Abstract
A vervet monkey model of trypanosomiasis was used to study inflammatory cytokine responses in serum and cerebrospinal fuid (CSF). Gamma interferon levels were transiently up-regulated in serum between days 6 and 8 of infection, followed by a sustained up-regulation of tumor necrosis factor alpha (TNF-α) and soluble TNF receptor 1. At no time were these cytokines detectable in the CSF.
Human African trypanosomiasis (HAT) is caused by the flagellate protozoan parasites Trypanosoma brucei rhodesiense and T. brucei gambiense, which are spread by tsetse flies of the genus Glossina. T. brucei rhodesiense causes an acute illness in eastern Africa, while T. brucei gambiense causes a chronic disease in west and central Africa. The parasites multiply extracellularly in the bloodstream and are disseminated via the blood to the lymph nodes, spleen, heart, and brain. The disease follows a two-stage clinical course manifesting an early-stage hemolymphatic trypanosome proliferation and a late-stage central nervous system (CNS) infection. The involvement of the CNS causes irreversible neurological damage culminating in coma and death in the absence of treatment (2).
Studies in mouse model infections suggest that the disease severity is associated with inflammatory responses. In vitro studies suggest that components of the glycosyl phosphatidylinositol anchor of the variant surface glycoprotein molecule when shed induce macrophage activation (7, 9), and in mice, the early stages of infections are characterized by up-regulated synthesis of the Th1 and proinflammatory cytokines, such as gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) (1, 11). Extended survival depends on a change of cytokine profile to a counterinflammatory and Th2 pattern (8). However, relatively little is known about the role of cytokines in the pathogenesis of human African trypanosome infections. IFN-γ and TNF-α were shown to be up-regulated in human T. brucei rhodesiense infection, but the timing of the onset of these responses was not amenable to study as patients reporting of infection duration is notoriously unreliable (5, 6). Also, after CNS infection with trypanosomes, high levels of interleukin-10 were detected in the plasma and cerebrospinal fluid (CSF) (6, 10), suggesting a late counterinflammatory response in HAT, but again the precise onset of this response in relation to the inflammatory response was unclear. A better understanding of the immunological events that occur in HAT will be essential in identifying the precise role of inflammatory responses in the pathophysiology of the infection. The vervet monkey model produces a disease that is clinically similar to that observed in humans and therefore is a potentially useful model of infection. In this study, the hypothesis that trypanosomiasis results in early upregulation of macrophage activation and proinflammatory cytokines in a primate model was tested.
All procedures were reviewed and approved by the KETRI Institutional Animal Care and Use Committee. Ten vervet monkeys (Cercopithecus aethiops) weighing between 2.5 and 4 kg were quarantined for 90 days and repeatedly screened for disease before enrollment into the study. Six of the animals were intravenously inoculated with a 104-organism T. brucei rhodesiense KETRI 2537 stabilate, and four remained as uninfected controls. The animals were maintained on a diet of fresh vegetables, fruits, green maize, and commercial primate pellets given twice daily and water ad libitum. The animals were clinically examined, and parasitemia was counted microscopically daily in ear-prick blood. Seven days before infection and on days 0, 2, 4, 6, 8, 10, 12, 14, 21, 28, 35, and 42 postinfection, the animals were anesthetized with Valium (1.0 mg/kg) and ketamine hydrochloride (10 to 15 mg/kg of body weight) before undergoing detailed clinical examination and sampling. Five milliliters of blood was withdrawn by inguinal venipuncture for serum preparation. In addition, 1 to 2 ml of CSF samples was removed via lumbar puncture at weekly intervals from day 7 preinfection up to day 42 postinfection. The samples were stored at −20°C before they were analyzed.
The levels of IFN-γ, TNF-α, and soluble TNF receptor 1 (sTNF-R1) in serum and CSF were determined by use of sandwich enzyme-linked immunosorbent assays. Human IFN-γ (Biosource International, Camarillo, Calif.) and primate (rhesus monkey)-specific TNF-α and sTNF-R1 (Bender MedSystems Diagnostics, Vienna, Austria) sets of capture and detection antibody pairs were used. The detection limits of the assays were <8 pg/ml, <9.375 U/ml, and <0.094 U/ml, respectively, for IFN-γ, TNF-α, and sTNF-R1. The cytokine levels were measured in duplicate for each sample. Values of cytokine concentrations in samples were calculated from a curve obtained from recombinant cytokine standards. Nitric oxide synthesis was measured by reduction of serum and CSF nitrate to nitrite with Aspergillus nitrate reductase followed by the Griess reaction (5).
The Kruskal-Wallis test was employed to assess quantitative changes in cytokine concentrations, while the Mann-Whitney U test was used to determine differences in the cytokine concentrations between uninfected and infected animals. Spearman's rank correlation coefficients were used to examine the relationships between cytokines and clinical variables during infection. P < 0.05 was considered statistically significant.
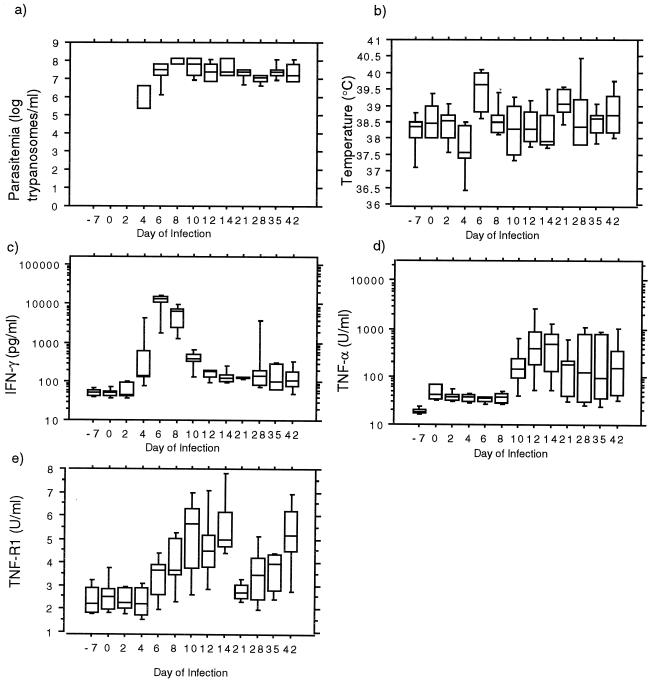
Trypanosomes were detected in all infected animals from day 4 (median, 2.5 × 105 parasites/ml) up to day 42 postinfection (Fig. 1a). The trypanosomes multiplied and reached peak levels on day 8 postinfection (median, 6.3 × 107 parasites/ml) and subsequently remained high with slight fluctuations over the course of the infection. The CSF parasitosis in all infected animals was detected on day 7 postinfection and for the entire duration of the infection. The body temperatures of infected animals varied intermittently during the infection (Fig. 1b) but were only significantly elevated above control group levels on day 6 postinfection (median, 39.7 versus 38.3°C; P < 0.05).
FIG. 1.
Parasitemia (a), body temperature (b), plasma IFN-γ (c), TNF-α (d), and sTNF-R1 (e) in T. brucei rhodesiense-infected vervet monkeys. Boxes show the median and 25th and 75th percentiles, with 10th and 90th percentiles represented by error bars.
Serum IFN-γ concentrations in infected animals significantly increased over control levels (P < 0.001, Fig. 1c), with levels increasing to peak on day 6 postinfection (median, 13,175.6 versus 54.2 pg/ml; P < 0.05). The increase in IFN-γ correlated with body temperature (r = 0.37; P < 0.005). CSF IFN-γ levels (median, 50 pg/ml) did not vary significantly between infected and control animals at any time during infection.
T. brucei rhodesiense infection also resulted in elevated concentrations of TNF-α in serum (P < 0.001, Fig. 1d). TNF-α levels increased from day 10 postinfection to peak at day 14 (median, 492.1 versus 40.4 U/ml; P < 0.05). The concentrations of TNF-α in serum then declined but remained elevated above the preinfection or control levels. In contrast, the TNF-α concentrations in the CSF were low (median, 18.8 U/ml) and did not show significant changes in both study animal groups. Interestingly, TNF-α levels showed no significant correlation to body temperature. Serum sTNF-R1 concentrations were also elevated compared to those of the controls in infected animals from day 8 (P < 0.05; Fig. 1e). The concentration of sTNF-R1 in the CSF did not vary significantly in either group of experimental animals (median, 5.98 U/ml).
The serum nitrate concentration in both infected and control animals fluctuated in this study, and there was no significant increase in infected animals at any time point. Also, nitrate was not detectable in the CSF in any animal.
This study provides new information on the early stages of HAT in a primate model. It confirms the hypothesis that the early response to infection is a systemic inflammatory response. We propose that the inflammatory response is driven by an initial pulse of high IFN-γ levels, which appears briefly at day 6 of infection, shortly after parasites are first detected microscopically in the blood. This is followed at day 8 of infection by steadily increasing concentrations of the proinflammatory cytokines TNF-α and sTNF-R1, which is released as a consequence of TNF interaction with and proteolytic cleavage of the p55 membrane receptor (12) and may act as an antagonist for the inflammatory activity of TNF. Plasma TNF levels decline after day 14 of infection but remain elevated over the control. Interestingly, significant pyrexia was only detected at day 6, and at no time point was plasma nitrate elevated. Recent data from HAT patients also failed to detect significant pyrexia or evidence of nitric oxide synthase activation (6). Invasion of the CSF is rapid in the primate model. Parasites were detectable from day 7 of infection. However, at no time point are any inflammatory cytokines or nitrate detectable in the CSF. This finding has an important bearing on models of pathogenesis in late-stage trypanosomiasis. In mouse models, CNS-produced inflammatory cytokines (3) and NO (4) have been detected and have been associated with late-stage neuropathology. The results in this primate suggest that this may not be the case in human infection, and indeed a study of the CSF in late-stage trypanosomiasis patients identified high levels of the counterinflammatory cytokine interleukin-10 (6).
Acknowledgments
This project was funded by the Wellcome Trust.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Darji, A., A. Beschin, M. Sileghem, H. Heremans, L. Brys, and P. De Baetselier. 1996. In vitro simulation of immunosuppression caused by Trypanosoma brucei: active involvement of gamma interferon and tumor necrosis factor in the pathway of suppression. Infect. Immun. 64:1937-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Atouguia, J. L. M., and P. G. E. Kennedy. 2000. Neurological aspects of human African trypanosomiasis, p. 321-372. In L. E. Davis and P. G. E. Kennedy (ed.), Infectious diseases of the nervous system. Butterworth-Heinemann, Oxford, United Kingdom.
- 3.Hunter, C. A., F. W. Jennings, P. G. E. Kennedy, and M. Murray. 1992. Astrocyte activation correlates with cytokine production in central nervous system of Trypanosoma brucei brucei-infected mice. Lab. Investig. 67:635-641. [PubMed] [Google Scholar]
- 4.Keita, M., P. Vincendeau, A. Buguet, R. Cespuglio, J.-M. Vallat, M. Dumas, and B. Bouteille. 2000. Inducible nitric oxide synthase and nitrotyrosine in the central nervous system of mice chronically infected with Trypanosoma brucei brucei. Exp. Parasitol. 95:19-27. [DOI] [PubMed] [Google Scholar]
- 5.MacLean, L., M. Odiit, D. Okitoi, and J. M. Sternberg. 1999. Plasma nitrate and interferon-gamma in Trypanosoma brucei rhodesiense infections: evidence that nitric oxide production is induced during both early blood-stage and late meningoencephalitic-stage infections. Trans. R. Soc. Trop. Med. Hyg. 93:169-170. [DOI] [PubMed] [Google Scholar]
- 6.MacLean, L., M. Odiit, and J. M. Sternberg. 2001. Nitric oxide and cytokine synthesis in human African trypanosomiasis. J. Infect. Dis. 184:1086-1090. [DOI] [PubMed] [Google Scholar]
- 7.Magez, S., B. Stijlemans, M. Radwanska, E. Pays, M. A. J. Ferguson, and P. De Baetselier. 1998. The glycosyl-inositol-phosphate and dimyristoyl glycerol moieties of the glycosylphosphatidylinositol anchor of the trypanosome variant-specific surface glycoprotein are distinct macrophage-activating factors. J. Immunol. 160:1949-1956. [PubMed] [Google Scholar]
- 8.Namangala, B., W. Noel, P. De Baetselier, L. Brys, and A. Beschin. 2001. Relative contribution of interferon-y and interleukin-10 to resistance to murine African trypanosomosis. J. Infect. Dis. 183:1794-1800. [DOI] [PubMed] [Google Scholar]
- 9.Paulnock, D. M., and S. P. Coller. 2001. Analysis of macrophage activation in African trypanosomiasis. J. Leukoc. Biol. 69:685-690. [PubMed] [Google Scholar]
- 10.Rhind, S. G., B. H. Sabiston, P. N. Shek, A. Buguet, G. Muanga, A. Stanghellini, M. Dumas, and M. W. Randomski. 1997. Effect of melarsoprol treatment on circulating IL-10 and TNF-alpha levels in human African trypanosomiasis. Clin. Immunol. Immunopathol. 83:185-189. [DOI] [PubMed] [Google Scholar]
- 11.Sternberg, J. M. 1998. Immunobiology of African trypanosomiasis. Chem. Immunol. 70:186-199. [DOI] [PubMed] [Google Scholar]
- 12.Truyens, C., F. Torrico, R. Lucas, P. De Baetselier, W. A. Burrman, and Y. Carlier. 1999. The endogenous balance of soluble tumor necrosis factor receptors and tumor necrosis factor modulates cachexia and mortality in mice acutely infected with Trypansoma cruzi. Infect. Immun. 67:5579-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]