Abstract
Human peripheral blood monocytes become apoptotic following phagocytosis and killing of Staphylococcus aureus. Although this type of monocyte apoptosis is known to be initiated by Fas-Fas ligand (FasL) interactions, the downstream signaling pathway has not been determined. In this work the involvement of mitochondria and the kinetics of caspase-8 and caspase-3 activation after phagocytosis of S. aureus were studied. Caspase-8 activity was measured in cell lysates by using the fluorogenic substrate Ac-IETD-AFC. Active caspase-3 levels and mitochondrial membrane potential (Δψm) were measured in whole cells by flow cytometry using monoclonal antibodies reacting with activated caspase-3 and chloromethyl-X-rosamine, respectively. The results show that caspase-8 was activated shortly after phagocytosis of bacteria. Caspase-8 activation was followed by progressive disruption of Δψm, which is associated with the production of reactive oxygen intermediates. The irreversible caspase-8 inhibitor zIETD-FMK prevented the disruption of Δψm and the release of cytochrome c from S. aureus-exposed monocytes. Caspase-3 activation occurred following disruption of Δψm. These results strongly suggest that apoptosis of monocytes that have phagocytosed and killed S. aureus is driven by the Fas-FasL-initiated pathway, which is typical for type II cells.
Phagocytosis and killing of bacteria by phagocytic cells constitute an important mechanism of innate immunity. Although professional phagocytes such as neutrophils and monocytes/macrophages are able to recognize, phagocytose, and destroy most of the bacteria, some may escape from killing and survive inside the cells, which leads to their apoptosis (12, 13, 36). Furthermore, extracellular bacteria, although efficiently killed by phagocytes, may also trigger apoptosis of phagocytes (4, 9, 12, 15). Recently, it has been shown that Staphylococcus aureus-induced apoptosis of human monocytes is triggered by CD95 (Fas)-Fas ligand (FasL) interaction and can be prevented by the use of antioxidants (5). Phagocytosis of S. aureus triggers the release of biologically active FasL that acts mostly in an autocrine manner by interacting with surface-expressed Fas/Apo1 (CD95). Reactive oxygen intermediates (ROI) are presumably involved, as phagocytosis of bacteria is followed by reduction of glutathione, and pretreatment of monocytes with N-acetylcysteine effectively blocks apoptosis of phagocytes (5). S. aureus is also known to infect nonphagocytic cells such as endothelial or epithelial cells, which leads to their apoptosis (7, 17, 19, 34, 35, 38) and activation of caspase-8 and -3 (38). However, there is no evidence for the involvement of Fas-FasL interactions in these cases. To characterize further the mechanisms responsible for the induction of monocyte apoptosis following engulfment of S. aureus, we studied the involvement of mitochondria. In particular, we asked whether changes in mitochondrial membrane potential (Δψm) occur before or after the activation of effector caspases.
The present report provides evidence that shortly after the peak of caspase-8 activity, changes in Δψm, followed by caspase-3 activation, are observed. These events are characteristic of type II Fas signaling pathways (31).
MATERIALS AND METHODS
Monocytes.
Peripheral blood mononuclear cells (PBMC) were isolated from EDTA-treated blood from healthy donors by standard Ficoll/Paque (Pharmacia, Uppsala, Sweden) gradient centrifugation. Thereafter, PBMC were suspended in Hanks' balanced salt solution supplemented with 1% autologous plasma and were subjected to countercurrent centrifugal elutriation (with a Beckman JE-6B elutriation system equipped with a 5-ml Sanderson separation chamber) to obtain monocytes, as previously described (5). After separation, monocytes were washed once in cold RPMI 1640 medium (GIBCO, Grand Island, N.Y.) and kept on ice (5 × 106/ml) in RPMI 1640 culture medium supplemented with l-glutamine and 10% fetal calf serum (all reagents were from GIBCO) until use. Monocyte enrichment was confirmed by the typical scatter pattern in flow cytometry and expression of CD14 antigen (85 to 92% Tük 4-positive cells; DAKO, Glostrup, Denmark).
Bacteria.
S. aureus (ATCC 25923) was grown for 18 h on sugar broth, washed twice with a large volume of saline, and opsonized (for 30 min at 37°C) in the presence of 10% fresh human serum (pooled fresh human serum stored in aliquots at −70°C). After additional washing, the density of bacteria was measured spectrophotometrically at 540 nm, and the number of cells was calculated by using a previously determined standard curve (based on CFU counts). Finally, the concentration of bacteria was adjusted to 109/ml in phosphate-buffered saline (PBS). To enable the quantitative analysis of phagocytosis by flow cytometry, in some experiments bacteria were incubated for 2 h at 37°C in PBS containing 0.1% fluorescein isothiocyanate (FITC) (BHD Chemicals Ltd., Poole, England) before opsonization. After labeling and two washes, bacteria were opsonized as described above.
Phagocytosis.
Monocytes (106/ml) were incubated (for 30 min at 37°C under 5% CO2) in Falcon 2054 tubes (Becton Dickinson Labware Europe, Le Pont De Croix, France) with suspensions of opsonized FITC-labeled or unlabeled S. aureus (at a 1:20 or 1:50 ratio) in a total volume of 0.5 ml of RPMI 1640 medium without antibiotics. Then antibiotics (penicillin at 100 U/ml and streptomycin at 100 μg/ml; GIBCO) were added, and the cells were cultured for as long as 24 h. Alternatively, after a 30-min incubation of monocytes with bacteria at 37°C, 1 ml of ice-cold medium with antibiotics was added, cells were centrifuged (at 110 × g for 5 min) to separate phagocytic cells from free bacteria, and the pellet was resuspended in medium with antibiotics. As a control, monocytes were incubated without bacteria. In some experiments monocytes were preincubated for 2 h at 37°C with the antioxidant n-acetyl-l-cysteine (NAC; Sigma, St. Louis, Mo.) at a final concentration of 25 mM before exposure to bacteria (5, 30). To block NADPH oxidase activity, monocytes were preincubated for 2 h with 10 μM diphenylene iodonium (DPI) or 1 mM apocynin (both purchased from Sigma) (5, 24, 32). Thereafter, monocytes were incubated with S. aureus as described above.
Determination of apoptosis and cell viability by flow cytometry.
To determine the proportion of apoptotic of monocytes, an annexin V-binding assay was performed. Monocytes cultured alone or together with bacteria were collected at the indicated time points, washed with staining buffer (HEPES buffer containing 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, and 1.8 mM CaCl2 [pH 7.4]), and labeled with annexin V-phycoerythrin (PE) (Bender MedSystems, Vienna, Austria) for 15 min on ice to detect phosphatidylserine expression on the outer cell membrane layer. After a wash with staining buffer, the cells were analyzed on a FACSCAlibur flow cytometer using CellQuest software (BD Biosciences, San Jose, Calif.). In some experiments the apoptosis or viability of monocytes was determined by detection of propidium iodide uptake, which occurs early after phagocytosis of S. aureus by monocytes and correlates with DNA laddering (14).
Caspase-8 activity.
Caspase-8 activity was measured by enzymatic cleavage of the fluorogenic substrate N-acetyl-Ile-Glu-Thr-Asp-(7-amino-4-trifluoromethylcoumarin) (Ac-IETD-AFC; BD Biosciences Pharmingen, San Diego, Calif.) according to the manufacturer's instructions. Briefly, nonincubated monocytes (107/ml) and monocytes incubated (at 37°C under 5% CO2) for different times either in the medium alone or with S. aureus were pelleted by centrifugation (at 450 × g for 5 min at 4°C) and resuspended in 100 μl of ice-cold distilled water. Cells were lysed by four cycles of freezing and thawing, and the lysates were added to 300 μl of HEPES buffer (Pharmingen). To each sample 2.5 μl of Ac-IETD-AFC was added, and lysates were incubated for 1 h at 37°C. In order to confirm the substrate cleavage specificity, the caspase-8 inhibitor Ac-IETD-aldehyde (Pharmingen) was added to some lysates incubated with Ac-IETD-AFC. AFC release was monitored at an excitation wavelength of 400 nm and an emission wavelength of 480 to 520 nm (with a Hitachi F-4500 fluorescence spectrophotometer; Hitachi, Tokyo, Japan). To exclude the possibility of caspase-8 substrate cleavage by S. aureus, the samples containing bacteria alone were run in parallel.
Detection of active caspase-3.
Activation of caspase-3 was determined by flow cytometry using a PE-conjugated rabbit monoclonal antibody against active caspase-3 (Active caspase-3 antibody apoptosis kit 1; BD Biosciences Pharmingen) according to the manufacturer's instructions.
Measurement of Δψm.
To measure Δψm, monocytes cultured alone and exposed to bacteria at a 1:20 ratio were loaded with the cell-permeable reagent8-(4′-chloromethyl)-phenyl-2,3,5,6,11,12,14,15-octahydro-1H,4H,10H,13H-diquinolizino-8H-xanthylium chloride (CMXRos; Molecular Probes, Leiden, The Netherlands) by incubation for 15 min at 37°C at a concentration of 40 nM. Thereafter, cells were washed in PBS and analyzed by flow cytometry. Accumulation of CMXRos in the mitochondria corresponds to their Δψm, which decreases in apoptotic cells (23, 25). To determine the involvement of ROI in Δψm changes, the cells were preincubated with DPI (10 μM), apocynin (1 mM), or NAC (25 mM) as described above. In some experiments the irreversible caspase-8 inhibitor z-Ile-Glu(methylester)-Thr-Asp(methylester)-fluoromethylketone (zIETD-FMK; ICN Biomedicals, Livermore, Calif.), at a final concentration of 25 μM, was added to monocytes 30 min before admixture of bacteria.
Measurement of cytochrome c in culture supernatants.
Cytochrome c concentrations in culture supernatants were measured with a human cytochrome c MODULE SET (Bender MedSystems) modified by the use of an anti-cytochrome c capture antibody (Pharmingen). This modification allowed us to increase the level of detection up to 40 pg/ml (K. Barczyk et al., submitted for publication). Further procedures followed the manufacturer's instructions.
RESULTS
Phagocytosis of S. aureus triggers activation of caspase-8.
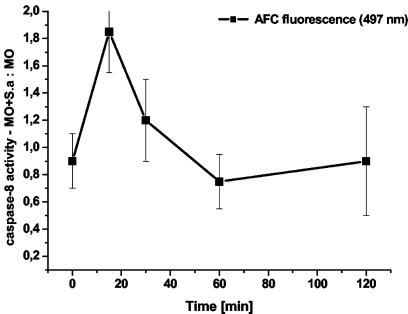
A previous report showed that Fas-FasL interactions trigger monocyte apoptosis as a result of phagocytosis and killing of S. aureus (5). Preliminary experiments also showed that phagocytosis of S. aureus triggers activation of caspase-8, which is the most proximal caspase of the death receptor-mediated apoptosis pathway. To characterize further the involvement of caspase-8, the kinetics of its activation was evaluated. The activation of caspase-8 occurred approximately 15 min after addition of bacteria to monocytes (Fig. 1). Cleavage was inhibited by addition of Ac-IETD-CHO to the cell lysates, indicating that the reaction was specific for caspase-8. Cleavage was not observed in control samples containing bacteria without monocytes (data not shown).
FIG. 1.
Caspase-8 activation in monocytes after phagocytosis of S. aureus. Ac-IETD-AFC was added to monocyte lysates prepared at various times after exposure to bacteria (MO+S.a). Control monocytes without bacteria (MO) were also sampled at the same time points. Ratios of fluorescence signals obtained in samples with S. aureus to those without S. aureus at the indicated time points are shown. Results obtained 15 min after exposure to bacteria are means ± standard deviations of five measurements from independent experiments; results for other time points are means ± standard deviations of measurements from three independent experiments.
Phagocytosis of bacteria leads to depolarization of monocytes' Δψm.
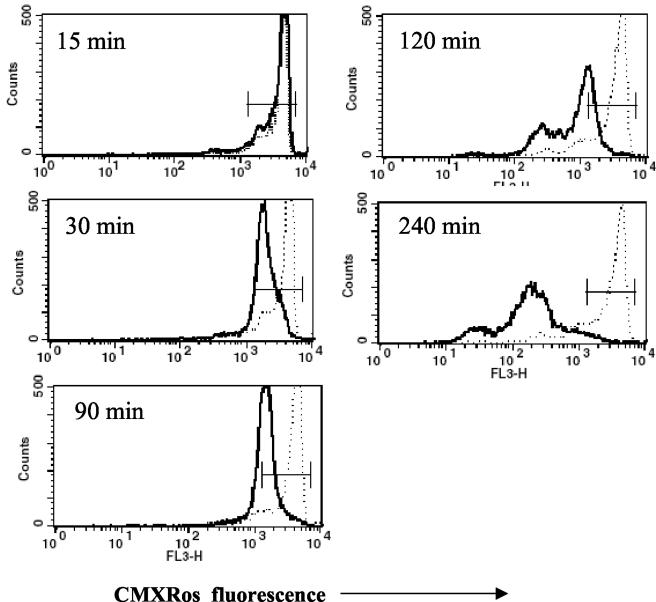
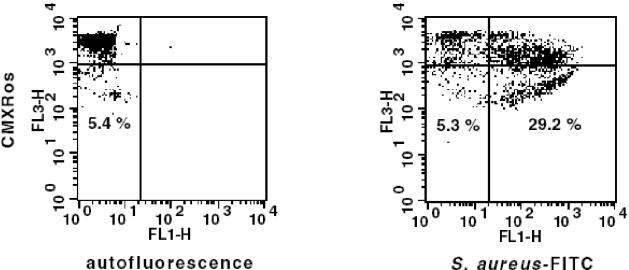
Many studies have suggested that a decline in Δψm may be an early event in apoptosis, including Fas-dependent signaling (8, 11, 33). To examine whether infection of monocytes with S. aureus causes a reduction in Δψm, cells were loaded with CMXRos at various time points after phagocytosis of bacteria and Δψm was analyzed by flow cytometry. Clear-cut reductions in Δψm were observed in monocytes 30 min after exposure to bacteria. The proportion of cells with low Δψm increased during culture, reaching about 80% cells with low Δψm around 4 h after phagocytosis (Fig. 2). To answer the question of whether observed changes in Δψm are associated with phagocytosis of bacteria, monocytes were allowed to phagocytose FITC-labeled S. aureus, which enabled discrimination of monocytes with and without bacteria by flow cytometry. At 2 h after addition of S. aureus, reduced Δψm was seen predominantly in those monocytes which had engulfed S. aureus, while the Δψm of bacteria-free cells remained unchanged (Fig. 3).
FIG. 2.
Evaluation of Δψm with the Δψm-sensitive probe CMXRos. Control (dotted lines) and S. aureus-exposed (solid lines) monocytes were incubated with CMXRos and analyzed by flow cytometry at the indicated time points. Note the early drop in Δψm, followed after 2 h by a dramatic reduction in the signal. Representative results (from one kinetic study out of four performed) are shown.
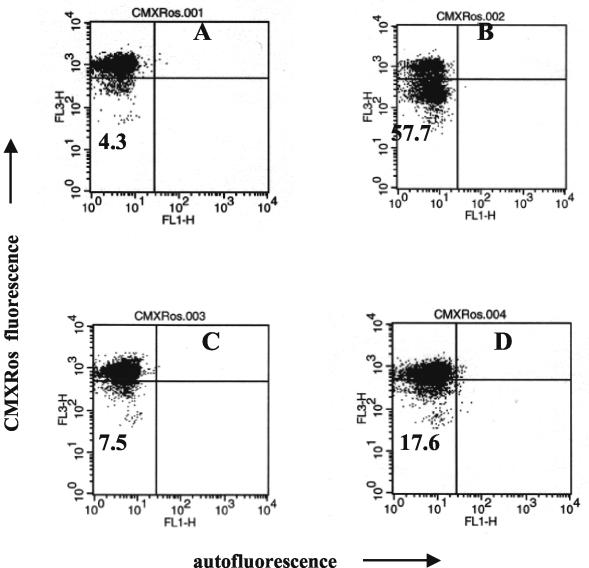
FIG. 3.
Reductions in Δψm are seen predominantly in monocytes that have engulfed bacteria. Monocytes were allowed to phagocytose FITC-labeled S. aureus bacteria, and their Δψms were evaluated by flow cytometry 2 h later. Quadrant statistics are shown to point at the proportions of cells with complete disruption of Δψm in both groups. Dot plots present results for control (left) and S. aureus-exposed (right) monocytes from a single experiment out of six performed.
Disruption of Δψm is associated with the production of ROI and activation of caspase-8.
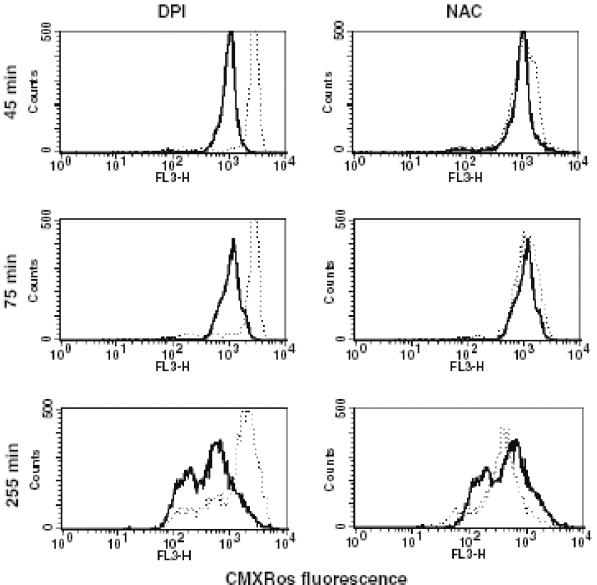
Mitochondrial membrane permeability is regulated by a redox equilibrium of ROI, pyridine nucleotides (NADH/NAD and NADPH/NADP), and the level of the reduced form of glutathione (GSH) (11). To test whether Δψm depolarization following phagocytosis of S. aureus is linked to the production of ROI, NAC, DPI, and apocynin, a specific inhibitor of NADPH oxidase (32), were used. NAC has been shown to act as an antioxidant by raising intracellular concentrations of cysteine and hence of GSH (11). DPI has frequently been used to inhibit ROI production mediated by flavoenzymes, particularly NADPH oxidase, and also inhibits mitochondrial ROI production (24). Monocytes were preincubated with these compounds for 2 h prior to addition of bacteria. NAC treatment only partially protected monocytes from Δψm depolarization (1/2-log-unit difference in CMXRos fluorescence intensity), while DPI almost completely blocked the disruption of Δψm observed after phagocytosis of bacteria (Fig. 4). Similarly, treatment of monocytes with apocynin almost completely prevented the decrease in Δψm in monocytes exposed to bacteria (Fig. 5). Therefore, it was concluded that NADPH oxidase and the production of ROI are essential for the reduction in Δψm.
FIG. 4.
Reductions in Δψm can be prevented by blocking of NADPH oxidase and by use of antioxidants. Δψms of S. aureus-exposed monocytes without pretreatment (solid lines) or pretreated with DPI or NAC (dotted lines) were evaluated at the indicated time points.
FIG. 5.
Reductions in Δψm can be prevented by blocking of NADPH oxidase. Shown is a dot plot analysis of monocytes probed with CMXRos 2 h after exposure to bacteria. (A and C) Monocytes cultured without bacteria; (B and D) monocytes exposed to bacteria. Monocytes for which results are shown in panels C and D were pretreated with apocynin. Quadrant statistics are shown to point at the proportions of cells with complete disruption of Δψm.
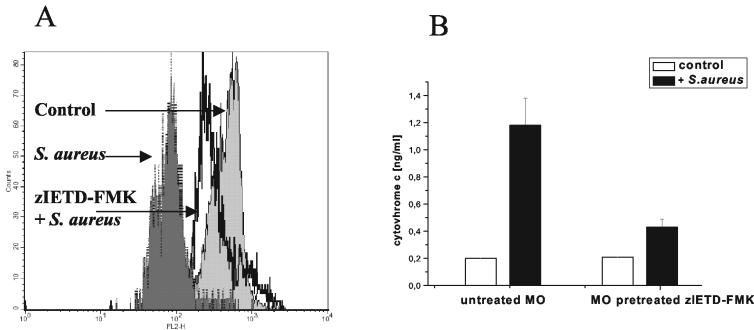
Because the blocking of NADPH oxidase with DPI reduces the release of FasL from S. aureus-exposed monocytes (5), the data described above suggest that Fas-FasL interactions and the resulting activation of caspase-8 are responsible for the disruption of Δψm. Further evidence was provided by the finding that the reduction in monocytes' Δψm, as measured by accumulation of CMXRos, was less pronounced when S. aureus-exposed cells were pretreated with the caspase-8 inhibitor zIETD-FMK (Fig. 6A). Such pretreatment also prevented the release of cytochrome c from monocytes (Fig. 6B). Since release of cytochrome c is considered the specific marker of apoptotic cell death (27), the data presented in Fig. 6 indicate that blocking of caspase-8 effectively protects monocytes from S. aureus-induced apoptosis.
FIG. 6.
Disruption of Δψm and release of cytochrome c by monocytes following phagocytosis of S. aureus does not occur in zIETD-FMK-pretreated cells. Monocytes were either preincubated with the caspase-8 inhibitor (25 μM) for 30 min or left untreated. Then bacteria were added. Δψm was measured by use of CMXRos after 60 min of culture. The concentration of cytochrome c in culture (106 monocytes/ml) was measured by enzyme-linked immunosorbent assay in supernatants harvested after 120 min of culture. (A) Overlay histogram shows fluorescence of control and zIETD-FMK-pretreated monocytes exposed to bacteria and of monocytes cultured alone, as indicated. Data from one representative experiment out of three performed are shown. (B) Cytochome c concentrations in culture supernatants. Means and standard deviations from three independent experiments are shown. MO, monocytes.
Disruption of Δψm precedes activation of caspase-3.
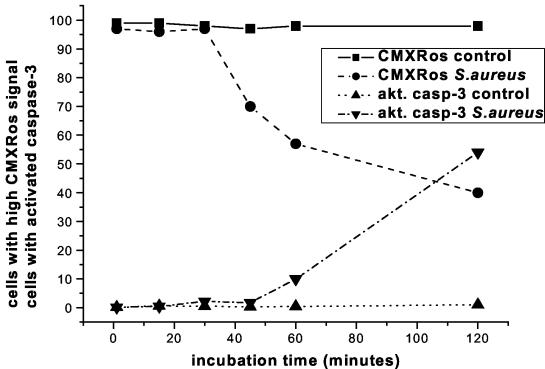
Although the changes in Δψm occurred shortly after caspase-8 activation and were blocked by a caspase-8 inhibitor, the possible involvement of the effector caspases could not be excluded. Therefore, to better characterize the pathways leading to apoptosis of monocytes which had engulfed bacteria, kinetic studies were performed to compare simultaneously changes in the Δψm and activation of caspase-3. Reductions in Δψm were observed in 30% of cells 45 min after the addition of bacteria, although at that time no activation of caspase-3 was detected (Fig. 7). At 60 min, 45% of cells showed reduced Δψm while 10% showed activated caspase-3. Finally, after 120 min of incubation, the proportions of cells with reduced Δψm and active caspase-3 were comparable (60%). These data clearly indicate that changes in Δψm precede activation of caspase-3 in monocytes exposed to S. aureus.
FIG. 7.
Time course of Δψm changes and of the appearance of activated caspase-3. Duplicate sets of samples of monocytes with and without bacteria were cultured and harvested at the indicated time points. In each pair, CMXRos was added to one sample 15 min before harvest while the second sample was stained for the presence of activated caspase-3 (akt. casp-3) at the indicated harvest time.
DISCUSSION
It is currently accepted that during Fas-induced apoptosis, caspase-8 becomes autoactivated in a death-inducing signaling complex (DISC) comprising procaspase-8, Fas, and the Fas-associated death domain protein. This can lead directly to a sequential activation of effector caspases, such as caspase-3 (2). On the other hand, a large body of evidence suggests that mitochondria are important participants in the pathways associated with cell damage (8). Many key regulators of apoptosis promote or inhibit the loss of mitochondrial integrity (23). Factors such as bongkretic acid, which prevents disruption of Δψm, are able to prevent several apoptotic events, including exposure of phosphatidylserine residues on the outer plasma cell membrane and oligonucleosomal DNA fragmentation (26). Changes in Δψm are considered a point of no return in the effector phase of apoptosis, since they lead to the release of cytochrome c from the mitochondrial intermembrane space into the cytoplasm (8, 27, 39).
The hierarchy of caspase activation and changes in Δψm appears complex. Previous reports showed that caspases may act upstream of mitochondria. Thus, inhibitors of serine proteases such as N-tosyl-1-lysyl chloromethylketone and degenerate tripeptidic inhibitors of interleukin-1-converting enzyme (ICE)-like proteases such as N-benzyloxycarbonyl-Val-Ala-Asp.fluoromethylketone (Z-VAD.fmk) prevent or retard glucocorticoid-induced Δψm disruption and subsequent apoptosis in various cell types (27, 29, 33). On the other hand, caspases may also act downstream of Δψm changes. Active caspase-8 can cleave Bid, yielding a truncated species that inserts into the outer mitochondrial membrane and facilitates cytochrome c release and Apaf-1-mediated activation of caspase-9, which in turn activates caspase-3 (23). To account for differences among individual cells in the apoptotic response to Fas ligation, it has been proposed that cells die by one of two signaling pathways (31). In type I cells, Fas receptor aggregation recruits signaling molecules to the plasma membrane via formation of the DISC, which promotes caspase-8 autoactivation, with subsequent activation of downstream effector caspases. In type II cells, DISC formation is strongly reduced relative to that in type I cells, and the small amounts of active caspase-8 are not sufficient to initiate a caspase cascade. However, the amount of active caspase-8 is sufficient to cleave Bid, which can then damage mitochondria, inducing cytochrome c release and caspase-9 activation via Apaf-1. It is suggested that both pathways contribute to different extents in various cell types (1).
In this work, evidence is presented that during monocyte apoptosis induced by phagocytosis and killing of S. aureus, caspase-8 activation precedes disruption of Δψm and caspase-3 activation occurs thereafter. Using flow cytometry analysis of Δψm and fluorometric measurement of caspase-8 activity, we have been able to show that caspase-8 activation occurs as early as 15 min after phagocytosis of S. aureus. These data are in agreement with earlier reports showing that activation of caspases is a rapid process that peaks at 15 to 30 min after Fas cross-linking (22). Thus, it precedes Δψm disruption, as quantified by the Δψm-sensitive dye CMXRos (25). Δψm collapse was noted in monocytes at 30 min after addition of bacteria to the cells. This is in keeping with the data of Susin et al. (33), who showed that Fas cross-linking provoked sequential activation of caspases, disruption of Δψm, and nuclear apoptosis with a kinetics similar to that observed in the present study. Disruption of Δψm. was prevented by the irreversible caspase-8 inhibitor zIETD-FMK, indicating that at least the early change in Δψm in monocytes that had engulfed bacteria was caused by caspase-8. Pretreatment of monocytes with zIETD-FMK also reduced the release of cytochrome c from monocytes exposed to bacteria. This indicates that the reduction in monocytes' Δψm is secondary to caspase-8 activation and that caspase-8 activation is not triggered by cytochrome c/Apaf-1-triggered caspase-9 (1). Furthermore, the blocking by zIETD-FMK of extracellular release of cytochrome c from monocytes, the specific marker of apoptotic cell death (27), indicates that under the conditions of the experiment, caspase-8 activation is necessary for the induction of monocyte apoptosis.
Disruption of monocyte Δψm after phagocytosis of S. aureus was also blocked by the use of antioxidants (NAC) or NADPH oxidase inhibitors (DPI and apocynin). Previously it has been shown that during phagocytosis of S. aureus, the release of FasL from monocytes is blocked by DPI (5). ROI may also lower the threshold of antiapoptotic defense within monocytes, which explains why monocytes that ingested bacteria preferentially show DNA strand breaks (4). ROI might also up-regulate FasL expression (6, 20) and modulate metalloproteinase activity (37). These findings and the data presented above strongly suggest the critical role of Fas-FasL interaction and the resulting caspase-8 activation in the disruption of Δψm. The time course of Δψm changes and of caspase-3 activation also point to the critical role of mitochondria in the effector phase of monocyte apoptosis after phagocytosis and killing of S. aureus. The majority of cells presented disrupted Δψm about 2 h after contact with S. aureus and, as judged from the intensity of the fluorescence signal (Fig. 2), from this time on, the damage to the mitochondrial membrane was more profound. This may suggest the additional damage of Δψm effected by caspase-3, which became activated around the same time. Such a possibility was recently documented by Ricci et al. (28), who showed a profound loss of Δψm in the presence of tBid and caspase-3.
Previously it was reported that after phagocytosis of S. aureus, monocytes release biologically active FasL and the blocking of CD95 prevents their apoptosis (5). Now we provide evidence for caspase-8 activation and its importance in the disruption of Δψm. S. aureus produces various toxins, of which alpha-toxin is known to induce apoptosis of different cell types, including monocytes (3, 10, 16, 18, 21). Activation of caspase-3, -8, and -9 has recently been shown in alpha-toxin-treated Jurkat cells and in PBMC (3, 10, 18). Furthermore, in Jurkat cells treated with alpha-toxin, cytochrome c release was induced both in intact and in isolated mitochondria, indicating an intrinsic pathway of caspase activation (3). Although we cannot exclude the involvement of alpha-toxin, the present data clearly differ from those reported for alpha-toxin, as induction of apoptosis by alpha-toxin does not require Fas-FasL interactions (3, 18). Apoptosis of monocytes treated with supernatants of S. aureus cultures was not inhibited by DPI, which prevents FasL release, although DPI was effective in blocking apoptosis induced by phagocytosis of S. aureus (5). Furthermore, in our hands, phagocytosis of the alpha-toxin-deficient S. aureus strain DU1090 by monocytes induces caspase-3 activation and disruption of Δψm (J. Pryjma, unpublished data). Finally, the differences may be due to our use of serum-opsonized bacteria for apoptosis induction, while washed bacteria were used in the other study (18). Under our experimental conditions, apoptosis was seen almost exclusively in those cells that had engulfed bacteria (4, 5; this report), and unopsonized S. aureus was much less effective at triggering monocyte apoptosis (4). It is of interest that unopsonized bacteria or bacteria opsonized with serum from hypogammaglobulinemic patients are much less efficiently taken up by monocytes and induce little ROI, as measured by chemiluminescence (Pryjma, unpublished).
In conclusion, our data provide further evidence for the crucial role of Fas-FasL interactions and hence of caspase-8 activation in triggering monocyte apoptosis following the phagocytosis and killing of S. aureus. This study also demonstrates the critical involvement of mitochondria between the times of caspase-8 and caspase-3 activation, indicating that monocyte apoptosis that occurs after phagocytosis of S. aureus follows the cascade of events typical for type II cells (1, 23, 31).
Acknowledgments
This work was supported by the State Committee for Scientific Research (grant no. 6P05A 123 21) and the Medical College of Jagiellonian University (grant W£/128/PKL/L). K.W. and J.P. were supported by the Foundation for Polish Science.
Editor: J. B. Bliska
REFERENCES
- 1.Adrain, C., E. M. Creagh, and S. J. Martin. 2002. Caspase cascades in apoptosis, p. 41-51. In M. Los and H. Walczak (ed.), Caspases: their role in cell death and cell survival. Kluwer Academic/Plenum Publishers, New York, N.Y.
- 2.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 3.Bantel, H., B. Sinha, W. Domschke, G. Peters, K. Schulze-Osthoff, and R. U. Jänicke. 2001. α-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155:637-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baran, J., K. Guzik, W. Hryniewicz, M. Ernst, H.-D. Flad, and J. Pryjma. 1996. Apoptosis of monocytes and prolonged survival of granulocytes as a result of phagocytosis of bacteria. Infect. Immun. 64:4242-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baran, J., K. Weglarczyk, M. Mysiak, K. Guzik, M. Ernst, H. D. Flad, and J. Pryjma. 2001. Fas (CD95)-Fas ligand interactions are responsible for monocyte apoptosis occurring as a result of phagocytosis and killing of Staphylococcus aureus. Infect. Immun. 69:1287-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer, M. K. A., M. Vogt, M. Los, J. Siegel, S. Wesselborg, and K. Schulze-Osthoff. 1998. Role of reactive oxygen intermediates in activation-induced CD95 (APO-1/Fas) ligand expression. J. Biol. Chem. 273:8048-8055. [DOI] [PubMed] [Google Scholar]
- 7.Bayles, K. W., C. A. Wesson, L. E. Liou, L. K. Fox, G. A. Bonach, and W. R. Trumble. 1998. Intracellular Staphylococcus aureus escapes the endosome and induces apoptosis in epithelial cells. Infect. Immun. 66:336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desagher, S., and J.-C. Martinou. 2000. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10:369-377. [DOI] [PubMed] [Google Scholar]
- 9.Dockrell, D. H., M. Lee, D. H. Lynch, and R. C. Read. 2001. Immune-mediated phagocytosis and killing of Streptococcus pneumoniae are associated with direct and bystander macrophage apoptosis. J. Infect. Dis. 184:713-722. [DOI] [PubMed] [Google Scholar]
- 10.Essmann, F., H. Bantel, G. Totzke, I. H. Engels, B. Sinha, K. Schulze-Osthoff, and R. U. Jänicke. 2003. Staphylococcus aureus alpha-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 10:1260-1272. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Checa, J. C. 2003. Redox regulation and signaling lipids in mitochondrial apoptosis. Biochem. Biophys. Res. Commun. 304:471-479. [DOI] [PubMed] [Google Scholar]
- 12.Fettucciari, K., E. Rosati, L. Scaringi, P. Cornacchione, G. Migliorati, R. Sabatini, I. Fetriconi, R. Rossi, and P. Marconi. 2000. Group B Streptococcus induces apoptosis in macrophages. J. Immunol. 165:3923-3933. [DOI] [PubMed] [Google Scholar]
- 13.Gao, L., and Y. Abu Kwaik. 2000. Hijacking of apoptotic pathways by bacterial pathogens. Microbes Infect. 2:1705-1719. [DOI] [PubMed] [Google Scholar]
- 14.Guzik, K., M. Bzowska, J. Dobrucki, and J. Pryjma. 1999. Heat-shocked monocytes are resistant to Staphylococcus aureus-induced apoptotic DNA fragmentation due to expression of HSP72. Infect. Immun. 67:4216-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hacker, H., C. Furmann, H. Wagner, and G. Hacker. 2002. Caspase-9/-3 activation and apoptosis are induced in mouse macrophages upon ingestion and digestion of Escherichia coli bacteria. J. Immunol. 169:3172-3179. [DOI] [PubMed] [Google Scholar]
- 16.Hameed, A., K. J. Olsen, M. Lee, M. G. Lichtenheld, and E. R. Podack. 1989. Cytolysis by Ca-permeable transmembrane channels. Pore formation causes extensive DNA degradation and cell lysis. J. Exp. Med. 169:765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamill, R. J., J. M. Vann, and R. A. Proctor. 1986. Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for post-adherence events in endovascular infections. Infect. Immun. 54:833-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haslinger, B., K. Strangfeld, G. Peters, K. Schulze-Osthoff, and B. Sinha. 2003. Staphylococcus aureus α-toxin induces apoptosis in peripheral blood mononuclear cells: role of endogenous tumour necrosis factor-α and the mitochondrial death pathway. Cell. Microbiol. 5:729-741. [DOI] [PubMed] [Google Scholar]
- 19.Hess, D. J., M. J. Henry-Stanley, E. A. Erickson, and C. L. Wells. 2003. Intracellular survival of Staphylococcus aureus within cultured enterocytes. J. Surg. Res. 114:42-49. [DOI] [PubMed] [Google Scholar]
- 20.Hugh, H., S. Strand, A. Grambihler, J. Galle, V. Hack, W. Stremmel, P. H. Kramer, and P. R. Galle. 1997. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J. Biol. Chem. 272:28191-28193. [DOI] [PubMed] [Google Scholar]
- 21.Jonas, D., I. Walev, T. Berger, M. Liebetrau, M. Palmer, and S. Bhakdi. 1994. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect. Immun. 62:1304-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Komoriya, A., B. Z. Packard, M. J. Brown, M. L. Wu, and P. A. Henkart. 2000. Assessment of caspase activities in intact apoptotic thymocytes using cell-permeable fluorogenic caspase substrates. J. Exp. Med. 191:1819-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 24.Li, Y., and M. A. Trush. 1998. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem. Biophys. Res. Commun. 253:295-299. [DOI] [PubMed] [Google Scholar]
- 25.Macho, A., D. Decaudin, M. Castedo, T. Hirsch, S. A. Susin, N. Zamzami, and G. Kroemer. 1996. Chloromethyl-X-rosamine is an aldehyde-fixable potential-sensitive fluorochrome for the detection of early apoptosis. Cytometry 25:333-340. [DOI] [PubMed] [Google Scholar]
- 26.Marchetti, P., M. Castedo, S. A. Susin, N. Zamzami, T. Hirsch, A. Macho, A. Haeffner, F. Hirsch, M. Geuskens, and G. Kroemer. 1996. Mitochondrial permeability transition is a central coordinating event of apoptosis. J. Exp. Med. 184:1155-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Renz, A., W. E. Berdel, M. Kreuter, C. Belka, K. Schulze-Osthoff, and M. Los. 2001. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood 98:1542-1548. [DOI] [PubMed] [Google Scholar]
- 28.Ricci, J. E., R. A. Gottlieb, and D. G. Green. 2003. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J. Cell Biol. 160:65-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rideout, H. J., E. Zang, M. Yeasmin, R. Gordon, O. Jabado, D. S. Park, and L. Stefanis. 2001. Inhibitors of trypsin-like serine proteases prevent DNA damage-induced neuronal death by acting upstream of the mitochondrial checkpoint and of p53 induction. Neuroscience 107:339-352. [DOI] [PubMed] [Google Scholar]
- 30.Såarnstrand, B. 1992. Is N-acetylcysteine a free radical scavenger in vivo? The effect of N-acetylcysteine in oxygen-induced lung injury. Eur. Respir. Rev. 2:11-15. [Google Scholar]
- 31.Scaffidi, C., S. Fulda, A. Srinivasan, C. Friesen, F. Li, K. J. Tomaselli, K. M. Debatim, P. H. Kramer, and M. E. Peter. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17:1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stolk, J., T. J. Hiltermann, J. H. Dijkman, and A. J. Verhoeven. 1994. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 11:95-102. [DOI] [PubMed] [Google Scholar]
- 33.Susin, S. A., N. Zamzami, M. Castedo, E. Daugas, H. G. Wang, S. Geley, F. Fassy, J. Reed, and G. Kroemer. 1997. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J. Exp. Med. 186:25-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tucker, K. A., S. S. Reilly, C. S. Leslie, and M. C. Hudson. 2000. Intracellular Staphylococcus aureus induces apoptosis in mouse osteoblasts. FEMS Microbiol. Lett. 186:151-156. [DOI] [PubMed] [Google Scholar]
- 35.Vann, J. M., and R. A. Proctor. 1987. Ingestion of Staphylococcus aureus by bovine endothelial cells results in time- and inoculum-dependent damage to endothelial cell monolayers. Infect. Immun. 55:2155-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weinrauch, Y., and A. Zychlinsky. 1999. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53:155-187. [DOI] [PubMed] [Google Scholar]
- 37.Weiss, S. J., G. Peppin, X. Ortiz, C. Ragsdalé, and S. T. Test. 1986. Oxidative autoactivation of latent collagenase by human neutrophils. Science 227:747-749. [DOI] [PubMed] [Google Scholar]
- 38.Wesson, C. A., J. Deringer, L. E. Liou, K. W. Bayles, G. A. Bohach, and W. R. Trumble. 2000. Apoptosis induced by Staphylococcus aureus in epithelial cells utilizes a mechanism involving caspases 8 and 3. Infect. Immun. 68:2998-3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamzami, N., P. Marchetti, M. Castedo, C. Zanin, J. L. Vayssiere, P. X. Petit, and G. Kroemer. 1995. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 181:1661-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]