Summary
Background and objectives
The term C3 glomerulopathy describes renal disorders characterized by the presence of glomerular deposits composed of C3 in the absence of significant amounts of Ig. On the basis of electron microscopy appearance, subsets of C3 glomerulopathy include dense deposit disease (DDD) and C3 glomerulonephritis (C3GN). The full spectrum of histologic change observed in C3 glomerulopathy has yet to be defined and pathologic predictors of renal outcome within this patient population remain largely unknown. This study thus characterized a large C3 glomerulopathy cohort and identified clinicopathologic predictors of renal outcome.
Design, setting, participants, & measurements
All patients with kidney biopsies fulfilling criteria for C3 glomerulopathy from two quaternary renal centers within the United Kingdom and Ireland between 1992 and 2012 were retrospectively reviewed. We recorded histologic, demographic, and clinical data and determined predictors of ESRD using the Cox proportional hazards model.
Results
Eighty patients with C3 glomerulopathy were identified: 21 with DDD and 59 with C3GN. Patients with DDD were younger, more likely to have low serum C3 levels, and more likely to have crescentic GN than patients with C3GN. Patients with C3GN were older and had more severe arteriolar sclerosis, glomerular sclerosis, and interstitial scarring than patients with DDD. Of 70 patients with available follow-up data, 20 (29%) progressed to ESRD after a median of 28 months. Age >16 years, DDD subtype, and crescentic GN were independent predictors of ESRD within the entire cohort. Renal impairment at presentation predicted ESRD only among patients with DDD.
Conclusions
Although detailed serologic and genetic data are lacking, this study nevertheless identifies important clinicopathologic distinctions between patients with DDD and C3GN. These include independent predictors of renal outcome. If replicated in other cohorts, these predictors could be used to stratify patients, enabling application of emerging mechanism-based therapies to patients at high risk for poor renal outcome.
Introduction
The complement system comprises a cascade of proteins integral to innate and acquired immunity (1). Dysregulation of any component of this pathway can result in the development of disease, including atypical hemolytic uremic syndrome (2) and age-related macular degeneration (3). The kidney is especially susceptible to complement-mediated injury, most commonly triggered by immune complex deposition and activation of the complement classic pathway (4). The term C3 glomerulopathy was recently coined to describe renal biopsy appearances characterized by the presence of glomerular deposits composed predominantly of C3 in the absence of significant amounts of Ig (5). The presence of C3 in the absence of Ig suggests activation of complement by antibody-independent pathways, typically the alternative pathway, and many patients with this type of renal lesion have evidence of genetic or acquired alternative pathway dysregulation (6). C3 glomerulopathy has been further divided into dense deposit disease (DDD) and C3 glomerulonephritis (C3GN) based on electron microscopy (EM) appearances. The underlying genetic defect has been identified in some hereditary forms of C3GN such as CFHR5 nephropathy (7,8). In contrast, most other reports of C3GN describe a diverse array of acquired and genetic abnormalities of complement regulation, in association with nonuniform histologic changes (6,9).
Detailed descriptions of renal biopsy findings in C3 glomerulopathy include a small case series (10) and reports on subtypes such as DDD and CFHR5 nephropathy (7,11,12). The full pathologic disease spectrum therefore remains to be characterized and histologic predictors of renal outcome are largely unknown.
To determine the spectrum of histologic change observed among patients with C3 glomerulopathy, we retrospectively reviewed all kidney biopsies from two quaternary renal centers within the United Kingdom and Ireland fulfilling pathologic criteria for C3 glomerulopathy. We analyzed clinical characteristics, pathologic findings, and long-term renal outcomes within this cohort and identified clinicopathologic predictors of renal outcome.
Materials and Methods
The reports of all renal biopsies referred to the West London Renal and Transplant Centre, Imperial College Healthcare National Health Service (NHS) Trust (February 1, 1992 to January 31, 2012), and the Department of Transplant, Urology, and Nephrology, Beaumont Hospital (January 1, 1995 to December 31, 2011) were manually reviewed. Patients satisfying pathologic criteria for a diagnosis of C3 glomerulopathy were identified. Patients were included if they demonstrated the presence of electron dense deposits on EM and glomerular staining for C3 without staining for IgG or IgA on immunohistology. Patients with IgM were included if the C3 was at least one order of magnitude more intense than the IgM staining. Patients were excluded if glomeruli were not available for EM or if EM images were of poor quality. Patients suspected of having postinfectious GN because of predominant hump-like subepithelial deposits on EM were only excluded if infective symptoms preceded or were coincident with clinical presentation and the patient made a full clinical and serologic recovery after resolution of the infectious episode without further disease relapse.
Light microscopy (LM), immunohistology, and EM findings were reviewed by one of two expert renal pathologists (H.T.C. or A.M.D.) at the time of the original reporting. To confirm study eligibility, EM images in conjunction with LM and immunohistology reports for all patients detected by initial screening were then centrally reviewed by a single renal pathologist with a particular interest in C3 glomerulopathy (H.T.C.). Recorded LM findings included the following: total number of glomeruli; number of sclerosed or necrotic glomeruli; number of cellular, fibrocellular, or fibrous crescents; extent of mesangial and endocapillary hypercellularity; presence of capillary wall double contours; percentage of cortical involvement by interstitial fibrosis and/or tubular atrophy; and the extent of arteriolar sclerosis. Recorded immunohistology findings included the strength of staining for C3, C1q, C4, and IgG, IgA, and IgM (graded 0–3+ on a semiquantitative scale), as well as the location and pattern of staining (linear, granular). Recorded EM findings included the location and morphology of electron dense deposits. Patients were labeled as having DDD if distinctive, highly electron dense, osmiophilic deposits were observed on EM, or as having C3GN if deposits did not fulfill these criteria. Crescentic GN was defined as GN with >50% of viable glomeruli containing crescents.
Demographic, clinical, and laboratory data at the time of the first renal biopsy were manually obtained from patient medical records and included age, sex, BP, hematuria, proteinuria, treatment, family history of renal disease, serum creatinine, serum albumin, serum C3 and C4, and antistreptolysin-O titer (ASOT). Renal status at the last clinical follow-up was recorded. Patients aged <16 years were regarded as children. Microscopic hematuria was defined as >5 red cells per high-power field on microscopic examination or positive blood by urine dipstick. Nephrotic-range proteinuria was defined as >3.5 g of protein in a 24-hour urine collection or a urine protein/creatinine ratio >350 mg/mmol. Significant renal impairment was defined as a serum creatinine >1.5 mg/dl.
All data were analyzed using STATA (version 10.0; StataCorp, College Station, TX). Categorical variables were expressed as the number and percentage and were compared using Fisher’s exact test. Continuous variables were expressed as the median and interquartile range and were compared using Wilcoxon rank-sum methods. Renal survival probabilities were determined using the Kaplan–Meier method and group comparisons for survival were performed using the log-rank test. Modeling for predictors of ESRD was conducted using Cox proportional hazards methods. A P value <0.05 was considered statistically significant.
Approval to perform this study was obtained from local ethics committees at both sites before study commencement.
Results
Disease Incidence
Eighty patients with C3 glomerulopathy (21 with DDD and 59 with C3GN) were identified. Five patients were previously described, comprising four patients with familial membranoproliferative GN (8) and one patient with persistent renal dysfunction and hypocomplementemia after streptococcal throat infection (13). No further familial cases were identified. C3 glomerulopathy was confirmed by native renal biopsy in 78 patients, and solely by transplant biopsy in two patents.
Within the Dublin cohort, 61 patients were identified among 4554 biopsies reviewed, representing an incidence of biopsy-proven C3 glomerulopathy with adequate tissue for diagnosis, including EM, of 1.34%. Assuming an estimated referral population of 2 million over the 17-year study duration, this equated to an annual incidence of biopsy-proven C3 glomerulopathy of 2 per million population. Within the London cohort, 19 patients were identified from a referral population of 500,000 over 14 years and 2 million over 6 years (after establishment of the Imperial College Healthcare NHS Trust), equating to an annual incidence of biopsy-proven C3 glomerulopathy of 1 per million population.
Clinical and Laboratory Features
At the time of the first diagnostic renal biopsy, the median age was 21 years, 32 patients (40%) were children, and 41 (51%) were male (Table 1). Patients typically presented with microscopic hematuria, proteinuria, and mild renal impairment (median creatinine 1.1 mg/dl), although 12 patients (15%) required dialysis.
Table 1.
Clinical and laboratory findings at the time of renal biopsy in patients with C3 glomerulopathy
| Category | Total (N=80) | DDD (n=21) | C3GN (n=59) | P Value |
|---|---|---|---|---|
| Age (yr) | ||||
| Median | 21 (10–47) | 12 (8–20) | 26 (12–53) | 0.002 |
| <16 | 32 (40) | 14 (68) | 18 (31) | |
| Male sex | 41 (51) | 9 (43) | 32 (54) | 0.45 |
| Race | ||||
| Caucasian | 73 (91) | 20 (95) | 53 (90) | 0.67 |
| Other | 7 (9) | 1 (5) | 6 (10) | |
| Native biopsies (n=71; 16 DDD, 55 C3GN) | 0.07 | |||
| None | 2 (3) | 1 (5) | 1 (2) | |
| 1 | 57 (71) | 11 (52) | 46 (78) | |
| 2 | 12 (15) | 4 (19) | 8 (13) | |
| Transplant biopsies (15 transplants; 7 DDD, 8 non-DDD) | 0.22 | |||
| None | 5 (33) | 1 (14) | 4 (50) | |
| 1 | 5 (33) | 4 (57) | 1 (12.5) | |
| 2 | 3 (20) | 2 (29) | 1 (12.5) | |
| ≥3 | 2 (14) | 0 | 2 (25) | |
| Treatment | ||||
| Steroids | 33 (41) | 11 (52) | 22 (37) | 0.10 |
| Dialysis | 12 (15) | 5 (24) | 7 (12) | 0.12 |
| Other immunosuppressiona | 14 (17.5) | 4 (19) | 10 (17) | 0.37 |
| ACEI/ARB | 42 (52.5) | 12 (57) | 30 (51) | 0.24 |
| Antibiotics | 11 (14) | 2 (9.5) | 9 (15) | 0.39 |
| No specific treatment | 12 (15) | 1 (5) | 11 (19) | 0.15 |
| Serum creatinine (mg/dl) | ||||
| Median | 1.1 (0.7–2.7) | 0.9 (0.7–2.3) | 1.4 (0.7–2.7) | 0.67 |
| >1.5 | 35 (44) | 6 (29) | 29 (50) | |
| Proteinuria (g/24 h) | ||||
| Median | 3.0 (0.95–4.5) | 3.0 (1.5–3.5) | 3.0 (0.7–4.5) | 0.72 |
| <500 mg | 13 (16) | 2 (9) | 11 (19) | |
| 500 mg to 3.5 g | 28 (35) | 9 (43) | 29 (32) | |
| >3.5 g | 35 (44) | 9 (43) | 26(44) | |
| Hematuria (n=78 total; 21 DDD, 57 C3GN) | 0.54 | |||
| None | 10 (13) | 3 (14) | 7 (12) | |
| Microscopic | 53 (68) | 16 (76) | 37 (65) | |
| Frank | 15 (19) | 2 (10) | 13 (23) | |
| Serum albumin (3.2–5.0 g/dl) | ||||
| Median (IQR) | 3.0 (2.4–3.5) | 3.0 (2.2–3.1) | 3.0 (2.6–3.5) | 0.23 |
| <3.0 | 33 (41) | 8 (38) | 25 (42) | |
| Hypertension (n=77 total; 20 DDD, 57 C3GN) | ||||
| Normotensive | 43 (56) | 8 (40) | 35 (61) | 0.18 |
| Hypertensive | 34 (44) | 12 (60) | 22 (39) | |
| C3 (n=69 total; 19 DDD, 50 C3GN) (0.65–1.65 g/L) | ||||
| Normal | 28 (41) | 2 (11) | 26 (52) | 0.003 |
| Low | 41 (59) | 17 (79) | 24 (48) | |
| C4 (n=60 total; 13 DDD, 47 C3GN) (0.16–0.6 g/L) | ||||
| Normal | 46 (77) | 11 (85) | 35 (74) | 0.25 |
| Low | 14 (23) | 2 (15 | 12 (36) | |
| ASOT (n=35 total; 7 DDD, 28 C3GN) | ||||
| High | 19 (54) | 3 (43) | 16 (57) | 0.53 |
| Normal | 16 (46) | 4 (57) | 12 (43) |
Data are presented as n (%) or the median (interquartile range). DDD, dense deposit disease; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; ASOT, antistreptolysin-O titer.
Other immunosuppressions include cyclophosphamide, mycophenolate mofetil, tacrolimus, and plasma exchange used alone and in combination.
Two patients had a monoclonal gammopathy at the time of the first renal biopsy. The dysproteinemia remained clinically silent in one patient and progressed to multiple myeloma many years later, after renal transplantation, in the other patient. No patient had viral hepatitis or relevant autoimmune disease. A majority of patients (59%) had low serum C3 levels and a minority of patients (23%) had low C4. Of the 35 patients in whom ASOT was measured, 19 (54%) had a raised ASOT, 4 (21.2%) of whom had a documented preceding respiratory tract infection.
Patients with DDD were significantly younger and more likely to have low serum C3 levels than patients with C3GN (Table 1). Otherwise, baseline clinical and laboratory characteristics were similar between groups.
Almost all patients received active treatment, including 42 (53%) who received angiotensin-converting enzyme inhibitor or angiotensin-II receptor blocker therapy, 33 (41%) who received steroids, and 14 (17.5%) who received other immunosuppression, including 5 treated with cyclophosphamide, 3 with mycophenolate mofetil (MMF), 2 with MMF and tacrolimus, 1 with rituximab, cyclophosphamide, and MMF, 2 with plasma exchange and cyclophosphamide, and 1 with plasma exchange and MMF.
Pathologic Features
The first native (n=78) or transplant (n=2) renal biopsy findings are summarized in Table 2. A diverse spectrum of pathologic patterns was observed. Membranoproliferative GN was predominant (55%) but not universal. Crescentic GN was more prevalent among patients with DDD than C3GN (19% versus 5%; P=0.05). Vascular disease and markers of chronicity, including glomerulosclerosis and interstitial fibrosis, were significantly more pronounced within the C3GN group.
Table 2.
Histopathologic findings in the C3 glomerulopathy cohort
| Feature | Total (N=80) | DDD (n=21) | C3GN (n=59) | P Value |
|---|---|---|---|---|
| Light microscopy findings | ||||
| Pattern of injury | ||||
| Mesangial proliferative GN | 17 (21) | 3 (14) | 14 (24) | 0.11 |
| Diffuse endocapillary proliferative GN | 12 (15) | 1 (5) | 11 (19) | |
| Crescentic GN | 7 (9) | 4 (19) | 3 (5) | |
| Membranoproliferative GN | 44 (55) | 13 (62) | 31 (52) | |
| Global sclerosis (% glomeruli) | ||||
| Absent | 37 (46) | 14 (66.5) | 23 (39) | 0.05 |
| <50 | 40 (50) | 6 (28.5) | 34 (58) | |
| >50 | 3 (4) | 1 (5) | 2 (3) | |
| Crescents present (%) | ||||
| None | 49 (61) | 9 (43) | 40 (68) | 0.05 |
| <50 | 24 (30) | 8 (38) | 16 (27) | |
| ≥50 | 7 (9) | 4 (19) | 3 (5) | |
| Interstitial fibrosis (%) | ||||
| None | 25 (31) | 14 (67) | 11 (19) | <0.001 |
| 1–25 | 30 (38) | 3 (14) | 27 (46) | |
| 26–50 | 17 (21) | 4 (19) | 13 (22) | |
| >50 | 8 (10) | 0 (0) | 8 (13) | |
| Arteriolar sclerosis | ||||
| None | 43 (54) | 18 (85.5) | 25 (42.4) | 0.003 |
| Mild | 21 (26) | 2 (9.5) | 19 (32.2) | |
| Moderate | 12 (15) | 1 (5) | 12 (20.4) | |
| Severe | 4 (5) | 0 (0) | 3 (5) | |
| Immunohistology | ||||
| C3 in mesangium | 6 (8) | 1 (5) | 5 (8.5) | 0.43 |
| C3 in capillary wall | 21 (26) | 8 (38) | 13 (22) | |
| C3 both | 53 (66) | 12 (57) | 41 (69.5) | |
| C3 and no IgM | 41 (51) | 7 (33) | 34 (58) | 0.02 |
| C3 and IgM trace | 8 (10) | 5 (24) | 3 (5) | |
| C3 and IgM 1+ | 31 (39) | 9 (43) | 22 (37) | |
| Electron microscopy findings | ||||
| Mesangial deposits | 57 (71) | 8 (38) | 49 (83) | <0.001 |
| Subepithelial humps | 28 (35) | 4 (19) | 24 (41) | 0.15 |
| Subepithelial deposits (any) | 39 (49) | 4 (19) | 36 (61) | <0.001 |
| Subendothelial deposits | 45 (56) | 3 (14) | 42 (71) | <0.001 |
| Intramembranous deposits, non-DDD | 28 (35) | 2 (9.5) | 29 (49) | <0.001 |
| Intramembranous deposits, DDD | 21 (26) | 21 (100) | 0 (0) | <0.001 |
| Deposits tubular basement membrane | 2 (3) | 2 (9.5) | 0 (0) | <0.001 |
| Deposits Bowman’s capsule | 2 (3) | 2 (9.5) | 0 (0) | <0.001 |
Data are presented as n (%). P values represent the differences between the DDD and C3GN subgroups. DDD, dense deposit disease.
Immunohistology showed that bright staining for C3 was evident within the mesangium and capillary wall in 66% of patients, in the capillary wall alone in 26% of patients, and in the mesangium alone in <10% of patients.
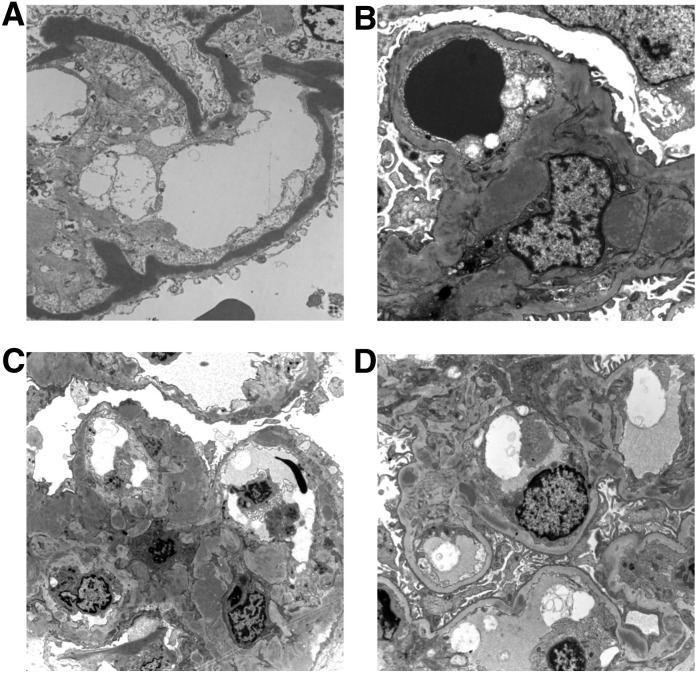
EM identified linear, hyperosmiophilic, intramembranous dense deposits occupying the lamina densa of the glomerular basement membrane in 21 patients, defining the DDD group (Figure 1). Intramembranous deposits were also found in C3GN biopsies, although they did not resemble classic DDD deposits (Figure 1). Subepithelial humps were more commonly observed within the C3GN group but this difference was not statistically significant (41% C3GN versus 19% DDD; P=0.15). All four patients with preceding respiratory infection had subepithelial humps on EM. Therefore, 24 of 28 patients (85.7%) with observed subepithelial humps had no documented preceding infection. Ten of the 19 (52.6%) patients with subepithelial humps and ASOT measured at the time of renal biopsy had a titer within the normal range. Subendothelial deposits were more common in the C3GN group (71% versus 14%; P<0.001). Tubular basement membrane and Bowman’s capsular basement membrane deposits were infrequent but exclusive to DDD.
Figure 1.
Electron micrographs showing a range of appearances that may be seen in C3 glomerulopathy. (A) A case of dense deposit disease showing the pathognomonic densely osmiophilic transformation of the glomerular basement membrane. (B) A case of C3GN showing poorly circumscribed electron dense material expanding the mesangium and some small deposits of similar material within the capillary basement membrane. (C) A case of C3GN showing expansion of the mesangium by electron dense material and irregular thickening of the glomerular capillary basement membrane by intramembranous and subepithelial electron dense deposits. (D) A case of C3GN showing scattered, relatively discrete, electron dense deposits in the mesangium and within the capillary wall basement membrane.
Twenty-six patients(33%) underwent more than one renal biopsy, including 12 with two native biopsies and 8 with three native biopsies. Aside from progressive interstitial scarring, LM findings were relatively preserved over time. No change in immunostaining pattern, or reproducible trend in deposit location, was observed on serial biopsies. Subjectively, deposits appeared to become more “dense” over time.
Renal Outcomes
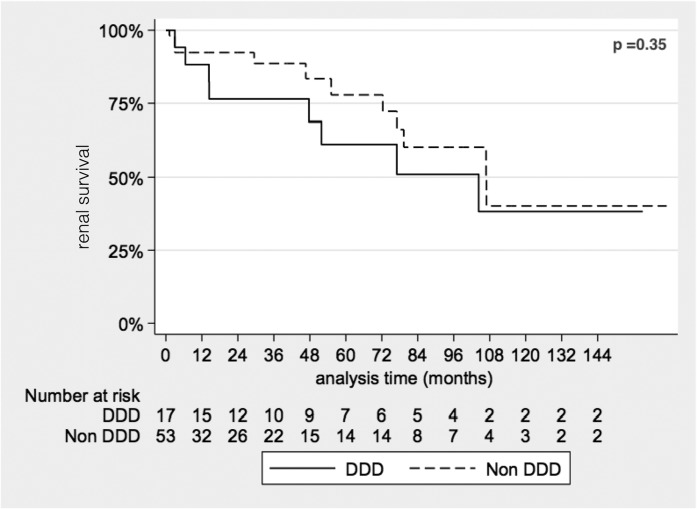
Patients without a native renal biopsy (n=2) or without adequate follow-up data (n=8) were excluded from outcome analyses. Median follow-up, censored for ESRD development, in the remaining cohort (n=70) was 28 months (interquartile range, 7–84) without any significant difference in median follow-up duration between subgroups. At last follow-up, 20 patients progressed to ESRD (47% of patients with DDD and 23% of patients with C3GN). Cumulative renal survival did not, however, differ between groups by Kaplan–Meier survival analysis (Figure 2).
Figure 2.
Kaplan–Meier analysis of renal survival by C3 glomerulopathy subtype. The analysis demonstrates no significant difference in ESRD free renal survival between dense deposit disease (DDD) and non-DDD (C3GN) C3 glomerulopathy subtypes.
Of patients without ESRD (n=50), a majority demonstrated ongoing hematuria (86%), proteinuria (78%), and varying degrees of renal impairment at last clinical follow-up, without significant differences between the DDD and C3GN subgroups. Of the 20 patients reaching ESRD, 6 patients with DDD (75%) and 7 patients with C3GN (58%) subsequently underwent renal transplantation. All six patients with DDD developed DDD recurrence in the transplant, contributing to allograft loss in three patients (50%). All three patients with allograft loss due to DDD recurrence were retransplanted: two developed DDD recurrence in a second transplant, leading to allograft loss in one patient. Four transplanted patients with C3GN (57%) developed C3GN recurrence within the allograft, contributing to allograft loss in three patients (75%). None were retransplanted. Allograft survival was estimated to be 94% at 1 year (95% confidence interval [95% CI], 65% to 99%), 69% at 5 years (95% CI, 41% to 86%), and 28% at 10 years (95% CI, 6% to 56%).
Two additional patients developed C3 glomerulopathy (one with DDD, one with C3GN) after renal transplantation but had not undergone native renal biopsy before transplantation. The first patient originally presented with advanced renal failure, partial lipodystrophy, and C3 nephritic factor (C3Nef) and was presumed to have DDD. A transplant biopsy performed to investigate graft dysfunction 1 year after transplantation demonstrated DDD. The second patient originally presented with advanced renal failure and active urinary sediment, supporting glomerular disease. He developed allograft dysfunction 3 months after renal transplantation. Five subsequent transplant biopsies supported a diagnosis of C3GN.
Univariate analysis showed that renal impairment at the time of the first renal biopsy, crescentic GN, and severe arteriolar sclerosis by LM were predictive of ESRD (Table 3). Age ≥16 years was of borderline significance. DDD did not appear to portend a worse prognosis. Multivariate analysis showed that DDD emerged as a strong and independent predictor of ESRD, along with age ≥16 years and crescentic GN (Table 3). Renal impairment at presentation predicted ESRD within the DDD group only (hazard ratio, 29.3; 95% CI, 1.18 to 727; P=0.04).
Table 3.
Predictors of ESRD in C3 glomerulopathy
| Feature | n | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|---|
| Univariate analysis | ||||
| Features at presentation | ||||
| Age >16 yr | 70 | 2.69 | 0.97 to 7.46 | 0.06 |
| Male sex | 70 | 0.88 | 0.36 to 2.14 | 0.78 |
| Steroid treatment | 67 | 0.81 | 0.31 to 2.12 | 0.66 |
| Other immunosuppressive treatment | 67 | 1.59 | 0.50 to 5.05 | 0.43 |
| Serum creatinine >1.5 mg/dl | 69 | 5.68 | 1.89 to 17.07 | 0.002 |
| Proteinuria >3.5 g/24 h | 68 | 1.77 | 0.72 to 4.37 | 0.22 |
| Low serum C3 | 61 | 0.53 | 0.20 to 1.43 | 0.21 |
| Biopsy features | 70 | |||
| DDD by EM | 1.52 | 0.62 to 3.75 | 0.36 | |
| Crescentic GN | 2.04 | 1.10 to 3.78 | 0.02 | |
| Arteriolar hyaline | 1.91 | 1.15 to 3.15 | 0.01 | |
| Interstitial fibrosis | 0.98 | 0.47 to 2.03 | 0.96 | |
| Multivariate analysis | 69 | |||
| Age >16 yr | 5.45 | 1.12 to 26.5 | 0.04 | |
| Male sex | 0.59 | 0.21 to 1.68 | 0.32 | |
| Serum creatinine >1.5 mg/dl | 2.49 | 0.52 to 11.9 | 0.26 | |
| DDD by EM | 4.70 | 1.22 to 18.1 | 0.03 | |
| Crescentic GN | 2.87 | 1.34 to 6.12 | 0.01 | |
| Arteriolar hyaline | 1.33 | 0.56 to 3.19 | 0.52 |
n is <70 if patients were excluded from analysis due to lack of clinical information. DDD, dense deposit disease; EM, electron microscopy.
Discussion
This study provides a detailed description of the histopathologic features observed in a large cohort of patients with C3 glomerulopathy. Consistent with previous reports (5,10), our analysis demonstrates that a membranoproliferative pattern of injury is not universal in C3 glomerulopathy and that both DDD and C3GN recur after renal transplantation, often resulting in allograft loss. We found low serum C3 levels with preserved serum C4 levels to be frequent but not universal among patients with C3 glomerulopathy.
We identified key clinicopathologic differences between patients with DDD and C3GN. Patients with DDD were younger, more likely to have low serum C3 levels, and more likely to have crescentic GN than patients with C3GN. Patients with C3GN were older and had more severe arteriolar sclerosis, glomerular sclerosis, and interstitial scarring on renal biopsy than patients with DDD. Older age, crescentic GN, and DDD subtype were independent predictors of ESRD. Renal impairment at the time of renal biopsy predicted a poor prognosis only in patients with DDD.
These findings have important clinical implications. Patients presenting with multiple poor prognostic markers (age ≥16 years, DDD, crescentic GN, and renal impairment) are at high risk for progression to ESRD. Such patients should be counseled appropriately and targeted for aggressive intervention. Patients with C3 glomerulopathy planning to undergo renal transplantation should be informed that their disease can recur and cause allograft failure after transplantation.
We were particularly interested in examining differences between patients with DDD and C3GN. Patients with DDD presented more acutely (with crescentic GN), at a younger age, and were more likely to progress to ESRD than patients with C3GN, demonstrating that C3 glomerulopathy is a heterogeneous disorder and suggesting that DDD is the clinically more aggressive subtype. This heterogeneity may result from a combination of genetic and environmental factors. In the case of DDD, the responsible acquired or genetic defect may cause particularly profound dysregulation of the alternative complement pathway, resulting in a rapidly progressive disease course. Conversely, as was observed in our cohort, patients with C3GN may present more indolently, allowing time for histologic markers of chronicity (interstitial fibrosis, glomerulosclerosis) to develop before renal biopsy.
We discovered some incidental EM findings of particular interest. The presence of intramembranous deposits lacking the “classic” DDD appearance in a substantial proportion of patients with C3GN may imply that deposit morphology better informs our understanding of disease pathogenesis than deposit location. The restriction of tubular basement membrane and Bowman’s capsular basement membrane deposits to the DDD subgroup may also provide a clue to differences in underlying pathogenesis.
Another interesting observation was the high frequency of elevated ASOT among patients with DDD and C3GN, suggesting that infection may trigger exacerbations of renal disease in patients with C3 glomerulopathy. We included some patients who were initially diagnosed as having postinfectious GN but with persistent renal or complement dysfunction in our analysis, because these patients are likely to represent true cases of C3 glomerulopathy (14). However, we excluded patients who achieved complete recovery of their renal function after resolution of their infection because, at this time, we feel that inclusion of such patients may compromise our study’s validity and clinical applicability. Our findings highlight the importance of considering a diagnosis of C3 glomerulopathy in patients with apparent postinfectious GN, however, particularly if the patient’s clinical history is atypical and regardless of the presence or absence of a history of preceding infective symptoms or subepithelial humps on EM. Our data also support a role for complement dysfunction in the pathogenesis of postinfectious GN and suggest that postinfectious GN could potentially be considered within the spectrum of C3 glomerulopathy (14).
The comparison of our patient cohort with previously reported cohorts of patients with C3 glomerulopathy reveals striking similarities. Our data are consistent with existing literature describing C3 glomerulopathy as a disease that presents with hematuria and proteinuria (6,13), predominantly in young adults (6,13), without a significant sex predilection (6,13), and with progression to ESRD in approximately one-third of patients (9). As was observed in our study, a previous report suggested that patients with DDD were younger, more likely to have low serum C3 levels, and more likely to progress to ESRD than patients with C3GN (13). Furthermore, age and serum creatinine at the time of biopsy were also identified as predictors of ESRD in a previously described cohort of patients with DDD (11). Finally, a study of Irish patients with membranoproliferative GN, some with C3 glomerulopathy, also identified serum creatinine at presentation and crescents on biopsy as strong predictors of ESRD (15).
Our study is novel in a number of respects. It provides a comprehensive description of the histologic features, including EM findings, observed among patients with heterogeneous forms of C3 glomerulopathy. It also provides a detailed comparison of pathologic differences between DDD and C3GN and associates histologic features with renal prognosis. Finally, it is the first report to provide an estimate of disease incidence, confirming the status of C3 glomerulopathy as a rare kidney disease.
This study is not without limitations. As a retrospective study, data may be incomplete. In particular, treatment details were inadequately documented in some cases, which may explain why, in contrast with previous reports (9,11), we did not identify an effect of treatment on renal outcome. It is also noteworthy that only three patients received plasma exchange, a therapeutic option for DDD patients with specific CFH mutations (16). Similarly, no patient received eculizumab, an anti-complement therapy recently reported to mitigate disease in selected patients with C3 glomerulopathy (17–20). Another limitation is that we were unable to collect a sufficient number of patients to allow for multiple intergroup comparisons. Consequently, important differences between subgroups or novel predictors of renal outcome may have gone undetected. Patients' height from the time of original presentation were not available, prohibiting calculation of estimated GFRs in children.
The diagnostic criteria we adopted for C3 glomerulopathy were stricter than those recently developed by a C3 glomerulopathy consensus group (Pickering et al., 2013, Unpublished data). Specifically, we excluded patients with any amount of IgG or IgA positivity on immunostaining and allowed only weak (1+) IgM staining. This contrasts with the consensus group statement that suggests that biopsies containing IgM, IgG, or IgA can still be considered to be C3 dominant glomerulonephritis if the intensity of the C3 staining is at least two orders of magnitude greater than that of any other immune reactant. Due to the inception of our study before the development of these consensus group criteria, combined with our desire to describe as well defined a cohort as possible, we accept that we may have excluded a small number of true cases of C3 glomerulopathy and consequently that our disease incidence estimate may be overly conservative. However, we feel the adoption of such strict exclusion criteria strengthens our findings because they are derived from a relatively pure population of patients who not only meet existing established criteria for a diagnosis of C3 glomerulopathy but who also now clearly meet the proposed consensus group criteria.
A major limitation of this study is the fact that few patients have undergone genetic and serologic testing for inherited and acquired abnormalities of alternative complement pathway regulation. Consequently, we cannot comment on underlying disease mechanisms or whether the presence of specific genetic or serologic markers can predict prognosis. This limitation reflects the rarity of C3 glomerulopathy and consequently our need to retrospectively collect patients from a large catchment population over 20 years, some of whom are no longer alive or contactable. We are at present inviting all surviving patients to centrally undergo serum and DNA collection and will report emerging results in due course. We at the same time recognize that such genotyping and detailed serologic analyses are not readily available at many centers, nor has the clinical utility of such testing been proven. Therefore, the absence of genetic and serologic data in this study may not necessarily detract from the clinical applicability of our findings.
In summary, we have described clinical and pathologic findings among a large cohort of patients with C3 glomerulopathy. We have observed important clinicopathologic distinctions between patients with DDD and C3GN and have identified independent predictors of renal outcome within this population. These data are particularly relevant when considering emerging complement therapeutic interventions, because understanding the natural history of this rare disease is a prerequisite for optimal trial design and patient selection.
Disclosures
None.
Acknowledgments
We acknowledge the physicians caring for the patients for allowing us access to these data.
This research was supported by the National Institute for Health Research Biomedical Research Centre Funding Scheme.
Footnotes
N.R.M.-T. and M.M.O. are joint first authors and P.J.C. and H.T.C. are joint senior authors.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Walport MJ: Complement. First of two parts. N Engl J Med 344: 1058–1066, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez de Córdoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sánchez-Corral P: The human complement factor H: Functional roles, genetic variations and disease associations. Mol Immunol 41: 355–367, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA: Complement factor H variant increases the risk of age-related macular degeneration. Science 308: 419–421, 2005 [DOI] [PubMed]
- 4.Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJ, Smoyer W, Tully HF, Tully SP, Walker P, Welsh M, Würzner R, Zipfel PF: Membranoproliferative glomerulonephritis type II (dense deposit disease): An update. J Am Soc Nephrol 16: 1392–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC: C3 glomerulopathy: A new classification. Nat Rev Nephrol 6: 494–499, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Barbour TD, Pickering MC, Cook HT: Recent insights into C3 glomerulopathy. Nephrol Dial Transplant 28: 1685–1693, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, Pusey CD, Pierides A, Kyriacou K, Athanasiou Y, Voskarides K, Deltas C, Palmer A, Frémeaux-Bacchi V, de Cordoba SR, Maxwell PH, Pickering MC: Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet 376: 794–801, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik TH, Lavin PJ, Goicoechea de Jorge E, Vernon KA, Rose KL, Patel MP, de Leeuw M, Neary JJ, Conlon PJ, Winn MP, Pickering MC: A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol 23: 1155–1160, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F, Moulin B, Grünfeld JP, Niaudet P, Lesavre P, Frémeaux-Bacchi V: Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 82: 454–464, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ: C3 glomerulonephritis: Clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int 82: 465–473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nasr SH, Valeri AM, Appel GB, Sherwinter J, Stokes MB, Said SM, Markowitz GS, D’Agati VD: Dense deposit disease: Clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol 4: 22–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Athanasiou Y, Voskarides K, Gale DP, Damianou L, Patsias C, Zavros M, Maxwell PH, Cook HT, Demosthenous P, Hadjisavvas A, Kyriacou K, Zouvani I, Pierides A, Deltas C: Familial C3 glomerulopathy associated with CFHR5 mutations: Clinical characteristics of 91 patients in 16 pedigrees. Clin J Am Soc Nephrol 6: 1436–1446, 2011 [DOI] [PMC free article] [PubMed]
- 13.Vernon KA, Goicoechea de Jorge E, Hall AE, Fremeaux-Bacchi V, Aitman TJ, Cook HT, Hangartner R, Koziell A, Pickering MC: Acute presentation and persistent glomerulonephritis following streptococcal infection in a patient with heterozygous complement factor H-related protein 5 deficiency. Am J Kidney Dis 60: 121–125, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi S, Fervenza FC, Zhang Y, Zand L, Meyer NC, Borsa N, Nasr SH, Smith RJ: Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int 83: 293–299, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little MA, Dupont P, Campbell E, Dorman A, Walshe JJ: Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int 69: 504–511, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Licht C, Weyersberg A, Heinen S, Stapenhorst L, Devenge J, Beck B, Waldherr R, Kirschfink M, Zipfel PF, Hoppe B, C L : Successful plasma therapy for atypical hemolytic uremic syndrome caused by factor H deficiency owing to a novel mutation in the complement cofactor protein domain 15. Am J Kidney Dis 45: 415–421, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz L, Stokes MB, Markowitz GS, D’Agati VD, Canetta PA, Radhakrishnan J, Appel GB: Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol 7: 748–756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daina E, Noris M, Remuzzi G: Eculizumab in a patient with dense-deposit disease. N Engl J Med 366: 1161–1163, 2012 [DOI] [PubMed] [Google Scholar]
- 19.McCaughan JA, O’Rourke DM, Courtney AE: Recurrent dense deposit disease after renal transplantation: An emerging role for complementary therapies. Am J Transplant 12: 1046–1051, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Vivarelli M, Pasini A, Emma F: Eculizumab for the treatment of dense-deposit disease. N Engl J Med 366: 1163–1165, 2012 [DOI] [PubMed] [Google Scholar]