Summary
A broad range of skin diseases occurs in patients with ESRD: from the benign and asymptomatic to the physically disabling and life-threatening. Many of them negatively impact on quality of life. Their early recognition and treatment are essential in reducing morbidity and mortality. The cutaneous manifestations can be divided into two main categories: nonspecific and specific. The nonspecific manifestations are commonly seen and include skin color changes, xerosis, half-and-half nails, and pruritus. The specific disorders include acquired perforating dermatosis, bullous dermatoses, metastatic calcification, and nephrogenic systemic fibrosis. This review article describes these conditions and considers the underlying pathophysiology, clinical presentations, diagnosis, and treatment options.
Introduction
In 2010 in the United States, 593,086 patients received treatment for ESRD (1). A broad range of skin diseases occurs in these patients: from the benign and asymptomatic to the physically disabling and life-threatening. Many of them negatively impact on quality of life. The prevalence of ESRD is increasing, and it is, therefore, likely that the incidence and prevalence of associated skin diseases will also increase. Their early recognition and treatment to reduce morbidity and mortality are essential.
Many factors are involved in the pathogenesis of the cutaneous manifestations of ESRD, including electrolyte imbalance, buildup of uremic substances, and comorbid disease (2). Some conditions are easily diagnosed but can be difficult to manage. Others are less straightforward to diagnose, largely because of clinical similarities with more common diseases, and they are notoriously difficult to manage.
In this review, we focus on two main categories of the cutaneous manifestations of ESRD: nonspecific and specific. Nonspecific manifestations include pruritus, which will be discussed in detail, as well as skin color changes, xerosis, and half-and-half nails, which are briefly described. Specific manifestations include acquired perforating dermatosis, bullous dermatoses, metastatic calcification, and nephrogenic systemic fibrosis, all of which will be considered in depth. Underlying pathophysiology, clinical presentations, diagnosis, and treatment options are considered.
Nonspecific Manifestations
Skin color changes, such as pallor and hyperpigmentation, are seen in approximately 40% and 20% of patients, respectively (3,4). Half-and-half nails (or Lindsay’s nails) are seen in approximately 20% of patients, and xerosis (dry and scaly skin) is seen in 50%–85% of patients with ESRD (3,4). The clinical manifestations, pathogenesis, treatment options, and dermatological sequelae of these nonspecific manifestations are set out in Table 1 (3–10).
Table 1.
Nonspecific cutaneous manifestations seen in ESRD patients (Figure 1)
| Disorder (Refs.) | Clinical Manifestations | Pathogenesis | Treatment |
|---|---|---|---|
| Skin color changes (3–6) | Pallor | Attributed to anemia of chronic disease | May improve with erythropoietin |
| Yellowing of the skin | Secondary to excessive deposition of urochrome and carotenoids | No skin treatment available | |
| Hyperpigmentation | Secondary to increased levels melanocyte-stimulating hormone | Daily sunscreen and sun protection | |
| Ecchymoses | Associated with platelet dysfunction from elevated urea and creatinine | Routine dialysis can reduce ecchymosis | |
| Xerosis (dry skin) (7–9) | Patients present with dry, scaly skin on the trunk and extensor surfaces of the extremities | Exact mechanism is not clear; it is believed that the xerosis is caused by dehydration of the stratum corneum and reduced sebum and sweat production secondary to sebaceous and sweat gland atrophy, respectively | Limit hand washing, showering, and bathing to reduce skin irritation |
| Pruritus is common and can range from mild to severe | Apply emollients daily | ||
| Avoid known skin irritants | |||
| Wear nonirritating fabrics | |||
| Half-and-half nails (also known as Lindsay’s nails) (4,10) | Seen in ∼20% of uremic patients; distal half of the nail appears normal or brown, and the proximal half appears white | Exact mechanism is unknown; one hypothesis is that there is an increased tissue concentration of melanocyte-stimulating hormone | No nail treatment available |
Pruritus is one of the most common symptoms associated with ESRD. The exact pathophysiology of uremic pruritus is unknown, but it is likely multifactorial. Possible etiological factors are presented in Table 2 (11–15). There is conflicting evidence regarding the relationship between the onset and severity of pruritus and the duration of ESRD and dialysis therapy (11,12,16). No clear association between pruritus and underlying renal disease has been established (12). The proportion of ESRD patients with uremic pruritus has decreased significantly with modern dialysis from approximately 85% in the 1970s to the present day rate of 40%–50% (11,17,18).
Table 2.
| Electrolyte Abnormalities | Endocrine Disorders | Neurocutaneous | Other |
|---|---|---|---|
| Hypervitaminosis A | Secondary hyperparathyroidism | Abnormal signaling within cutaneous C nerve fibers | Xerosis (dry skin) |
| Hypercalcemia | Peripheral neuropathy | Elevated levels of inflammatory cytokines IL-2 and -6 | |
| Hyperphosphatemia | Increased serum β-endorphin, a μ-receptor agonist, in hemodialysis patients | Iron deficiency anemia | |
| Hypermagnesemia | Substance P | ↑ Amounts of dermal mast cells and histamine | |
| ↑ Serum C-reactive protein | |||
| ↑ γ-Aminobutyric acid |
Uremic pruritus may be localized or generalized. The frequency, severity, and clinical characteristics vary widely, ranging from mildly irritating, to severely debilitating (19). Patients frequently present with excoriations, lichen simplex chronicus, or prurigo nodularis from continuous scratching (Figure 1B) (11).
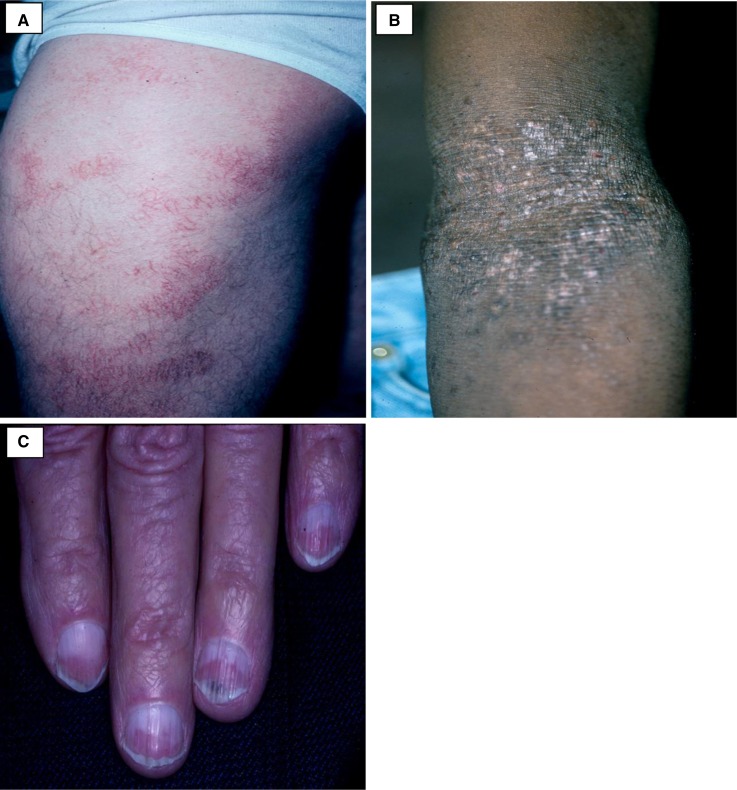
Figure 1.
The nonspecific cutaneous manifestations seen in ESRD. (A) Patches of xerotic eczema on the lateral thigh. (B) Lichenified plaque with few excoriations on the antecubital fossa from repeated scratching. (C) Half-and-half nails with the characteristic proximal white half of the nail and the distal red half of the nail. (Courtesy of Timothy G. Berger.)
Before making the diagnosis of uremic pruritus, other causes of pruritus must be ruled out. Uremic pruritus is difficult to treat. A summary of effective treatments that decrease the severity of pruritus is presented in Table 3. No single agent has been shown to be universally effective. Topical therapy, aimed principally at alleviating the xerosis that many ESRD patients have, has been of moderate benefit. Only a few oral medications have shown any significant efficacy, and ultraviolet B phototherapy has been used with some reported success. Optimal treatment is individualized and usually requires combination therapy (43).
Table 3.
Effective treatments to decrease the severity of uremic pruritus in dialysis patients
| Drug or Therapy | Dose Range | Ref. | Year | Number of Patients | Outcome: Percent Decrease in Mean Pruritus Score (Based on VAS) | Comments |
|---|---|---|---|---|---|---|
| Gabapentin | 100–300 mg given PO three times per week after each dialysis session | 20 | 2004 | 25 | 86% decrease in the treatment group versus 9.5% in controls | Used 300 mg PO three times per week for 1 mo |
| 21 | 2007 | 34 | 92.8% decrease in the treatment group versus 21% in controls | Used 400 mg PO two times per week after each dialysis session for 1 mo | ||
| 22 | 2009 | 34 | 93.5% decrease in the treatment group versus 18% in controls | Used 100 mg PO three times per week for 1 mo | ||
| Pregabalin | 25–75 mg PO daily | 23 | 2010 | 16 | 77.2% decrease | Prospective trial without control group; used 25 mg PO daily for 1 mo |
| 24 | 2012 | 50 | 79.2% decrease in pregabalin group versus 77.9% decrease in gabapentin group (equivalent efficacy) | All participants had established peripheral neuropathy and/or neuropathic pain | ||
| Crossover trial: 25 participants on pregabalin 75 mg PO daily for 6 wks followed by a 2-wk washout period and then 6 wks of gabapentin 300 mg PO given three times per week post-HD sessions; 25 participants started with gabapentin | ||||||
| 25 | 2012 | 12 | 69% decrease | Prospective trial without control group; used 25 mg PO three times per week (if ineffective, dose ↑ to 25–50 mg PO daily for 24 wks) | ||
| Nalfurafine | 5 µg iv three times per week directly after each HD session | 26 | 2005 | 144 | 40% decrease in treatment group versus 19% in control group | Study I: Parallel group design, 1-mo duration, 5 µg iv three times per week directly after each HD session |
| Study II: Crossover design, 2-wk treatment period, 3-wk washout period, 1-wk run-in period, and 2-wk treatment period; results of both studies similar | ||||||
| 2.5–5 µg PO nightly | 27 | 2010 | 337 | 35.4% decrease in treatment group versus 20% in control group | Used 2.5 or 5 µg PO nightly for 2 wks; results between different dosing groups were the same | |
| 28 | 2012 | 211 | 58.9% decrease | Open-label, single-arm, prospective trial; used 5 µg PO nightly for 52 wks | ||
| Cromolyn | 135 mg PO TID | 29 | 2010 | 62 | 89.6% decrease in treatment group versus 34.2% in control group | 8-wk trial |
| Topical cromolyn sodium 4% cream daily | 30 | 2012 | 60 | 88% decrease in treatment group versus 51.6% in control group | 4-wk trial | |
| Sericin cream | Sericin 8% cream BID | 31 | 2012 | 50 | 68.4% decrease in treatment group | Intersubject control using a split-body biometrological assessment. |
| 6-wk trial | ||||||
| High-permeability HD | High-permeability dialyzers | 32 | 2009 | 116 | 69.3% decrease in treatment group versus 11.5% in control group | HD was performed three times per week for 12 wks; high-permeability dialyzers (F60; Fresenius) were used, with polysulphone membranes of 1.3 m2 and an ultrafiltrate coefficient of 40 ml/h per mmHg |
| Capsaicin | Capsaicin 0.03% ointment | 33 | 2010 | 34 | Not based on VAS; 84.3% decrease in treatment group versus 52% in control group | Crossover design with 4 wks of treatment, a 2-wk washout period, and 4 wks of treatment; because of the burning sensation with the initial use of capsaicin, it is highly likely that those patients knew their group assignment. |
| Four times per day | Did not use VAS; scored pruritus based on severity, distribution, and sleep disorder | |||||
| Pramoxine | Pramoxine 1% lotion BID | 34 | 2009 | 28 | 61% decrease in treatment group versus 12% in control group | 4-wk trial |
| Narrow-band UVB therapy | Narrow-band UVB to whole-body surface | 35 | 2005 | 20 | 70.8% decrease in treatment group | 6-wk treatment period; open pilot trial, with only 10 patients completing the trial |
| Three times per week | 36 | 2007 | 46 | 54.2% decrease in group 1 after a mean of 22 treatments; 67.9% decrease in group 2 after a mean of 22 treatments | Open-label trial with two groups: Group 1 had 17 patients with uremic pruritus, and group 2 had 29 patients with idiopathic pruritus | |
| 37 | 2011 | 21 | 54.9% decrease in treatment group | Single-blind, randomized, controlled trial; 6-wk treatment period | ||
| Versus 59.3% in control groupa | Control group received matched UVA treatments | |||||
| Sertraline | 50 mg PO daily | 38 | 2012 | 19 | Not based on VAS; 57.8% of patients had improved pruritus severity from severe or moderate to weak | Open-label, single-arm, prospective trial for 4 mos of active treatment; severity graded by a researcher-developed 30-item inventory (content validity for this form was 0.82) |
| γ-Linolenic acid | Topical γ-linolenic acid 2.2% cream to entire body daily and TID to pruritic areas | 39 | 2006 | 17 | 51.2% decrease in treatment group (group A) versus 15% in control group (group B) | Crossover design with two groups randomized into treatment versus urea cream control; 2-wk treatment periods with a 2-wk washout period |
| After crossover, 45.51% decrease in treatment group (group B) versus 11.2% in control group (group A) | ||||||
| Thalidomide | 100 mg PO | 40 | 1994 | 29 | Not based on VAS | Crossover design; 1-wk treatment periods with a 1-wk washout period (pruritus intensity was scored TID from zero to three) |
| QHS | 78% decrease in treatment group versus no change in control group | Response in control group could be caused by placebo effect versus carryover effect | ||||
| After crossover, 81% decrease in treatment group versus 54% in control group | ||||||
| Activated charcoal | Activated powdered charcoal 6 g | 41 | 1995 | 23 | Not based on VAS | Nonrandomized, single-blind, controlled trial for 6 wks |
| PO daily | 70.4% decrease | Pruritus intensity scored from one to six; 10 patients initially given a placebo for 1 wk before all patients received treatment (data from 10 patients only) | ||||
| Glycerin and paraffin emulsion | Glycerin 15% and paraffin | 42 | 2011 | 100 | Did not compare change in VAS in period I | Period I: 7-d comparative period of test product versus plain emulsion intraindividually (left leg versus right leg) |
| 10% in an oil and water emulsion BID | 75% overall decrease at the end of open-label period (period II) | Period II: 49-d noncomparative, open-label trial of the test product to all xerotic areas BID |
All therapies listed showed a statistically significant reduction in the mean pruritus score. All are randomized, double-blind, and controlled trials unless otherwise noted in the comments. VAS, visual analog scale; PO, per os; HD, hemodialysis; iv, intravenous; TID, three times daily; BID, two times daily; UVB, ultraviolet B; UVA, ultraviolet A; QHS, nightly.
Lack of statistical significance could be attributed to small sample size, effect of UVA on pruritus, or placebo effect.
Specific Manifestations
Acquired Perforating Dermatosis
Perforating disorders are a heterogeneous group of dermatoses characterized by transepidermal elimination of dermal structures. The perforating disorders seen in ESRD, known as acquired perforating dermatosis (APD), are both clinically and histologically similar to the primary perforating dermatoses. The majority of affected patients are African American, and there is a strong association with CKD and diabetes mellitus (44–46). APD usually develops after dialysis treatment has started (44–47). The prevalence in dialysis patients is between 2% and 11% (45,48).
The exact pathophysiology is unknown. Localized skin irritation, typically from scratching, may cause an inflammatory cutaneous reaction to uremic substrates in the dermis, leading to lesion formation (45,46). Patients present with moderate to severe pruritus in the affected skin areas and characteristically have pruritic, firm, dome-shaped papules or nodules with a central keratotic plug distributed on the extensor surfaces of the extremities and trunk (Figure 2). Koebnerization (formation of lesions in areas of skin trauma, most notably from scratching) is common. Spontaneous resolution can occur (44–46).
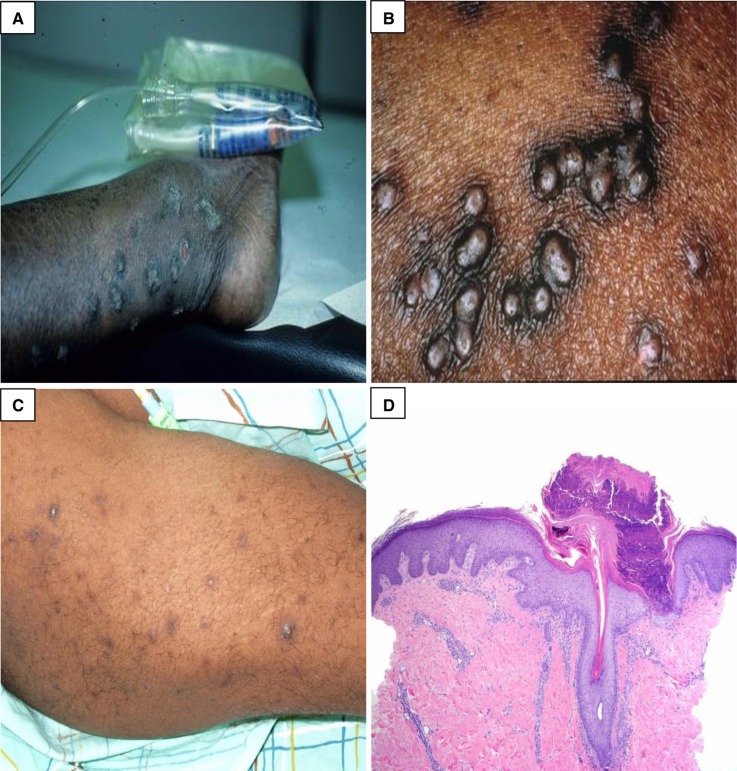
Figure 2.
Acquired perforating dermatosis. (A and B) Multiple clustered, hyperpigmented, dome-shaped nodules and coalescing plaques with a central keratotic plug. (C) Hyperpigmented macules and patches from lesions that have resolved. (D) There is a cup-shaped epidermal depression filled with parakeratosis and neutrophilic debris. At the base of the depression, the epidermis is thinned, and degenerated collagen fibers are noted protruding through this attenuated epidermis. (Courtesy of Timothy G. Berger, Anna K. Haemel, and Thaddeus W. Mully.)
Histologically, these disorders are characterized by transepidermal elimination of dermal substrates, such as collagen, keratin, and elastic fibers (Figure 2) (44,47). The diagnosis of APD is made clinically and confirmed with a lesional skin biopsy. Individual case reports and small case series have provided evidence of effective treatment with topical, systemic, and other therapies, such as phototherapy and cryotherapy. This information is presented in Table 4. To date, there has not been a randomized controlled trial to evaluate the efficacy of a single treatment.
Table 4.
Effective therapies for the acquired perforating dermatosis
| Topical Therapy | Oral Therapy | Other Therapies |
|---|---|---|
| Clobetasol or β-methasone and/or a keratolytic: salicylic acid, urea, or ammonium lactate (45,46,49) | Antihistamines: hydroxyzine, fexofenadine, and doxepin (49,50) | Narrow-band ultraviolet B (51,52) |
| Retinoid (45,53) | Allopurinol 100 mg (49,54,55) | Photodynamic therapy with 5-aminolevulenic acida (56) |
| Cantharidina (57) | Acitretin (49,50) | Cryotherapy with liquid nitrogen (58) |
| Doxycycline 100 mg (59,60) or minocycline 100–200 mg (61) |
Data are from small case series and multiple case reports.
Single case report.
Bullous Diseases
Bullous diseases manifest with vesicular/bullous eruptions on the skin and/or mucous membranes. In ESRD, porphyria cutanea tarda and pseudoporphyria may be seen (4,62). Both are caused by the accumulation of photosensitive molecules in the skin that, on ultraviolet light exposure, leads to skin fragility and vesiculation on sun-exposed areas (4).
Porphyria cutanea tarda (PCT) is characterized by uroporphyrinogen-decarboxylase (URO-D) deficiency or dysfunction. Alcohol abuse, hepatitis C virus, HIV infection, and iron supplementation contribute to decreased URO-D activity (63). Porphyrins may form complexes with high-molecular weight proteins, which are poorly dialyzed by conventional methods. In hemodialysis (HD) patients, PCT occurs secondary to decreased URO-D activity and poor clearance of the plasma porphyrins (63,64). PCT is uncommon with peritoneal dialysis (PD), most probably because PD achieves more effective clearance of larger molecules (63).
Pseudoporphyria is clinically and histologically identical to PCT without the serum and urine porphyrin abnormalities. The pathophysiology in ESRD patients is unknown. Patients with PCT and pseudoporphyria have skin photosensitivity and fragility, and they present with tense vesicles/bullae, erosions, and crusts distributed on the face, extensor surface of the forearms, and dorsal hands (Figure 3). Lesions typically heal with atrophic scarring and milial cysts (Figure 3C). Sclerodermatous plaques, hypertrichosis, and hyperpigmentation in sun-exposed areas are also seen in patients with PCT but not patients with pseudoporphyria. Increased serum levels of iron and ferritin, increased urinary uroporphyrin excretion, and increased levels of plasma uroporphyrin in anuric patients are typical in PCT (63,64).
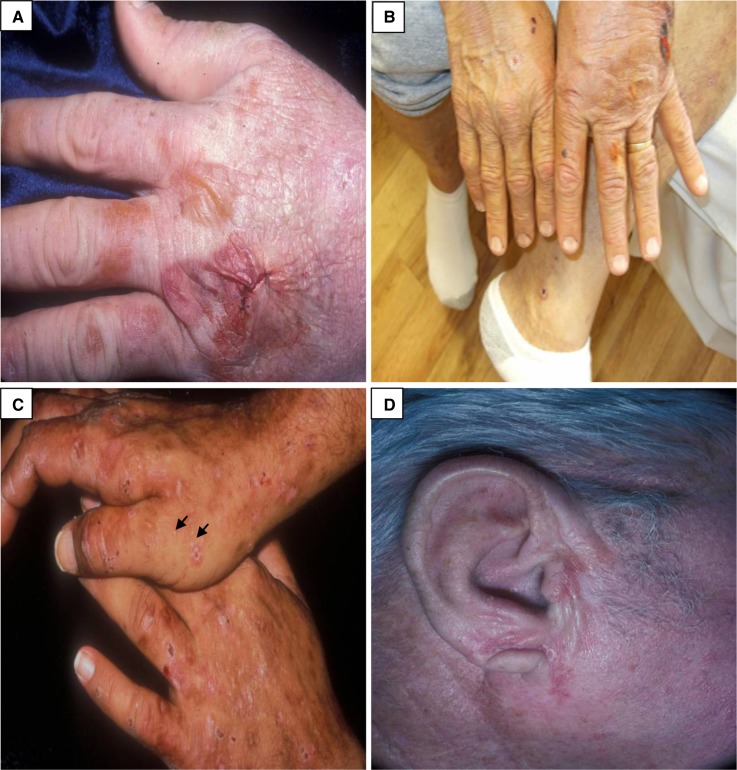
Figure 3.
Porphyria cutanea tarda. (A and B) Tense and ruptured bullae on the dorsal hand. (C) Erosions, crusts, atrophic scarring, and milia (at the tips of the black arrows) on the dorsal hands. (D) Sclerodermatous plaque on the right ear. (Courtesy of Timothy G. Berger and Anna K. Haemel.)
Treatment of PCT includes avoiding triggers, like alcohol, hepatotoxic medications, and sun exposure, and using sunscreen. Decreasing iron overload is effective for symptom management. The combination of erythropoiesis-stimulating agents with small volume phlebotomy (50–100 ml once or twice weekly) to decrease hepatic iron stores can induce remission within several months. Alternatively, deferoxamine can be used intravenously with each dialysis session in those patients unable to tolerate phlebotomy (63,64). High-flux membrane HD, which is more effective at removing plasma porphyrins, is an effective adjunct (63). Treatment of pseudoporphyria involves stopping/avoiding any of the medications known to induce the disease (for example, diuretics, antibiotics, and antifungals) as well as sun avoidance and sun protection. N-Acetylcysteine, a metabolic precursor of glutathione that increases antioxidant activity and clearing of free radicals, is an effective treatment for pseudoporphyria (62).
Metastatic Calcification
Metastatic calcification is the deposition of calcium salts into the cutaneous and/or subcutaneous tissues that occurs as a result of elevated serum levels of calcium. It can also affect the blood vessels and viscera (4,65). In ESRD, metastatic calcification is characterized by abnormal calcium and/or phosphate metabolism and can present as either calcinosis cutis or calcific uremic arteriolopathy.
Calcinosis Cutis.
Calcinosis cutis, also known as benign nodular calcification, consists of calcium deposition within the cutaneous and subcutaneous tissues without tissue necrosis. Hyperphosphatemia is typical and secondary to diminished renal clearance of phosphate and inadequate clearance by dialysis. Secondary hyperparathyroidism, with increased levels of intact parathyroid hormone (iPTH), mobilizes the release of calcium and phosphate from the bone into the serum. When the levels of serum calcium and phosphate reach a solubility threshold, calcinosis cutis can occur (65,66).
Patients present with firm white papules/plaques and nodules that are most commonly distributed in the periarticular areas and fingertips (Figure 4). Periarticular lesions are normally asymptomatic, but lesions on the fingertips are usually painful (65). A whitish discharge can be eliminated from these lesions. The number and size of lesions correlate with the serum phosphate level. Dietary phosphate restriction, phosphate binders, and if necessary in patients with elevated iPTH levels, parathyriodectomy may help lower serum phosphate levels (65–67). Lesions tend to regress after the hyperphosphatemia resolves (66).
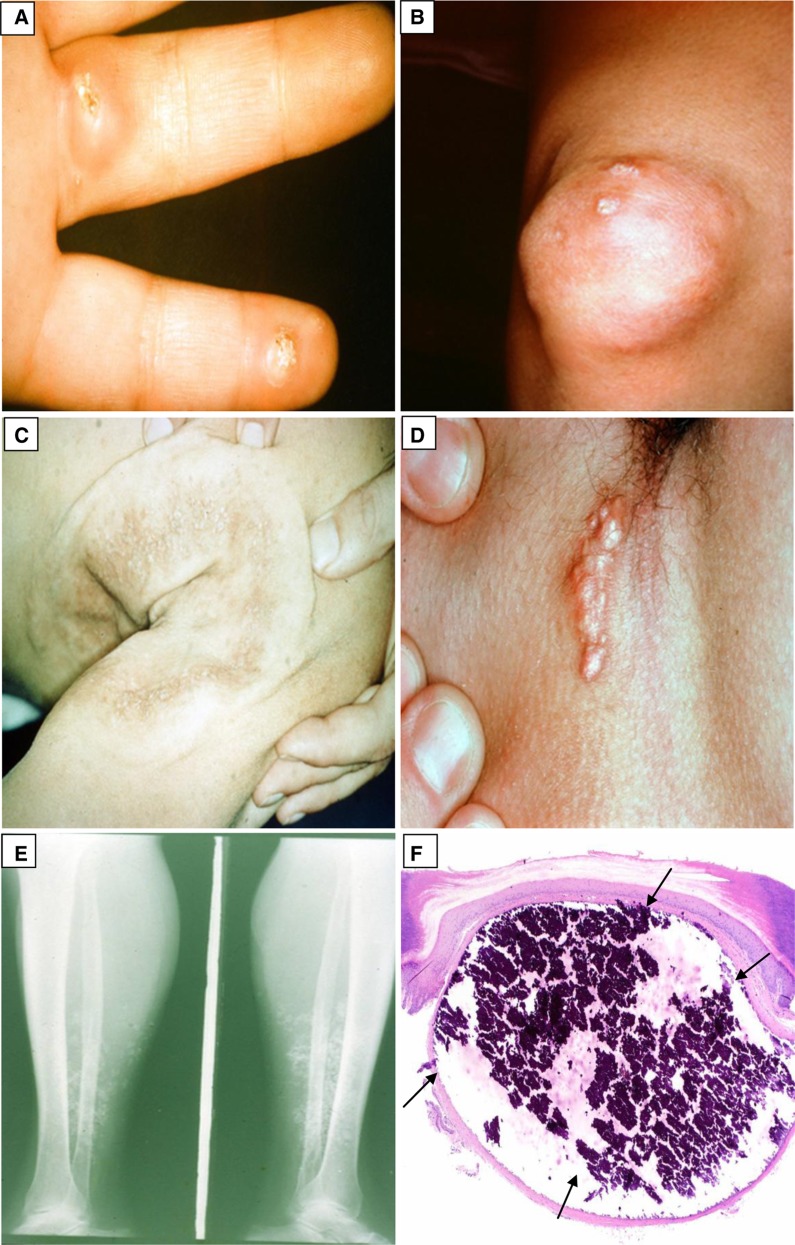
Figure 4.
Calcinosis cutis. (A) Firm nodules on the palmar aspect of the fingers with central white calcium globules. (B) A calcified nodule around the knee. (C) A large, white, indurated calcified plaque on the right posterior shoulder and arm. (D) A linear, white calcified plaque in the axilla. (E) A plain radiograph of the lower extremity showing radio-opaque soft tissue calcium deposits. (F) There is a nodular deposit of basophilic refractile calcium in the dermis (black arrows). Minimal inflammation is present. (Courtesy of Timothy G. Berger and Thaddeus W. Mully.)
Calcific Uremic Arteriolopathy.
Calcific uremic arteriolopathy (CUA), also known as calciphylaxis, is characterized by thrombosis of calcified small- and medium-sized arteries and arterioles as well as fibrosis, which most commonly affects the dermis and subcutaneous fat. Extravascular calcification may also be present (65,68). It is rare and potentially life-threatening. It has an estimated incidence of 1% and prevalence of 1%–4% in the ESRD population. Patients typically present in their 50s (69–72). CUA has a mortality rate of 60%–80%. Death is most commonly secondary to sepsis and organ failure (4,69,70).
The exact pathogenesis of CUA is unclear but multifactorial. Metabolic factors, systemic inflammation, oxidative stress, and endothelial injury along with certain triggers have been implicated. Risk/triggering factors include local trauma, women (73,74), Caucasian ethnicity (75), hypoalbuminemia (72,74,75), therapy with calcium salts, vitamin D supplements, erythropoiesis-stimulating agents (69,70,76), warfarin (72–74,77), and iron supplements (78). Other independent risk factors include obesity (body mass index>30 kg/m2) (69,73,74), liver disease (69,79), and systemic corticosteroids (65,69,73,74).
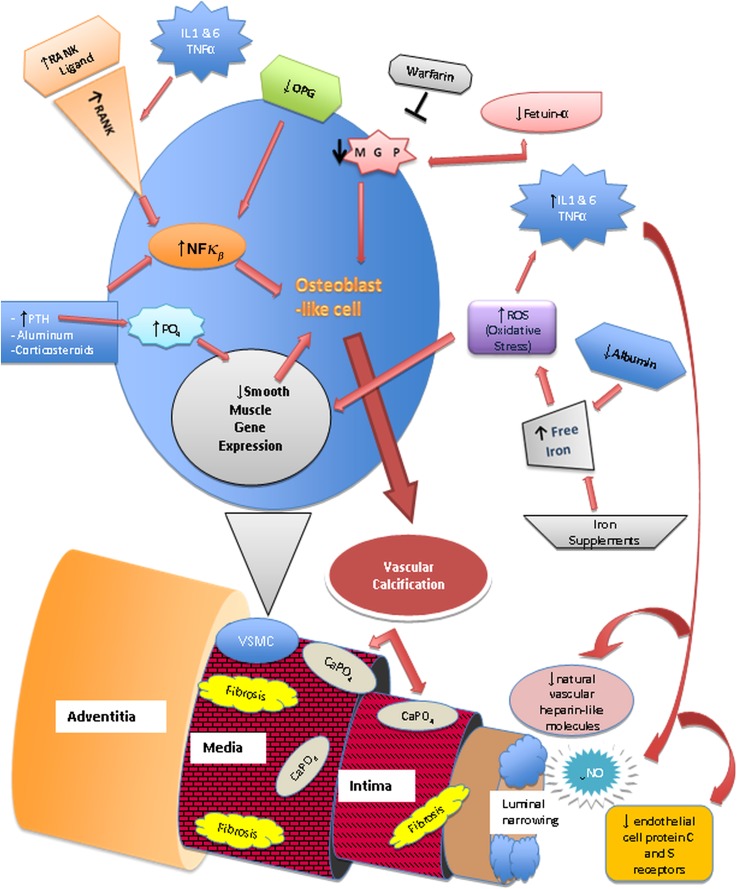
CKD/ESRD, dialysis therapy, and commonly occurring comorbid conditions, like hypertension, hypercholesterolemia, obesity, and diabetes mellitus, increase oxidative stress (69,74,76,80). Vascular calcification is promoted in the presence of increased oxidative stress by reactive oxygen species that also leads to a state of systemic inflammation, which is evidenced by increased levels of TNF-α, IL-1, and IL-6 (76). The body’s natural antioxidants, such as glutathione, are depleted when these disorders are chronic. Vascular calcification causes endothelial injury and luminal narrowing. The low-flow rate within cutaneous vessels, combined with luminal narrowing, leads to decreased blood flow and can cause blood stasis (73). It creates a procoagulant state in the narrowed vessels, which increases the risk of thrombus formation and can lead to localized tissue ischemia and necrosis (73,76). The relationship between the implicated etiologic factors in CUA and its pathogenesis is schematically represented in Figure 5.
Figure 5.
Pathophysiology of calcific uremic arteriolopathy. Nuclear factor κβ (NFκβ), a transcription factor involved in the production of cytokines and inflammatory mediators, the receptor activator of NFκβ (RANK), and its ligand are vital for bone mineral resorption and development (68,73,76). They can be activated by chronic inflammatory states, parathyroid hormone (PTH), aluminum, corticosteroids, free radicals, and infectious agents (73,76). Increased NFκβ activity leads to bone mineral loss and vascular calcification. Osteoprotegerin (OPG) is an antagonist of RANKL. RANK/RANKL and OPG are expressed on endothelial cells, osteoblasts/osteoclasts, and vascular smooth muscle cells (VSMCs) (73,76). Transformation of VSMCs into osteoblast-like cells is initiated by metabolic disturbances (particularly hyperphosphatemia), reactive oxygen species (ROS), and decrease of local vascular calcification inhibitors, such as matrix γ-carboxy-glutamate protein (MGP), a vitamin K-dependent protein (68,76). Fetuin-A, a hepatically synthesized protein that functions systemically to inhibit vascular calcification, is reduced in inflammatory states, renal failure, and calcific uremic arteriolopathy (68,76). Progressive subintimal fibrosis and medial-arteriolar calcification lead to endothelial injury and luminal narrowing. The low-flow rate within the cutaneous vessels combined with luminal narrowing leads to decreased blood flow and may lead to blood stasis (73). Systemic inflammation also causes endothelial dysfunction. It creates a procoagulant state in the narrowed vessels and increases the risk of thrombus formation, which can lead to localized tissue ischemia and necrosis (73,76,78).
Clinically, patients initially experience pain or localized tenderness associated with erythema, violaceous mottling, or reticulate discoloration of the skin that resembles livedo reticularis. Lesions progress over days to weeks to become severely painful plaques or nodules surrounded by a reticular purple discoloration (Figure 6). They tend to be distributed bilaterally and symmetrically, and they are most commonly located on the lower extremities, which may relate to poor circulation, as well as the abdomen and buttocks, both of which have large amounts of subcutaneous fat (69–72). Patients can present with hemorrhagic vesicles/bullae that precede the progression to necrosis and commonly develop painful, irregularly shaped, nonhealing ulcers. Lesion distribution, specifically proximal lesions (lesions on the trunk, thighs, and upper arms) versus distal lesions (lesions on the calves and forearms), does not seem to be a clear prognostic indicator as previously thought (69,74,80). Systemic symptoms secondary to vascular and extravascular calcification of internal organs and soft tissues may also occur. Patients may develop cardiac valve or electrical conduction dysfunction, myocardial, pulmonary, or cerebrovascular infarction (80,81), myositis, muscle weakness, and bowel infarction (70,80).
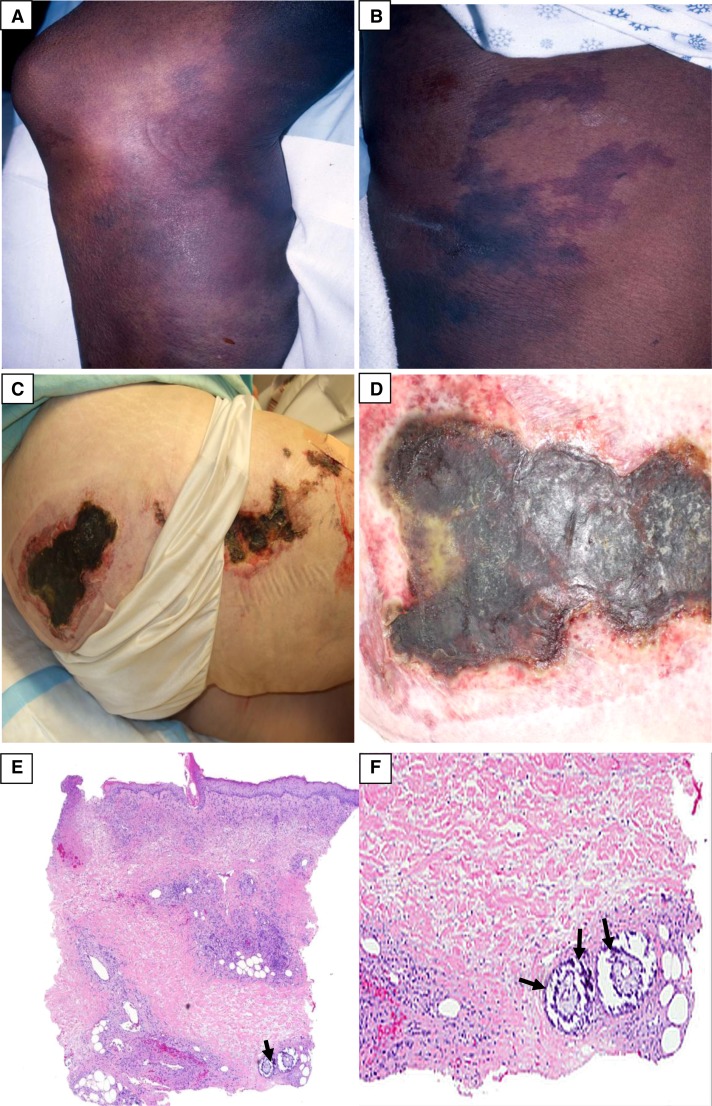
Figure 6.
Calcific uremic arteriolopathy. (A) Violaceous, indurated plaque with surrounding reticular purple discoloration. (B) Violaceous reticulation resembling livedo reticularis. (C) Necrotic ulcerations on the right buttock and thigh. (D) Close view of a necrotic ulcer with a violaceous border. (E and F) Low- and high-power magnification views showing basophilic calcium deposits (at the tips of the black arrows) in the deep dermal blood vessels. Minimal associated inflammation is noted. More superficially, congested blood vessels are identified. Focal epidermal necrosis is present. (Courtesy of Timothy G. Berger, Anna K. Haemel, and Thaddeus W. Mully.)
Histologically, calcification of small- and medium-sized blood vessels is seen in the dermis and subcutaneous tissue, although this finding can be quite subtle and may be missed. Typically, there is little or no inflammatory infiltrate, but panniculitis (inflammation of adipose tissue) is often seen (65,70). Fibrin thrombi within vessels, epidermal ulceration, and ischemic necrosis of the epidermis, dermis, and/or subcutaneous tissue may occur (65,70). Close examination, multiple tissue sections, and Von Kossa staining, highlighting the calcium deposits, may be required for an accurate diagnosis.
Patients may present with hypercalcemia, hyperphosphatemia, elevated calcium-phosphate product, and elevated parathyroid hormone (PTH) levels. However, more recent data suggest that calcium, phosphate, and PTH levels are often normal at presentation (69,72,74,80). Patients may also have raised inflammatory markers, hyperglycemia, and hypercholesterolemia (68,69,72–74). The typical serum biochemistry profile of a patient with ESRD presenting with CUA is set out in Table 5. In isolation, these metabolic abnormalities lack the sensitivity for predicting disease, but their presence should be noted, because they increase the patient’s risk for vascular calcification. Radiographic imaging may be helpful in making the diagnosis. Small vascular calcifications and a net-like pattern of soft tissue calcification have been described on plain radiography, although this finding has not been validated. Computed tomography can also identify calcified arterioles in the soft tissues as well as calcification of the viscera. These findings and other radiographic findings seen in CUA are summarized in Table 5 (81–83).
Table 5.
Common serum biochemistry laboratory tests and radiographic findings in calcific uremic arteriolopathy
| Laboratory Tests (68,71,74,80) | Values Seen | Normal Values | Radiographic Study | Findings (Refs.) |
|---|---|---|---|---|
| Phosphate | >5.0 mg/dl | 3.0–4.5 mg/dl | Plain x-ray | Small vascular calcificationsa (81,82) |
| Calcium | >10.0 mg/dl | 9.0–10.5 mg/dl | Net-like pattern of soft tissue calcification (82) | |
| iPTH | >300 ng/L | 12–65 ng/L | ||
| Calcium-phosphate product | >70 mg2/dl2 | <50 mg2/dl2 | CT scan | Calcified arterioles in the soft tissues as well as calcification of visceral vessels (81–83) |
| Alkaline phosphatase | >300 IU/L | 44–147 IU/L | Mammography | Soft tissue arteriolar calcificationsb (82) |
| ESR | >30 mm/h | 0–20 mm/h | ||
| CRP | >5 mg/dl | <0.5 mg/dl | Bone scan | Increased tracer uptake in affected localized areas of subcutaneous tissues (82) |
| Aluminum | >25 ng/ml | 0–6 ng/ml (nondialysis); <60 ng/ml (dialysis) | ||
| Albumin | <3.5 g/dl | 3.5–5.5 g/dl |
Early recognition, diagnosis, and treatment of CUA are paramount to halt disease progression. However, there is a paucity of controlled, prospective clinical trial data to guide treatment, which is often difficult and supportive. Pain relief takes weeks, and lesion resolution can take several months.
Management includes correcting serum calcium and phosphate levels. Serum electrolyte levels should be, where possible, maintained at target per National Kidney Foundation guidelines (84). Lowering the calcium concentration in the dialysate to 1.0–1.5 mEq/L as tolerated and intensifying dialysis to four to six times per week have been used (76,77). Bisphosphonates, both intravenous and oral preparations, have been used successfully, resulting in pain relief within days and lesion resolution within weeks (75,85,86). Although bisphosphonate use is controversial in those patients with a GFR<30 ml/min because of the potential risk of adynamic bone disease and direct nephrotoxicity (87), the benefit of decreasing morbidity and/or mortality in the case of CUA outweighs the risks. The use of parathyroidectomy in patients with normal or mildly elevated iPTH (<200 ng/L) is controversial and may even increase morbidity and/or mortality. Medical management for patients with normal or mildly elevated iPTH is the gold standard and should be used in preference to parathyroidectomy, which should only be considered in this setting if medical management fails. However, parathyroidectomy may be required for those patients with significant iPTH elevations (>300 ng/L). Survival benefit has not been consistently shown in small case series or larger retrospective studies (74,75,88,89).
Sodium thiosulfate is a dialyzable calcium chelator that increases the solubility of calcium deposits. Sodium thiosulfate also enhances antioxidant glutathione production, has vasodilatory properties by increasing endothelial nitric oxide production, and can reduce inflammation (75,80,90). It has been shown to relieve pain within days and lead to wound healing in approximately 8 weeks (75).
Several recent case reports suggest that Cinacalcet, a calcimimetic agent that increases parathyroid cell calcium sensitivity, can be used successfully in patients with normal and elevated PTH levels to normalize electrolyte levels and may serve as a medical alternative to parathyroidectomy (75,76,91,92). Optimizing the nutritional status and if needed, albumin replacement in select patients may be beneficial (75). Wound care, with gentle surgical debridement, has been shown to improve survival in retrospective studies. However, its use is controversial because of an increased risk of infection and the potential to induce new lesions or cause additional progression of lesions if the disease process is still active (69,70,74,75). Antibiotics, skin grafting, and biologic dressings can be used to prevent infection (70,89). A recent analysis of tissue plasminogen activator as an adjunctive therapy did not show a survival benefit. It did show variable degrees of wound healing but with some significant risks (93). More data are needed to assess the use of tissue plasminogen activator. Hyperbaric oxygen therapy (HBO) promotes wound healing by improving tissue oxygenation. HBO has been used successfully to reverse CUA progression (70,75,76,89). Reported side effects are minimal, but access and cost of HBO limit its use (75).
The clinical management of CUA is challenging. Interventions focused on treating the different causes of vascular calcification and hypercoagulability have been shown to be effective in one case series that used a standardized treatment approach (77). Controlling and minimizing the triggering factors are also important adjuncts (70,76,89). Additional investigation to evaluate the use of a multi-intervention standardized protocol for calciphylaxis is required.
Nephrogenic Systemic Fibrosis
Nephrogenic systemic fibrosis (NSF) is a scleroderma-like disorder caused by exposure to gadolinium-based contrast agents (GBCAs) used in diagnostic imaging. NSF is seen almost exclusively in patients with renal insufficiency with a GFR<30 ml/min; however, NSF in normouremic patients has been reported. Patients with a GFR<15 ml/min and severe AKI on dialysis and patients with ESRD on dialysis, especially PD, are at the highest risk of developing NSF (94,95). It can also occur in renal transplant patients with abnormal graft function. The international NSF registry contains over 335 confirmed cases (96); however, this number is likely an underestimate of the total number of cases, because misdiagnosis was common before NSF was well described and widely known. Increased awareness and routine screening of patients undergoing GBCA-enhanced magnetic resonance imaging/angiography have led to a reduction in the incidence of NSF over the last few years (94). NSF affects men and women equally. It has no known association with ethnicity or etiology of the patient’s primary renal disease. Most cases are seen in middle-aged adults, with an average age at onset of 51 years (94).
The pathogenesis of NSF is related to gadolinium (Gd) exposure, GFR, type and frequency of dialysis, and presence of a concomitant triggering event. Gd is renally eliminated. In patients with normal renal function, Gd has a half-life of 1–2 hours but is markedly increased (up to 60 hours) in patients with renal insufficiency or on dialysis (94,97). A prolonged half-life allows Gd to disassociate into its toxic ionic form, which can form precipitates with anions, such as phosphates, and lead to tissue deposition. After deposited in the tissues, dissociated Gd is phagocytized by macrophages, which may lead to the recruitment and/or activation of circulating fibrocytes (CFs) into the area (98–100).
CFs, are fibroblast-like collagen-producing spindle cells that circulate within the peripheral blood and enter areas of inflammation and tissue injury (98–100). They are involved in wound repair, fibrosis, and cytokine/chemokine production, including TGF-β1 and PDGF, both of which are involved in tissue fibrosis (98,99,101). CFs have been implicated in causing pathologic fibrosis, such as in keloid formation and scleroderma (98,99). The rapid development and symmetry of NSF lesions and the proliferation of dermal spindle cells typically seen on biopsy specimens support the pathogenic role of CFs in NSF (98,99,101).
Patients with ESRD on PD, which is less effective at removing Gd than HD, and patients with an elevated serum phosphate level are at an increased risk of developing NSF (94,95). ESRD patients who receive a high dose (>0.2 mmol/kg) or multiple cumulative doses of GBCA (with an overall GBCA volume>0.2 mmol/kg) versus a single standard dose of GBCA (0.1 mmol/kg) and the use of linear or nonionic chelating agents pose the highest risk of NSF (94,101,102). Although no GBCA is risk-free, most cases have been associated with gadodiamide (Omniscan), a linear nonionic agent, which has less in vitro stability than the ionic and macrocylcic GBCAs (94,95,101).
Concomitant triggering factors include vascular injury (such as thrombosis and acute ischemia), infection, and high-dose erythropoietin therapy. These triggering factors are proinflammatory and stimulate the production of bone marrow-derived CFs, which increases CF migration (94,97,103).
Patients initially present with a severely painful, symmetric erythema and edema that evolve into sclerodermatous and erythematous induration of the skin with firm papules/plaques and nodules (Figure 7). Lesions are commonly symmetrical, distributed on the lower extremities, and less often on the arms and trunk. Patients can have hyper- or hypopigmentation of the skin, skin thickening, and swelling, especially of the hands. Progression of skin fibrosis can lead to flexion contractures, causing limited joint mobility (94,100,101). Although rare, patients can have rapidly progressive fibrosis and fulminant disease, sometimes within weeks of disease onset (97). Patients may also complain of pain, burning, and pruritus at sites of fibrosis. Fibrosis can affect the lungs, heart, liver, kidneys, intestines, and skeletal muscle (94,97).
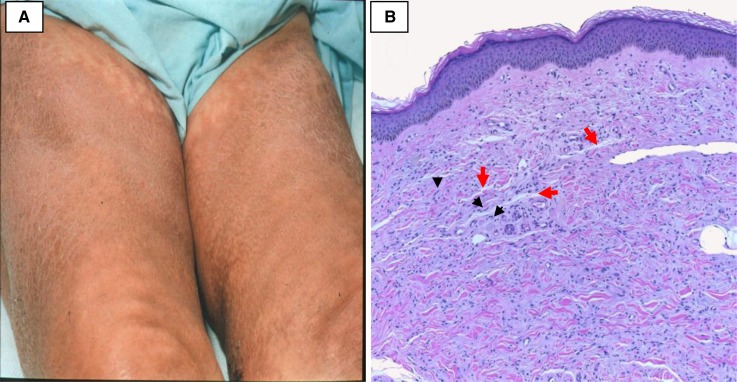
Figure 7.
Nephrogenic systemic fibrosis. (A) Hyperpigmentation and skin thickening of the thighs bilaterally. (B) Increased number of small spindle cells (at the tips of the black arrowheads) and collagen bundles within the dermis. The spaces that are normally seen between collagen bundles in the reticular dermis are effaced by mucin deposition (red arrows) and fine collagen bundles. (Courtesy of Philip E. LeBoit.)
The prominent histologic feature of NSF on deep skin biopsy is dermal fibrosis with thickened collagen bundles. Proliferation of dermal spindle cells, increased stromal mucin, and minimal inflammatory infiltrate are common. The epidermis is usually unaffected (98,99), and patients may have abnormal calcification in the dermis and subcutaneous tissue (99,104). The entire dermis may be involved, with the fibrocytes extending through the subcutaneous tissue. Fibrosis can extend through the fascia and into the skeletal muscle, which can lead to atrophy (98).
Diagnosis is made after medical history and examination and confirmed by the histologic features. Diagnosis can be difficult, because the initial clinical presentation is often subtle and can mimic scleroderma. Clinicians must have a high index of suspicion in ESRD patients recently exposed to Gd. Avoiding linear and ionic GBCAs in patients with a GFR<30 ml/min or AKI, avoiding high-dose GBCA, and dialyzing patients as soon as is reasonably possible after GBCA exposure is recommended to prevent NSF (94).
Spontaneous improvement of NSF has been reported in a few cases of AKI that resolved quickly (94,97,100). Improving renal function seems to slow NSF progression and may improve the signs and symptoms of disease (96,105). Renal transplant for patients with NSF does not guarantee disease improvement, but the majority of cases has had successful outcomes, with skin softening and increased joint mobility (94,97,105,106). Treatments with limited benefit include oral corticosteroids, ultraviolet A phototherapy, plasmapheresis, sirolimus, high-dose intravenous Ig, methotrexate, and pentoxifylline (97,100,104,107). Several case reports of extracorporeal photopheresis have shown moderate clinical improvement (100,108,109). Physical therapy can also be used to decrease the extent of joint contractures and improve joint mobility (96,97).
Imatinib mesylate (Gleevec) blocks Abelson murine leukemia viral oncogene homolog 1 and PDGF receptor signal transduction involved in mediating fibrosis. Skin softening, improvement in lesion size, decreased pain, and increased mobility were reported in three cases with imatinib treatment (110,111). An open-label, nonrandomized, uncontrolled clinical trial of imatinib in four patients showed improvement in disease progression and symptom severity (112). Alefacept, which acts to inhibit the activation of CD4+/CD8+ T lymphocytes and also induces the apoptosis of memory effector T lymphocytes, has been shown to be effective in treating graft-versus-host disease, which has sclerodermoid features. Alefacept improved the cutaneous manifestations of three patients in a single case series (113). Sodium thiosulfate was shown to increase joint mobility, lead to skin softening, and decrease pain in one case series and one case report (114,115). These therapies are promising, but additional investigation is needed.
Conclusion
There is a broad range of cutaneous manifestations of ESRD. They can usefully be divided into nonspecific and specific categories. Although often benign, they may decrease patients’ quality of life and can be life-threatening. Increasing clinical awareness and implementing preventive strategies combined with early detection and treatment are all required to decrease the morbidity and mortality of dermatological disorders in ESRD patients.
Disclosures
T.A.G. and K.S.L. have no disclosures. A.J.C. has received research funding from the National Institute for Health Research Biomedical Research Centre, Guy's and St. Thomas' National Health Service Foundation Trust, and King's College London.
Acknowledgments
We would like to thank Dr. Timothy G. Berger, Dr. Anna K. Haemel, Dr. Philip E. LeBoit, and Dr. Thaddeus W. Mully for allowing us to use their clinical and histological photographs for this manuscript.
The views expressed are the views of the authors and not necessarily the views of the National Health Service, the National Institute for Health Research, or the Department of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases; US Renal Data System: Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, 2012. Available at: http://www.usrds.org/2012/pdf/v2_ch1_12.pdf Accessed April 10, 2013
- 2.Goldman, L, Schafer AI : Chronic Kidney Disease. In: Goldman’s Cecil Medicine, Expert Consult Premium Edition, 24th Ed., Philadelphia, Elsevier Saunders, 2012 [Google Scholar]
- 3.Attia EA, Hassan SI, Youssef NM: Cutaneous disorders in uremic patients on hemodialysis: An Egyptian case-controlled study. Int J Dermatol 49: 1024–1030, 2010 [DOI] [PubMed] [Google Scholar]
- 4.Markova A, Lester J, Wang J, Robinson-Bostom L: Diagnosis of common dermopathies in dialysis patients: A review and update. Semin Dial 25: 408–418, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Airaghi L, Garofalo L, Cutuli MG, Delgado R, Carlin A, Demitri MT, Badalamenti S, Graziani G, Lipton JM, Catania A: Plasma concentrations of alpha-melanocyte-stimulating hormone are elevated in patients on chronic haemodialysis. Nephrol Dial Transplant 15: 1212–1216, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Kaw D, Malhotra D: Platelet dysfunction and end-stage renal disease. Semin Dial 19: 317–322, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Szepietowski JC, Reich A, Schwartz RA: Uraemic xerosis. Nephrol Dial Transplant 19: 2709–2712, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Yosipovitch G, Reis J, Tur E, Sprecher E, Yarnitsky D, Boner G: Sweat secretion, stratum corneum hydration, small nerve function and pruritus in patients with advanced chronic renal failure. Br J Dermatol 133: 561–564, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Löffler H, Effendy I: Prevention of irritant contact dermatitis. Eur J Dermatol 12: 4–9, 2002 [PubMed] [Google Scholar]
- 10.Salem A, Al Mokadem S, Attwa E, Abd El Raoof S, Ebrahim HM, Faheem KT: Nail changes in chronic renal failure patients under haemodialysis. J Eur Acad Dermatol Venereol 22: 1326–1331, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Yosipovitch G: New insights into the pathophysiology and treatment of chronic itch in patients with end-stage renal disease, chronic liver disease, and lymphoma. Int J Dermatol 49: 1–11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mistik S, Utas S, Ferahbas A, Tokgoz B, Unsal G, Sahan H, Ozturk A, Utas C: An epidemiology study of patients with uremic pruritus. J Eur Acad Dermatol Venereol 20: 672–678, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Narita I, Alchi B, Omori K, Sato F, Ajiro J, Saga D, Kondo D, Skatsume M, Maruyama S, Kazama JJ, Akazawa K, Gejyo F: Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int 69: 1626–1632, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Keithi-Reddy SR, Patel TV, Armstrong AW, Singh AK: Uremic pruritus. Kidney Int 72: 373–377, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Kimmel M, Alscher DM, Dunst R, Braun N, Machleidt C, Kiefer T, Stülten C, van der Kuip H, Pauli-Magnus C, Raub U, Kuhlmann U, Mettang T: The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol Dial Transplant 21: 749–755, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Dyachenko P, Shustak A, Rozenman D: Hemodialysis-related pruritus and associated cutaneous manifestations. Int J Dermatol 45: 664–667, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Patel TS, Freedman BI, Yosipovitch G: An update on pruritus associated with CKD. Am J Kidney Dis 50: 11–20, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Pisoni RL, Wikström B, Elder SJ, Akizawa T, Asano Y, Keen ML, Saran R, Mendelssohn DC, Young EW, Port FK: Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 21: 3495–3505, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Szepietowski JC, Balaskas E, Taube KM, Taberly A, Dupuy P, Uraemic Xerosis Working Group : Quality of life in patients with uraemic xerosis and pruritus. Acta Derm Venereol 91: 313–317, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Gunal AI, Ozalp G, Yoldas TK, Gunal SY, Kirciman E, Celiker H: Gabapentin therapy for pruritus in haemodialysis patients: A randomized, placebo-controlled, double-blind trial. Nephrol Dial Transplant 19: 3137–3139, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Naini AE, Harandi AA, Khanbabapour S, Shahidi S, Seirafiyan S, Mohseni M: Gabapentin: A promising drug for the treatment of uremic pruritus. Saudi J Kidney Dis Transpl 18: 378–381, 2007 [PubMed] [Google Scholar]
- 22.Razeghi E, Eskandari D, Ganji MR, Meysamie AP, Togha M, Khashayar P: Gabapentin and uremic pruritus in hemodialysis patients. Ren Fail 31: 85–90, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Aperis G, Paliouras C, Zervos A, Arvanitis A, Alivanis P: The use of pregabalin in the treatment of uraemic pruritus in haemodialysis patients. J Ren Care 36: 180–185, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Solak Y, Biyik Z, Atalay H, Gaipov A, Guney F, Turk S, Covic A, Goldsmith D, Kanbay M: Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: A prospective, crossover study. Nephrology (Carlton) 17: 710–717, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Shavit L, Grenader T, Lifschitz M, Slotki I: Use of pregabalin in the management of chronic uremic pruritus. J Pain Symptom Manage 45: 776–781, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, Ueno Y: Kappa-opioid system in uremic pruritus: Multicenter, randomized, double-blind, placebo-controlled clinical studies. J Am Soc Nephrol 16: 3742–3747, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kumagai H, Ebata T, Takamori K, Muramatsu T, Nakamoto H, Suzuki H: Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: A Phase III, randomized, double-blind, placebo-controlled study. Nephrol Dial Transplant 25: 1251–1257, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Kumagai H, Ebata T, Takamori K, Miyasato K, Muramatsu T, Nakamoto H, Kurihara M, Yanagita T, Suzuki H: Efficacy and safety of a novel ĸ-agonist for managing intractable pruritus in dialysis patients. Am J Nephrol 36: 175–183, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Vessal G, Sagheb MM, Shilian S, Jafari P, Samani SM: Effect of oral cromolyn sodium on CKD-associated pruritus and serum tryptase level: A double-blind placebo-controlled study. Nephrol Dial Transplant 25: 1541–1547, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Feily A, Dormanesh B, Ghorbani AR, Moosavi Z, Kouchak M, Cheraghian B, Mousavi SSB, Mehrabian A, Ranjbari N: Efficacy of topical cromolyn sodium 4% on pruritus in uremic nephrogenic patients: A randomized double-blind study in 60 patients. Int J Clin Pharmacol Ther 50: 510–513, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Aramwit P, Keongamaroon O, Siritientong T, Bang N, Supasyndh O: Sericin cream reduces pruritus in hemodialysis patients: A randomized, double-blind, placebo-controlled experimental study. BMC Nephrol 13: 119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen ZJ, Cao G, Tang WX, Lv XY, Huang SM, Qin W, Ping F, Ye T: A randomized controlled trial of high-permeability haemodialysis against conventional haemodialysis in the treatment of uraemic pruritus. Clin Exp Dermatol 34: 679–683, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Makhlough A, Ala S, Haj-Heydari Z, Kashi Z, Bari A: Topical capsaicin therapy for uremic pruritus in patients on hemodialysis. Iran J Kidney Dis 4: 137–140, 2010 [PubMed] [Google Scholar]
- 34.Young TA, Patel TS, Camacho F, Clark A, Freedman BI, Kaur M, Fountain J, Williams LL, Yosipovitch G, Fleischer AB, Jr.: A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. J Dermatolog Treat 20: 76–81, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Ada S, Seçkin D, Budakoğlu İ, Özdemir FN: Treatment of uremic pruritus with narrowband ultraviolet B phototherapy: An open pilot study. J Am Acad Dermatol 53: 149–151, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Seckin D, Demircay Z, Akin O: Generalized pruritus treated with narrowband UVB. Int J Dermatol 46: 367–370, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Ko M-J, Yang J-Y, Wu H-Y, Hu F-C, Chen S-I, Tsai P-J, Jee S-H, Chiu H-C: Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: A randomized controlled trial. Br J Dermatol 165: 633–639, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Shakiba M, Sanadgol H, Azmoude HR, Mashhadi MA, Sharifi H: Effect of sertraline on uremic pruritus improvement in ESRD patients. Int J Nephrol 2012: 363901, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y-C, Chiu W-T, Wu M-S: Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. Am J Kidney Dis 48: 69–76, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Silva SR, Viana PC, Lugon NV, Hoette M, Ruzany F, Lugon JR: Thalidomide for the treatment of uremic pruritus: A crossover randomized double-blind trial. Nephron 67: 270–273, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Giovannetti S, Barsotti G, Cupisti A, Dani L, Bandini S, Angelini D, Antonelli A, Salvadori M, Urti DA: Oral activated charcoal in patients with uremic pruritus. Nephron 70: 193–196, 1995 [DOI] [PubMed] [Google Scholar]
- 42.Balaskas E, Szepietowski JC, Bessis D, Ioannides D, Ponticelli C, Ghienne C, Taberly A, Dupuy P: Randomized, double-blind study with glycerol and paraffin in uremic xerosis. Clin J Am Soc Nephrol 6: 748–752, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feramisco JD, Berger TG, Steinhoff M: Innovative management of pruritus. Dermatol Clin 28: 467–478, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Hong S-B, Park J-H, Ihm C-G, Kim N-I: Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci 19: 283–288, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morton CA, Henderson IS, Jones MC, Lowe JG: Acquired perforating dermatosis in a British dialysis population. Br J Dermatol 135: 671–677, 1996 [PubMed] [Google Scholar]
- 46.Hari Kumar KV, Prajapati J, Pavan G, Parthasarathy A, Jha R, Modi KD: Acquired perforating dermatoses in patients with diabetic kidney disease on hemodialysis. Hemodial Int 14: 73–77, 2010 [DOI] [PubMed] [Google Scholar]
- 47.Saray Y, Seçkin D, Bilezikçi B: Acquired perforating dermatosis: Clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol 20: 679–688, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Weng C-H, Hu C-C, Ueng S-H, Yu C-C, Hui C-Y, Lin J-L, Yang C-W, Hung C-C, Hsu C-W, Yen T-H: Predictors of acquired perforating dermatosis in uremic patients on hemodialysis: A case-control study. ScientificWorldJournal 2012: 158075, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karpouzis A, Giatromanolaki A, Sivridis E, Kouskoukis C: Acquired reactive perforating collagenosis: Current status. J Dermatol 37: 585–592, 2010 [DOI] [PubMed] [Google Scholar]
- 50.Satchell AC, Crotty K, Lee S: Reactive perforating collagenosis: A condition that may be underdiagnosed. Australas J Dermatol 42: 284–287, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Ohe S, Danno K, Sasaki H, Isei T, Okamoto H, Horio T: Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol 50: 892–894, 2004 [DOI] [PubMed] [Google Scholar]
- 52.Gambichler T, Altmeyer P, Kreuter A: Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol 52: 363–364, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Berger RS: Reactive perforating collagenosis of renal failure/diabetes responsive to topical retinoic acid. Cutis 43: 540–542, 1989 [PubMed] [Google Scholar]
- 54.Hoque SR, Ameen M, Holden CA: Acquired reactive perforating collagenosis: Four patients with a giant variant treated with allopurinol. Br J Dermatol 154: 759–762, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Gnanaraj P, Venugopal V, Sangitha C, Rajagopalan V, Pandurangan CN: A giant variant of acquired reactive perforating collagenosis associated with hydronephrosis: Successful treatment with allopurinol. Int J Dermatol 48: 204–206, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Sezer E, Erkek E: Acquired perforating dermatosis successfully treated with photodynamic therapy. Photodermatol Photoimmunol Photomed 28: 50–52, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Wong J, Phelps R, Levitt J: Treatment of acquired perforating dermatosis with cantharidin. Arch Dermatol 148: 160–162, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L: Acquired disorders of elastic tissue: Part I. Increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol 51: 1–21, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Gönül M, Cakmak SK, Gül U, Kiliç A, Ergül G: Two cases of acquired perforating dermatosis treated with doxycycline therapy. Int J Dermatol 45: 1461–1463, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Brinkmeier T, Schaller J, Herbst RA, Frosch PJ: Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol 82: 393–395, 2002 [DOI] [PubMed] [Google Scholar]
- 61.Tsuboi H, Katsuoka K: Characteristics of acquired reactive perforating collagenosis. J Dermatol 34: 640–644, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Kurban MS, Boueiz A, Kibbi A-G: Cutaneous manifestations of chronic kidney disease. Clin Dermatol 26: 255–264, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Ryali ME, Whittier WL: Bullous skin lesions in a patient undergoing chronic hemodialysis. Semin Dial 23: 83–87, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Balwani M, Desnick RJ: The porphyrias: Advances in diagnosis and treatment. Blood 120: 4496–4504, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reiter N, El-Shabrawi L, Leinweber B, Berghold A, Aberer E: Calcinosis cutis: Part I. Diagnostic pathway. J Am Acad Dermatol 65: 1–12, 2011 [DOI] [PubMed] [Google Scholar]
- 66.Saliba W, El-Haddad B: Secondary hyperparathyroidism: Pathophysiology and treatment. J Am Board Fam Med 22: 574–581, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Martin KJ, González EA: Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: What is normal, when to start, and how to treat? Clin J Am Soc Nephrol 6: 440–446, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Rivet J, Lebbé C, Urena P, Cordoliani F, Martinez F, Baglin AC, Aubert P, Aractingi S, Ronco P, Fournier P, Janin A: Cutaneous calcification in patients with end-stage renal disease: A regulated process associated with in situ osteopontin expression. Arch Dermatol 142: 900–906, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Weenig RH, Sewell LD, Davis MDP, McCarthy JT, Pittelkow MR: Calciphylaxis: Natural history, risk factor analysis, and outcome. J Am Acad Dermatol 56: 569–579, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Daudén E, Oñate M-J: Calciphylaxis. Dermatol Clin 26: 557–568, 2008 [DOI] [PubMed] [Google Scholar]
- 71.Fine A, Zacharias J: Calciphylaxis is usually non-ulcerating: Risk factors, outcome and therapy. Kidney Int 61: 2210–2217, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, Sato Y, Japanese Calciphylaxis Study Group : A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant 27: 1580–1584, 2012 [DOI] [PubMed] [Google Scholar]
- 73.Weenig RH: Pathogenesis of calciphylaxis: Hans Selye to nuclear factor κ-B. J Am Acad Dermatol 58: 458–471, 2008 [DOI] [PubMed] [Google Scholar]
- 74.Lal G, Nowell AG, Liao J, Sugg SL, Weigel RJ, Howe JR: Determinants of survival in patients with calciphylaxis: A multivariate analysis. Surgery 146: 1028–1034, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Vedvyas C, Winterfield LS, Vleugels RA: Calciphylaxis: A systematic review of existing and emerging therapies. J Am Acad Dermatol 67: e253–e260, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Sowers KM, Hayden MR: Calcific uremic arteriolopathy: Pathophysiology, reactive oxygen species and therapeutic approaches. Oxid Med Cell Longev 3: 109–121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baldwin C, Farah M, Leung M, Taylor P, Werb R, Kiaii M, Levin A: Multi-intervention management of calciphylaxis: A report of 7 cases. Am J Kidney Dis 58: 988–991, 2011 [DOI] [PubMed] [Google Scholar]
- 78.Neven E, De Schutter TM, Behets GJ, Gupta A, D’Haese PC: Iron and vascular calcification. Is there a link? Nephrol Dial Transplant 26: 1137–1145, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Videla LA, Pettinelli P: Misregulation of PPAR functioning and its pathogenic consequences associated with nonalcoholic fatty liver disease in human obesity. PPAR Res 2012: 107434, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zitt E, Konig M, Vychytil A, Auinger M, Wallner M, Lingenhel G, Schilcher G, Rudnicki M, Salmhofer H, Lhotta K: Use of sodium thiosulphate in a multi-interventional setting for the treatment of calciphylaxis in dialysis patients. Nephrol Dial Transplant 28: 1232–1240, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Li Y-J, Tian Y-C, Chen Y-C, Huang S-F, Huang C-C, Fang J-T, Yang C-W: Fulminant pulmonary calciphylaxis and metastatic calcification causing acute respiratory failure in a uremic patient. Am J Kidney Dis 47: e47–e53, 2006 [DOI] [PubMed] [Google Scholar]
- 82.Shmidt E, Murthy NS, Knudsen JM, Weenig RH, Jacobs MA, Starnes AM, Davis MDP: Net-like pattern of calcification on plain soft-tissue radiographs in patients with calciphylaxis. J Am Acad Dermatol 67: 1296–1301, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Tom CW, Talreja DR: Heart of stone. Mayo Clin Proc 81: 335, 2006 [DOI] [PubMed] [Google Scholar]
- 84.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 85.Hanafusa T, Yamaguchi Y, Tani M, Umegaki N, Nishimura Y, Katayama I: Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol 57: 1021–1025, 2007 [DOI] [PubMed] [Google Scholar]
- 86.Torregrosa J-V, Durán CE, Barros X, Blasco M, Arias M, Cases A, Campistol JM: Successful treatment of calcific uraemic arteriolopathy with bisphosphonates. Nefrologia 32: 329–334, 2012 [DOI] [PubMed] [Google Scholar]
- 87.Toussaint ND, Elder GJ, Kerr PG: Bisphosphonates in chronic kidney disease; balancing potential benefits and adverse effects on bone and soft tissue. Clin J Am Soc Nephrol 4: 221–233, 2009 [DOI] [PubMed] [Google Scholar]
- 88.Duffy A, Schurr M, Warner T, Chen H: Long-term outcomes in patients with calciphylaxis from hyperparathyroidism. Ann Surg Oncol 13: 96–102, 2006 [DOI] [PubMed] [Google Scholar]
- 89.Ross EA: Evolution of treatment strategies for calciphylaxis. Am J Nephrol 34: 460–467, 2011 [DOI] [PubMed] [Google Scholar]
- 90.Auriemma M, Carbone A, Di Liberato L, Cupaiolo A, Caponio C, De Simone C, Tulli A, Bonomini M, Amerio P: Treatment of cutaneous calciphylaxis with sodium thiosulfate: Two case reports and a review of the literature. Am J Clin Dermatol 12: 339–346, 2011 [DOI] [PubMed] [Google Scholar]
- 91.Robinson MR, Augustine JJ, Korman NJ: Cinacalcet for the treatment of calciphylaxis. Arch Dermatol 143: 152–154, 2007 [DOI] [PubMed] [Google Scholar]
- 92.Mohammed IA, Sekar V, Bubtana AJ, Mitra S, Hutchison AJ: Proximal calciphylaxis treated with calcimimetic “Cinacalcet.” Nephrol Dial Transplant 23: 387–389, 2008 [DOI] [PubMed] [Google Scholar]
- 93.el-Azhary RA, Arthur AK, Davis MD, McEvoy MT, Gibson LE, Weaver AL, Camilleri MJ, Wetter DA, Weenig RH: Retrospective analysis of tissue plasminogen activator as an adjuvant treatment for calciphylaxis. JAMA Dermatol 149: 63–67, 2013 [DOI] [PubMed] [Google Scholar]
- 94.Zou Z, Zhang HL, Roditi GH, Leiner T, Kucharczyk W, Prince MR: Nephrogenic systemic fibrosis: Review of 370 biopsy-confirmed cases. JACC Cardiovasc Imaging 4: 1206–1216, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Elmholdt TR, Pedersen M, Jørgensen B, Søndergaard K, Jensen JD, Ramsing M, Olesen AB: Nephrogenic systemic fibrosis is found only among gadolinium-exposed patients with renal insufficiency: A case-control study from Denmark. Br J Dermatol 165: 828–836, 2011 [DOI] [PubMed] [Google Scholar]
- 96.Cowper S: ICNSFR, 2012. Available at: http://www.icnsfr.org/ Accessed April 24, 2013
- 97.Schieren G, Wirtz N, Altmeyer P, Rump LC, Weiner SM, Kreuter A: Nephrogenic systemic fibrosis—a rapidly progressive disabling disease with limited therapeutic options. J Am Acad Dermatol 61: 868–874, 2009 [DOI] [PubMed] [Google Scholar]
- 98.Thakral C, Abraham JL: Nephrogenic systemic fibrosis: Histology and gadolinium detection. Radiol Clin North Am 47: 841–853, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Quan TE, Cowper S, Wu S-P, Bockenstedt LK, Bucala R: Circulating fibrocytes: Collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 36: 598–606, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Introcaso CE, Hivnor C, Cowper S, Werth VP: Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: A case series of nine patients and review of the literature. Int J Dermatol 46: 447–452, 2007 [DOI] [PubMed] [Google Scholar]
- 101.Broome DR: Nephrogenic systemic fibrosis associated with gadolinium based contrast agents: A summary of the medical literature reporting. Eur J Radiol 66: 230–234, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Bhave G, Lewis JB, Chang SS: Association of gadolinium based magnetic resonance imaging contrast agents and nephrogenic systemic fibrosis. J Urol 180: 830–835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS: Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17: 2359–2362, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Song J, Volkov S, Shea CR, Alegre M-L, Salgia R, Gregg K, Curran JJ, Woodruff J, Krausz T, Levine JS, Sweiss NJ: Nephrogenic systemic fibrosis associated with stromal and vascular calcification, report of two cases. J Cutan Pathol 36[Suppl 1]: 31–34, 2009 [DOI] [PubMed] [Google Scholar]
- 105.Cuffy MC, Singh M, Formica R, Simmons E, Abu Alfa AK, Carlson K, Girardi M, Cowper SE, Kulkarni S: Renal transplantation for nephrogenic systemic fibrosis: A case report and review of the literature. Nephrol Dial Transplant 26: 1099–1101, 2011 [DOI] [PubMed] [Google Scholar]
- 106.Panesar M, Banerjee S, Barone GW: Clinical improvement of nephrogenic systemic fibrosis after kidney transplantation. Clin Transplant 22: 803–808, 2008 [DOI] [PubMed] [Google Scholar]
- 107.Swaminathan S, Arbiser JL, Hiatt KM, High W, Abul-Ezz S, Horn TD, Shah SV: Rapid improvement of nephrogenic systemic fibrosis with rapamycin therapy: Possible role of phospho-70-ribosomal-S6 kinase. J Am Acad Dermatol 62: 343–345, 2010 [DOI] [PubMed] [Google Scholar]
- 108.Richmond H, Zwerner J, Kim Y, Fiorentino D: Nephrogenic systemic fibrosis: Relationship to gadolinium and response to photopheresis. Arch Dermatol 143: 1025–1030, 2007 [DOI] [PubMed] [Google Scholar]
- 109.Mathur K, Morris S, Deighan C, Green R, Douglas KW: Extracorporeal photopheresis improves nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: Three case reports and review of literature. J Clin Apher 23: 144–150, 2008 [DOI] [PubMed] [Google Scholar]
- 110.Chandran S, Petersen J, Jacobs C, Fiorentino D, Doeden K, Lafayette RA: Imatinib in the treatment of nephrogenic systemic fibrosis. Am J Kidney Dis 53: 129–132, 2009 [DOI] [PubMed] [Google Scholar]
- 111.Kay J, High WA: Imatinib mesylate treatment of nephrogenic systemic fibrosis. Arthritis Rheum 58: 2543–2548, 2008 [DOI] [PubMed] [Google Scholar]
- 112.Elmholdt TR, Buus NH, Ramsing M, Olesen AB: Antifibrotic effect after low-dose imatinib mesylate treatment in patients with nephrogenic systemic fibrosis: An open-label non-randomized, uncontrolled clinical trial. J Eur Acad Dermatol Venereol 27: 779–784, 2013 [DOI] [PubMed] [Google Scholar]
- 113.Robinson MR, Routhouska SB, Paspulati RM, Korman NJ: Alefacept therapy for nephrogenic systemic fibrosis: A case series. J Drugs Dermatol 10: 922–924, 2011 [PubMed] [Google Scholar]
- 114.Kadiyala D, Roer DA, Perazella MA: Nephrogenic systemic fibrosis associated with gadoversetamide exposure: Treatment with sodium thiosulfate. Am J Kidney Dis 53: 133–137, 2009 [DOI] [PubMed] [Google Scholar]
- 115.Yerram P, Saab G, Karuparthi PR, Hayden MR, Khanna R: Nephrogenic systemic fibrosis: A mysterious disease in patients with renal failure—role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol 2: 258–263, 2007 [DOI] [PubMed] [Google Scholar]