Abstract
Objective
The aim of this study is to evaluate whether contrast enhanced computed tomography (CECT) attenuation, using a cationic contrast agent (CA4+), correlates with the equilibrium compressive modulus (E) and coefficient of friction (μ) of ex vivo bovine articular cartilage.
Methods
Correlations between CECT attenuation and E (Group 1, n=12) and μ (Group 2, n=10) were determined using 7mm diameter bovine osteochondral plugs from the stifle joints of six freshly slaughtered, skeletally mature cows. The equilibrium compressive modulus was measured using a 4-step, unconfined, compressive stress relaxation test, and the coefficients of friction were determined from a torsional friction test. Following mechanical testing, samples were immersed in CA4+, imaged using μCT, rinsed, and analyzed for glycosaminoglycan (GAG) content using the DMMB assay.
Results
The CECT attenuation was positively correlated with the GAG content of bovine cartilage (R2= 0.87, p<0.0001 for Group 1 and R2= 0.74, p=0.001 for Group 2). Strong and significant positive correlations were observed between E and GAG content (R2= 0.90, p<0.0001) as well as CECT attenuation and E (R2= 0.90, p<0.0001). The CECT attenuation was negatively correlated with the three coefficients of friction: CECT vs. μstatic (R2=0.71, p=0.002), CECT vs. μstatic_equilibrium (R2=0.79, p<0.001), and CECT vs. μkinetic (R2=0.69, p=0.003).
Conclusions
CECT with CA4+ is a useful tool for determining the mechanical properties of ex vivo cartilage tissue as the attenuation significantly correlates with the compressive modulus and coefficient of friction.
Keywords: Computed tomography, friction, compressive modulus, cartilage, osteoarthritis, imaging
INTRODUCTION
Articular cartilage is the soft, hydrated tissue located at the ends of long bones, serving to distribute load while reducing friction and wear during joint articulation. Comprised primarily of collagen type II, proteoglycans, chondrocytes, and water, articular cartilage’s composition and structure directly affect its mechanical properties and function [1]. The extracellular matrix (ECM) of cartilage confers its resistance to compressive loads and enhanced lubricating abilities by providing a porous structure that regulates and retains water. The collagen matrix, a crucial component of the cartilage ECM, is specifically oriented throughout the tissue, permitting it to resist tensile stresses, prevent the expansion of the ECM during compression, and resist shear stresses at the articular surface [1-3]. The proteoglycans[4] principally located in the middle and deep zones, consist of a protein core with many attached glycosaminoglycans (GAGs) that also influence the mechanical properties of cartilage. These negatively-charged GAGs repel each other and form non-covalent interactions with water affording a swelling pressure in the ECM that contributes to compressive stiffness [1] and lubrication between cartilage surfaces [5-7]. Articular cartilage chondrocytes are responsible for the maintenance and homeostasis of the cartilage ECM and thus its biological and mechanical function. These cells have different size, shape, orientation, and biosynthetic activity in the different zones of articular cartilage.[8] In the superficial zone, the cells are flattened and parallel to the articular surface, and primarily synthesize collagen. Chondrocytes in the middle and deep zones are rounder, have more synthetic activity, and tend to synthesize more proteoglycan and larger collagen fibrils. Deep zone chondrocytes are often arranged in columns perpendicular to the articular cartilage surface.
Healthy cartilage maintains a balanced process of synthesis and degradation with an intact ECM, however, during osteoarthritis (OA), this balance is disturbed and matrix degradation prevails [1]. OA is a multifactorial disease which manifests in the clinic at its later stages as patient pain and reduced mobility due to cartilage thinning, lesions, osteophytes, and synovial inflammation.[9, 10] Cartilage lesions arise from the loss of GAGs and disruption of the collagen network,[11] although the precise timing of these biological events in sequence is being actively investigated. Loss of GAG, or the fixed negative charge content, is a key event prior to advanced OA[12-21] along with loss or alteration of other biochemical markers like denatured collagen or aggrecan TEGE fragments. However, some studies suggest that cartilage responds to the early disease process with compensatory events (such as cartilage hypertrophy) which may maintain or increase GAG content,[22-25] complicating the time chosen to measure GAG or the degree to which GAG quantification can be used as an indicator of early stage OA. For example, Stubendorff et al have recently reported no difference between GAG content in cartilage samples taken from OA and healthy patients. Importantly, many studies have established that cartilage samples with reduced GAG content and/or degraded collagen matrix have increased tissue pore size, hydraulic permeability, and water content as well as alteration of the organized matrix structure.[3, 26-32] These biological and physical changes lead to reduced mechanical integrity[26, 33, 34] of the cartilage and diminished lubrication in the joint, resulting in accelerated wear and cartilage degradation.
Consequently, characterizing and understanding the biomechanical properties of cartilage is important from both a basic science and clinical perspective, and various mechanical testing regimens have been developed. These approaches typically use either excised cartilage disks or osteochondral plugs cored from various joint surfaces. Unconfined compressive stress relaxation tests using osteochondral plugs allow facile computation of the equilibrium compressive modulus (E) of cartilage by fitting a line to the resulting equilibrium stress-strain data [34-36]. Additionally, torsional friction tests consisting of static compression followed by relaxation and then rotation are used for evaluating coefficients of friction (μ) of cartilage [37, 38]. Even though both tests are non-destructive and allow for subsequent evaluation of the same samples, additional minimally-invasive methods to quantify cartilage mechanics are highly desired, especially imaging-based techniques that can be used in pre-clinical animal or clinical studies.
Several quantitative imaging methods are being developed to evaluate biochemical changes in cartilage, specifically GAG content changes [39-42]. Most of these techniques indirectly determine changes in the GAG content of cartilage tissue utilizing an anionic contrast agent probe that partitions in inverse proportion to the GAG content of the cartilage matrix. For example, Gadopentetic acid (charge -2) is used for delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) [17, 43-48] while ioxaglate [49-55] (charge -1) and iothalamate [34, 56] (charge -1) are used for contrast-enhanced computed tomographic (CECT) imaging of cartilage. dGEMRIC can quantify changes in GAG content in both in vitro [43, 44, 47, 48] as well as in in vivo [17, 45] models. In this technique, the changes in MRI T1 relaxation time in the presence of GdDTPA2- reflect variations in both the structure and composition of the cartilage ECM, including GAGs. Similarly for CECT, changes in the x-ray attenuation of cartilage in the presence of ioxaglate or iothalamate can be used to quantify the GAG content of normal as well as degraded articular cartilage [34, 49-56]. Additionally, CECT attenuation of bovine cartilage plugs, obtained using iothalamate at high concentrations, was correlated to the compressive modulus [34], thus providing motivation to further explore this technique for the assessment of cartilage tissue mechanical properties.
Previously, CECT using a novel cationic contrast agent (CA4+) was reported as a sensitive technique for monitoring changes in cartilage GAG content and distribution at considerably lower concentrations than anionic contrast agents [49, 56, 57]. Since cartilage biomechanical properties are related to GAG content, we hypothesize that x-ray attenuations obtained from CECT imaging of cartilage using CA4+ will correlate with two important articular cartilage biomechanical properties: compressive modulus and coefficient of friction. Therefore, the aim of this study was to test this hypothesis, and herein, we describe the strong positive correlation between CECT attenuation and compressive modulus, as well as the strong negative correlation between CECT attenuation and coefficient of friction in a bovine osteochondral plug model.
METHODS
Material/specimen preparation
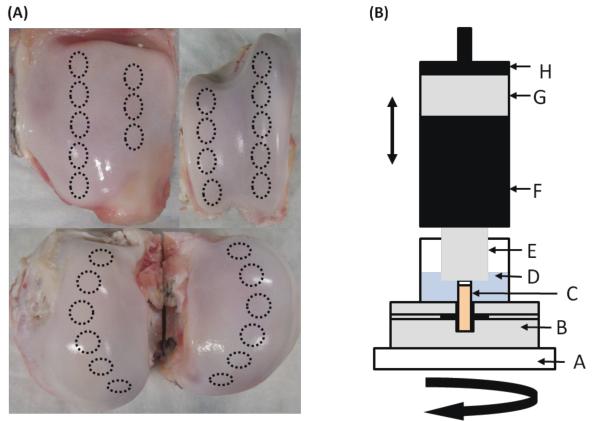
Twenty-two osteochondral plugs (7mm diameter) were cored from the stifle joints of six freshly slaughtered, skeletally mature cows using a diamond-tipped cylindrical cutter, irrigated with 0.9% saline at room temperature. Twelve plugs from the femoral condyles were used to test for a correlation between CECT attenuation and E (Group 1). Five of these plugs were degraded using Chondroitinase ABC (Sigma C3667, St. Louis, MO) [0.1 U/mL in 50 mM Tris, 60 mM NaOAc, 0.02% BSA, pH 8.0] at 37 °C for 24 hrs. The degraded plugs were then rinsed twice for 4 hrs each in 10 mL of saline at room temperature before a final rinse overnight in 10 mL of saline at 4 °C to ensure any remaining Chondroitinase ABC was removed. A separate ten plugs were harvested from the tibial, femoral, and patellar surfaces (Figure 1A) to test for a correlation between CECT attenuation and μ (Group 2). All the samples were then frozen at −20 °C in 0.9% saline with protease inhibitors, antibiotics, and antimycotics for later use. GIBCO Anti-Anti stock solution (Invitrogen, Grand Island, NY), 5mM of EDTA (Sigma, St. Louis, MO), and benzamidine HCl (Sigma B6506, St. Louis, MO) were included in all the solutions that were exposed to the cartilage to prevent nonspecific degradation of the cartilage during the study.
Figure 1.
(A) Photos showing locations where osteochondral plugs were harvested from bovine patellae, femoral grooves, and femoral condyles. Plugs were randomly selected after freezing. (B) Schematic of mechanical testing setup. A- Frame of machine, B- Plug fixture with set screws to anchor plug by its subchondral bone, C- 7mm diameter osteochondral plug, D- physiologic saline, E- aluminum platen, F- Torque Cell, G- Load Cell, H- Actuator. Each osteochondral plug from both groups was subjected to a 4- step compression against the aluminum platen while immersed in saline. Plugs from Group 2 were also subjected to a 720° rotation following the 45-min relaxation after the final compressive step.
Compressive Modulus (E) and Coefficient of Friction (μ) Testing
The samples in both groups were evaluated using similar mechanical testing procedures. Briefly, a pre-load was applied to establish complete contact between each sample’s surface and a polished aluminum platen (Figure 1 B). While immersed in saline, each sample was compressed using a 4-step unconfined stress-relaxation regimen consisting of four 5% strain steps at a displacement rate of 0.005 mm/sec (Enduratec 3230, BOSE, Eden Prairie, MN), each followed by a 45-min relaxation period [34]. For Group 1, E was then computed by fitting a linear regression line to the resulting equilibrium stress-strain data [34-36]. The samples in Group 2 were rotated 720 degrees at 5 degrees/sec (effective velocity of 0.3 mm/sec) [38] immediately following the last stress-relaxation period. The compressive force, torque, displacement, and rotational data were collected at a sampling rate of 10 Hz. We computed three torsional coefficients of friction (μ) representing the performance of articular cartilage [38] using the equation μ = T/(RN), where T is the torque, N is the normal force, and R is radius of the sample. Specifically, we calculated:
μstatic: the maximum value of μ for the first 10 degrees of rotation.
μstatic_eq: computed using the maximum value of T from the first 10 degrees of rotation and the normal force as the force at the end of the last relaxation period.
μkinetic: the average value of μ during the second revolution.
CA4+ Contrast Agent Solution
CA4+ was synthesized as previously reported [57]. The contrast agent solution was prepared by dissolving the dry compound in deionized water, balancing the pH to 7.4 using concentrated 4.0M NaOH and adjusting the osmolality to 400 mOsm/kg using sodium chloride to match the in situ osmolality of articular cartilage (350-450 mOsm/kg [58]). Similarly to the preparation of saline, protease inhibitors, antibiotics, and antimycotics were added before plug immersion.
CECT Imaging
Following mechanical testing for both groups, the plugs recovered in saline for at least 12 hrs at 4 °C prior to exposure to the contr ast agent. Each sample was then immersed in a 0.9 mL solution of the CA4+ contrast agent at 12 mgI/mL for 24 hrs at room temperature. Following immersion, each sample was gently blotted to remove excess contrast agent, and the plugs were positioned in a μCT imaging system (μCT40, Scanco Medical AG, Brüttisellen, Switzerland) using a custom, airtight holder that maintained a humid environment to prevent drying of the cartilage. Sequential transaxial μCT images of the cartilage and subchondral bone were acquired at an isotropic voxel resolution of 36 μm3, 70-kVP tube voltage, 113-μAmp current, and 300-ms integration time for all samples. The μCT data were converted to DICOM format using the proprietary software from Scanco Medical before being imported for post-processing using Analyze™ (BIR, Mayo Clinic, Rochester, MN). The cartilage was segmented from the subchondral bone using a semi-automatic, threshold-based algorithm. To perform accurate cartilage segmentation, multiple techniques, such as thresholding and component labeling, were utilized. The mean CECT attenuation value for each cartilage sample was obtained by averaging the x-ray attenuation over all transaxial μCT images corresponding to cartilage tissue and is reported in this study as grayscale intensities in the Hounsfield Units (HU).
Biochemical Assay
Each plug was immersed in saline at 4 °C for 24 hrs to wash out the contrast agent before the cartilage was carefully excised from the subchondral bone using a scalpel. Care was taken to remove all of the cartilage tissue, including the entire deep zone, and the wet mass of the cartilage was obtained. Following lyophilization for 24 hrs, the dry weight of each sample was also measured, and the samples were digested in papain (0.5 mg/mL in a buffer solution of 50 mM sodium phosphate, 5 mM EDTA, 2 mM DTT, pH 6.8) at 65 °C for 24 hrs. The GAG conten t of each cartilage sample was determined using the 1,9-dimethylmethylene blue (DMMB) colorimetric assay [59]. Briefly, each cartilage digestion solution was diluted 40 to 60 times for the assay. To convert from absorbance to GAG content, a linear calibration curve was generated using chondroitin-4-sulfate (Sigma 27042, St. Louis, MO) dissolved in the same buffer as above at concentrations ranging from 10-100 μg/mL. Ten microliters of each chondroitin-4-sulfate calibration solution and each diluted sample digestion solution were separately combined with 100 μL of DMMB dye solution in a 96-well plate. The absorbance of each resulting solution at 520 nm was measured in triplicate using a plate reader (Beckman Coulter AD340, Fullerton, CA). The total GAG mass of each sample was calculated using the calibration curve and normalized to total mg of GAG per mg wet weight of the cartilage for each sample.
Statistics
Univariate linear regression analysis (SPSS 17.0, Chicago, IL) was applied to evaluate whether the CECT attenuation correlated with the GAG content in both groups. Similarly, for Groups 1 and 2, the correlations between E and GAG content, between CECT attenuation and E, and between CECT attenuation and each of the three μ values were evaluated using univariate linear regression models. The coefficient of determination (R2) was used to assess the strength of the correlations. Significance level was set as two-tailed P-value < 0.05.
RESULTS
CECT vs. GAG Content (Groups 1 and 2)
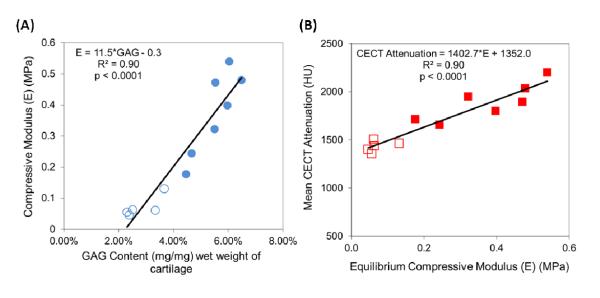
The CA4+ enhanced CT attenuation and GAG content for the samples tested for compressive modulus (Group 1; Figure 2A) and those tested for coefficient of friction (Group 2; Figure 2B) were strongly and significantly correlated with each other: R2= 0.87, p<0.0001 and R2= 0.74, p=0.001, respectively. Color maps of samples from Group 2 with low (Figure 2C) and high (Figure 2D) GAG content illustrate the differences in GAG contents measured with CECT.
Figure 2.
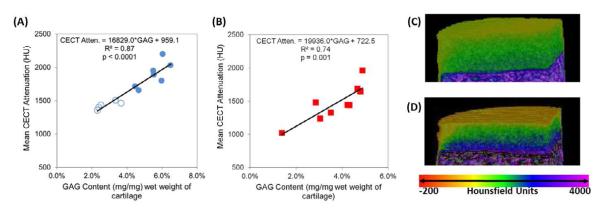
Correlations between CECT Attenuation (HU) and GAG content (mg/mg) of cartilage samples for (A) CECT vs. E samples (Group 1, unfilled data points indicate degraded samples) and (B) CECT vs. μ samples (Group 2). Both correlations were strong (coefficients of variation greater than or equal to 0.74) and statistically significant (p≤0.001). Color maps of representative, non-degraded samples with (C) low (2.86%) and (D) high (4.88%) GAG contents.
CECT vs. E (Group 1)
Strong positive correlations were observed between E and GAG content as well as between CECT attenuation and E: E vs. GAG (R2= 0.90, p<0.0001) (Figure 3A) and CECT vs. E (R2= 0.90, p<0.0001) (Figure 3B).
Figure 3.
Correlations between (A) Equilibrium Compressive Modulus (E) (MPa) and GAG content and (B) CECT attenuation (HU) and Equilibrium Compressive Modulus (E) (MPa) for Group 1(unfilled data points indicate degraded samples). Both correlations were strong (coefficients of variation equal to 0.90) and statistically significant (p<0.0001).
CECT vs. μ (Group 2)
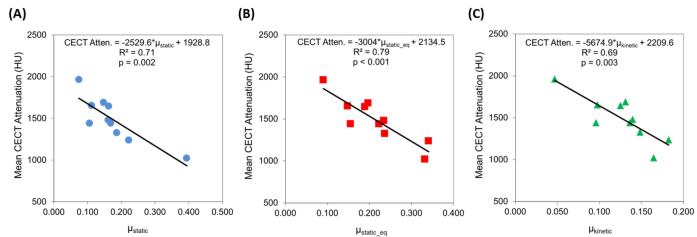
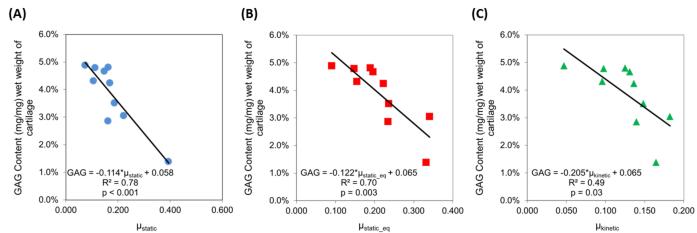
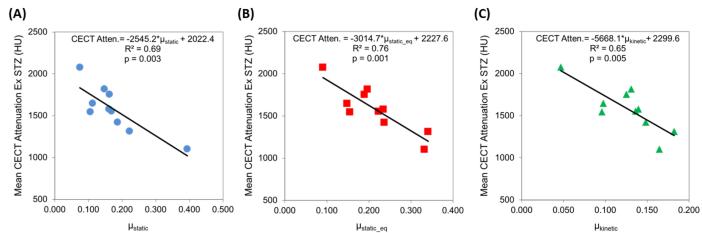
Additionally, the CA4+ enhanced CT attenuation was negatively correlated with the three coefficients of friction, accounting for up to 79% of the variation in μ (Figure 4): CECT vs. μstatic (R2=0.71, p=0.002), CECT vs. μstatic_equilibrium (R2=0.79, p<0.001), and CECT vs. μkinetic (R2=0.69, p=0.003). These correlations were similar to those achieved when comparing GAG content to the three coefficients of friction (Figure 5), with the correlation strength being ~30% lower for μkinetic (R2=0.49 vs. R2=0.69). To evaluate the effect of the cartilage superficial tangential zone (STZ) on the frictional properties, we excluded the CECT attenuation of the STZ and correlated the resulting CECT attenuation with the coefficients of friction (Figure 6). A small percent decrease in the R2 values (ΔR2) was observed, but the resulting correlations were still statistically significant for μstatic (ΔR2= −2.2%, p=0.003 for resulting correlation), μstatic_equilibrium (ΔR2 = −3.5%, p=0.001) and μkinetic (ΔR2 = −3.7%, p=0.005).
Figure 4.
Correlations between CECT attenuation (HU) and three different experimentally-determined coefficients of friction (μ): (A) μstatic, (B) μstatic_eq, (C) μkinetic for Group 2.
Figure 5.
Correlations between GAG content of each sample and three different experimentally-determined coefficients of friction (μ): (A) μstatic, (B) μstatic_eq, (C) μkinetic for Group 2.
Figure 6.
Correlations between CECT attenuation (HU) excluding the STZ of each sample and three different experimentally-determined coefficients of friction (μ): (A) μstatic, (B) μstatic_eq, (C) μkinetic for Group 2.
DISCUSSION
The objective of this study was to determine if the biomechanical properties, namely equilibrium compressive modulus and coefficient of friction, of bovine articular cartilage can be evaluated using CECT imaging with a cationic contrast agent. As mentioned earlier, a decrease in GAG content is an indicator of OA, and GAGs contribute to the equilibrium compressive properties of cartilage. Figures 2A & 2B show strong, significant correlations between CECT attenuation and GAG content for all samples used in this study, again establishing the well-known link between CECT attenuation and GAG content. Figures 2C & 2D show color maps of representative samples with low (2.86%) and high (4.88%) GAG contents. As shown in Figures 3A & 3B, the equilibrium compressive modulus was strongly and positively correlated with GAG content and mean CECT attenuation (R2= 0.90 and p<0.0001 for both). The CECT attenuation values ranged from 1300 to 2200 HU with GAG contents from 2 to 7% and E from 0.02 to 0.55 MPa. These findings generally agree with previous results [34, 49-56] using an anionic contrast agent, except that the correlations are inversely related. For example, Bansal et al [34] reported a negative, linear correlation between CECT attenuation and E of bovine osteochondral plugs from the patella and femoral groove using iothalamate [Cysto-Conray II (CCII)]. The specific correlations and comparisons are: E vs. GAG: R2=0.89 (CCII) vs. R2=0.90 (CA4+), and CECT vs. E: R2=0.93 (CCII) vs. R2=0.90 (CA4+). However, the magnitude of the slope obtained for the CECT vs. E correlation (slope = 1403) in this study is greater than that reported by Bansal et al [34] (slope = −856), indicating an increased sensitivity to changes in compressive modulus compared to the anionic contrast agent. Furthermore, the results obtained with the CA4+ were achieved with a smaller sample size (n=12 vs. n=30 for CECT vs. GAG and n=12 vs. n=15 for E vs. GAG and CECT vs. E) and a considerably lower concentration of contrast agent (12 vs. 81 mgI/mL). With the CA4+, the electrostatic attraction between the contrast agent and the negatively charged GAGs results in high contrast agent uptake in cartilage [49], a positive linear correlation between CECT attenuation and both GAG content and E, and a steeper slope for the correlation between CECT and E.
Although there are no reports of the correlation between CECT attenuation and cartilage coefficient of friction, previous studies have shown that cartilage GAG content affects its frictional properties [5-7]. GAGs contribute to the frictional performance of cartilage through both hydrostatic and elastohydrodynamic lubrication [1, 38]. Hydrostatic lubrication typically occurs at the onset of loading and for a prolonged period thereafter, during which the cartilage interstitial fluid becomes pressurized and supports most of the load transmitted across the contact interface. On the other hand, elastohydrodynamic lubrication occurs as the cartilage ECM is further compressed during motion, during which the cartilage interstitial fluid becomes increasingly pressurized and more of the fluid is exuded at the tissue interface. When the interstitial water is pressurized, the frictional load of the collagen-proteoglycan matrix is considerably reduced, resulting in a lower μ. Since GAGs bind water in cartilage, contributing to the interstitial fluid pressure, the GAG content of cartilage affects frictional performance, and this was confirmed by Basalo et al [7], who showed that the depletion of GAGs from cartilage results in increased μ. Additionally, the same group also demonstrated that removal of the superficial tangential layer of cartilage did not increase μ [60], indicating that the frictional response of cartilage is not limited to a surface phenomenon, rather it is also affected by the GAG content deeper in the tissue.
As shown in Figures 4 & 5, both the CECT attenuation and GAG content were strongly and significantly correlated with the three torsional coefficients of friction. The μstatic values ranged from 0.05 to 0.4, μstatic_equilibrium values ranged from 0.1 to 0.35, and μkinetic values ranged from 0.05 to 0.2, with GAG contents from 1 to 5% and CECT attenuation from 1000 to 2000 HU. The discrepancy in correlation strengths for the CECT vs. μkinetic and GAG vs. μkinetic plots (R2=0.69 vs. R2=0.49, respectively) may reflect that frictional properties are influenced by more than GAG. Other factors, such as permeability, could affect both the diffusion of the contrast agent into the cartilage tissue and elastohydrodynamic lubrication. Thus, GAG content may not be the only contributor influencing cartilage frictional performance, especially as the tissue is continually deformed during the μkinetic testing. Since structural changes are associated with GAG loss in cartilage during OA, future studies are planned to investigate the effect of permeability and collagen content on cartilage biomechanical properties using CECT.
Next, the effect of excluding the superficial-tangential zone (STZ) of cartilage on the three correlations between CECT attenuation and frictional coefficients was investigated. This was predicated on the Krishnan et al report that demonstrated that removal of the superficial layer did not increase μ [60], indicating that the frictional response of cartilage is not limited to a surface phenomenon, rather it is also affected by the GAG content deeper in the tissue. Since this was demonstrated by performing frictional tests, removing the STZ layer with a microtome, and re-testing, which required altering the tissue, we sought to examine if a similar result could be obtained nondestructively by removing the STZ layer via image processing. We excluded the 10% of the CECT image slices closest to the articular surface and repeated the regression analyses. The effect of removing the STZ voxels was minimal (Figure 6), affording a small percent decrease in the R2 values (ΔR2), but the resulting correlations were still significant for μstatic (ΔR2 = −2.2%, p=0.003 for resulting correlation), μstatic_equilibrium (ΔR2 = −3.5%, p=0.001) and μkinetic (ΔR2 = −3.7%, p=0.005). This result corroborates the fact that frictional properties are not purely a surface phenomenon. Although current clinical CT scanners do not have sufficient resolution to examine only the STZ layer of cartilage, the scanners do have enough resolution to examine the cartilage tissue as a whole, which as we have shown in an ex vivo setting is sufficient for correlating cartilage frictional performance to CECT attenuation.
The slight discrepancy in correlation strength between the two CECT vs. GAG correlations (Figures 2A & 2B) is likely due to the way the samples were selected and prepared. For comparing CECT attenuation to compressive modulus (Group 1), the samples were selected from the same surface from multiple knees and then five of them were degraded using Chondroitinase ABC to selectively cleave GAG. This procedure was selected to enable comparison to previous studies comparing CECT attenuation and GAG content to compressive modulus. Degrading the samples to obtain a more continuous range of GAG content rather than selecting intact tissue samples is likely to improve the correlation strength for the CECT vs. GAG data for Group 1. The samples used to compare CECT attenuation to μ (Group 2) were selected from different surfaces in multiple knees to generate a sample set with varying GAG content without enzymatic degradation. This approach for obtaining a series of samples with different GAG contents was chosen, as opposed to using Chondroitinase ABC, to minimize any possible surface alterations that could be introduced with degradation via Chondroitinase ABC, as it has been shown that the degradation process begins at the articular surface and progresses deeper into the tissue [34].
In this study, the samples were compressed against an aluminum platen, which does not represent the in vivo conditions whereby two opposing cartilage surfaces are loaded against each other. However, previous studies have demonstrated reliable compressive modulus measurements using unconfined compression [35, 36] and frictional testing [7, 60-66] against non-biological surfaces (e.g., metal or glass). These results are also similar to those reported when two cartilage surfaces are tested against each other [34, 38]. The frictional samples were subjected to a 4-step compression before torsional loading to measure the coefficient of friction. This loading regimen allows sufficient time for the interstitial fluid to depressurize, which could affect the frictional performance of the samples. However, the samples supported loads typical of what we observe at the end of a shorter compression regimen [67], indicating that the interstitial fluid is likely still pressurized at the end of the 45-min dwell after the 4th step. Additionally, the normal force increased during the rotations as the tissue was further deformed, indicating a further pressurization of the interstitial fluid [66]. All the samples were also mechanically tested in saline, which is not the native environment of cartilage. Synovial fluid contains various biomacromolecules that function as boundary lubricants that affect the frictional performance of cartilage [68, 69]. Since our goal was to isolate the effects of hydrostatic and elastohydrodynamic lubrication, which are linked with GAG content [5-7], saline was used to prevent confounding the results from the presence of these lubricants. However, we are now in a position to look at the effects of lubricants on μ, E, and GAG degradation in future studies.
In osteoarthritis, cartilage progressively breaks down, resulting in a loss of proteoglycans, increased hydration, and fibrillation of the extracellular matrix [32]. As a result of these compositional alterations, the biomechanical performance of osteoarthritic cartilage reduces affecting the functionality of the tissue. Since CECT attenuation has been shown to correlate with GAG content [34, 49-56], compressive modulus [34] (and shown here), and now, for the first time, with frictional performance, CECT is a valuable tool for determining not only the GAG content of the cartilage tissue, but also its overall mechanical integrity. Additionally, a recent study demonstrated that CA4+ remains in the knee cartilage of an in vivo rabbit model for up to 2 hrs and is eliminated from the cartilage and joint space within 24 hrs; thus indicating CA4+ may be suitable for evaluating cartilage in animal OA models [70]. Since the subchondral bone is thought to play a role in OA [71-73], CECT can evaluate both cartilage and bone tissue, leading to a more thorough monitoring and diagnosis of the disease. Future studies using healthy and osteoarthritic human cartilage tissue are planned to validate our findings and further challenge this cationic CECT imaging technique for the assessment of overall cartilage health.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Vahid Entezari for his discussions on the study and Mr. Luai Zakaria for his help with image processing.
FUNDING SOURCES
This work was supported in part by the Coulter Foundation, the Harvard Catalyst Program, the NIH (R01GM098361), the Boston University Undergraduate Research Opportunities Program (D.J.G.), NIH (T32 GM008541) Pharmacology Training Grant (J.D.F.), and the Henry Luce Foundation (R.C.S).
COMPETING INTERESTS M.W.G. and B.S.D. have a conflict of interest as they have submitted a patent application on the composition and use of the CA4+ contrast agent as well as have grant funding from the NIH and Coulter Foundation. D.J.G., J.D.F., and R.C.S. also have received funding for their stipend support from the Boston University Undergraduate Research Opportunities Program, NIH (T32 GM008541) Pharmacology Training Grant, and the Henry Luce Foundation, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTIONS B.A. Lakin contributed to the experiments, data interpretations, data analysis, and manuscript writing.
D.J. Grasso contributed to the experiments, data analysis, and review of the manuscript.
S.S. Shah contributed to the experiments, data analysis, and review of the manuscript.
R.C. Stewart contributed to the experiments, data interpretations, and manuscript writing.
P.N. Bansal contributed to the experiment design, data interpretations, and manuscript writing.
J.D. Freedman contributed to the experiments, data interpretations, and review of the manuscript.
M.W. Grinstaff contributed to the experiment design, data interpretations, data analysis, and manuscript writing as well as resources to perform the experiments.
B.D. Snyder contributed to the experiment design, data interpretations, data analysis, and manuscript writing as well as resources to perform the experiments.
REFERENCES
- 1.Mow VC, Huiskes R. Basic orthopaedic biomechanics & mechano-biology. 3rd Edition Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 2.Athanasiou KA, Rosenwasser MP, Buckwalter JA, Malinin TI, Mow VC. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 1991;9:330–40. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 3.Maroudas A. Balance Between Swelling Pressure and Collagen Tension in Normal and Degenerated Cartilage. Nature. 1976:808–09. doi: 10.1038/260808a0. [DOI] [PubMed] [Google Scholar]
- 4.Heinegard D. Proteoglycans and more – from molecules to biology. Int. J. Exp. Path. 2009;90:575–86. doi: 10.1111/j.1365-2613.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katta J, Stapleton T, Ingham E, Jin ZM, Fisher J. The effect of glycosaminoglycan depletion on the friction and deformation of articular cartilage. Proceedings of the Institution of Mechanical Engineers. Part H, Journal of Engineering in Medicine. 2008;222:1–11. doi: 10.1243/09544119JEIM325. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2004;22:565–70. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basalo IM, Raj D, Krishnan R, Chen FH, Hung CT, Ateshian GA. Effects of enzymatic degradation on the frictional response of articular cartilage in stress relaxation. Journal of Biomechanics. 2005;38:1343–49. doi: 10.1016/j.jbiomech.2004.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zehbea R, Riesemeier H, Kirkpatrickc CJ, Brochhausenc C. Imaging of articular cartilage – Data matching using X-ray tomography, SEM, FIB slicing and conventional histology. Micron. 2012;43:1060–67. doi: 10.1016/j.micron.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and Cartilage. 2002;10:432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 10.Lohmander LS. What can we do about osteoarthritis? Arthritis Research. 2000;2:95–100. doi: 10.1186/ar74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis and Cartilage. 2001;10:432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 12.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instructional Course Lectures. 1998;47:487–504. [PubMed] [Google Scholar]
- 13.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Annals of Internal Medicine. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 14.Maroudas A, Leaback DH, Stockwell RA. Glycosaminoglycan Turn-Over in Articular Cartilage [and Discussion] Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1975;271:293–313. doi: 10.1098/rstb.1975.0054. [DOI] [PubMed] [Google Scholar]
- 15.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. Journal of Bone and Joint Surgery. American Volume. 1971;53:523–37. [PubMed] [Google Scholar]
- 16.Heinegård D, Inerot S, Olsson SE, Saxne T. Cartilage proteoglycans in degenerative joint disease. J Rheumatol. 1987;14:110–12. [PubMed] [Google Scholar]
- 17.Kim YJ, Jaramillo D, Millis MB, Gray ML, Burstein D. Assessment of early osteoarthritis in hip dysplasia with delayed gadolinium-enhanced magnetic resonance imaging of cartilage. The Journal of Bone and Joint Surgery. American Volume. 2003;85-A:1987–92. doi: 10.2106/00004623-200310000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Squires GR, Okouneff S, Ionescu M, Poole AR. The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis. Arthr Rheum. 2003;48:1261–70. doi: 10.1002/art.10976. [DOI] [PubMed] [Google Scholar]
- 19.Hoch DH, Grodzinsky AJ, Koob TJ, Albert ML, Eyre DR. Early changes in material properties of rabbit articular cartilage after meniscectomy. Journal of Orthopaedic Research. 1983;1:4–12. doi: 10.1002/jor.1100010102. [DOI] [PubMed] [Google Scholar]
- 20.Yagi R, McBurney D, Laverty D, Weiner S, Horton JWE. Intrajoint comparisons of gene expression patterns in human osteoarthritis suggest a change in chondrocyte phenotype. Journal of Orthopaedic Research. 2005;23:1128–38. doi: 10.1016/j.orthres.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 21.Grushko G, Schneiderman R, Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connective Tissue Research. 1989;19:149–76. doi: 10.3109/03008208909043895. [DOI] [PubMed] [Google Scholar]
- 22.Matyas JR, Ehlers PF, Huang D, Adams ME. The Early Molecular Natural History of Experimental Osteoarthritis. Arthritis and Rheumatism. 1999;42:993–1002. doi: 10.1002/1529-0131(199905)42:5<993::AID-ANR19>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Lorenz H, Wenz W, Ivancic M, Steck E, Richter W. Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Research and Therapy. 2005;7:R156–65. doi: 10.1186/ar1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams ME, Brandt KD. Hypertrophic repair of canine articular cartilage in osteoarthritis after anterior cruciate ligament transection. Journal of Rheumatology. 1991;18:428–35. [PubMed] [Google Scholar]
- 25.Mankin HJ, Lippiello L. The Glycosaminoglycans of Normal and Arthritis. Journal of Clinical Investigation. 1971;50:1712–19. doi: 10.1172/JCI106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. Journal of Orthopaedic Research. 2000;18:739–48. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- 27.Comper WD, Zampario O. Hydrodynamic properties of connective-tissue polysaccharides. Biochemical Journal. 1990;269:561–64. doi: 10.1042/bj2690561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Annals of the Rheumatic Diseases. 1977;36:121–29. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue H. Alterations in the collagen framework of osteoarthritic cartilage and subchondral bone. Int Orthop. 1981;5:47–52. doi: 10.1007/BF00286099. [DOI] [PubMed] [Google Scholar]
- 30.Xie T, Guo S, Zhang J, Chen Z, Peavy GM. Determination of characteristics of degenerative joint disease using optical coherence tomography and polarization sensitive optical coherence tomography. Lasers Surg Med. 2006;38:852–65. doi: 10.1002/lsm.20394. [DOI] [PubMed] [Google Scholar]
- 31.Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Altered structure-function relationships for articular cartilage in human osteoarthritis and an experimental canine model. Agents Actions Suppl. 1993;39:27–48. doi: 10.1007/978-3-0348-7442-7_3. [DOI] [PubMed] [Google Scholar]
- 32.Brandt KD. Cartilage changes in osteoarthritis. Indiana University School of Medicine; 1990. [Google Scholar]
- 33.Kempson G, Muir H, Swanson S, Freeman M. Correlations between stiffness and the chemical constituents of cartilage on the femoral head. Biochimica et Biophysica Acta. 1976;428:741–46. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- 34.Bansal PN, Joshi NS, Entezari V, Grinstaff MW, Snyder BD. Contrast Enhanced Computed Tomography can Predict the Glycosaminoglycan Content of and Biomechanical Properties of Articular Cartilage. Osteoarthritis and Cartilage. 2010;18:184–89. doi: 10.1016/j.joca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen AM, Levenston ME. Comparison of osmotic swelling influences on meniscal fibrocartilage and articular cartilage tissue mechanics in compression and shear. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2011 doi: 10.1002/jor.21493. [DOI] [PubMed] [Google Scholar]
- 36.Korhonen RK, Laasanen MS, Töyräs J, Rieppo J, Hirvonen J, Helminen HJ, et al. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. Journal of Biomechanics. 2002;35:903–09. doi: 10.1016/s0021-9290(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL, Sah RL. Boundary lubrication of articular cartilage: role of synovial fluid constituents. Arthritis and Rheumatism. 2007;56:882–91. doi: 10.1002/art.22446. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt TA, Sah RL. Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis and Cartilage. 2007;15:35–47. doi: 10.1016/j.joca.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 39.Link TM, Stahl R, Woertler K. Cartilage imaging: motivation, techniques, current and future significance. European Radiology. 2007;17:1135–46. doi: 10.1007/s00330-006-0453-5. [DOI] [PubMed] [Google Scholar]
- 40.Kneeland JB, Reddy R. Frontiers in musculoskeletal MRI: Articular cartilage. Journal of Magnetic Resonance Imaging. 2007;25:339–44. doi: 10.1002/jmri.20811. [DOI] [PubMed] [Google Scholar]
- 41.Winalski CS, Rajiah P. The evolution of articular cartilage imaging and its impact on clinical practice. Skeletal Radiology. 2011;40:1197–222. doi: 10.1007/s00256-011-1226-z. [DOI] [PubMed] [Google Scholar]
- 42.Bekkers JEJ, Creemers LB, Dhert WJA, Saris DFB. Diagnostic Modalities for Diseased Articular Cartilage—From Defect to Degeneration. Cartilage. 2010;1:157–64. doi: 10.1177/1947603510364539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bashir A, Gray ML, Burstein D. Gd-DTPA2- as a measure of cartilage degradation. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1996;36:665–73. doi: 10.1002/mrm.1910360504. [DOI] [PubMed] [Google Scholar]
- 44.Bashir A, Gray ML, Hartke J, Burstein D. Nondestructive imaging of human cartilage glycosaminoglycan concentration by MRI. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1999;41:857–65. doi: 10.1002/(sici)1522-2594(199905)41:5<857::aid-mrm1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 45.Eckstein F, Buck RJ, Wyman BT, Kotyk JJ, Le Graverand MP, Remmers AE, et al. Quantitative imaging of cartilage morphology at 3.0 Tesla in the presence of gadopentate dimeglumine (Gd-DTPA) Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007;58:402–6. doi: 10.1002/mrm.21290. [DOI] [PubMed] [Google Scholar]
- 46.Gray ML, Burstein D, Kim YJ, Maroudas A. 2007 Elizabeth Winston Lanier Award Winner. Magnetic resonance imaging of cartilage glycosaminoglycan: basic principles, imaging technique, and clinical applications. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society. 2008;26:281–91. doi: 10.1002/jor.20482. [DOI] [PubMed] [Google Scholar]
- 47.Lammentausta E, Kiviranta P, Nissi MJ, Laasanen MS, Kiviranta I, Nieminen MT, et al. T2 relaxation time and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) of human patellar cartilage at 1.5 T and 9.4 T: Relationships with tissue mechanical properties. Journal of Orthopaedic Research. 2006;24:366–74. doi: 10.1002/jor.20041. [DOI] [PubMed] [Google Scholar]
- 48.Taylor C, Carballido-Gamio J, Majumdar S, Li X. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1rho, dGEMRIC and contrast-enhanced computed tomography. Magnetic Resonance Imaging. 2009;27:779–84. doi: 10.1016/j.mri.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bansal PN, Stewart RC, Entezari V, Snyder BD, Grinstaff MW. Contrast Agent Electrostatic Attraction Rather than Repulsion To Glycosaminoglycans Affords a Greater Contrast Uptake Ratio and Improved Quantitative CT Imaging in Cartilage. Osteoarthritis and Cartilage. 2011;19:970–76. doi: 10.1016/j.joca.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Kokkonen HT, Jurvelin JS, Tiitu V, Töyräs J. Detection of mechanical injury of articular cartilage using contrast enhanced computed tomography. Osteoarthritis and Cartilage. 2011;19:295–301. doi: 10.1016/j.joca.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 51.Kallioniemi AS, Jurvelin JS, Nieminen MT, Lammi MJ, Toyras J. Contrast agent enhanced pQCT of articular cartilage. Physics in Medicine and Biology. 2007;52:1209–19. doi: 10.1088/0031-9155/52/4/024. [DOI] [PubMed] [Google Scholar]
- 52.Palmer AW, Guldberg RE, Levenston ME. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:19255–60. doi: 10.1073/pnas.0606406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Siebelt M, van Tiel J, Waarsing JH, Piscaer TM, van Straten M, Booij R, et al. Clinically applied CT arthrography to measure the sulphated glycosaminoglycan content of cartilage. Osteoarthritis and Cartilage. 2011;19:1183–89. doi: 10.1016/j.joca.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Silvast TS, Jurvelin JS, Aula AS, Lammi MJ, Toyras J. Contrast agent-enhanced computed tomography of articular cartilage: association with tissue composition and properties. Acta Radiologica. 2009;50:78–85. doi: 10.1080/02841850802572526. [DOI] [PubMed] [Google Scholar]
- 55.Silvast TS, Jurvelin JS, Lammi MJ, Toyras J. pQCT study on diffusion and equilibrium distribution of iodinated anionic contrast agent in human articular cartilage - associations to matrix composition and integrity. Osteoarthritis and Cartilage. 2009;17:26–32. doi: 10.1016/j.joca.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Bansal PN, Joshi NS, Entezari V, Stewart RC, Malone B, Grinstaff MW, et al. Cationic contrast agents improve quantification of glycosaminoglycan (GAG) content by Contrast Enhanced CT imaging of cartilage. Journal of Orthopaedic Research. 2011;29:704–09. doi: 10.1002/jor.21312. [DOI] [PubMed] [Google Scholar]
- 57.Joshi NS, Bansal PN, Stewart RC, Snyder BD, Grinstaff MW. Effect of Contrast Agent Charge on Visualization of Articular Cartilage Using Computed Tomography: Exploiting Electrostatic Interactions for Improved Sensitivity. Journal of the American Chemical Society. 2009;131:13234–35. doi: 10.1021/ja9053306. [DOI] [PubMed] [Google Scholar]
- 58.Urban JP, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. Journal of Cellular Physiology. 1993;154:262–70. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- 59.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et Biophysica Acta. 1986;883:173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 60.Krishnan R, Caligaris M, Mauck RL, Hung CT, Costa KD, Ateshian GA. Removal of the superficial zone of bovine articular cartilage does not increase its frictional coefficient. Osteoarthritis and Cartilage. 2004;12:947–55. doi: 10.1016/j.joca.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Basalo IM, Chahine NO, Kaplun M, Chen FH, Hung CT, Ateshian GA. Chondroitin sulfate reduces the friction coefficient of articular cartilage. Journal of Biomechanics. 2007;40:1847–54. doi: 10.1016/j.jbiomech.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Caligaris M, Ateshian GA. Effects of sustained interstitial fluid pressurization under migrating contact area, and boundary lubrication by synovial fluid, on cartilage friction. Osteoarthritis and Cartilage. 2008;16:1220–27. doi: 10.1016/j.joca.2008.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Forster H, Fisher J. The influence of continuous sliding and subsequent surface wear on the friction of articular cartilage. Proceedings of the Institution of Mechanical Engineers. Part H, Journal of Engineering in Medicine. 1999;213:329–45. doi: 10.1243/0954411991535167. [DOI] [PubMed] [Google Scholar]
- 64.Kumar P, Oka M, Toguchida J, Kobayashi M, Uchida E, Nakamura T, et al. Role of uppermost superficial surface layer of articular cartilage in the lubrication mechanism of joints. Journal of Anatomy. 2001;199:241–50. doi: 10.1046/j.1469-7580.2001.19930241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schiavinato A, Whiteside RA. Effective lubrication of articular cartilage by an amphiphilic hyaluronic acid derivative. Clinical Biomechanics. 2011 doi: 10.1016/j.clinbiomech.2011.11.012. In press. [DOI] [PubMed] [Google Scholar]
- 66.Wang H, Ateshian GA. The normal stress effect and equilibrium friction coefficient of articular cartilage under steady frictional shear. Journal of Biomechanics. 1997;30:771–76. doi: 10.1016/s0021-9290(97)00031-6. [DOI] [PubMed] [Google Scholar]
- 67.Bansal PN, Wathier M, Stoddardt SS, Shah SS, Snyder BD, Grinstaff MW. A Synthetic Polymer for Efficacious Boundary Lubrication of Articular Cartilage; Orthopedic Research Society Annual Meeting; New Orleans. 2010. [Google Scholar]
- 68.Jay GD, Haberstroh K, Cha CJ. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and Healon. Journal of Biomedical Materials Research. 1998;40:414–18. doi: 10.1002/(sici)1097-4636(19980605)40:3<414::aid-jbm11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 69.Swann DA, Silver FH, Slayter HS, Stafford W, Shore E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. The Biochemical Journal. 1985;225:195–201. doi: 10.1042/bj2250195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart RC, Bansal PN, Entezari V, Lusic H, Nazarian R, Snyder BD, et al. Contrast Enhanced Computed Tomography using a High Affinity Cationic Contrast Agent for Imaging Ex vivo Bovine, Intact Ex vivo Rabbit, and In vivo Rabbit Cartilage. Radiology. 2012 doi: 10.1148/radiol.12112246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brandt KD, Radin EL, Dieppe PA, van de Putte L. Yet more evidence that osteoarthritis is not a cartilage disease. Annals of the Rheumatic Diseases. 2006;65:1261–4. doi: 10.1136/ard.2006.058347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radin EL. Who gets osteoarthritis and why? An update. Journal of Rheumatology. 2005;32:1136–8. [PubMed] [Google Scholar]
- 73.Radin EL, Burr DB, Caterson B, Fyhrie D, Brown TD, Boyd RD. Mechanical determinants of osteoarthrosis. Seminars in Arthritis and Rheumatism. 1991;21:12–21. doi: 10.1016/0049-0172(91)90036-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.