Abstract
Previously, orbA, the gene encoding the outer membrane receptor for ferric-ornibactin, was identified in Burkholderia cenocepacia K56-2, a strain which produces ornibactin, salicylic acid, and negligible amounts of pyochelin. A K56-2 orbA mutant was less virulent than the parent strain in a rat agar bead infection model. In this study, an orbA mutant of B. cenocepacia Pc715j which produces pyochelin in addition to ornibactin and salicylic acid was constructed. The gene encoding the outer membrane receptor for ferric-pyochelin (fptA) was also identified. An fptA mutant was constructed in Pc715j and shown to be deficient in [59Fe]pyochelin uptake. A 75-kDa iron-regulated protein was identified in outer membrane preparations of Pc715j that was absent in outer membrane preparations of Pc715jfptA::tp. Pc715jfptA::tp and Pc715jorbA::tp produced smaller amounts of their corresponding siderophores. Both Pc715jorbA::tp and Pc715jfptA::tp were able to grow in iron starvation conditions in vitro. In the agar bead model, the Pc715jorbA::tp mutant was cleared from the lung, indicating that the pyochelin uptake system does not compensate for the absence of a functional ornibactin system. Pc715jfptA::tp persisted in rat lung infections in numbers similar to those of the parent strain, indicating that the ferric-ornibactin uptake system could compensate for the defect in ferric-pyochelin uptake in vivo. These studies suggest that the ornibactin uptake system is the most important siderophore-mediated iron transport system in B. cenocepacia lung infections.
Burkholderia cenocepacia (formerly B. cepacia genomovar III) is an opportunistic organism which has emerged as a major respiratory pathogen for patients with cystic fibrosis or chronic granulomatous disease (25, 26, 38). Infections with B. cenocepacia are a major concern because of the high patient-to-patient transmissibility and the multidrug resistance of the organism. Colonization is often associated with a rapid and fatal pneumonia (25, 26, 38). Recently, it has been determined that the B. cepacia complex which infects cystic fibrosis patients consists of nine biochemically similar but genetically distinct species (13, 14, 38). B. cenocepacia (70) is the most common Burkholderia species in populations of cystic fibrosis patients, causing more than 50% of the infections in these patients (38).
Iron is an essential requirement for bacterial growth because it is required for many biological processes (46). However, little free iron is available in the human host because much of it is bound by proteins such as lactoferrin, transferrin, and heme (17, 45). Under physiological conditions, iron exists in the ferric state and is insoluble, present at not more than 10−18 M, while for optimal bacterial growth 10−6 to 10−8 M is required (27, 46). Pathogenic bacteria have developed specific systems to acquire iron. One mechanism is the production of low-molecular-weight ferric iron chelators known as siderophores (27, 46). Siderophores facilitate the solubilization and transport of ferric iron into the cell by a specific outer membrane receptor-mediated transport system in which TonB, ExbB, and ExbD provide energy for transport across the inner membrane into the cell (reviewed in references 17, 27, and 45).
The B. cepacia complex has been shown to produce four siderophores: ornibactins, salicylic acid (SA), pyochelin, and cepabactin (42, 43, 66, 68, 72). Ornibactins and SA are the predominant siderophores found in cystic fibrosis isolates, but there is species and strain variation (19). Ornibactins are linear hydroxamate/hydroxycarboxylate siderophores similar in structure to the pyoverdines produced by fluorescent pseudomonads, yet they lack a chromophore (43, 68). Pyochelin is a structurally unique siderophore of the phenolate class (16). SA, a biosynthetic precursor of pyochelin, has been shown to possess siderophore activity in Pseudomonas aeruginosa, Pseudomonas fluorescens (41, 72), and Burkholderia cepacia (66, 72).
In previous studies, genes involved in the synthesis (pvdA and pvdD) (65) and uptake (orbA) (63) of ornibactin have been identified. pvdA and orbA allelic exchange mutants were less virulent than the parent strain in a chronic respiratory infection model. K56orbA::tp and K56pvdA::tp both showed reduced histopathology as well as reduced persistence, indicated by the fact that they were cleared from the lungs (63, 65). These mutants also demonstrated a reduced ability to grow in iron-restricted conditions in vitro (63, 65). These studies demonstrated a requirement for ornibactin in growth and virulence; however, these studies were performed with a strain that produces negligible amounts of pyochelin.
The objectives of the present study were to construct mutants defective in either pyochelin-mediated iron uptake or ornibactin-mediated iron uptake in a strain that produced both siderophores to determine if a single functional siderophore system was sufficient for growth in iron-restricted conditions and for survival in a chronic respiratory infection model.
MATERIALS AND METHODS
Strains and plasmids.
The bacterial strains and plasmids used in this study are described in Table 1. K56-2 produces ornibactins, SA, and negligible amounts of pyochelin, while Pc715j produces ornibactins, SA, and pyochelin (19). When appropriate, antibiotics were added at the following concentrations: 100 μg of ampicillin, 15 μg of tetracycline, 50 μg of kanamycin, and 1.2 mg of trimethoprim per ml for Escherichia coli and 300 μg of tetracycline and 100 μg of trimethoprim per ml for B. cenocepacia. A 100-mg/ml stock of trimethoprim was prepared in N,N-dimethylacetamide.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Source or reference(s) |
|---|---|---|
| E. coli | ||
| DH5α | F− φ80 lacZΔM15 Δ(lacZYA-argF) recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 deoRU169 | Invitrogen |
| HB101 | F− Δ(gpt-proA)62 leuB6 glnV44 ara-14 galK2 lacY1 Δ(mcrC-mrr) rpsL20 (Strr) xyl-5 mtl-1 recA13 | 57 |
| TOP10 | F−mcrA Δ (mrr-hsdRMS-mcrBC) φ80 lacZΔM15 Δ lacX74 deoR recA1 araD139 Δ (ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| SM10 | Mobilizing strain, RP4 genes integrated in chromosome, Kmr | 62 |
| B. cenocepacia | ||
| Pc715j | CF respiratory isolate; Orn, Pch, SA | 19, 40 |
| Pc715jorbA::tp | orbA::Tpr derivative of Pc715j; Tpr | This study |
| Pc715jfptA::tp | fptA::Tpr derivative of Pc715j; Tpr | This study |
| K56-2 | CF respiratory isolate; Orn, SA | 19, 39 |
| K56orbA::tp | orbA::Tpr derivative of K56-2; Tpr | 63 |
| Plasmids | ||
| pRK2013 | ColE1 tra(RK2)+; Kmr | 23 |
| pCC524T | pEX18Tc with a 3-kb EcoRI-BglII orbA fragment from K56-2 containing a 0.6-kb Tpr cassette inserted into the orbA gene; Tcr Tpr | 63 |
| pCRR2.1TOPO | Cloning vector for PCR products; Kmr Apr | Invitrogen |
| pEX18Tc | Suicide vector; sacB Tcr | 31 |
| p34E-Tp | Source of trimethoprim resistance cassette; Tpr | 20 |
| pEKH103 | pCRR2.1TOPO containing a 2.35-kb fptA PCR product; Apr Kmr | This study |
| pEKH107 | pEKH103 with a 0.67-kb SalI fragment containing the Tpr cassette from p34E-Tp inserted into the SalI site in the fptA gene; Apr Kmr Tpr | This study |
| pEKH113 | pEX18Tc with a 2.2-kb HindIII-PstI fptA::tp fragment from pEKH107; Tcr Tpr | This study |
Tc, tetracycline; Tp, trimethoprim; Km, kanamycin; Ap, ampicillin; Orn, ornibactin; Pch, pyochelin; Str, streptomycin; CF, cystic fibrosis.
DNA manipulations.
For genetic manipulations, cultures were grown in Luria-Bertani (LB) broth (Invitrogen, Burlington, Canada) or on 1.5% LB agar at 37°C. Molecular biology techniques were generally performed as described by Sambrook et al. (57). Genomic DNA was isolated from Pc715j as described by Ausubel et al. (5). Restriction enzymes and oligonucleotide primers were purchased from Invitrogen (Burlington, Canada), while T4 DNA ligase was purchased from Promega (Madison, Wis.). PCR products were cloned with the pCRR2.1TOPO kit according to the manufacturer's directions (Invitrogen). Recombinant plasmids were electroporated into E. coli DH5α or TOP10 with a Gene Pulser (Bio-Rad, Richmond, Calif.) as recommended by the manufacturer.
To construct an orbA allelic exchange mutant of strain Pc715j, pCC524T (63), containing an insertionally inactivated orbA gene, was used. This plasmid was transferred to B. cenocepacia Pc715j by triparental mating with pRK2013 (23) as previously described (63). Insertional inactivation was confirmed by PCR and Southern hybridization.
To amplify the fptA gene from Pc715j, the fptA gene sequence from Pseudomonas aeruginosa PAO1 (4) was used to search the B. cepacia genome sequence of B. cenocepacia strain J2315 produced by the sequencing group at the Sanger Institute (http://www.sanger.ac.uk/Projects/B_cenocepacia). Primers fptAup (5′-GTGACGAGCTCAATACGGGCCG-3′) and fptAdo (5′-CCCCAAGCTTGACGCCATCAGA-3′) were used to amplify a 2.3-kb product containing the fptA gene from Pc715j. This product was cloned into pCRR2.1TOPO to create pEKH103.
For the construction of an fptA allelic-exchange mutant of Pc715j, a 0.92-kb internal SalI fragment was removed from the fptA gene and replaced with a 0.67- kb SalI fragment from p34E-Tp (20) containing the trimethoprim resistance cassette to create pEKH107. A 2.2-kb HindIII-PstI fragment from pEKH107 consisting of the insertionally inactivated fptA gene was inserted into the HindIII and PstI sites in pEX18Tc (31) to form pEKH113. This plasmid was transferred to Pc715j by triparental mating with pRK2013 (23) as described above. Insertional inactivation of fptA was confirmed by both Southern hybridization and PCR.
Iron uptake assays.
For pyochelin uptake assays, cultures were grown overnight at 32°C with maximum aeration in 20 ml of Casamino Acids-phosphate-sulfate (CPS) medium (2) supplemented with 5 μg of pyochelin per ml. For SA uptake assays, cultures were grown as above in tryptic soy broth medium treated with Chelex 100 (Bio-Rad, Richmond, Calif.) and subsequently dialyzed (TSB-DC) (48). Cultures were washed with 10 ml of medium and resuspended to an A600 of 0.3. The iron binding efficiency of the pyochelin stock was determined with the Chrome Azurol S (CAS) assay (60). 59FeCl3 (3.6 nmol) was mixed with 4.8 nmol of actively binding pyochelin in a volume of 100 μl and equilibrated for 60 min. Salicylic acid (7.2 nmol) was mixed with 3.6 nmol of 59FeCl3 in a volume of 100 μl and equilibrated as above. Uptake reactions were initiated by the addition of 100 μl of 59Fe-siderophore mixture to 10 ml of cells. Samples (1 ml) were then removed at selected time points, filtered through cellulose acetate filters (0.45 μm), and washed with 3 ml of 10 mM Tris (pH 7.5)-0.9%NaCl. The amount of accumulated 59Fe on the filters was measured with an LKB gamma counter (66).
Growth determination.
To determine the effects of siderophores on growth, cultures were grown overnight in 10 ml of iron-deficient TSB-DC at 32°C with maximum aeration and subcultured into 15 ml of medium to an A600 of 0.02. Pyochelin and ornibactins were added to a final concentration of 10 μg/ml. To determine the effects of nonsiderophore iron chelators on growth, 20 μM ethylenediamine-di(o-hydroxyphenylacetic acid) (EDDHA) (Sigma, Oakville, Canada) was added to the medium in one experiment. Growth experiments were performed in triplicate, and growth was measured by determining A600.
Siderophore assays.
For the determination of siderophore activity via CAS assays (60) and ornibactin production assays, cultures were grown at 32°C for 40 h in succinate medium (44) supplemented with 10 mM ornithine (43). Ornibactins were purified by Sephadex LH-20 chromatography and quantitated in CAS assays as previously described (35, 36). To determine the amount of SA and pyochelin produced, cultures were grown in deferrated Casamino Acids medium (64) at 32°C for 24 h. SA and pyochelin were isolated by thin-layer chromatography and quantitated as previously described (36, 65).
Outer membrane protein isolation.
For outer membrane protein isolation, Pc715j, Pc715jorbA::tp, and Pc715jfptA::tp were grown overnight in CPS medium (2) at 32°C, subcultured into 500 ml of fresh medium, and grown to an A600 of approximately 1.0. Outer membranes were isolated as described previously. Some cultures were supplemented with 10 μg of pyochelin per ml and 50 μM FeCl3 as required.
Animal studies.
Animal infection experiments were performed in the chronic respiratory infection model in rats as described by Cash et al. (10). For the preparation of agar beads, cultures were grown overnight in 20 ml of TSB-DC at 32°C with maximum aeration. Groups of 10 male Sprague-Dawley rats weighing 150 to 170 g (Charles River Canada, Inc.) were tracheotomized under anesthesia and inoculated with approximately 105 CFU of the appropriate strain embedded in agar beads as previously described. On days 7 and 14 postinfection, the lungs from four or five animals in each group were removed aseptically and homogenized (Polytron homogenizer; Brinkmann Instruments, Westbury, N.Y.) in 3 ml of phosphate-buffered saline (0.05 M, pH 7.2, containing 0.9% saline). Serial dilutions were plated on Trypticase soy agar (TSA) (Difco, Detroit, Mich.) and TSA plus the appropriate antibiotic or B. cepacia selection agar (30), and bacterial counts were performed.
RESULTS
Construction of orbA and fptA mutants.
An orbA mutant of Pc715j was constructed as previously described (63) and designated Pc715jorbA::tp. Outer membrane profiles were prepared for Pc715j and Pc715jorbA::tp grown in both low- and high-iron conditions. Two iron-regulated proteins migrated near the 75-kDa molecular mass marker and are marked with arrows in Fig. 1. The upper band, believed to be the ornibactin receptor, is present in Pc715j but absent in Pc715jorbA::tp (Fig. 1, compare lanes 2 and 5) and absent in the presence of 50 μM FeCl3 in both Pc715j and Pc715jorbA::tp (Fig. 1, compare lanes 2 and 4 and lanes 5 and 6). These results are similar to those reported for K56-2 and K56orbA::tp (63).
FIG. 1.
Outer membrane protein profiles of Pc715j, Pc715jfptA::tp, and Pc715jorbA::tp. Ten micrograms of outer membrane preparations from cultures grown in CPS medium was electrophoresed on an SDS-10% PAGE gel and stained with Coomassie brilliant blue. Lanes 1 and 9, 150-, 100-, 75-, and 50-kDa molecular mass markers; lane 2, Pc715j in medium with no additions; lane 3, Pc715j in medium plus 10 μg of pyochelin per ml; lane 4, Pc715j in medium plus 50 μM FeCl3; lane 5, Pc715jorbA::tp in medium with no additions; lane 6, Pc715jorbA::tp in medium plus 50 μM FeCl3; lane 7, Pc715jfptA::tp in medium with no additions; lane 8, Pc715jfptA::tp in medium plus 50 μM FeCl3.
The ferric-pyochelin outer membrane receptor gene fptA has been identified in P. aeruginosa PAO1 (4). The P. aeruginosa PAO1 FptA amino acid sequence was used to search the B. cenocepacia J2315 sequence (http://www.sanger.ac.uk/Projects/B.cenocepacia). The deduced amino acid sequence of B. cenocepacia J2315 FptA was determined to be 64% identical to that of P. aeruginosa FptA (4). This amino acid sequence was also used to search the GenBank database with the BLASTP algorithm (1, 37) for similar matches. Identity ranged from between 34 and 35% for various hydroxamate-type siderophore receptors, including the PupB (32) and PupA (6) pseudobactin receptors of P. putida; FpvA, the pyoverdine receptor of P. aeruginosa (50); and coprogen-, rhodotorulic acid-, and ferrioxamine-type receptors such as E. coli FhuE (58). FptA was also 35% identical to a Bordetella sp. FauA (7), the receptor for the siderophore alcaligin. Alcaligin is a macrocyclic dihydroxamate-type siderophore that requires ornithine as a precursor (8).
B. cenocepacia fptA is predicted to encode a 737-amino-acid polypeptide with a molecular mass of 80,766 Da and a pI of 8.67. A probable signal sequence cleavage site was detected between amino acids 41 and 42 with the SignalP program (47). The mature form of FptA is predicted to have a molecular mass of 76,040 Da and a pI of 6.95. A TonB-dependent signature sequence 2 was identified at the C-terminal end from residues 721 to 738 by a Prosite search. Ten of 18 residues in this region matched the consensus sequence.
The sequence surrounding fptA was searched for open reading frames with Artemis (56; www.sanger.ac.uk/Software/Artemis), and BLASTP was used to search for similarities to the amino acid sequences encoded by selected open reading frames. It appears that upstream of fptA, gene organization is similar to that in P. aeruginosa. Open reading frames that showed identity to genes involved in pyochelin synthesis (pchG) and ABC transporter proteins with export functions (pchH and pchI) (54) were identified. Downstream of fptA was an open reading frame with approximately 35% homology to a gene encoding iron-regulated membrane proteins of unknown function, as reported for P. aeruginosa (4).
An fptA mutant of Pc715j was constructed and designated Pc715jfptA::tp. Outer membrane preparations isolated from Pc715j and Pc715jfptA::tp in both low-iron and high-iron conditions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The smaller of the two iron-regulated proteins in the 75-kDa range was absent in Pc715jfptA::tp (Fig. 1, compare lanes 2 and 7) and migrated close to the expected mass for mature FptA. Although a faint protein with a molecular mass similar to that of FptA was detectable in lane 7, this was probably another protein of similar size, since it was present in much smaller amounts than the FptA band observed in Pc715 or Pc715jorbA::tp (lanes 2, 3, and 5). Efforts to clearly separate these two proteins by SDS-PAGE with different percentages of acrylamide were not successful.
The protein corresponding to FptA was not expressed in the presence of 50 μM FeCl3 in either Pc715j or Pc715jfptA::tp (Fig. 1, compare lanes 2 and 4 and lanes 7 and 8). Expression of the pyochelin receptor in P. aeruginosa has been shown to be inducible by the presence of pyochelin (24). The effect of the addition of pyochelin on FptA expression in Pc715j was examined. In low-iron conditions, the addition of pyochelin resulted in no observable differences in protein profiles (Fig. 1, compare lanes 2 and 3). Since Pc715j produces pyochelin, it is not surprising that an increase in the amount of pyochelin in the medium did not result in a further increase in expression of the receptor. Addition of 50 μM FeCl3 to the medium resulted in altered expression of several proteins in addition to FptA and OrbA and appeared to slightly retard the migration of others (Fig. 1. lanes 4, 6, and 8). These proteins have not yet been identified.
Role of fptA and orbA in iron transport and siderophore production.
OrbA has previously been confirmed to be required for ferric ornibactin uptake (63). In order to confirm that fptA was required for pyochelin uptake in B. cenocepacia, the ability of Pc715jfptA::tp to take up [59Fe]pyochelin was compared to that of Pc715j. Pc715jfptA::tp was unable to accumulate [59Fe]pyochelin in the assay period (Fig. 2A). The ability of Pc715jorbA::tp to take up [59Fe]pyochelin was also determined. Pc715jorbA::tp was able to take up [59Fe]pyochelin at a rate similar to that of Pc715j, indicating that pyochelin uptake is not affected by an orbA mutation (Fig. 2A).
FIG. 2.
Uptake of 59Fe-labeled siderophore complexes by Pc715j, Pc715jfptA::tp, and Pc715jorbA::tp. (A) [59Fe]pyochelin; (B) [59Fe]SA. Reactions were initiated by the addition of the 59Fe-labeled siderophore complex. Samples were removed at selected intervals, and the amount of 59Fe accumulated was determined. Values are the means ± standard deviations for triplicate assays. The cultures in panel A were grown in CPS medium, and the cultures in panel B were grown in TSB-DC medium.
SA is a biosynthetic precursor to pyochelin (3) in addition to functioning as a siderophore in P. aeruginosa and B. cepacia species in vitro (66, 72). It is not known whether a specific receptor protein is involved in the uptake of SA (55). To determine the effects of an fptA mutation on SA uptake, the ability of Pc715fptA::tp to take up [59Fe]SA was examined. Pc715j and Pc715jfptA::tp were able to take up [59Fe]SA at similar rates (Fig. 2B), indicating that an fptA mutation does not affect the ability to acquire SA. These data suggest the presence of a specific outer membrane receptor for SA uptake.
The effects of orbA and fptA mutations on siderophore production were also determined (Table 2). Pc715jorbA::tp produced significantly less ornibactin than Pc715 (P < 0.05). These results were similar to those reported previously for strain K56-2 (63). Pyochelin production by Pc715jfptA::tp was significantly less than that by Pc715j (P < 0.05), whereas the amount of pyochelin produced by Pc715jorbA::tp was similar to that by Pc715j. Interestingly, SA production by Pc715jfptA::tp was increased fivefold compared to that by Pc715j (P < 0.05).
TABLE 2.
Effect of fptA and orbA mutations on siderophore production in B. cenocepacia Pc715j
| Strain | Amta (μg/ml of supernatant)
|
||
|---|---|---|---|
| Salicylic acid | Ornibactin | Pyochelin | |
| Pc715j | 0.1 ± 0.01 | 23.5 ± 8.2 | 4.8 ± 2.1 |
| Pc715j fptA::tp | 0.5 ± 0.1b | 26.0 ± 1.3 | 0.1 ± 0.2b |
| Pc715jorbA::tp | 0.3 ± 0.3 | 5.5 ± 2.5b | 3.3 ± 2.2 |
Values are the means ± standard deviations for triplicate assays. Assays were performed three times with similar results.
Significantly different from results for Pc715j (P < 0.05) by analysis of variance, Dunnett multiple-comparisons test.
Effect of fptA and orbA mutations on growth in the presence of siderophores or EDDHA.
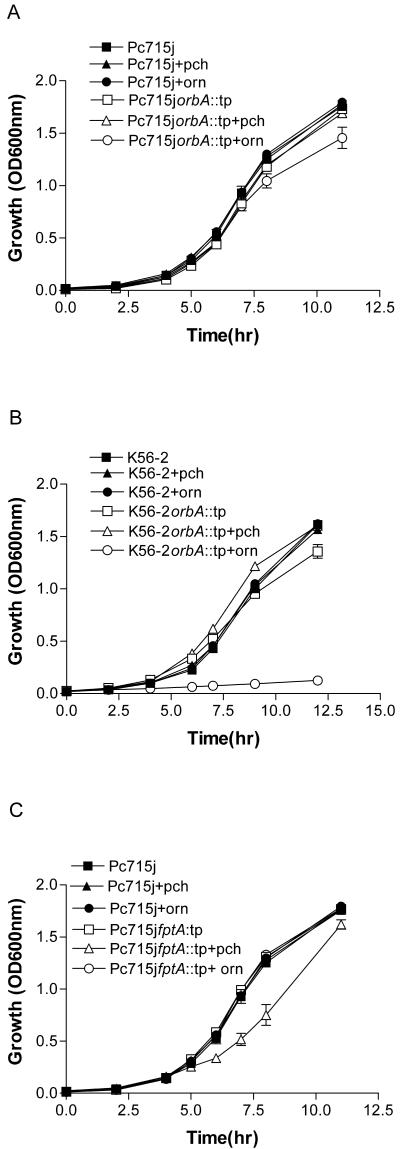
The ability of Pc715jorbA::tp and K56orbA::tp to utilize ornibactins and pyochelin for growth in vitro in iron-deficient medium was examined. Wild-type and mutant strains grew at similar rates in the absence of added siderophores (compare Fig. 3A and B). The addition of pyochelin had no effect on the growth of any of the strains. When ornibactins were added, Pc715jorbA::tp had a slight decrease in growth rate between 6 and 12 h (Fig. 3A). This was markedly different from that observed for K56orbA::tp, which grew very poorly with the addition of ornibactins to the medium (Fig. 3B). This indicates that Pc715jorbA::tp can acquire iron effectively with the pyochelin transport system. To determine the effects of other iron chelators on growth, 20 μM EDDHA was added to the growth medium instead of siderophores. Pc715j and Pc715jorbA::tp grew at similar rates in the presence and absence of EDDHA (data not shown), which also suggests that pyochelin is an effective siderophore in vitro. This is in contrast to the previously reported effects of EDDHA on the growth of the non-pyochelin-producing strain K56orbA::tp (63).
FIG. 3.
Effect of orbA and fptA mutations on growth in the presence of siderophores. Cultures were grown in iron-deficient TSB-DC medium containing either 10 μg of pyochelin (pch) or ornibactin (orn)per ml or no additions. (A) Comparison of growth rates of Pc715j and Pc715jorbA::tp. (B) Comparison of growth rates of K56-2 and K56orbA::tp. (C) Comparison of growth rates of Pc715j and Pc715jfptA::tp. Optical density (OD) was determined at various intervals. Values are the means ± standard deviations for triplicate assays.
The ability of Pc715jfptA::tp to utilize pyochelin and ornibactins for growth in iron-deficient medium was also examined. Pc715j and Pc715jfptA::tp grew at similar rates in the absence of siderophores (Fig. 3C). The addition of ornibactins had no effect on the growth of either strain. When pyochelin was added to the growth medium, a slight decrease in growth was observed for Pc715jfptA::tp between 5 and 12 h. Pc715j and Pc715fptA::tp grew at similar rates in both the absence and presence of EDDHA (data not shown). These studies suggest that although pyochelin can function as a siderophore in vitro, it is not required for in vitro growth in iron-limited conditions.
Effects of orbA and fptA mutations on virulence.
Previous studies have indicated that ornibactin biosynthesis and uptake are important factors in the ability of at least some strains of B. cenocepacia to persist in experimental lung infections (63). To determine the relative importance of pyochelin uptake in virulence, the persistence of Pc715jorbA::tp and Pc715jfptA::tp was compared to that of Pc715j in a chronic respiratory infection model. Rats were infected with Pc715j, Pc715jorbA::tp, and Pc715jfptA::tp, and on days 7 and 14 postinfection, quantitative bacteriological analysis was performed on the lungs. On days 7 and 14 postinfection, approximately 3 logs fewer Pc715jorbA::tp than parent strain organisms were recovered from the lungs, while there was no significant difference in the number of Pc715jfptA::tp organisms recovered from the lungs (Table 3). These results demonstrate that although a functional ferric-ornibactin uptake system is sufficient for persistence in the lung, a functional ferric-pyochelin uptake system is unable to compensate for a nonfunctional ferric-ornibactin uptake system in vivo.
TABLE 3.
Effect of orbA and fptA mutations on persistence of B. cenocepacia Pc715j in the rat lung chronic-infection model
| Expt | Strain | Inoculum (log CFU) | Log CFU/ml of lung homogenatea
|
|
|---|---|---|---|---|
| Day 7 p.i. | Day 14 p.i. | |||
| 1 | Pc715j | 4.7 | 5.5 ± 0.82 | 3.8 ± 1.6 |
| Pc715jorbA::tp | 4.6 | 2.0 ± 2.5b | 0.4 ± 0.7b | |
| 2 | Pc715j | 5.8 | 5.0 ± 0.3 | 3.5 ± 1.5* |
| Pc715jfptA::tp | 6.0 | 5.2 ± 0.5 | 4.9 ± 0.7* | |
Values are means ± standard deviations for four animals per group or, if marked with an asterisk, five animals per group at 7 and 14 days postinfection (p.i.).
Significantly different from results for Pc715j (P < 0.05) by analysis of variance, Bonferroni multiple-comparisons test.
DISCUSSION
In this study, we compared the importance of the ferric-ornibactin and ferric-pyochelin siderophore-mediated transport systems in B. cenocepacia in vitro and in a chronic lung infection model. Pc715jorbA::tp was able to overcome iron limitation in vitro and grew at rates similar to those of Pc715j. The addition of ornibactin to Pc715jorbA::tp cultures resulted in only a slight decrease in growth rate compared to the parent (Fig. 3A). This is markedly different than the result in K56-2, where an orbA mutation severely hindered growth under both of these conditions (Fig. 3B) (63). Pc715jorbA::tp is likely able to overcome these limitations by producing sufficient pyochelin to effectively acquire iron in vitro, even though pyochelin has a lower binding affinity for iron (16, 72) than ornibactin. Although an affinity constant for iron has not been reported for ornibactin, based on its structural similarity to pyoverdine, which has a binding coefficient for iron of 1033 (44), ornibactin likely has a higher affinity for iron than pyochelin (5 × 105) (16). Although ornibactin would bind much of the iron, some could be bound by pyochelin and still be available for iron accumulation by Pc715jorbA::tp, which was not inhibited by the addition of ornibactin to the medium to the same extent that K56orbA::tp was (Fig. 3A and B).
Pc715jorbA::tp was less able to persist in the lung (Table 3,) and the number of bacteria surviving in the lung was similar to the number observed previously for K56orbA::tp (65). Even though the amount of pyochelin produced in vitro by Pc715jorbA::tp was similar to that produced by Pc715j, this mutant does not appear to compete effectively for iron and survive in the lungs. Although we have not measured pyochelin production directly in vivo, these data suggest that a functional ferric-pyochelin system is unable to compensate for a nonfunctional ferric-ornibactin system. In an immunosuppressed mouse infection model, a P. aeruginosa PAO1 pyoverdine-deficient mutant grew poorly in the lung and was recovered in lower numbers in the blood, even though pyochelin production was similar to that of strain PAO1. These data suggest that pyoverdine is more important than pyochelin in P. aeruginosa infections in vivo (69).
In contrast to Pc715orbA::tp, Pc715jfptA::tp appeared to be able to effectively acquire iron both in vitro and in vivo. Infection with Pc715jfptA::tp in a chronic lung infection model resulted in the same level of persistence as infection with Pc715j (Table 3), suggesting that a functional ferric-ornibactin uptake system is able to compensate for a nonfunctional ferric-pyochelin uptake system in vivo. No difference in the ability of a P. aeruginosa PAO1 pyochelin-deficient strain and PAO1 to persist in the lungs and blood of immunosuppressed mice was observed, which also provides evidence that a pyochelin uptake system is less important in vivo (69). Although pyochelin is not sufficient for B. cenocepacia to acquire iron in vivo, it can enhance virulence. In the agar bead model, exogenously supplied pyochelin was shown to increase the virulence of pyochelin-negative B. cepacia strains (67). In addition to its role in iron acquisition, pyochelin also plays a role in tissue injury. Iron bound to pyochelin has been shown to be an efficient catalyst for hydroxyl radical formation and to increase injury to pulmonary artery endothelial cells and epithelial cells resulting from exposure to superoxide and hydrogen peroxide (9, 15).
Previously we demonstrated that a strain with a functional SA uptake system but lacking the ability to synthesize or take up ornibactin was unable to survive in the lung (63, 65). The effect of mutations in the SA uptake system has not been examined directly.
Interestingly, SA production was increased approximately fivefold and pyochelin production was decreased by a similar amount in Pc715jfptA::tp. A decrease in pyochelin production might be expected because, in the absence of the pyochelin receptor protein, pyochelin is not needed. Although we have not ruled out the possibility of polar effects due to the fptA mutation, we have confirmed that the decrease in ornibactin synthesis observed in the K56orbA::tp mutant (65) can be complemented by the orbA gene (data not shown), suggesting that the orbA mutation is not polar on the downstream biosynthetic genes. Unfortunately, complementation studies with Pc715j are problematic due to the lack of useful antibiotic resistance markers. Pyochelin biosynthesis has been shown to be positively regulated in P. aeruginosa PAO through the action of a transcriptional regulator, PchR (28, 29). PvdS, an alternative sigma factor, has been identified as a positive regulatory protein of pyoverdine biosynthesis in P. aeruginosa PAO (18). Iron-responsive regulators that affect the production of pyoverdine-like siderophores have also been identified in P. putida WS358 (33, 71) and P. fluorescens M114 (61). Additional factors or regulatory proteins may also be involved in the PvdS regulatory network (34). The mechanisms of regulation of pyochelin and ornibactin biosynthesis in B. cenocepacia are not yet known, but our studies suggest that there may be a complex network of regulation similar to that of the pseudomonads.
Hyperproduction of SA has also been observed in a K56-2 pvdA mutant (65). The rate of [59Fe]SA uptake was also increased in K56orbA::tp (63), suggesting that the increase in SA production is a compensation mechanism for the decreased amount of pyochelin and ornibactin produced. Mutants of P. fluorescens CHAO that were unable to synthesize pyoverdines produced SA in much larger amounts (41).
SA has also been shown to act as an endogenous siderophore under iron-limiting conditions in vitro in organisms such as Mycobacterium spp. (51) and Azospirillum lipoferum (59) in addition to P. aeruginosa (72), P. fluorescens (41), and B. cenocepacia (66, 72). SA has been shown to be an intermediate in the biosynthesis of pyochelin as well as being present in other siderophores (11, 22, 49, 52). Our data suggest that even though SA is a precursor in pyochelin synthesis, there is a specific receptor for SA. The ability of Pc715jfptA::tp to take up [59Fe]SA as effectively as the parent strain (Fig. 2B) indicates that Fe-SA complexes are not taken up by the pyochelin receptor.
It is interesting that SA is effective at binding ferric iron only in the absence of other ions such as phosphate. However, phosphate ions are abundant in culture media as well as in host tissues and fluids (53). Recently, a theoretical study to calculate the concentration and speciation of ions was used to conclude that SA is not a bacterial siderophore (12). In the presence of 10−4 M SA, the concentration of free ferric iron increased only slightly, by approximately 8.4 × 10−9 M, compared to the value in the absence of SA. SA also had little effect on the solubilization of iron in the presence of 40 mM phosphate.
A requirement for mycobactin T, a salicylate-derived siderophore, for growth of M. tuberculosis in macrophages has been determined (21). Mutation of the mbtB gene, involved in mycobactin biosynthesis, interrupted all salicylate-derived siderophore production. This mutant was deficient in growth in low-iron medium as well as in a macrophage cell line, providing evidence for the ability of SA to act as a siderophore.
B. cenocepacia produces three siderophores, ornibactin, pyochelin, and SA. In this study, we identified the siderophore receptor for pyochelin and provided additional evidence that the ferric-ornibactin uptake system is the most important iron acquisition system in chronic B. cenocepacia lung infections.
Acknowledgments
This study was supported by a grant from the Canadian Cystic Fibrosis Foundation.
We thank C. D. Kooi, B. Pohorelic, and R. Chen for excellent technical assistance.
Editor: J. T. Barbieri
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ankenbauer, R. G. 1992. Cloning of the outer membrane high-affinity Fe(III)-pyochelin receptor of Pseudomonas aeruginosa. J. Bacteriol. 174:4401-4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ankenbauer, R. G., and C. D. Cox. 1988. Isolation and characterization of Pseudomonas aeruginosa mutants requiring salicylic acid for pyochelin biosynthesis. J. Bacteriol. 170:5364-5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ankenbauer, R. G., and H. N. Quan. 1994. FptA, the Fe(III)-pyochelin receptor of Pseudomonas aeruginosa: a phenolate siderophore receptor homologous to hydroxamate siderophore receptors. J. Bacteriol. 176:307-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology, vol. 1. John Wiley & Sons, Inc., New York, N.Y.
- 6.Bitter, W., J. D. Marugg, L. A. de Weger, J. Tommassen, and P. J. Weisbeek. 1991. The ferric-pseudobactin receptor PupA of Pseudomonas putida WCS358: homology to TonB-dependent Escherichia coli receptors and specificity of the protein. Mol. Microbiol. 5:647-655. [DOI] [PubMed] [Google Scholar]
- 7.Brickman, T. J., and S. K. Armstrong. 1999. Essential role of the iron-regulated outer membrane receptor FauA in alcaligin siderophore-mediated iron uptake in Bordetella species. J. Bacteriol. 181:5958-5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brickman, T. J., and S. K. Armstrong. 1996. The ornithine decarboxylase gene odc is required for alcaligin siderophore biosynthesis in Bordetella spp.: putrescine is a precursor of alcaligin. J. Bacteriol. 178:54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Britigan, B. E., G. T. Rasmussen, and C. D. Cox. 1997. Augmentation of oxidant injury to human pulmonary epithelial cells by the Pseudomonas aeruginosa siderophore pyochelin. Infect. Immun. 65:1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cash, H. A., D. E. Woods, B. McCullough, W. G. Johanson, Jr., and J. A. Bass. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119:453-459. [DOI] [PubMed] [Google Scholar]
- 11.Chambers, C. E., D. D. McIntyre, M. Mouck, and P. A. Sokol. 1996. Physical and structural characterization of yersiniophore, a siderophore produced by clinical isolates of Yersinia enterocolitica. Biometals 9:157-167. [DOI] [PubMed] [Google Scholar]
- 12.Chipperfield, J. R., and C. Ratledge. 2000. Salicylic acid is not a bacterial siderophore: a theoretical study. Biometals 13:165-168. [DOI] [PubMed] [Google Scholar]
- 13.Coenye, T., and P. Vandamme. 2003. Diversity and significance of Burkholderia species occupying diverse ecological niches. Environ. Microbiol. 5:719-729. [DOI] [PubMed] [Google Scholar]
- 14.Coenye, T., P. Vandamme, J. R. Govan, and J. J. LiPuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 39:3427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coffman, T. J., C. D. Cox, B. L. Edeker, and B. E. Britigan. 1990. Possible role of bacterial siderophores in inflammation. Iron bound to the Pseudomonas siderophore pyochelin can function as a hydroxyl radical catalyst. J. Clin. Investig. 86:1030-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cox, C. D., and R. Graham. 1979. Isolation of an iron-binding compound from Pseudomonas aeruginosa. J. Bacteriol. 137:357-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crosa, J. H. 1989. Genetics and molecular biology of siderophore-mediated iron transport in bacteria. Microbiol. Rev. 53:517-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunliffe, H. E., T. R. Merriman, and I. L. Lamont. 1995. Cloning and characterization of pvdS, a gene required for pyoverdine synthesis in Pseudomonas aeruginosa: PvdS is probably an alternative sigma factor. J. Bacteriol. 177:2744-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darling, P., M. Chan, A. D. Cox, and P. A. Sokol. 1998. Siderophore production by cystic fibrosis isolates of Burkholderia cepacia. Infect. Immun. 66:874-877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeShazer, D., and D. E. Woods. 1996. Broad-host-range cloning and cassette vectors based on the R388 trimethoprim resistance gene. BioTechniques 20:762-764. [DOI] [PubMed] [Google Scholar]
- 21.De Voss, J. J., K. Rutter, B. G. Schroeder, H. Su, Y. Zhu, and C. E. Barry III. 2000. The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages. Proc. Natl. Acad. Sci. USA 97:1252-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drechsel, H., H. Stphan, R. Lotz, H. Hagg, H. Zanner, K. Hankte, and G. Jung. 1995. Structure elucidation of yersiniabactin, a siderophore from highly virulent Yersinia strains. Liebigs Ann. 1727-1733.
- 23.Figurski, D. H., and D. R. Helenski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gensberg, K., K. Hughes, and A. W. Smith. 1992. Siderophore-specific induction of iron uptake in Pseudomonas aeruginosa. J. Gen. Microbiol. 138:2381-2387. [DOI] [PubMed] [Google Scholar]
- 25.Govan, J. R., and V. Deretic. 1996. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 60:539-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govan, J. R., J. E. Hughes, and P. Vandamme. 1996.Burkholderia cepacia: medical, taxonomic and ecological issues. J. Med. Microbiol. 45:395-407. [DOI] [PubMed] [Google Scholar]
- 27.Guerinot, M. L. 1994. Microbial iron transport. Annu. Rev. Microbiol. 48:743-772. [DOI] [PubMed] [Google Scholar]
- 28.Heinrichs, D. E., and K. Poole. 1993. Cloning and sequence analysis of a gene (pchR) encoding an AraC family activator of pyochelin and ferripyochelin receptor synthesis in Pseudomonas aeruginosa. J. Bacteriol. 175:5882-5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinrichs, D. E., and K. Poole. 1996. PchR, a regulator of ferripyochelin receptor gene (fptA) expression in Pseudomonas aeruginosa, functions both as an activator and as a repressor. J. Bacteriol. 178:2586-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry, D. A., M. E. Campbell, J. J. LiPuma, and D. P. Speert. 1997. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J. Clin. Microbiol. 35:614-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 32.Koster, M., J. van de Vossenberg, J. Leong, and P. J. Weisbeek. 1993. Identification and characterization of the pupB gene encoding an inducible ferric-pseudobactin receptor of Pseudomonas putida WCS358. Mol. Microbiol. 8:591-601. [DOI] [PubMed] [Google Scholar]
- 33.Koster, M., W. van Klompenburg, W. Bitter, J. Leong, and P. Weisbeek. 1994. Role for the outer membrane ferric siderophore receptor PupB in signal transduction across the bacterial cell envelope. EMBO J. 13:2805-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leoni, L., N. Orsi, V. de Lorenzo, and P. Visca. 2000. Functional analysis of PvdS, an iron starvation sigma factor of Pseudomonas aeruginosa. J. Bacteriol. 182:1481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewenza, S., B. Conway, E. P. Greenberg, and P. A. Sokol. 1999. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J. Bacteriol. 181:748−756. [DOI] [PMC free article] [PubMed]
- 36.Lewenza, S., and P. A. Sokol. 2001. Regulation of ornibactin synthesis and N-acyl-l-homoserine lactone production by CepR in Burkholderia cepacia. J. Bacteriol. 183:2212-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madden, T. L., R. L. Tatusov, and J. Zhang. 1996. Applications of network BLAST server. Methods Enzymol. 266:131-141. [DOI] [PubMed] [Google Scholar]
- 38.Mahenthiralingam, E., A. Baldwin, and P. Vandamme. 2002. Burkholderia cepacia complex infection in patients with cystic fibrosis. J. Med. Microbiol. 51:533-538. [DOI] [PubMed] [Google Scholar]
- 39.Mahenthiralingam, E., T. Coenye, J. W. Chung, D. P. Speert, J. R. Govan, P. Taylor, and P. Vandamme. 2000. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J. Clin. Microbiol. 38:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKevitt, A. I., S. Bajaksouzian, J. D. Klinger, and D. E. Woods. 1989. Purification and characterization of an extracellular protease from Pseudomonas cepacia. Infect. Immun. 57:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer, J. M., P. Azelvandre, and C. Georges. 1992. Iron metabolism in Pseudomonas: salicylic acid, a siderophore of Pseudomonas fluorescens CHAO. Biofactors 4:23-27. [PubMed] [Google Scholar]
- 42.Meyer, J. M., D. Hohnadel, and F. Halle. 1989. Cepabactin from Pseudomonas cepacia, a new type of siderophore. J. Gen. Microbiol. 135:1479-1487. [DOI] [PubMed] [Google Scholar]
- 43.Meyer, J. M., V. T. Van, A. Stintzi, O. Berge, and G. Winkelmann. 1995. Ornibactin production and transport properties in strains of Burkholderia vietnamiensis and Burkholderia cepacia (formerly Pseudomonas cepacia). Biometals 8:309-317. [DOI] [PubMed] [Google Scholar]
- 44.Meyer, J. M., and M. A. Abdullah. 1978. The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physico-chemical properties. J. Gen. Microbiol. 107:319-328. [Google Scholar]
- 45.Mietzner, T. A., and S. A. Morse. 1994. The role of iron-binding proteins in the survival of pathogenic bacteria. Annu. Rev. Nutr. 14:471-493. [DOI] [PubMed] [Google Scholar]
- 46.Neilands, J. B. 1995. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723-26726. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 48.Ohman, D. E., J. C. Sadoff, and B. H. Iglewski. 1980. Toxin A-deficient mutants of Pseudomonas aeruginosa PA103: isolation and characterization. Infect. Immun. 28:899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okujo, N., M. Saito, S. Yamamoto, T. Yoshida, S. Miyoshi, and S. Shinoda. 1994. Structure of vulnibactin, a new polyamine-containing siderophore from Vibrio vulnificus. Biometals 7:109-116. [DOI] [PubMed] [Google Scholar]
- 50.Poole, K., S. Neshat, K. Krebes, and D. E. Heinrichs. 1993. Cloning and nucleotide sequence analysis of the ferripyoverdine receptor gene fpvA of Pseudomonas aeruginosa. J. Bacteriol. 175:4597-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ratledge, C., and F. G. Winder. 1962. The accumulation of salicylic acid by Mycobacteria during growth on an iron-deficient medium. Biochem. J. 84:501-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ratledge, C., and M. J. Hall. 1970. Uptake of salicylic acid into mycobactin S by growing cells of Mycobacterium smegmatis. FEBS Lett. 10:309-312. [DOI] [PubMed] [Google Scholar]
- 53.Ratledge, C., L. P. Macham, K. A. Brown, and B. J. Marshall. 1974. Iron transport in Mycobacterium smegmatis: a restricted role for salicylic acid in the extracellular environment. Biochim. Biophys. Acta 372:39-51. [DOI] [PubMed] [Google Scholar]
- 54.Reimmann, C., H. M. Patel, L. Serino, M. Barone, C. T. Walsh, and D. Haas. 2001. Essential pchG-dependent reduction in pyochelin biosynthesis of Pseudomonas aeruginosa. J. Bacteriol. 183:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reimmann, C., L. Serino, M. Beyeler, and D. Haas. 1998. Dihydroaeruginoic acid synthetase and pyochelin synthetase, products of the pchEF genes, are induced by extracellular pyochelin in Pseudomonas aeruginosa. Microbiology 144:3135-3148. [DOI] [PubMed] [Google Scholar]
- 56.Rutherford, K., J. Parkhill, J. Crook, T. Horsnell, P. Rice, M. A. Rajandream, and B. Barrell. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944-945. [DOI] [PubMed] [Google Scholar]
- 57.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 58.Sauer, M., K. Hantke, and V. Braun. 1990. Sequence of the fhuE outer-membrane receptor gene of Escherichia coli K12 and properties of mutants. Mol. Microbiol. 4:427-437. [DOI] [PubMed] [Google Scholar]
- 59.Saxena, B., M. Modi, and V. V. Modi. 1986. Isolation and characterization of siderophores from Azospirillum lipoferum D-2. J. Gen. Microbiol. 132:2219-2224. [Google Scholar]
- 60.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 61.Sexton, R., P. R. Gill, Jr., M. J. Callanan, D. J. O'Sullivan, D. N. Dowling, and F. O'Gara. 1995. Iron-responsive gene expression in Pseudomonas fluorescens M114: cloning and characterization of a transcription-activating factor, PbrA. Mol. Microbiol. 15:297-306. [DOI] [PubMed] [Google Scholar]
- 62.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic enginerering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 63.Sokol, P., P. Darling, S. Lewenza, C. Corbett, and C. Kooi. 2000. Identification of a siderophore receptor required for ferric-ornibactin uptake in Burkholderia cepacia. Infect. Immun. 68:6554-6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sokol, P. A. 1986. Production and utilization of pyochelin by clinical isolates of Pseudomonas cepacia. J. Clin. Microbiol. 23:560-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sokol, P. A., P. Darling, D. E. Woods, E. Mahenthiralingam, and C. Kooi. 1999. Role of ornibactin biosynthesis in the virulence of Burkholderia cepacia: characterization of pvdA, the gene encoding l-ornithine N5-oxygenase. Infect. Immun. 67:4443-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sokol, P. A., C. J. Lewis, and J. J. Dennis. 1992. Isolation of a novel siderophore from Pseudomonas cepacia. J. Med. Microbiol. 36:184-189. [DOI] [PubMed] [Google Scholar]
- 67.Sokol, P. A., and D. E. Woods. 1988. Effect of pyochelin on Pseudomonas cepacia respiratory infections. Microb. Pathog. 5:197-205. [DOI] [PubMed] [Google Scholar]
- 68.Stephan, H., S. Freund, W. Beck, G. Jung, J. M. Meyer, and G. Winkelmann. 1993. Ornibactins—a new family of siderophores from Pseudomonas. Biometals 6:93-100. [DOI] [PubMed] [Google Scholar]
- 69.Takase, H., H. Nitanai, K. Hoshino, and T. Otani. 2000. Impact of siderophore production on Pseudomonas aeruginosa infections in immunosuppressed mice. Infect. Immun. 68:1834-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vandamme, P., B. Holmes, T. Coenye, J. Goris, E. Mahenthiralingam, J. J. LiPuma, and J. R. Govan. 2003. Burkholderia cenocepacia sp. nov.—a new twist to an old story. Res. Microbiol. 154:91-96. [DOI] [PubMed] [Google Scholar]
- 71.Venturi, V., C. Ottevanger, M. Bracke, and P. Weisbeek. 1995. Iron regulation of siderophore biosynthesis and transport in Pseudomonas putida WCS358: involvement of a transcriptional activator and of the Fur protein. Mol. Microbiol. 15:1081-1093. [DOI] [PubMed] [Google Scholar]
- 72.Visca, P., A. Ciervo, V. Sanfilippo, and N. Orsi. 1993. Iron-regulated salicylate synthesis by Pseudomonas spp. J. Gen. Microbiol. 139:1995-2001. [DOI] [PubMed] [Google Scholar]