Abstract
About 20-40% of breast cancer patients eventually develop recurrences in distant organs, which are often not detected until years to decades after the primary tumor diagnosis. This phenomenon is especially pronounced in ER+ breast cancer, suggesting that ER+ cancer cells may stay dormant for a protracted period of time, despite adjuvant therapies. Multiple mechanisms have been proposed to explain how cancer cells survive and remain in dormancy , and how they become reactivated and exit dormancy. These mechanisms include angiogenic switch, immunosurveillance, and interaction with extracellular matrix (ECM) and stromal cells. How to eradicate or suppress these dormant cancer cells remains a major clinical issue because of the lack of knowledge about the biological and clinical nature of these cells. Herein, we review the clinical manifestation of metastasis dormancy in ER+ tumors, the current biological insights of tumor dormancy obtained from various experimental models, and the clinical challenges to predict, detect, and treat dormant metastases. We also discuss future research directions toward a better understanding of the biological mechanisms and clinical management of ER+ dormant metastasis.
Keywords: ER+ breast cancer, metastasis, dormancy
Introduction
Nearly all breast cancer-related deaths are caused by metastases rather than the primary tumor. Different subtypes of breast cancer exhibit distinct metastasis behaviors in terms of the temporal kinetics and anatomic sites. ER+ breast cancer, in particular, predominantly recurs in bone, often years to occasionally decades after the diagnosis of the primary tumor. This protracted latency suggests a dormant stage of metastasis progression wherein cancer cells either stay quiescent or proliferate very slowly. Although late recurrences (> 5 years) occur to over 50% of patients, our knowledge about tumor dormancy is extremely limited. This is largely due to the fact that dormant cancer cells are rare and difficult to detect and isolate from clinical specimens. Moreover, ideal animal models that can fully recapitulate the natural history of dormant tumors are also lacking. In spite of these difficulties, several hypotheses have been proposed as major mechanisms underlying tumor dormancy. The common theme of these mechanisms is the crosstalk between tumor cells and the microenvironment they encounter. The processes and factors that have been implicated in dormancy include angiogenesis (1, 2), immunosurveillance (3-5), and a wide variety of microenvironment cues such as extracellular matrix, growth factors and cytokines (Figure 1). Although illuminating, very few, if any, studies have been conducted using ER+ breast cancer models, despite the fact that metastasis dormancy is most common in this tumor subtype. In this review, we will describe the clinical manifestation of ER+ dormant metastasis. We will then discuss the urgent need, possible solutions, and the conceptual challenges faced by basic and clinical scientists who want to study metastasis dormancy in ER+ breast cancer. Several other excellent reviews of tumor dormancy are also recommended (2, 4-9).
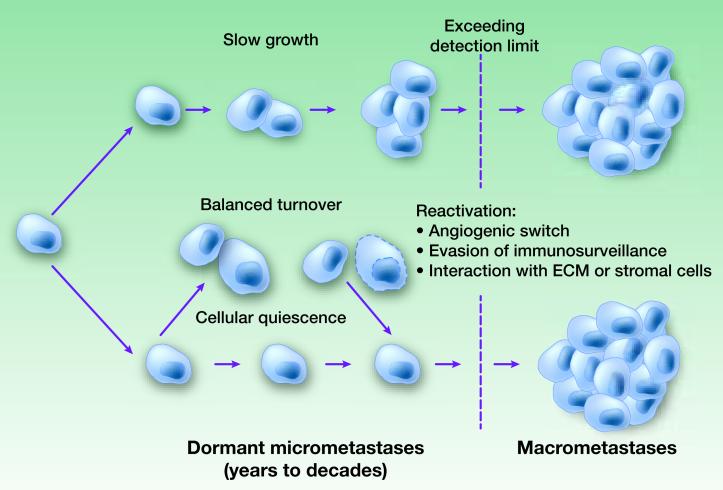
Figure 1. Hypothetic mechanisms underlying metastasis dormancy.
During dormancy, metastatic cancer cells may undergo very slow proliferation (“Slow growth”), a balanced turnover due to equal rates of cell deaths and proliferation (“Balanced turnover”), or G0/G1 arrest (“Cellular quiescence”). The termination of dormancy, or the detection of metastases, may result from the accumulation of tumor mass that eventually exceeds detection limit, the onset of successful angiogenesis (“angiogenic switch”), evasion of immunosurveillance, and/or the initiation of interaction with certain ECM or stromal cells (e.g., Tenascin C and VCAM-1).
CLINICAL MANIFESTATION OF DORMANCY OF ER+ BREAST CANCER
Tumor dormancy is defined as clinically undetectable microscopic metastases that eventually progress to overt cancer after a long period of time. In breast cancer, metastases usually become evident asynchronously with the primary tumor, and they demonstrate variable lengths of time to become clinically apparent. This lag time depends to some extent on the initial tumor volume or stage at first diagnosis. At one end of the spectrum, neglected locally advanced cancers are often diagnosed with metastases already evident or appearing soon. At the other end, metastases of low-stage breast cancer may occur many years after the diagnosis. Other factors also relate to the time until metastases are identified. Such factors as the rate of proliferation or the estrogen receptor (ER) status of the tumor are also related to the time to distant recurrence. Indeed, late recurrences sometimes decades after the initial primary diagnosis indicating a long dormant period present a significant clinical challenge mainly for ER+ breast cancer. Over half of the recurrences of ER+ tumors occur five years or longer after diagnosis and surgical removal of the primary tumor, and some patients suffer recurrence after more than 20 years (10-12). This is in sharp contrast to ER- tumors, for which the recurrence rate peaks at around two years but diminishes to a low rate after five years (13). Current prognostic markers often focus on and are reasonably good at predicting early recurrences within five years (14-17), but the risk of late recurrences remains poorly predictable. Is late recurrence merely a reflection of very slowly proliferating ER+ cancer cells lying in distant sites or due to cancer cells actually entering a period of total dormancy in which they stop proliferating until they are activated to grow again by some as yet unknown stimulus. We know that many patients with early breast cancer harbor micrometastases in their bone marrow. While the presence of these cells predicts a slightly worse prognosis, still the majority of the patients with these disseminated cells never suffer a recurrence, at least within the time of follow up on the studies (7, 18).
Systemic therapy given to patients with early breast cancer after surgery in an attempt to eradicate micrometastases (adjuvant therapy) could also influence the time it takes for a patient to demonstrate a recurrence by slowing cell proliferation or by killing the majority but not all of the metastatic cells thereby delaying their clinical appearance. Adjuvant chemotherapy is effective in reducing recurrences within the first five years, but ineffective in preventing late recurrences between five and 15 years (1). These data suggest that late recurrences arise from residual disease that is treatment resistant.
Further insights may be obtained from studies of adjuvant endocrine therapies in patients with ER+ tumors. These studies have shown that longer and longer durations of treatment are better at reducing recurrence and prolonging survival than shorter durations. Early clinical trials indicated that five years of tamoxifen treatment was more effective than one or two years (1). Recent studies of prolonging adjuvant tamoxifen from 5 to 10 years demonstrate a reduction in recurrence and death between years 10 to 15, suggesting that longer treatment continued to suppress proliferation of micrometastases still viable after just 5 years of treatment (see Table 1 and references therein). These results indicate that inhibition of ER signaling may suppress the exit of cancer cells from dormancy or growth inhibition, but may not kill them. Whether ER+ micrometastases are ever totally eradicated by treatment will require very long follow-up of patients to ascertain.
Table 1.
Clinical trials of extended hormonal therapies.
| Clinical Trial | Patient Accrual |
Design | Results | Reference |
|---|---|---|---|---|
| NCIC-MA17 | 5,187 | Tamoxifen 5 years + letrozole 5 years vs. tamoxifen 5 years + placebo |
Extended letrozole treatment prolonged DFS (HR=0.58 [0.45- 0.76], p < 0.001) and distant DFS (HR=0.60 [0.43-0.84], p < 0.002) |
(60) |
| ATLAS | 12,894 | Tamoxifen 10 years vs.
stopping at 5 years |
10-year treatment significantly reduced recurrences (HR= 0.75 [0.62-0.90], p = 0.003) and mortality (HR= 0.71 [0.58-0.88], p = 0.001). |
(61) |
| NSABP B−33 | 1,598 | Tamoxifen 5 years + exemestane 5 years vs. tamoxifen 5 years + placebo |
Extended exemestane treatment prolonged DFS with a borderline significance (2% absolute improvement, p = 0.07). |
(62) |
| ABCSG 6a | 856 | Tamoxifen 5 years + anastrozole 3 years vs. tamoxifen 5 years + placebo |
Extended anastrozole treatment reduces risk of recurrences (HR=0.62[0.40-0.96], p=0.031). |
(63) |
BIOLOGICAL INSIGHTS INTO ER+ METASTASIS DORMANCY
Biological models of metastasis dormancy
Before this discussion, it is necessary to define the key parameters of an experimental model for ER+ metastasis dormancy. Such models need to have several key features. 1) Recapitulation of several characteristics of human ER+ tumors including estrogen-dependence, growth inhibition by anti-estrogen strategies, as well as the potential to develop resistance to these treatments. 2) Recapitulation of the natural progression of ER+ tumors, including tumorigenesis, local invasion and intravasation, and the temporal kinetics and anatomical site of metastasis (predominantly bone). 3) Opportunities to investigate the roles of major cell types that may be involved in dormancy. In subsequent paragraphs, we will go through the major models/techniques that have been used in breast cancer and point out their strengths and weaknesses for dormancy research. It needs to be noted here that although the abovementioned properties are highly desirable, models lacking these features may still generate useful information. For instance, late recurrences are not exclusively ER+, and the mechanistic insights obtained from ER- models may also be relevant to ER+ diseases.
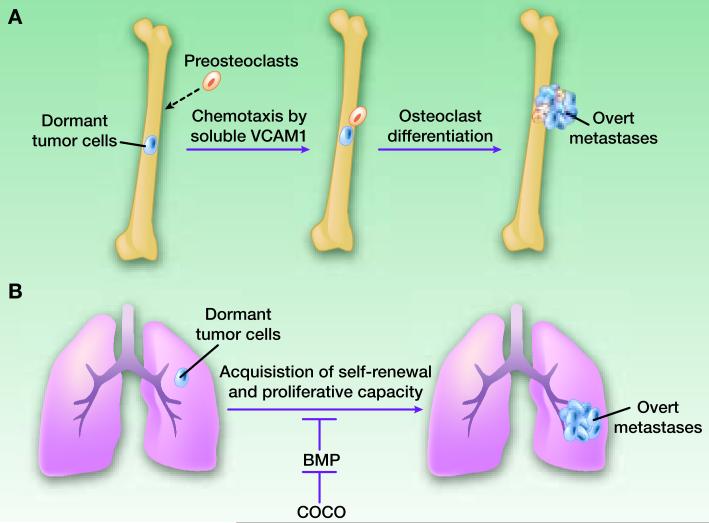
The most widely used breast cancer models are human breast cancer cell lines that are largely derived from pleural effusions of advanced breast cancer patients. Recent genomic studies support the validity of cell lines as breast cancer models by demonstrating the common genomic/gene expression profiles shared between primary tumors and cohorts of cell lines (19). In particular, ER+ breast cancer cell lines have been essential for the elucidation of steroid hormone-stimulated signaling and resistance mechanisms of anti-hormonal therapies (20). When transplanted into the mammary glands of immunodeficient mice, ER+ cancer cells can generate orthotopic xenograft tumors, which are typically dependent on estradiol supplementation. However such tumors rarely give rise to spontaneous metastases during the relatively short lifespan of the mouse, and therefore fall short of serving as models of metastasis dormancy. As an alternative approach, direct introduction of ER+ cancer cells via intracardiac injection delivers them throughout the entire arterial circulation, and can result in colonization in multiple organs including bone. Other limitations of xenograft models include the lack of an intact immune system in immunodeficient mice, the use of already fully-transformed and metastatic cancer cells, and the absence of potential influence from the primary tumor (21). Despite these caveats, important discoveries pertinent to metastasis dormancy were made using this approach. In one example, a subpopulation of MDA-MB-231 cells that outgrew after a long dormancy in animals was compared to the parental population. VCAM1 was identified as a key molecule that could serve as a chemo-attractant for osteoclast precursors which in turn trigger the proliferation and exit from dormancy of cancer cells (22) (Figure 2A). The role of VCAM1 was verified in several other models including MCF-7 cells (22). Therefore, the observation may be relevant to ER+ breast cancer.
Figure 2. The roles of Coco and VCAM-1 in metastasis dormancy.
A. Dormant cancer cells in the bone marrow may acquire the ability to secrete soluble VCAM-1, which will form concentration gradient and chemotract pre-osteoclasts. The interaction between cancer cells and pre-osteoblasts will accelerate the differentiation of latter into activated osteoclasts (multinucleated cells depicted in light yellow) and drive the progression toward overt bone metastases. B. Coco antagonizes BMP signaling in the lung microenvironment and foster the self-renewal and proliferation of dormant cancer cells.
Murine cell lines derived from mouse mammary tumors have also been widely used to investigate breast cancer. As opposed to xenograft models, these models provide the advantage of using immune-competent syngeneic mice. Considering the growing evidence for the role of immune cells in tumor progression (23), this is a potential major advantage for such models. The disadvantage of the vast majority of murine cell line models is the lack of estrogen dependence. Nevertheless, important progress has been made in understanding metastasis dormancy using murine cell lines. One recent study using this model discovered the BMP-regulator Coco as a mediator driving the outgrowth of micrometastases into macrometastases in lungs (24) (Figure 2B). Another study identified Irf7 as a suppressor of spontaneous bone metastases, apparently by regulating the adaptive immune system (25). How relevant these findings are to ER+ human breast cancer remains to be tested.
Transgenic and other genetically engineered mouse models (GEMM) have also been developed to understand mechanisms driving tumor progression and metastases. These models possess the obvious strengths of capturing the full course of tumor progression from a pre-malignant stage to the terminal metastastatic stage. They also preserve the native interactions between cancer cells and their microenvironment at every stage. Like murine cell lines, however, GEMMs are rarely ER+ and estrogen-dependent, and, therefore, cannot be directly used for studies of ER+ breast cancer dormancy. Moreover, with few exceptions, GEMMs, unlike ER+ breast cancers, predominantly metastasize to lungs but not bones within the lifespan of the tumor-bearing mice (26). Despite these weaknesses, several groups have created elegant models that exhibit relevant features including local and metastasis dormancy. In a series of studies, de-induction of an inducible MMTV-Neu oncogene resulted in tumor regression. The regressed tumor, however, still contains viable tumor cells that remain dormant but can be activated by several processes and factors including expression of snail, a driver of epithelial-mesenchymal transition (EMT). This finding provided a connection between dormancy and a cellular program that was later linked to traits of cancer progenitor (stem) cells (27, 28). A more recent study demonstrated that Ron receptor kinase suppresses T-cells and promotes the outgrowth of micrometastases in lungs, highlighting the important role of the immune system in tumor dormancy (29). Again, whether these findings can be applied to human ER+ tumors is not known.
In addition to these models, patient-derived primary xenografts generated by directly transplanting pieces of surgically removed tumors to immunocompromised mice preserve histopathological features, genomic and transcriptomic similarities, and pharmacological responses similar to the original tumors in the patient. A minority of these primary xenografts maintain ER expression and ER-dependence. Importantly, many such xenografts spontaneously spread to the bone marrow, and therefore provide relevant sources of quiescent disseminated tumor cells (DTCs) (30-32).
In summary, although significant progress has been made in our understanding of metastasis dormancy, individual biological models cannot cover every essence of this process, especially for ER+ breast tumors. To overcome this barrier, researchers may need to combine different types of models and create novel ones. Like many other biological questions, the establishment of better models may benefit from our deeper understanding of the objectives. One possible solution is to divide the dormancy process into separate steps, conquer each of them separately, and finally synthesize our knowledge to construct an ideal experimental model. In the next section, we divide the problem of dormancy into a few conceptual components, summarize our current understanding of each, and propose future directions.
Biological mechanisms underlying metastasis dormancy
We envision that the dormancy process is comprised conceptually of several components including cell survival mechanisms that constantly sustain the viability of cancer cells, self-renewal mechanisms that maintain tumorigenesis capacity, and activation/suppression mechanisms that restore/prevent aggressive outgrowth. Very importantly, all of these mechanisms are likely to involve crosstalk between dormant cells and their microenvironment. We will focus on bone as the host tissue of dormant cancer cells because it is the most frequent metastatic site of ER+ breast cancer, and is the major reservoir of DTCs.
Upon arrival in the bone marrow, cancer cells need to extravasate from the blood circulation and enter the area close to the interface between the bone marrow and bone matrix. The extravasation process is not expected to be a major hurdle for cancer cells because of the sinusoid structures in the bone marrow, which are extensively fenestrated with 1 μm-wide pores. The cancer cells need to exploit the foreign microenvironment for survival. This can be viewed as a Darwinian selection process during which only a small fraction will succeed. Our previous study suggested that Src is a survival-mediator specifically in bone colonization in that it potentiates PI3K/AKT activation in cancer cells by CXCL12 and IGF1, both of which abound in the bone marrow microenvironment. Although the cell models we utilized were ER-, it is noteworthy that most ER+ tumors also exhibit high Src activity in part due to the interaction between ER and Src (33). Thus, our results provide one possible mechanism underlying the survival of cancer cells in bones. This mechanism links the intrinsic survival machinery to unique features of the bone microenvironment. Further investigations are needed to confirm this mechanism or to discover additional ones, because the survival mediators represent ideal therapeutic targets to permanently eradicate dormant cancer cells. As a result, short term intervention could generate long-lasting effects thereby alleviating concerns about side effects and prohibitive cost of treatment.
It has long been suspected that dormant cells are also tumor-initiating cells or cancer stem cells, although direct evidence is still lacking. Conceptually, if a dormant cell maintains the potential to eventually re-initiate a tumor, it should be a tumor-initiating cell by definition. However, are dormant tumor initiating cells that ultimately initiate bone metastases the same population of tumor-initiating cell that maintain the tumorigenesis potential of the primary tumor? Many recent studies suggest that the potential of initiating a tumor can be dynamically acquired or lost, and therefore represent a cellular status rather than a cellular entity (34-36). In this scenario, the tumor initiating potential needs to be suppressed to ensure dormancy, or activated to exit dormancy. A recent study discovered that BMP2 signaling in the lung microenvironment inhibits tumor initiating potential. The acquisition of Coco expression terminates metastasis dormancy and drives lung metastasis outgrowth (24) (Figure 2B). In another study, cancer cells disseminated to lungs were shown to induce the expression of Periostin (POSTN) in fibroblasts which will reciprocally increase WNT signaling and promote tumor initiating potential and tumor outgrowth (37). Similarly, lung micrometastases can also produce Tenascin C (TNC), an extracellular matrix protein that facilitates maintenance of tumor initiating potential by activating WNT and Notch pathways in cancer cells (38). The abovementioned molecules and the underlying pathways may represent therapeutic targets to diminish tumor initiating potential of dormant cancer cells. However, whether similar mechanisms are operative in bone and for ER+ breast cancer cells remains unknown.
The termination of dormancy not only needs the restoration of tumor initiating potential, but also requires strong proliferative signals, which may already be in place but actively suppressed by the host (e.g., immunosurveillance) or need to be acquired de novo. Several recent studies provide interesting examples of this process. In one study, dormant cancer cells are proposed to activate osteoclast progenitors via soluble VCAM1 as a non-conventional chemotractant. The activated osteoclasts in turn initiate the osteolytic vicious cycle and trigger the outgrowth of cancer cells (22) (Figure 2A). In a second study, the authors demonstrated that cancer cells in lungs need to form filopodia-like protrusions with extracellular matrix in order to grow out. The formation of these structures activates the integrin-FAK-ERK signaling cascades and drives cancer cell proliferation (39). The perivascular niche may also play a role in regulating the maintenance and exit of dormancy. Cyrus et al., showed that endothelial cells secrete thrombospondin 1 to suppress cancer cell proliferation, and this suppression is lost in sprouting neovasculature, which leads to outgrowth (40). Again, whether and how these processes are similar in ER+ tumors in bones after long-term dormancy remains to be investigated.
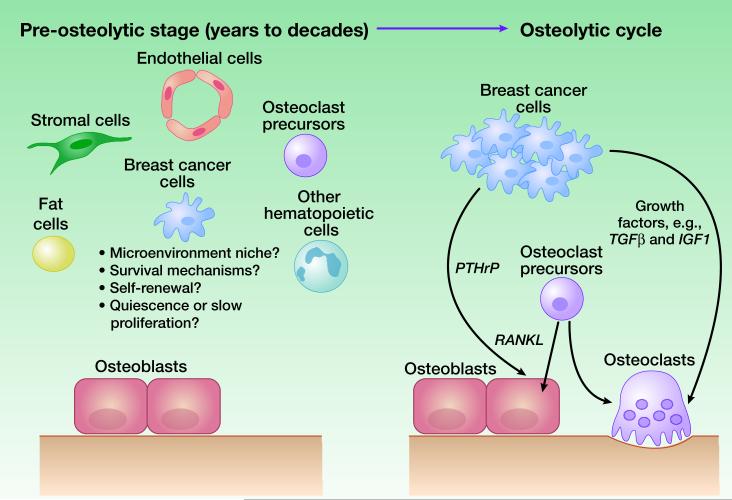
In summary, although insights have been gained into the dormancy process, it remains unexplored whether these discoveries can be applied to ER+ models under a dormant setting in bone. A key piece of information missing in our understanding of ER+ metastasis is the biology of bone micrometastases. It is well established that in the advanced stage, bone metastases are typically osteolytic and driven by a vicious cycle between cancer cells and osteoclasts. However, it is also evident that many patients do not exhibit symptoms of osteolysis for many years before bone relapses. How cancer cells exist as microscopic lesions and how they crosstalk with the bone microenvironment in the pre-osteolytic stage remains completely elusive. DTCs may represent cancer cells in this stage, but direct evidence is lacking. Several questions need to be addressed regarding pre-osteolytic micrometastases (Figure 3). First, do they have preferential microenvironment niches in bone and bone marrow? Some recent studies using prostate cancer models suggest that cancer cells tend to lodge into the same niches as hematopoietic stem cells (HSCs) (41). However, the definition of the HSC niche is itself a biological question under intensive study. Second, are these micrometastases truly quiescent or are they slowly proliferating (Figure 1)? Third, are these micrometastases dormant or indolent because they are enriched with tumor-initiating cells or because they are restricted by the bone microenvironment. Finally, are there biological pathways that specifically mediate their survival and proliferation? The answers to these questions will shed light on the dormancy behavior of ER+ breast cancer and they warrant intensive investigation.
Figure 3. Bone metastasis progression from a pre-osteolytic stage to the osteolytic vicious cycle.
Left, A diagram showing cancer cells and various types of cells in bones before the initiation of the vicious cycle. Conceptual questions that remain to be answered are listed. Right, A simplified diagram showing major cell types and a few molecular players that have been known involved in the osteolytic cycle.
CLINICAL CHALLENGES OF ER+ DORMANCY
Detection of micrometastases
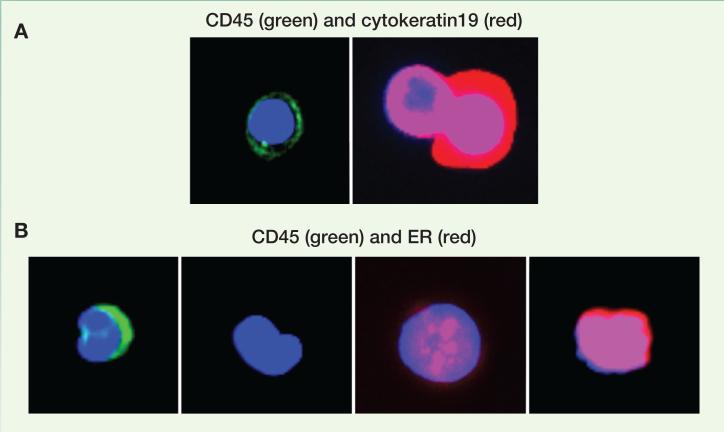
The current detection of metastases in the clinic primarily relies on the appearance of symptoms caused by macrometastases. As mentioned above, bone metastases cause osteolysis in their advanced stage, which are usually manifested by skeletal related events such as bone pain, fractures, and hypercalcemia. At the time of diagnosis, the vicious cycle between cancer cells and osteoclasts has already started, and the dormancy stage has been terminated. Some metastases are asymptomatic, and are detected by tests such as X-rays, computed tomography (CT), or Positron emission tomography with fluorodeoxyglucose (FDG-PET). However, microscopic metastases comprised of only a few cancer cells are below the detection threshold of these technologies. Thus, we have not been able to identify micrometastases in the clinic unless they are discovered “accidently” in a patient undergoing a bone marrow biopsy for research or unrelated purpose. We may be approaching the ability to detect sub-millimeter or even single-cell metastases because of the development of several new technologies. For instance, high-resolution hyperpolarized 3He MRI can detect micrometastases that are 300μm in diameter in pre-clinical models (42). Circulating tumor cells and tumor DNA in patient peripheral blood may represent useful surrogates of micrometastases in distant organs. Recently, therapeutic and biological insights have been obtained by monitoring and characterizing CTCs (43) and their released DNAs (44) in small cohorts of patients Molecular characterization of CTCs from the same blood sample has shown heterogeneous subpopulations with different transcriptional profiles and hormone receptor phenotypes (43, 45-47). Heterogeneity in ER expression of CTCs co-existing within a single blood draw from our study (Figure 4). The exact relation between CTCs and dormant micrometastases remains to be experimentally elucidated. DTCs in the bone marrow have been considered as dormant bone metastases, and mark a poorer prognosis (7, 48, 49). However, the correlation between these cells and bone relapses has not been firmly established. In some studies, patients with detectable DTCs and those with CTCs only partially overlap and exhibit different prognosis (50-52). Further investigations are needed to provide a deep understanding of the biological nature of DTCs and CTCs, and to answer the question of whether they represent or can be used as surrogates for dormant micrometastases.
Figure 4. Identification of CTCs and ER expression in peripheral blood samples of patients with metastatic breast cancer.
A. CTC enrichment post depletion of red and white blood cells (using RosetteSep®, StemCell Technologies): Immunofluorescence analysis defines residual leukocytes as CD45-positive/cytokeratin19-negative cells (left) and CTCs as CD45-negative/cytokeratin19-positive cells (right). B. Evaluation of ER expression in CTCs isolated from a single draw of another patient with ER-positive metastatic breast cancer reveals the coexistence of ER-negative, ER-weakly positive and ER-highly positive CTCs.
Prediction of late recurrences
Early relapses within five years can be predicted with reasonable accuracy using histopathological features and/or gene expression profiles. Current successful predictors share the commonality of having some components reflecting proliferation, suggesting that early relapses may be caused by the rapid proliferation of residual tumor cells (14-17). Late recurrences beyond five years, however, apparently are not associated with proliferation markers, and have been difficult to predict (53). The ratio of two individual mRNAs, HOXB13/IL17BR, has been demonstrated to be a prognostic factor in ER+ and node negative patients, which is the major population giving rise to late recurrences (54, 55). Gene expression signatures that are retrospectively associated with late recurrences have recently been derived by comparing the gene expression profiles of primary tumors of early vs. late recurrences (56, 57), or using dormant cancer cells in experimental systems (58). Whether these signatures can prospectively predict late recurrences remains to be tested. The difficulty of predicting recurrences beyond five years may be anticipated for biological reasons. Using information derived from the primary tumor to predict recurrences is based on the assumption that the traits driving recurrences are encoded in the bulk of primary tumors. While this assumption has been validated for early recurrences in numerous studies, it might need to be reconsidered in the case of late recurrences. The fact that dormant micrometastases stay in distant organs for many years suggests a long evolutionary process of these cells after their departure from the primary tumor. During this time, independent genetic and epigenetic traits may arise and drive the recurrences which will not be present the original primary tumors (59). Thus, it may be necessary to examine the metastases from patients with late relapses and compare them to the primary tumor and early metastases to decipher the genetic or epigenetic alterations that lead to late recurrences. Other factors may also confound the prediction of late recurrences. Some late recurrences may exceed the detection threshold, but are asymptomatic and undetected till the patients are deceased. These cases may therefore be wrongly excluded from the late recurrence category. On the other hand, patients who succumb to early recurrences may harbor dormant metastases at different sites which would progress further had the patient survived. Therefore, it remains a challenge to accurately classify primary tumors as to their propensity for late recurrences.
Treatment and prevention of late recurrences
As mentioned earlier roughly 20-40% of ER+ breast cancer patients eventually develop distant metastases, and half of these events occur five years or later after diagnosis of the primary tumor. However, the proportion of patients carrying dormant cells may be much higher if in some patients these cells never progress to become macrometastases during the patient’s lifetime, and even they do, the resultant slowly growing metastases may remain asymptomatic and undetected. A recent example of a patient cared for by one of the authors (CKO) who was found on a CT scan ordered for a kidney stone to have a 5cm asymptomatic osteolytic lesion in the pelvis proven on biopsy to be an ER+ breast cancer metastasis developing 22 years after her primary tumor diagnosis illustrates this possibility. Even proposing to evaluate asymptomatic patients looking for occult metastasis presents practical and ethical dilemmas. With the rapid development of imaging and other detection technologies, it is foreseeable that we will be able to detect sub-millimeter or even single cancer cells in the future. However, key questions remain 1) how many of the detected dormant metastases will progress to threaten patients’ lives; 2) how can we distinguish the dormant metastases that are likely to progress from those that are not; and 3) what therapeutic strategies can be used to eradicate them or prevent their progression? The answers to these questions require us to directly investigate dormant cancer cells. Thus, there are urgent needs to establish biological models of dormant metastases in the laboratory, and obtain patient-derived dormant cancer cells from the clinic. These efforts will open several diagnostic and therapeutic avenues. First, characterization of dormant cancer cells may lead to identification of highly-specific serum markers of dormant cancer cells, including DNA, RNA, proteins, and metabolites that are released to the circulation. Second, identification and investigation of survival pathways that sustain dormant cancer cells may lead to therapies that eradicate dormant cancer cells. Finally, elucidation of how dormant cancer cells progress to resume aggressive growth may help the prevention of overt metastases, and facilitate the prediction of which dormant metastases need to be proactively managed in the clinic. Our current knowledge is far from sufficient to achieve these goals. Therefore, pre-clinical and clinical studies on dormant micrometastases are imperatively needed to promote our understanding of these critical issues and ultimately to prevent late recurrences and further improve the outcome of breast cancer patients.
ACKNOWLEDGEMENTS
C.K.O and R.S. are supported by NIH SPORE Grant P50CA058183 and Cancer Center Grant P30CA125123, the EIF/Lee Jeans Breast Cancer Research Program, Breast Cancer Research Foundation, Komen Promise Grant PG12221410 from Susan G. Komen for the Cure Foundation and Stand Up 2 Cancer (SU2C-AACR-DT0409). R.S. and T.V.T. are supported by U54CA149196 pilot project. X. H.-F. Z. is supported by NCI CA151293, Breast Cancer Research Foundation, and McNair Medical Institute.
Footnotes
Conflict of Interest: No potential conflicts of interest are disclosed.
Financial Support: C.K.O and R.S. are supported by NIH SPORE Grant P50CA058183 and Cancer Center Grant P30CA125123, Komen Promise Grant PG12221410 from Susan G. Komen for the Cure Foundation. The EIF/Lee Jeans Breast Cancer Research Program, Breast Cancer Research Foundation, and a Stand Up To Cancer Dream Team Breast Cancer Research Grant, a Program of the Entertainment Industry Foundation (SU2C-AACR-DT0409). R.S. and T.V.T. are supported by U54CA149196 pilot project. X. H.-F. Z. is supported by NCI CA151293, Breast Cancer Research Foundation, and McNair Medical Institute.
REFERENCES
- 1.Gao D. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 2.Favaro E, Amadori A, Indraccolo S. Cellular interactions in the vascular niche: implications in the regulation of tumor dormancy. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2008;116:648–59. doi: 10.1111/j.1600-0463.2008.01025.x. [DOI] [PubMed] [Google Scholar]
- 3.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–7. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 4.Quesnel B. Tumor dormancy and immunoescape. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2008;116:685–94. doi: 10.1111/j.1600-0463.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 5.Disis ML, Stanton SE. Can immunity to breast cancer eliminate residual micrometastases? Clin Cancer Res. 2013;19:xx–xx. doi: 10.1158/1078-0432.CCR-13-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–46. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wikman H, Vessella R, Pantel K. Cancer micrometastasis and tumor dormancy. APMIS. 2008;116:754–70. doi: 10.1111/j.1600-0463.2008.01033.x. [DOI] [PubMed] [Google Scholar]
- 8.Retsky MW, Demicheli R, Hrushesky WJ, Baum M, Gukas ID. Dormancy and surgery-driven escape from dormancy help explain some clinical features of breast cancer. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2008;116:730–41. doi: 10.1111/j.1600-0463.2008.00990.x. [DOI] [PubMed] [Google Scholar]
- 9.Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology. 2012;26:688–94. 96. [PubMed] [Google Scholar]
- 10.Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11.Demicheli R, Abbattista A, Miceli R, Valagussa P, Bonadonna G. Time distribution of the recurrence risk for breast cancer patients undergoing mastectomy: further support about the concept of tumor dormancy. Breast Cancer Res Treat. 1996;41:177–85. doi: 10.1007/BF01807163. [DOI] [PubMed] [Google Scholar]
- 12.Karrison TG, Ferguson DJ, Meier P. Dormancy of mammary carcinoma after mastectomy. J Natl Cancer Inst. 1999;91:80–5. doi: 10.1093/jnci/91.1.80. [DOI] [PubMed] [Google Scholar]
- 13.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–32. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 16.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 17.Metzger Filho O, Ignatiadis M, Sotiriou C. Genomic Grade Index: An important tool for assessing breast cancer tumor grade and prognosis. Crit Rev Oncol Hematol. 2011;77:20–9. doi: 10.1016/j.critrevonc.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 18.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329–40. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 19.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–27. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer Cell. 2011;20:701–14. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 24.Gao H, Chakraborty G, Lee-Lim AP, Mo Q, Decker M, Vonica A, et al. The BMP inhibitor Coco reactivates breast cancer cells at lung metastatic sites. Cell. 2012;150:764–79. doi: 10.1016/j.cell.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bidwell BN, Slaney CY, Withana NP, Forster S, Cao Y, Loi S, et al. Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med. 2012;18:1224–31. doi: 10.1038/nm.2830. [DOI] [PubMed] [Google Scholar]
- 26.Kretschmann KL, Welm AL. Mouse models of breast cancer metastasis to bone. Cancer Metastasis Rev. 2012;31:579–83. doi: 10.1007/s10555-012-9378-4. [DOI] [PubMed] [Google Scholar]
- 27.Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–61. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 28.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, et al. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Eyob H, Ekiz HA, Derose YS, Waltz SE, Williams MA, Welm AL. Inhibition of Ron kinase blocks conversion of micrometastases to overt metastases by boosting anti-tumor immunity. Cancer Discov. 2013;3:751–60. doi: 10.1158/2159-8290.CD-12-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeRose YS, Wang G, Lin YC, Bernard PS, Buys SS, Ebbert MT, et al. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat Med. 2011;17:1514–20. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Claerhout S, Prat A, Dobrolecki L, Petrovic I, Lai Q, et al. A Renewable Tissue Resource of Phenotypically Stable, Biologically and Ethnically Diverse, Patient-derived Human Breast Cancer Xenografts. Cancer research. 2013;73:4885–97. doi: 10.1158/0008-5472.CAN-12-4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cottu P, Marangoni E, Assayag F, de Cremoux P, Vincent-Salomon A, Guyader C, et al. Modeling of response to endocrine therapy in a panel of human luminal breast cancer xenografts. Breast Cancer Res Treat. 2012;133:595–606. doi: 10.1007/s10549-011-1815-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizuno H, Spike BT, Wahl GM, Levine AJ. Inactivation of p53 in breast cancers correlates with stem cell transcriptional signatures. Proc Natl Acad Sci U S A. 2010;107:22745–50. doi: 10.1073/pnas.1017001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–40. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malanchi I, Santamaria-Martinez A, Susanto E, Peng H, Lehr HA, Delaloye JF, et al. Interactions between cancer stem cells and their niche govern metastatic colonization. Nature. 2012;481:85–9. doi: 10.1038/nature10694. [DOI] [PubMed] [Google Scholar]
- 38.Oskarsson T, Acharyya S, Zhang XH, Vanharanta S, Tavazoie SF, Morris PG, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17:867–74. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibue T, Brooks MW, Inan MF, Reinhardt F, Weinberg RA. The outgrowth of micrometastases is enabled by the formation of filopodium-like protrusions. Cancer Discov. 2012;2:706–21. doi: 10.1158/2159-8290.CD-11-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013 doi: 10.1038/ncb2767. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121:1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Branca RT, Cleveland ZI, Fubara B, Kumar CS, Maronpot RR, Leuschner C, et al. Molecular MRI for sensitive and specific detection of lung metastases. Proc Natl Acad Sci U S A. 2010;107:3693–7. doi: 10.1073/pnas.1000386107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 45.Nadal R, Fernandez A, Sanchez-Rovira P, Salido M, Rodriguez M, Garcia-Puche JL, et al. Biomarkers characterization of circulating tumour cells in breast cancer patients. Breast Cancer Res. 2012;14:R71. doi: 10.1186/bcr3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell AA, Talasaz AH, Zhang H, Coram MA, Reddy A, Deng G, et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS One. 2012;7:e33788. doi: 10.1371/journal.pone.0033788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayes DF, Paoletti C. Circulating tumour cells: insights into tumour heterogeneity. J Intern Med. 2013;274:137–43. doi: 10.1111/joim.12047. [DOI] [PubMed] [Google Scholar]
- 48.Pantel K. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst. 1993;85:1419–24. doi: 10.1093/jnci/85.17.1419. [DOI] [PubMed] [Google Scholar]
- 49.Sienel W, Seen-Hibler R, Mutschler W, Pantel K, Passlick B. Tumour cells in the tumour draining vein of patients with non-small cell lung cancer: detection rate and clinical significance. Eur J Cardiothorac Surg. 2003;23:451–6. doi: 10.1016/s1010-7940(02)00865-5. [DOI] [PubMed] [Google Scholar]
- 50.Benoy IH, Elst H, Philips M, Wuyts H, Van Dam P, Scharpe S, et al. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer. 2006;94:672–80. doi: 10.1038/sj.bjc.6602985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiedswang G, Borgen E, Karesen R, Qvist H, Janbu J, Kvalheim G, et al. Isolated tumor cells in bone marrow three years after diagnosis in disease-free breast cancer patients predict unfavorable clinical outcome. Clin Cancer Res. 2004;10:5342–8. doi: 10.1158/1078-0432.CCR-04-0245. [DOI] [PubMed] [Google Scholar]
- 52.Molloy TJ, Bosma AJ, Baumbusch LO, Synnestvedt M, Borgen E, Russnes HG, et al. The prognostic significance of tumour cell detection in the peripheral blood versus the bone marrow in 733 early-stage breast cancer patients. Breast Cancer Res. 2011;13:R61. doi: 10.1186/bcr2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burstein HJ, Griggs JJ. Deep time: the long and the short of adjuvant endocrine therapy for breast cancer. J Clin Oncol. 2012;30:684–6. doi: 10.1200/JCO.2011.40.1455. [DOI] [PubMed] [Google Scholar]
- 54.Ma XJ, Hilsenbeck SG, Wang W, Ding L, Sgroi DC, Bender RA, et al. The HOXB13:IL17BR expression index is a prognostic factor in early-stage breast cancer. J Clin Oncol. 2006;24:4611–9. doi: 10.1200/JCO.2006.06.6944. [DOI] [PubMed] [Google Scholar]
- 55.Sgroi DC, Carney E, Zarrella E, Steffel L, Binns SN, Finkelstein DM, et al. Prediction of Late Disease Recurrence and Extended Adjuvant Letrozole Benefit by the HOXB13/IL17BR Biomarker. J Natl Cancer Inst. 2013;105:1036–42. doi: 10.1093/jnci/djt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu MC, Dixon JM, Xuan JJ, Riggins RB, Chen L, Wang C, et al. Molecular Signaling Distinguishes Early ER Positive Breast Cancer Recurrences Despite Adjuvant Tamoxifen. 34th San Antonio Breast Cancer Symposium. 2011 2011. [Google Scholar]
- 57.Saghatchian M, Mittempergher L, Michiels S, Casinius S, Glas A, Lazar V, et al. Characterization of Breast Cancer Distant Metastasis Based on Outcome over Time Using a Gene Expression Profiling Approach and Identification of Pathway Activities of Late Relapse; 34th San Antonio Breast Cancer Symposium; San Antonio. 2011. 2011. [Google Scholar]
- 58.Kim RS, Avivar Valderas A, Estrada Y, Bragado P, Sosa MS, Aguirre-Ghiso JA, et al. Dormancy signatures and metastasis in estrogen receptor positive and negative breast cancer. PLoS One. 2012;7:e35569. doi: 10.1371/journal.pone.0035569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah SP, Morin RD, Khattra J, Prentice L, Pugh T, Burleigh A, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–13. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 60.Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97:1262–71. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 61.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013;381:805–16. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol. 2008;26:1965–71. doi: 10.1200/JCO.2007.14.0228. [DOI] [PubMed] [Google Scholar]
- 63.Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, et al. Extended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a. J Natl Cancer Inst. 2007;99:1845–53. doi: 10.1093/jnci/djm246. [DOI] [PubMed] [Google Scholar]