Abstract
Purpose
Dabigatran is approved for prevention of stroke or systemic embolism in patients with nonvalvular atrial fibrillation (AF). The safety and effectiveness of periprocedural dabigatran in left atrial ablation for AF are unknown.
Methods
We performed a meta-analysis of all studies comparing periprocedural dabigatran with warfarin for anticoagulation in AF ablation. Studies of >100 patients with post-procedure follow-up were included. Outcomes were compared by calculating maximum likelihood estimates with confidence intervals. The co-primary endpoints were neurological events and major bleeding.
Results
Ten cohort studies were included, including a total of 1,501 patients receiving dabigatran and 2,356 receiving warfarin. The mean age was 59–64 years and inclusion of women varied (10–33 %). Intra-procedural unfractionated heparin and irrigated ablation catheters were used routinely. Adverse events were low overall; however, the dabigatran group demonstrated a numerical excess of neurological events (10/1,501 [0.7 %] versus 4/2,356 [0.2 %]), but equivalent major bleeding outcomes (24/1,501 [1.6 %] versus 40/2,356 [1.7 %]). In the meta-analysis, there was a nonsignificant trend towards higher rates of the composite primary endpoints (any neurological event or major bleeding) in the dabigatran group. Dabigatran demonstrated a significantly higher rate of neurological events (estimated absolute risk difference 0.0047, 95 % confidence interval 0.0007 to 0.0099).
Conclusions
Compared with warfarin, dabigatran may be associated with a higher frequency of periprocedural neurological events following radiofrequency ablation of AF. Randomized clinical trials are needed to definitively assess the safety and efficacy of novel oral anticoagulant use for periprocedural anticoagulation for ablation of AF.
Keywords: Atrial fibrillation, Ablation, Dabigatran, Warfarin, Meta-analysis
1 Introduction
Pulmonary vein isolation (PVI) by endocardial radiofrequency ablation is an effective therapy for drug-refractory, symptomatic atrial fibrillation (AF) [1, 2]. In appropriately selected patients, catheter ablation provides significantly longer duration of freedom from AF than medical therapy at 1 year [2]. However, catheter ablation carries short-term risks including stroke and bleeding complications. International survey data suggest that AF ablation procedures are complicated by stroke in approximately 0.5–1.0 % of cases and death in less than 0.2 % of cases [3]. The overall incidence of vascular and bleeding complications ranges from 1 to 13 % [2]. The desire to minimize bleeding events is carefully balanced against the overriding need to prevent stroke and other thromboembolic complications of performing left atrial ablation. Irrespective of pre-ablation stroke risk, current guidelines recommend periprocedural anticoagulation with intravenous unfractionated heparin and systemic oral anticoagulation for a minimum of 2 months post-procedure (independent of rhythm) [2, 4]. Currently, the majority of patients receive warfarin as the anticoagulant of choice before, during, and after PVI [3].
In 2009, the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial demonstrated that dabigatran at 150 mg twice daily was superior to warfarin for the prevention of stroke or systemic embolism in patients with nonvalvular AF, with equivalent rates of bleeding [5]. Dabigatran was subsequently approved for this indication [6]. However, the safety and efficacy of dabigatran for periprocedural anticoagulation in the setting of PVI are not well studied and no randomized data are available assessing the use of dabigatran in this setting. Currently, several small, observational cohorts have been published describing outcomes in patients receiving dabigatran during and after PVI. The objective of this meta-analysis is to estimate the safety and effectiveness of periprocedural dabigatran versus warfarin for thromboembolic prophylaxis in patients with AF undergoing PVI.
2 Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used for the current study to search for and identify appropriate publications [7, 8]. PubMed was searched for the terms “dabigatran AND ablation” (MeSH terms), and no filters were used in the query. A preliminary search on July 16, 2012 yielded 24 abstracts. The same query was conducted in EMBASE and the abstract supplements of major cardiology scientific sessions since dabigatran approval (October 19, 2010). These included the American Heart Association Scientific Sessions (2010, 2011), the Heart Rhythm Society Scientific Sessions (2011, 2012), the European Society of Cardiology World Congress (2011), and the American College of Cardiology Scientific Sessions (2011, 2012). Additional studies were identified in the course of peer review.
Inclusion criteria for the studies were: (1) use of dabigatran periprocedure, in patients undergoing radiofrequency ablation in the left atrium, (2) report of a vitamin K antagonist comparator group, (3) report of thromboembolic and major bleeding outcomes, (4) at least 100 total patients studied (including both treatment and comparator groups), and (5) post-procedure follow-up. Major exclusion criteria were: (1) inclusion of patients with only right atrial ablation (e.g., atrial flutter) or (2) inclusion of patients without an active comparator.
For the purpose of this analysis, the co-primary endpoints were the incidence of (1) any neurological event (stroke or transient ischemic attack) and (2) any major bleeding within 30 days following the procedure. Secondary endpoints focused on the safety of dabigatran periprocedurally and included: (1) all-cause mortality, (2) pericardial effusion with and without tamponade (components of the major bleeding endpoint), and (3) a composite of the primary safety and effectiveness outcomes (any neurological event or any major bleeding event).
2.1 Data abstraction
Full-text abstracts and manuscripts (where applicable) were obtained for those citations which were deemed appropriate for inclusion in the study. After review of the full text, the data elements and endpoints were recorded by the investigators (BS and JP). Disagreements were resolved by review and consensus.
2.2 Statistical methods
Baseline study and patient demographics were described as reported by the original source data, where available. Summary event rates were tabulated and summed across all studies. We subsequently computed a meta-analysis of the complication rates using the methods described by Hasselblad et al. [9]. Because the usual normal approximation to the likelihood function will not work for very small event counts or rare events, it was necessary to compute the exact likelihood function for the difference. These likelihood functions can be combined numerically to give maximum likelihood estimates. Outcome measures were reported as absolute risk differences with 95 % confidence intervals. These metrics also allow estimation of numbers needed to treat (or harm). A sensitivity analysis was performed excluding studies where low-molecular weight heparin bridging was used.
All statistical analyses of the aggregate, de-identified data were performed by the authors using FAST*PRO Software. The analysis was granted a common rule exemption by the Duke University Institutional Review Board. The authors take full responsibility for the initiation of the analysis and the data presented herein, as well as the drafting of the manuscript.
3 Results
3.1 Search results
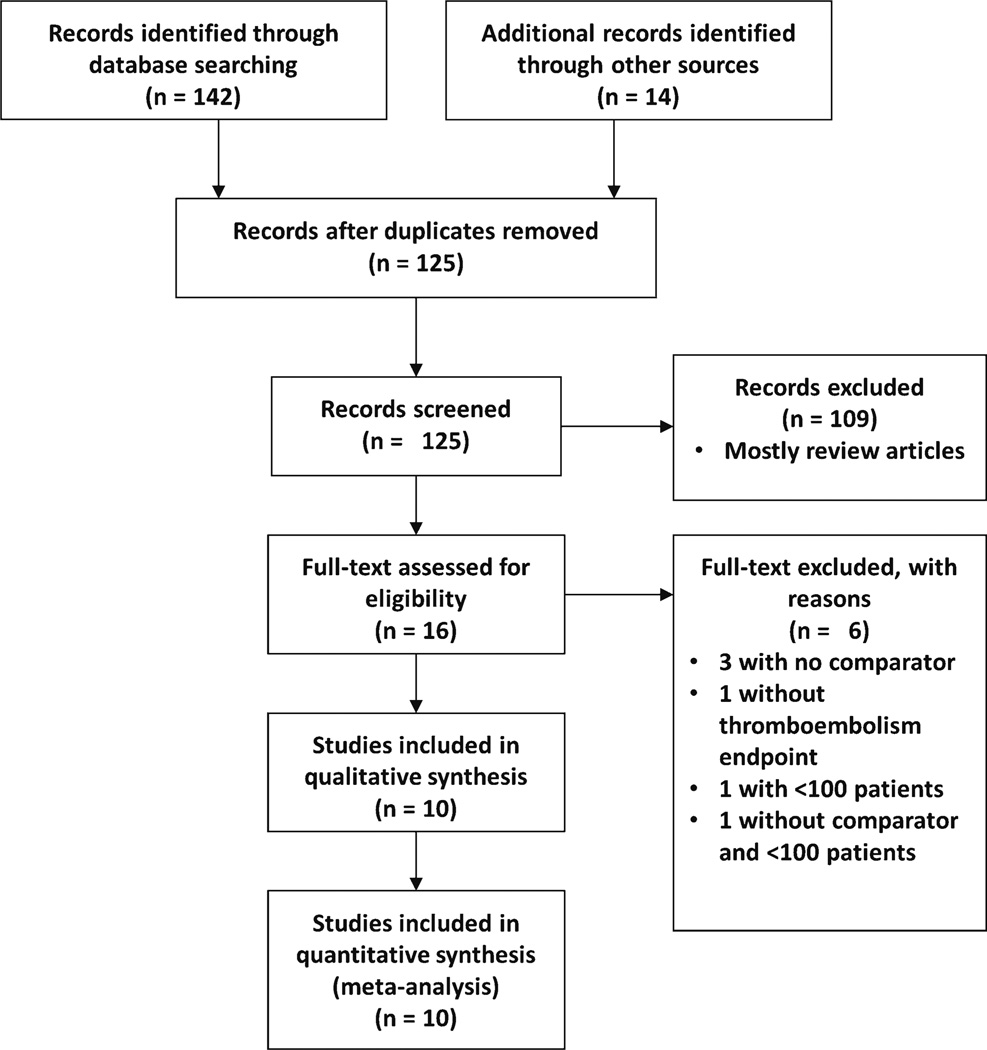
After excluding duplicates, 125 unique studies were identified by online database search and other sources (Fig. 1). These were screened and 109 were subsequently excluded based upon the inclusion and exclusion criteria. Subsequently, 16 full-text manuscripts or abstracts were retrieved and 6 were excluded: 3 were excluded for lack of a comparator group [10–12], 1 did not have thromboembolic endpoints [13], 1 had fewer than 100 total patients [14], and 1 had no comparator and was under 100 patients [15]. Ten studies that met inclusion criteria without any exclusions were subsequently identified [16–25]. Of the 10 studies identified, six were available as full-length manuscripts [19–23, 25] and three were available only in abstract form [16–18]. The full manuscript from one abstract is subsequently in press, and the endpoint data were verified with the authors by personal communication [24]. Despite attempts, data from the remaining abstracts could not be further verified [16–18]. Overall, the studies included single- and multicenter cohorts within the previous 2 years.
Fig. 1.
PRISMA diagram of meta-analysis to identify studies [8]. PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
3.2 Trial characteristics and study quality
All 10 studies consisted of comparisons between two observational cohorts. No randomized groups were available. Numbers of patients receiving dabigatran and warfarin were balanced in all studies except three, where warfarin patients outnumbered dabigatran patients (Table 1). The warfarin groups consistently had an INR goal of 2–3, and three cohorts included patients who received low-molecular weight heparin as a bridge. Cohorts from the USA consistently used the 150-mg twice-daily dosing in the dabigatran group [16, 19, 21–23, 25], while the single international trial used 110 mg twice daily [20]. The majority stopped dabigatran 12–24 h prior to the procedure. Unfractionated heparin titrated to activated clotting time was used during PVI consistently across all studies. Timing of restarting dabigatran post-procedure commonly occurred within 12 h of the procedure.
Table 1.
Studies of dabigatran for anticoagulation before, during, and after pulmonary vein isolation
| Mendoza et al. [16] |
Rowley et al. [17] |
Ellis et al. [18] | Lakkireddy et al. [19] |
Kaseno et al. [20] |
Snipelisky et al. [21] |
Kim, et al. [22] |
Maddox et al. [23] |
Haines et al. [24] |
Bassiouny et al. [25] |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Publication/meeting | HRS | HRS | HRS | JACC | Circ J | J Interv Card Electrophysiol | Heart Rhythm | J Cardiovasc Electrophysiol | J Interv Card Electrophysiol | Circ Arrhythm Electrophysiol |
| Year | 2012 | 2012 | 2012 | 2012 | 2012 | 2012 | 2013 | 2013 | 2013 | 2013 |
| Number of centers | 1 | 1 | 1 | 8 | 1 | 1 | 1 | 1 | 5 | 1 |
| Total patients | 118 | 282 | 171 | 290 | 211 | 156 | 763 | 463 | 404 | 999 |
| Pre-procedure | ||||||||||
| INR goal (warfarin group) | 2–3 | 2.0 | 2–3.5 | 2–3 | 2–3a | 2–3 | 2–3 | – | – | |
| Dabigatran dose | 150 mg bid | – | – | 150 mg bid | 110 mg bid | 150 mg bid | 150 mg bid | 150 mg bid | – | 150 mg bid |
| Time of last dabigatran dose pre-procedure | Held in AM | Held in AM | 12–24 h prior | Held in AM | Held in AM | Held in AM | Held night before | Taken in AM | 2.5–48 h prior | 1–2 doses held |
| Periprocedure | ||||||||||
| Irrigated catheter | – | – | – | Yes (ThermoCool) | Yes (ThermoCool) | – | Yes (ThermoCool) | Yes | Mixed | Yes |
| ACT goal (for UFH) | 300–350 s | – | 300–350 s | 300–400 s | 300–350 sb | ≥350 s | 300–350 s | 350–400 s | 300–350 s | 350–450 s |
| Bridging with LMWH | No | Yes | Yes | No | No | No | No | No | Yes | No |
| Post-procedure | ||||||||||
| Dabigatran restart timing | After sheath removal | Next day | 4–24 h post-procedure | 0–3 h post-procedure | Morning after procedure | Evening after procedure | 4 h post-procedure | Evening after procedure | 4–12 h post-procedure | Immediately post-procedure |
| Follow-up duration | 1 month | – | 1 month | 30 days | 2 months | 1 week | 3 months | – | – | 3 months |
Dashes indicate unreported data
HRS Heart Rhythm Society; JACC Journal of the American College of Cardiology; Circ J Circulation Journal: Official Journal of the Japanese Circulation Society; Circ Arrhythm Electrophysiol: Circulation: Arrhythmia and Electrophysiology; INR international normalized ratio; ACT activated clotting time natriuretic peptide; UFH unfractionated heparin; LMWH low-molecular weight heparin
Patients on warfarin held the dose the evening prior to the procedure
Patients in each group received 10,000 units of UFH for 24 h after the procedure
3.3 Patient and procedure characteristics
A total of 3,857 patients are included in our analysis, 1,501 who received dabigatran and 2,356 treated with warfarin. Patient characteristics across studies, stratified by anticoagulation strategy, are show in Table 2. The mean age was approximately 60 years with a predominance of male patients. The majority had CHADS2 scores of 1 or 2, whereas mean HAS-BLED bleeding risk scores varied among studies, but were similar between treatment groups.
Table 2.
Patients undergoing pulmonary vein isolation with dabigatran versus warfarin
| Mendoza et al. [16] |
Rowley et al. [17] |
Ellis et al. [18] |
Lakkireddy et al. [19] |
Kaseno et al. [20] |
Snipelisky et al. [21] |
Kim et al. [22] |
Maddox et al. [23] |
Haines et al. [24] |
Bassiouny et al. [25] |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | W | D | W | D | W | D | W | D | W | D | W | D | W | D | W | D | W | D | W | |
| N | 60 | 52 | 113 | 169 | 61 | 110 | 145 | 145 | 110 | 101 | 31 | 125 | 191 | 572 | 212 | 251 | 202 | 202 | 376 | 623 |
| Age (year, mean) | 63 | 64 | 63 | – | – | 60 | 60 | 59 | 62 | 61 | 65 | 61 | 61 | 62 | 63 | 60 | 60 | 59 | 63 | |
| Male (%) | 90 | 88 | – | – | – | – | 79 | 79 | 75 | 79 | 81 | 74 | 80 | 74 | 76 | 67 | 74 | 69 | 75 | 73 |
| Chronic renal insufficiency (%) | – | – | – | – | – | – | 1 | 2 | – | – | ||||||||||
| Creatinine clearance (mL/min, mean) | – | – | – | – | – | – | 97 | 98 | – | – | 81 | 84 | 93 | 93 | ||||||
| Serum creatinine (mg/dL, mean) | – | – | – | – | – | – | 0.9 | 0.9 | 1.0 | 1.1 | 0.9 | 0.9 | ||||||||
| Paroxysmal AF (%) | – | – | – | – | – | – | 57 | 57 | 83 | 55 | 68 | 46 | 53 | 48 | 63 | 57 | 58 | 52 | 57 | 55 |
| CHADS2 score (mean overall) | 1.32 | 1.29 | 1.3 | 1.2 | 0.84 | 1.22 | 1.0 | 1.1 | 0.9 | 0.9 | – | – | ||||||||
| 0 (%) | 34 | 40 | 61 | 46 | 22 | 20 | 43 | 29 | ||||||||||||
| 1 (%) | 43 | 41 | 31 | 41 | 27 | 31 | 37 | 42 | ||||||||||||
| >2 (%) | 23 | 19 | 8 | 14 | 51 | 49 | 21 | 29 | ||||||||||||
| CHADS-Vase score (mean) | – | – | – | – | – | – | 1.6 | 1.5 | – | – | – | – | 1.6 | 1.7 | 1.7 | 1.7 | 1.6 | 1.9 | – | – |
| HAS-BLED score (mean) | 1.47 | 1.63 | – | – | – | – | 1.2 | 1.1 | 0.5 | 0.6 | – | – | 1.0 | 1.1 | – | – | – | – | ||
Single values across treatment groups indicate that only the overall population value was provided. Dashes indicate unreported data
D patients treated with dabigatran, W patients treated with warfarin
3.4 Outcomes
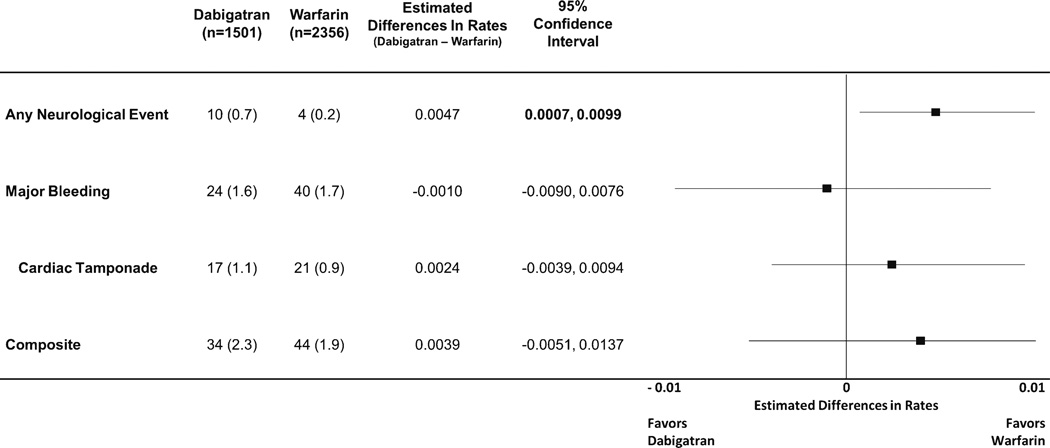
Adverse outcomes in the setting of PVI were uncommon in the selected cohorts (Table 3). No deaths following PVI were reported in any of the cohorts. Unadjusted rates of thrombo-embolic and bleeding outcomes are shown in Fig. 2. The majority of major bleeding complications consisted of cardiac tamponade (n=17/24 for dabigatran and n=21/40 for warfarin). There were 10 neurological events in the dabigatran group (10/1,501, 0.7 %) versus 4 in the warfarin group (4/2,356, 0.2 %).
Table 3.
Outcomes in patients undergoing atrial fibrillation ablation by study
| Mendoza et al. [16] |
Rowley et al. [17] |
Ellis, et al. [18] |
Lakkireddy et al. [19] |
Kaseno et al. [20] |
Snipelisky et al. [21] |
Kim, et al. [22] |
Maddox et al. [23] |
Haines et al. [24] |
Bassiouny et al. [25] |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | W | D | W | D | W | D | W | D | W | D | W | D | W | D | W | D | W | D | W | |
| Any neurological event | 0 | 1 | 2 | 1 | 2 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 1 |
| Major bleeding | 1 | 0 | 0 | 1 | 1 | 5 | 9 | 1 | 0 | 2 | 1 | 1 | 4 | 12 | 2 | 6 | 2 | 2 | 4 | 10 |
| Cardiac tamponade | 0 | 0 | 0 | 0 | 0 | 0 | 9 | 1 | 0 | 2 | 0 | 0 | 2 | 7 | 1 | 2 | 2 | 2 | 3 | 7 |
| Composite | 1 | 1 | 2 | 2 | 3 | 6 | 12 | 1 | 0 | 2 | 1 | 1 | 4 | 12 | 3 | 6 | 4 | 2 | 4 | 11 |
D events in patients treated with dabigatran, W events in patients treated with warfarin
Fig. 2.
Combined rates of raw outcomes across studies, with estimated absolute risk differences and forest plot of outcomes. Composite outcome includes any major bleeding or neurological event. Reported as total numbers of events (percent) for raw outcomes
Absolute differences in event rates from the 10 studies were combined using maximum likelihood methods. The results of this meta-analysis are shown in Fig. 2. There was a nonsignificant trend towards higher rates of the composite endpoint of neurological event or major bleeding in the dabigatran group (34/1,501 [2.3 %] versus 44/2,356 [1.9 %], estimated difference in rate 0.0039, 95 % confidence interval [CI] −0.0051 to 0.0137). This was largely attributable to a significant increased risk of neurological event in the dabigatran group (10/1,501 [0.7 %] versus 4/2,356 [0.2 %], estimated difference in rate 0.0047, 95 % CI 0.0007–0.0099). Major bleeding events (24/1,501 [1.6 %] versus 40/2,356 [1.7 %], estimated difference in rates −0.0010, 95 % CI −0.0090 to 0.0076), including cardiac tamponade (17/1,501 [1.1 %] versus 21/2,356 [0.9 %], and estimated difference in rate 0.0024, 95 % CI −0.0039 to 0.0094) were similar between the two groups.
In sensitivity analysis, three studies were excluded for use of a bridging strategy [17, 18, 24]. The results are shown in the Appendix (Table 4) and demonstrated a numerical excess of events in the dabigatran group without statistical significance.
4 Discussion
In this meta-analysis of nearly 4,000 patients with AF undergoing PVI, dabigatran appears to be associated with a higher risk of neurological events compared to uninterrupted warfarin. Overall rates of complications were numerically higher, with equivalent rates of major bleeding.
There are several potential explanations for these findings. One of the major distinctions of dabigatran is its pharmaco-dynamic profile [26]. While warfarin’s anticoagulant effect will fluctuate gradually over a period of days (due to its effect on synthesis of clotting factors), direct thrombin inhibition by dabigatran is maximal within 1–2 h of oral dosing. Similarly, the half-life of dabigatran is 12–17 h, and its anticoagulant effect is no longer detectable within 24–36 h of the last dose (prescribing information recommends longer pre-procedure discontinuation periods in patients with renal dysfunction) [26]. Differences in timing of dabigatran dosing, before and after PVI, could have a significant impact on outcomes, an effect which would be exaggerated by impaired renal function.
Additionally, patients receiving dabigatran may have had more fluctuation in anticoagulation effect through the periprocedural period (with the potential for significant interruption during a high-risk period), and this may have contributed to rates of thromboembolic events. Studies of parenteral anticoagulants in patients with acute coronary syndromes have demonstrated varying outcomes of bleeding and ischemic events, when compared with a consistent approach [27, 28]. There are currently limited data available regarding the risk of bleeding or ischemic events with transitions to and from novel oral anticoagulants.
Several other factors are important to consider in the setting of dabigatran use. First, significant renal dysfunction can dramatically alter the in vivo drug effect (potentially increasing the risk of bleeding). Second, compared with warfarin, compliance with dabigatran cannot be as readily assessed, and missed doses may convey an added risk of thromboembolic events. Lastly, the impact of drug–drug interactions on the anticoagulant effect of dabigatran could also account for observed differences—such interactions with dabigatran (including dronedarone and amiodarone) may well be present but are not identified for a variety of reasons, including lack of an easily accessible assay. The frequency of use for these concomitant medications, or others (e.g., antiplatelet drugs), is not known in the study population.
While each of the studies included cohorts of similar patients undergoing PVI, differences in the underlying populations could have contributed to the difference in outcomes. Many potential factors inform the clinical decision to use dabigatran versus warfarin, and these may have varying influences on the endpoints. Preliminary prescribing patterns for dabigatran suggest that patients (a) with difficulty complying with warfarin, (b) able to pay for dabigatran, and/or (c) who are treated by a cardiologist are more likely to receive dabigatran [29]. Alternatively, it is certainly possible that pre-scribers are selecting dabigatran as an alternative in patients who have had prior bleeding and/or thromboembolic events under another prophylaxis strategy (either warfarin or antiplatelet therapy). Nonetheless, the data available from the present analysis suggest largely similar distributions of age, gender, thromboembolic risk (by CHADS2 and CHA2DS2-VASc scores), and bleeding risk (by HAS-BLED score) between the two treatment groups.
Dosing of dabigatran was consistent across studies (150 mg bid), with the exception of a lower dose in one international study [20]. The 110-mg dosing used in Kaseno et al. is not approved in the USA, though it was tested in RE-LY and was non-inferior to warfarin for the prevention of thromboembolism, with a lower risk of bleeding [5, 30]. In the Kaseno et al. study, the outcomes were identical between the dabigatran and warfarin groups. Inclusion of the Kaseno et al. study may have biased our treatment estimates to lower the risk of bleeding. Using a lower dose of dabigatran at 110 mg bid could reduce bleeding complications after PVI (potentially at the expense of thromboembolic events); however, this hypothesis remains untested.
Our data are distinct from the subgroup analysis of the RE-LY trial that looked at periprocedural bleeding in patients on dabigatran [31]. In that study, the vast majority of patients were undergoing non-cardiac procedures (~90 %) and none had a catheter ablation. Furthermore, all anticoagulants were interrupted—dabigatran was stopped at a mean of 49 h prior to procedure, and warfarin was stopped at a mean of 114 h prior. There were equivalent rates of bleeding and thromboembolic events between the two different doses of dabigatran and warfarin in the RE-LY subgroup analysis. While the RE-LY data support the safety and efficacy of interrupting dabigatran for invasive procedures (in comparison with interrupted warfarin), our data support a dedicated study of dabigatran for periprocedural anticoagulation in catheter ablation of AF.
The use of dabigatran as a periprocedural anticoagulant certainly has appeal for both patient and provider. It has a predictable half-life in patients with normal renal function and thus can be stopped shortly prior to the procedure. Similarly, it is quick in onset and therefore can provide therapeutic anticoagulation effect within hours. Among patients on long-term dabigatran for nonvalvular AF, continuing dabigatran rather than transitioning to warfarin periprocedurally may avoid significant logistical challenges. Our data suggest that the “ease of use” of dabigatran must be weighed against a potential for more adverse events. Ultimately, this choice requires consideration of the circumstances unique to each ablation center and each patient being taken to the lab. The data support careful attention to the implementation of dabigatran before and after PVI, and some clinicians may choose to avoid this agent in patients undergoing PVI who are at high risk of thromboembolic events, in favor of warfarin.
Lastly, dabigatran represents the first of several novel oral anticoagulants emerging as alternatives to warfarin for the prevention of stroke or systemic embolism in patients with non-valvular AF [32]. Both rivaroxaban and apixaban are approved for this indication in the USA; however, there remain no data comparing either agent to warfarin following PVI. Due to differences in pharmacodynamics, mechanism of action, metabolism, and dosing, the results of the present analysis cannot be extrapolated to other novel anticoagulants. However, given the risks associated with suboptimal anticoagulation following PVI, prospective, randomized clinical trials are needed to address the safety and efficacy of all novel oral agents relative to uninterrupted warfarin. We would advocate for multicenter, broadly applicable trials, with meaningful clinical endpoints, performed using contemporary AF ablation techniques.
5 Limitations
This meta-analysis has several limitations. First, the treatment groups were not randomized. While attempts were made at matching similar patients in most studies, patient-level data are not available and it is likely that residual confounding is present. Second, three of the included studies have only been published in abstract form. While they each met the inclusion and exclusion criteria, complete data were not available despite attempts to contact the authors. Lastly, the present analysis included a relatively small number of studies. However, this represents the entirety of comparative data available with regard to the periprocedural use of dabigatran in PVI. Therefore, some caution should be exercised when generalizing these data.
6 Conclusions
Periprocedural anticoagulation with either warfarin or dabigatran is associated with a low risk of major adverse events in AF ablation. The use of dabigatran for periprocedural anticoagulation may be associated with a slightly higher risk of neurological events compared with uninterrupted warfarin. Additional, randomized data are needed to definitively assess the safety and efficacy of dabigatran, and other novel oral anticoagulants, for thromboem-bolism prevention during and after PVI.
Acknowledgments
This project was supported (in part) by funding from the Agency of Healthcare Research and Quality through cooperative agreement number 1U19 HS021092. Dr. Steinberg was funded by NIH T-32 training grant #5 T32 HL 7101-37.
Dr. Atwater receives grants for clinical research from the American Heart Association and Medtronic and serves on an advisory board for Medtronic. Dr. Bahnson receives research funds from Medtronic and St. Jude Medical and participates as a consultant and/or speaker for Boehringer Ingelheim, ChanRX, Sequel Pharma, and Sanofi-Aventis. Dr. Alexander receives institutional research support from Bristol Myers Squibb, Pfizer, and Regado Biosciences and is a consultant to Bayer, Bristol Myers Squibb, Daiichi Sankyo, Janssen Pharmaceuticals, and Pfizer. Dr. Daubert receives research support from Boston Scientific, St. Jude, Medtronic, and Biosense Webster; institutional fellowship support from Boston Scientific, St. Jude, Medtronic, Biosense Webster, and Bard; and honoraria for advisory board participation or lectures from Biosense Webster, Sanofi-Aventis, Boston Scientific, and Sorin Medical. Dr. Piccini receives grants for clinical research from Johnson & Johnson and Boston Scientific and is a consultant to Forest Research Laboratories, Janssen Pharmaceuticals, Medtronic, and Titan Pharmaceuticals.
Appendix
Table 4.
Sensitivity meta-analysis after excluding studies where low-molecular weight heparin bridging was used
| Estimated differences in rates (Dabigatran– warfarin) |
95 % confidence interval |
|
|---|---|---|
| Any neurological event | 0.0022 | −0.0010, 0.0071 |
| Major bleeding | 0.0016 | −0.0079, 0.0120 |
| Cardiac tamponade | 0.0032 | −0.0045, 0.0119 |
| Composite | 0.0040 | −0.0061, 0.0152 |
Footnotes
Conflict of interest Drs. Steinberg and Hasselblad have no relevant disclosures. A full list of Dr. Piccini and Dr. Alexander’s disclosures can be found at https://www.dcri.org/about-us/conflict-of-interest.
Contributor Information
Benjamin A. Steinberg, Electrophysiology Section, Duke Center for Atrial Fibrillation, Duke University Medical Center, Duke Clinical Research Institute, PO Box 17969, Durham, NC 27710, USA Duke Clinical Research Institute, Durham, NC, USA.
Vic Hasselblad, Duke Clinical Research Institute, Durham, NC, USA.
Brett D. Atwater, Electrophysiology Section, Duke Center for Atrial Fibrillation, Duke University Medical Center, Duke Clinical Research Institute, PO Box 17969, Durham, NC 27710, USA
Tristram D. Bahnson, Electrophysiology Section, Duke Center for Atrial Fibrillation, Duke University Medical Center, Duke Clinical Research Institute, PO Box 17969, Durham, NC 27710, USA Duke Clinical Research Institute, Durham, NC, USA.
Jeffrey B. Washam, Duke Heart Center, Durham, NC, USA
John H. Alexander, Electrophysiology Section, Duke Center for Atrial Fibrillation, Duke University Medical Center, Duke Clinical Research Institute, PO Box 17969, Durham, NC 27710, USA Duke Clinical Research Institute, Durham, NC, USA.
James P. Daubert, Electrophysiology Section, Duke Center for Atrial Fibrillation, Duke University Medical Center, Duke Clinical Research Institute, PO Box 17969, Durham, NC 27710, USA Duke Clinical Research Institute, Durham, NC, USA.
Jonathan P. Piccini, Email: jonathan.piccini@duke.edu, Electrophysiology Section, Duke Center for Atrial Fibrillation, Duke University Medical Center, Duke Clinical Research Institute, PO Box 17969, Durham, NC 27710, USA; Duke Clinical Research Institute, Durham, NC, USA.
References
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123(10):e269–e367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 2.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: Recommendations for Patient Selection, Procedural Techniques, Patient Management and Follow-up, Definitions, Endpoints, and Research Trial Design: a report of the Heart Rhythm Society (HRS) Task Force on Catheter and Surgical Ablation of Atrial Fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC) and the European Cardiac Arrhythmia Society (ECAS); and in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), the Asia Pacific Heart Rhythm Society (APHRS), and the Society of Thoracic Surgeons (STS). Endorsed by the governing bodies of the American College of Cardiology Foundation, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, the Asia Pacific Heart Rhythm Society, and the Heart Rhythm Society. Heart Rhythm. 2012;9(4):632–696. doi: 10.1016/j.hrthm.2011.12.016. e621. [DOI] [PubMed] [Google Scholar]
- 3.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3(1):32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 4.Natale A, Raviele A, Arentz T, Calkins H, Chen SA, Haissaguerre M, et al. Venice Chart international consensus document on atrial fibrillation ablation. Journal of Cardiovascular Electrophysiology. 2007;18(5):560–580. doi: 10.1111/j.1540-8167.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Beasley BN, Unger EF, Temple R. Anticoagulant options—why the FDA approved a higher but not a lower dose of dabigatran. The New England Journal of Medicine. 2011;364(19):1788–1790. doi: 10.1056/NEJMp1103050. [DOI] [PubMed] [Google Scholar]
- 7.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasselblad V, Mosteller F, Littenberg B, Chalmers TC, Hunink MG, Turner JA, et al. A survey of current problems in meta-analysis. Discussion from the Agency for Health Care Policy and Research inter-PORT Work Group on Literature Review/Meta-Analysis. Medical Care. 1995;33(2):202–220. [PubMed] [Google Scholar]
- 10.Bunch TJ, Crandall BG, Weiss JP, Osborn JS, May HT, Day JD. Pradaxa can be safely used as monotherapy or as a bridge to therapeutic warfarin after atrial fibrillation ablation. Circulation. 2011;124(21) [Google Scholar]
- 11.Eitel C, Hindricks G, Piorkowski C. Dabigatran in patients post atrial fibrillation ablation. Journal of the American College of Cardiology. 2012;59(13):E603. [Google Scholar]
- 12.Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. The use of dabigatran immediately after atrial fibrillation ablation. Journal of Cardiovascular Electrophysiology. 2012;23(3):264–268. doi: 10.1111/j.1540-8167.2011.02175.x. [DOI] [PubMed] [Google Scholar]
- 13.Yamaji H, Murakami T, Kawamura H, Murakami M, Hina K. Safety of dabigatran for atrial fibrillation ablation as a periprocedural anticoagulation strategy. Heart Rhythm. 2012;9(5):S170. [Google Scholar]
- 14.Nin T, Sairaku A, Yoshida Y, Kamiya H, Tatematsu Y, Nanasato M, et al. A randomized controlled trial of dabigatran versus warfarin for periablation anticoagulation in patients undergoing ablation of atrial fibrillation. Pacing and Clinical Electrophysiology. 2012 doi: 10.1111/pace.12036. [DOI] [PubMed] [Google Scholar]
- 15.Gangireddy S, Coffey JO, Calenda BW, Teh A, Miller M, Skipitaris NT, et al. The incidence of stroke after atrial fibrillation ablation in patients on dabigatran. Heart Rhythm. 2012;9(5):S472. [Google Scholar]
- 16.Mendoza I, Helguera M, Baez-Escudero J, Reina J, Pinski SL. Atrial fibrillation ablation on uninterrupted anticoagulation with dabigatran versus warfarin. Heart Rhythm. 2012;9(5):S270–S271. Abstract. [Google Scholar]
- 17.Rowley CP, Bradford NS, Bernard ML, Sidney DS, Brabham WW, Netzler PC, et al. Complications of atrial fibrillation ablation in patients anticoagulated with dabigatran compared to warfarin. Heart Rhythm. 2012;9(5):S201. [Google Scholar]
- 18.Ellis CR, Streur MM, Nagarakanti R. Safety and efficacy of dabigatran versus warfarin in patients undergoing left atrial catheter ablation. Heart Rhythm. 2012;9(5):S421. [Google Scholar]
- 19.Lakkireddy D, Reddy YM, Di Biase L, Vanga SR, Santangeli P, Swarup V, et al. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation results from a multicenter prospective registry. Journal of the American College of Cardiology. 2012;59(13):1168–1174. doi: 10.1016/j.jacc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Kaseno K, Naito S, Nakamura K, Sakamoto T, Sasaki T, Tsukada N, et al. Efficacy and safety of periprocedural dabigatran in patients undergoing catheter ablation of atrial fibrillation. Circulation journal. 2012;76(10):2337–2342. doi: 10.1253/circj.cj-12-0498. [DOI] [PubMed] [Google Scholar]
- 21.Snipelisky D, Kauffman C, Prussak K, Johns G, Venkatachalam K, Kusumoto F. A comparison of bleeding complications post-ablation between warfarin and dabigatran. Journal of Interventional Cardiac Electrophysiology. 2012;35(1):29–33. doi: 10.1007/s10840-012-9708-z. [DOI] [PubMed] [Google Scholar]
- 22.Kim JS, She F, Jongnarangsin K, Chugh A, Latchamsetty R, Ghanbari H, et al. Dabigatran vs warfarin for radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2012 doi: 10.1016/j.hrthm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Maddox W, Kay GN, Yamada T, Osorio J, Doppalapudi H, Plumb VJ, et al. Dabigatran versus warfarin therapy for uninterrupted oral anticoagulation during atrial fibrillation ablation. Journal of Cardiovascular Electrophysiology, n/a-n/a. 2013 doi: 10.1111/jce.12143. [DOI] [PubMed] [Google Scholar]
- 24.Haines DE, Mead-Salley M, Salazar M, Marchlinski FE, Zado E, Calkins H, et al. Dabigatran versus warfarin anticoagulation before and after catheter ablation for the treatment of atrial fibrillation. J Interv Card Electrophysiol. 2013 doi: 10.1007/s10840-013-9800-z. in press. [DOI] [PubMed] [Google Scholar]
- 25.Bassiouny M, Saliba W, Rickard J, Shao M, Sey A, Diab M, et al. Use of dabigatran for peri-procedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circulation Arrhythm Electrophysiology. 2013 doi: 10.1161/CIRCEP.113.000320. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boehringer-Ingelheim. [Accessed 14 May 2011];Dabigatran prescribing information. 2010 http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf.
- 27.Cohen M, Mahaffey KW, Pieper K, Pollack CV, Jr, Antman EM, Hoekstra J, et al. A subgroup analysis of the impact of prerandomization antithrombin therapy on outcomes in the SYNERGY trial: enoxaparin versus unfractionated heparin in non-ST-segment elevation acute coronary syndromes. Journal of the American College of Cardiology. 2006;48(7):1346–1354. doi: 10.1016/j.jacc.2006.05.058. [DOI] [PubMed] [Google Scholar]
- 28.White HD, Chew DP, Hoekstra JW, Miller CD, Pollack CV, Jr, Feit F, et al. Safety and efficacy of switching from either unfractionated heparin or enoxaparin to bivalirudin in patients with non-ST-segment elevation acute coronary syndromes managed with an invasive strategy: results from the ACUITY (Acute Catheterization and Urgent Intervention Triage strategY) trial. Journal of the American College of Cardiology. 2008;51(18):1734–1741. doi: 10.1016/j.jacc.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 29.Huang C, Siu M, Vu L, Wong S, Shin J. Factors influencing doctors’ selection of dabigatran in non-valvular atrial fibrillation. Journal of Evaluation in Clinical Practice. 2012 doi: 10.1111/j.1365-2753.2012.01886.x. Epub. [DOI] [PubMed] [Google Scholar]
- 30.Eikelboom JW, Wallentin L, Connolly SJ, Ezekowitz M, Healey JS, Oldgren J, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123(21):2363–2372. doi: 10.1161/CIRCULATIONAHA.110.004747. [DOI] [PubMed] [Google Scholar]
- 31.Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S, et al. Peri-procedural bleeding and thrombo-embolic events with dabigatran compared to warfarin: results from the RE-LY Randomized Trial. Circulation. 2012;126(3):343–348. doi: 10.1161/CIRCULATIONAHA.111.090464. [DOI] [PubMed] [Google Scholar]
- 32.Schirmer SH, Baumhakel M, Neuberger HR, Hohnloser SH, van Gelder IC, Lip GY, et al. Novel anticoagulants for stroke prevention in atrial fibrillation: current clinical evidence and future developments. Journal of the American College of Cardiology. 2010;56(25):2067–2076. doi: 10.1016/j.jacc.2010.09.017. [DOI] [PubMed] [Google Scholar]