 40%–79% in women with COD and 74%–93% in women with CBD (4,6,19). The differing live birth rates between CBD and COD cohorts were influenced by rates of early pregnancy loss. The COD group had higher rates of surgical termination and spontaneous abortion. This could reflect reporting bias because women who had pregnancy loss (or any pregnancy outcome) before commencing dialysis are not included in this study even if they require dialysis later in life. However, in the COD cohort, most spontaneous abortions occurred between 14 and 20 weeks’ gestation. The mean gestational age at which dialysis was commenced in the CBD group was 3.2 months; therefore, second-trimester pregnancy losses were likely to be captured equally in both groups. Comparison of the live birth rate in pregnancies reaching ≥14 weeks and ≥20 weeks suggests that the longer the pregnancy progresses, the smaller the difference between live birth rates in the CBD and COD groups and the better the chances of live birth.
Elective termination of pregnancy does not represent natural pregnancy loss, and inclusion in the denominator affects the live birth rate calculation and estimation of pregnancy success. Therefore, we analyzed live birth rates including and excluding termination of pregnancy, in keeping with other reports (4,6,9,19). Of note, the termination rate fell during the period of the study, but the reason for termination was unknown (possible reasons include mother’s wish, fetal abnormality, high-risk drug exposure, medical advice, and pregnancy complication). Women with CBD had lower surgical termination rates than the COD group. The reason is unclear but may represent a reporting bias as women with renal failure but not requiring renal replacement therapy who have early pregnancy loss or surgical termination will not be captured by ANZDATA, and these early pregnancy events may have occurred before dialysis was commenced.
Women with CBD may have higher residual renal function during pregnancy, which is important for pregnancy outcome. We did not have data on residual function of women with CBD, although they had higher eGFR at dialysis entry. Luders et al. reported that women with CBD had a greater urine output, required shorter dialysis time, and required a smaller dialysis dose to maintain predialysis urea levels; however, residual function was not formally quantified (6). Measuring urine output and creatinine clearance regularly to assess residual function, in addition to predialysis urea levels, may assist in titrating dialysis dose in these patients.
Factors crucial to successful pregnancy outcome include increased dialysis delivery to optimize biochemical measures, particularly urea (6–12,16,18), control of hypertension, correction of anemia (7,15), close fetal monitoring (8,10), and a multidisciplinary approach to patient care (2,3,8). An important limitation of ANZDATA data are limited detail regarding such individual patient care, which would assist in further interpreting the differing outcomes observed in our cohort. Details of dialysis schedules and delivery cannot be addressed in this study. Most women in this study received hemodialysis. Although pregnancy rates are lower in women treated with peritoneal dialysis (5), successful pregnancies in peritoneal dialysis patients in Australia have been previously reported (24). In our study, dialysis modality was not associated with outcome, although analysis was limited as the peritoneal dialysis cohort was small and pregnant women generally started on hemodialysis. Given the predominance of hemodialysis, it would be useful to know whether women received intensive hemodialysis (>20 hours/week), which is now standard care and strongly associated with better outcomes (3,11). ANZDATA collects dialysis data annually, and this does not document altered care during pregnancy. It would be important to note the duration and frequency of dialysis, reasons for changing dialysis modality, biochemical measures, weight adjustments, urine output and residual renal function, BP data, and incidence of hypotension and other dialysis complications. Obstetric management and infant follow-up beyond 28 days are also not collected but are of great interest. This type of information would require detailed case reviews from individual institutions, prospective collection of an expanded dataset by ANZDATA, or collection by a separate parenthood registry.
The number of pregnancies reported to the registry doubled in 2001–2011 compared with the era before 2000, confirming previous findings from ANZDATA showing increasing pregnancy rates over time (5). This may relate to a true increase in pregnancy rates in women with CKD/ESRD but could reflect increased reporting with the introduction of a specific ANZDATA parenthood form in 2001. Reporting bias is the main limitation of registry studies. Pregnancies lasting beyond the first trimester or reaching a birth outcome are more likely to be reported, whereas early pregnancy loss may be undiagnosed or under-reported. Despite such limitations, registry data remains a powerful source of information. There is a strong argument for the development of a separate registry for women receiving renal replacement therapy (including renal transplant) in pregnancy in Australia and New Zealand.
While these remain high-risk pregnancies, we demonstrated some encouraging findings. The incidence of fetal death after 20 weeks was 9%, and no babies died in utero beyond 24 weeks’ gestation. Extreme prematurity (GA <28 weeks) occurred in 11% of our cohort, but 41% of pregnancies reached GA of 35 weeks. Although prematurity is very likely, a large proportion do progress to a satisfactory duration. Other studies from the recent era report a median GA of 31–33 weeks (2,6,9,10,20), with superior GA in patients treated with nocturnal hemodialysis (16,18). Most infants born were of low BW (<2500 g), which correlated with prematurity, although a large proportion of babies were on or below the 10th centile of weight for GA. Our median BW was similar to that in other recent studies, which reported median or mean BW ranging from 1483 to 1765 g (6–10,20), but it remains substantially less than the mean BW (± SD) of 2417.5±657 g reported in pregnancies managed with nocturnal hemodialysis (16).
It is important to note that while CBD led to a higher live birth rate, no differences in BW or GA were noted between the CBD and COD groups. This in keeping with findings from Luders et al. (6) but in contrast to those of Chou et al., in which BW was superior in the CBD group (20). The reasons for this are unclear but again may relate to factors influencing pregnancy outcomes not recorded by the registry. Additionally, the diminishing discrepancy in live birth rates between the CBD and COD groups as gestational age advances suggests that once established beyond 20 weeks, these pregnancies are similar.
The rates of prematurity and low BW in dialysis pregnancies are concerning because these factors have been associated with numerous health issues (25). The long-term outcomes of infants born to women dialyzed during pregnancy are undefined. One preliminary study found children of dialyzed mothers are at risk of nephron loss or albuminuria (26). This is yet to be fully explored, and further studies are required.
Pre-eclampsia in women with CKD or receiving dialysis is a risk factor for adverse outcomes, associated with poorer GA and BW and higher rate of prenatal death (6). The pre-eclampsia rate in our population was 15%, with an 80% live birth rate. The diagnosis of pre-eclampsia is inherently difficult in women with pre-existing renal dysfunction, proteinuria, and hypertension. ANZDATA reports the occurrence of pre-eclampsia (yes/no), but the diagnostic criteria are at the discretion of the clinician completing the form. Therefore, it is possible that pre-eclampsia rates are underrepresented, and more detailed data could be collected in a specific pregnancy registry.
Finally, half of the pregnancies in women established on dialysis occurred within the first year of starting dialysis. Counseling all women of child-bearing age with CKD/ESRD regarding pregnancy options and contraception is important to avoid unplanned pregnancy. The timing of pregnancy in relation to CKD severity may influence pregnancy outcomes because of residual renal function. Deferring pregnancy until a renal transplant is achieved is the ideal option, although complications remain high (27). However, given prolonged waiting times for organs, this may occur after the reproductive years have passed. With studies such as ours demonstrating improved outcomes for women with CKD who commence dialysis in pregnancy, as well as the growing body of evidence regarding intensive dialysis regimens to improve outcomes (6–10,16), there may be a shift in the way women are counseled regarding pregnancy on dialysis, toward a less discouraging attitude (2). This may provide an avenue for motherhood in women who otherwise may not have an opportunity.
Disclosures
None.
Acknowledgments
The authors would like to thank all the renal units, staff and patients who contribute to the ANZDATA registry and Dr. Catherine Henry for preliminary data collation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Nevis IF, Reitsma A, Dominic A, McDonald S, Thabane L, Akl EA, Hladunewich M, Akbari A, Joseph G, Sia W, Iansavichus AV, Garg AX: Pregnancy outcomes in women with chronic kidney disease: A systematic review. Clin J Am Soc Nephrol 6: 2587–2598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Piccoli GB, Conijn A, Consiglio V, Vasario E, Attini R, Deagostini MC, Bontempo S, Todros T: Pregnancy in dialysis patients: Is the evidence strong enough to lead us to change our counseling policy? Clin J Am Soc Nephrol 5: 62–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hladunewich M, Hercz AE, Keunen J, Chan C, Pierratos A: Pregnancy in end stage renal disease. Semin Dial 24: 634–639, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Bagon JA, Vernaeve H, De Muylder X, Lafontaine JJ, Martens J, Van Roost G: Pregnancy and dialysis. Am J Kidney Dis 31: 756–765, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Shahir AK, Briggs N, Katsoulis J, Levidiotis V: An observational outcomes study from 1966-2008, examining pregnancy and neonatal outcomes from dialysed women using data from the ANZDATA Registry. Nephrology (Carlton) 18: 276–284, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Luders C, Castro MC, Titan SM, De Castro I, Elias RM, Abensur H, Romão JE, Jr: Obstetric outcome in pregnant women on long-term dialysis: A case series. Am J Kidney Dis 56: 77–85, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Asamiya Y, Otsubo S, Matsuda Y, Kimata N, Kikuchi K, Miwa N, Uchida K, Mineshima M, Mitani M, Ohta H, Nitta K, Akiba T: The importance of low blood urea nitrogen levels in pregnant patients undergoing hemodialysis to optimize birth weight and gestational age. Kidney Int 75: 1217–1222, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Haase M, Morgera S, Bamberg C, Halle H, Martini S, Hocher B, Diekmann F, Dragun D, Peters H, Neumayer HH, Budde K: A systematic approach to managing pregnant dialysis patients—the importance of an intensified haemodiafiltration protocol. Nephrol Dial Transplant 20: 2537–2542, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chao AS, Huang JY, Lien R, Kung FT, Chen PJ, Hsieh PC: Pregnancy in women who undergo long-term hemodialysis. Am J Obstet Gynecol 187: 152–156, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Bamberg C, Diekmann F, Haase M, Budde K, Hocher B, Halle H, Hartung J: Pregnancy on intensified hemodialysis: Fetal surveillance and perinatal outcome. Fetal Diagn Ther 22: 289–293, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Hou S: Pregnancy in women treated with dialysis: Lessons from a large series over 20 years. Am J Kidney Dis 56: 5–6, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Hou S: Pregnancy in women on dialysis: Is success a matter of time? Clin J Am Soc Nephrol 3: 312–313, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou S: Daily dialysis in pregnancy. Hemodial Int 8: 167–171, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Prasad S, Parkhurst D, Morton MR, Henning P, Lawton J, Bannister K: Increased delivery of haemodialysis assists successful pregnancy outcome in end-stage renal failure. Nephrology (Carlton) 8: 311–314, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Reddy SS, Holley JL: The importance of increased dialysis and anemia management for infant survival in pregnant women on hemodialysis. Kidney Int 75: 1133–1134, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Barua M, Hladunewich M, Keunen J, Pierratos A, McFarlane P, Sood M, Chan CT: Successful pregnancies on nocturnal home hemodialysis. Clin J Am Soc Nephrol 3: 392–396, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craig KL, Podymow T, Pauly RP: Intensifying renal replacement therapy during pregnancy: The role for nocturnal home hemodialysis. Int Urol Nephrol 42: 137–139, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Thompson S, Marnoch CA, Habib S, Robinson H, Pauly RP: A successful term pregnancy using in-center intensive quotidian hemodialysis. Hemodial Int 15[Suppl 1]: S59–S63, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Okundaye I, Abrinko P, Hou S: Registry of pregnancy in dialysis patients. Am J Kidney Dis 31: 766–773, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Chou CY, Ting IW, Lin TH, Lee CN: Pregnancy in patients on chronic dialysis: A single center experience and combined analysis of reported results. Eur J Obstet Gynecol Reprod Biol 136: 165–170, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Shahir AK, Briggs N, Katsoulis J, Levidiotis V: An observational outcomes study from 1966-2008, examining pregnancy and neonatal outcomes from dialysed women using data from the ANZDATA registry. Nephrology (Carlton) 18: 276–284, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Dobbins TA, Sullivan EA, Roberts CL, Simpson JM: Australian national birthweight percentiles by sex and gestational age, 1998-2007. Med J Aust 197: 291–294, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Grace BS, Clayton P, McDonald SP: Increases in renal replacement therapy in Australia and New Zealand: Understanding trends in diabetic nephropathy. Nephrology (Carlton) 17: 76–84, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Jefferys A, Wyburn K, Chow J, Cleland B, Hennessy A: Peritoneal dialysis in pregnancy: A case series. Nephrology (Carlton) 13: 380–383, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Luyckx VA, Brenner BM: Low birth weight, nephron number, and kidney disease. Kidney Int Suppl S68–S77, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Abou-Jaoude P, Dubourg L, Bessenay L, Pincon A, Jolivot A, Guebre-Egziabher F, Cochat P, Bacchetta J: What about the renal function during childhood of children born from dialysed mothers? Nephrol Dial Transplant 27: 2365–2369, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Richman K, Gohh R: Pregnancy after renal transplantation: A review of registry and single-center practices and outcomes. Nephrol Dial Transplant 27: 3428–3434, 2012 [DOI] [PubMed] [Google Scholar]