Abstract
Platelet aggregation plays an important role in the pathogenesis of infective endocarditis induced by viridans streptococci or staphylococci. Aggregation induced in vitro involves direct binding of bacteria to platelets through multiple surface components. Using platelet aggregometry, we demonstrated in this study that two Streptococcus mutans laboratory strains, GS-5 and Xc, and two clinical isolates could aggregate platelets in an irreversible manner in rabbit platelet-rich plasma preparations. The aggregation was partially inhibited by prostaglandin I2 (PGI2) in a dose-dependent manner. Whole bacteria and heated bacterial cell wall extracts were able to induce aggregation. Cell wall polysaccharides extracted from the wild-type Xc strain, containing serotype-specific polysaccharides which are composed of rhamnose-glucose polymers (RGPs), could induce platelet aggregation in the presence of plasma. Aggregation induced by the serotype-specific RGP-deficient mutant Xc24R was reduced by 50% compared to the wild-type strain Xc. In addition, cell wall polysaccharides extracted from Xc24R failed to induce platelet aggregation. The Xc strain, but not the Xc24R mutant, could induce platelet aggregation when preincubated with plasma. Both Xc and Xc24R failed to induce platelets to aggregate in plasma depleted of immunoglobulin G (IgG), but aggregation was restored by replenishment of anti-serotype c IgG. Analysis by flow cytometry showed that S. mutans RGPs could bind directly to rabbit and human platelets. Furthermore, cell wall polysaccharides extracted from the Xc, but not the Xc24R, strain could induce pseudopod formation of both rabbit and human platelets in the absence of plasma. Distinct from the aggregation of rabbit platelets, bacterium-triggered aggregation of human platelets required a prolonged lag phase and could be blocked completely by PGI2. RGPs also trigger aggregation of human platelets in a donor-dependent manner, either as a transient and reversible or a complete and irreversible response. These results indicated that serotype-specific RGPs, a soluble product of S. mutans, could directly bind to and activate platelets from both rabbit and human. In the presence of plasma containing IgG specific to RGPs, RGPs could trigger aggregation of both human and rabbit platelets, but the degree of aggregation in human platelets depends on the donors.
Members of the viridans streptococci are common causes of infective endocarditis in humans (1, 23). Streptococcus mutans, a primary etiological agent of human dental caries (30), accounts for 1.7 to 14% of cases of streptococcal endocarditis (51). In Taiwan, Streptococcus oralis and Streptococcus sanguis are isolated most frequently from blood cultures in patients with endocarditis, but S. mutans is responsible for the highest incidence of endocarditis in bacteremia-associated pyogenic infections (6). S. mutans and other oral streptococci could enter the bloodstream following dental extractions, brushing of teeth, and chewing (16) and cause transient bacteremia in humans . Transient bacteremia facilitated colonization of valve tissues by oral streptococci, particularly in patients with preexisting valvular damage (37). The development of endocarditis depends upon the ability of the colonizing streptococci to induce the formation of vegetations, a fibrin-platelet matrix, inside of which the bacteria are embedded and evade immune clearance by the host.
Various species of oral streptococci have been demonstrated in vitro to possess the ability to induce the aggregation of platelets from various species, including rats, rabbits, and humans (20, 27, 31). The induction of platelet aggregation and formation of bacterial thrombotic vegetations are considered to be important virulence traits in the pathogenesis of endocarditis (20, 44). Direct binding of bacteria to platelets is essential for triggering platelet aggregation, and multiple components from the bacteria and of plasma origin were involved in the subsequent triggering of platelet activation. Bacterial components, such as platelet aggregation-associated protein (PAAP) in S. sanguis or PblA, PblB, and PblT from Streptococcus mitis could mediate direct binding of the bacteria to platelets (3, 21, 22). Direct binding of bacteria to platelets also was demonstrated for Staphylococcus aureus, which causes endocarditis with acute and massive valvular destruction in patients with intact, undamaged heart valves (11). Diminished platelet binding by S. aureus in vitro has been associated with reduced virulence, based on testing in an animal model of endocarditis and manifested by decreased concentrations of bacteria within vegetations (44). S. aureus could bind rabbit platelets directly in a plasma-independent manner (52), mediated through the interaction of multiple bacterial surface components, clumping factor A interacting with a 118-kDa platelet membrane protein (42) and protein A interacting with platelet gC1qR (32). Antibodies specific to S. aureus bound to bacterial antigens could induce platelet aggregation into thrombus formation in vitro (43).
Similar to the phenomenon found in S. aureus, platelet aggregation by either S. sanguis or Streptococcus salivarius also requires the plasma components, including specific immunoglobulin G (IgG) and others (45). The aggregation of human platelets induced in vitro by S. sanguis or S. salivarius was characterized by lag times ranging from 6 to 23 min in a donor-specific manner, before reaching a final abrupt and irreversible response, detectable by aggregometry (46). In addition, aggregation by these two species required direct platelet-bacterial interaction and was not mediated exclusively by soluble bacterial products. Aggregation could be completely blocked by apyrase but not by indomethacin, suggesting that an ADP-mediated mechanism is involved and is independent of cyclooxygenase function (46). These studies also suggested that plasma components in addition to IgG are needed as cofactors to trigger aggregation.
The ability of various species of viridans streptococci to induce aggregation in vitro suggested that some common properties, or even structurally related components shared by these related bacteria, were involved in this bacterium-platelet interaction. On the other hand, members of the viridans streptococci group are phenotypically and genetically distinct species. Therefore, distinct bacterial components and different strategies might be adopted during the complex mechanisms involved in platelet aggregation. Using aggregometry, as well as fluorescence microscopy, we demonstrated that serotype-specific rhamnose-glucose polymers (RGPs) of S. mutans are involved in the adherence of bacteria to both human and rabbit platelets and are capable of triggering platelet aggregation in the presence of plasma. The common structural unit composed of a rhamnose backbone, which can be found on several members of viridans streptococci, is essential for this interaction. In addition, we also demonstrated that the serotype-specific RGPs alone could bind directly to platelets and could induce changes in the shape of purified, plasma-free platelets from rabbits and humans.
MATERIALS AND METHODS
Bacteria and growth conditions.
Two S. mutans laboratory strains, GS-5 and Xc (28, 29), and two clinical isolates, NTU-5526 and NTU-4312, were grown and maintained in brain heart infusion (BHI) broth (Difco Laboratories Inc., Detroit, Mich.). Strain NTU-5526 was isolated from the peripheral blood of a patient suffering from infective endocarditis at the Department of Infectious Diseases, National Taiwan University Hospital (NTUH). Strain NTU-4312 is an oral isolate from a patient with rampant caries from the Department of Dentistry, NTUH. Strain Xc24R is an isogenic mutant derived from the parental Xc strain and is defective in the synthesis of serotype-specific RGPs (48). Strain Xc25 is also derived from strain Xc and carries an insertional mutation at mutX, immediately downstream of the rmlB locus (48). Strain NHS1DD, an isogenic mutant of GS-5, is deficient in the expression of the glucosyltransferase B, C, and D enzymes (50). Xc24R and Xc25 were grown in BHI broth supplemented with erythromycin (10 μg/ml), and NHS1DD was grown in BHI broth supplemented with both erythromycin (10 μg/ml) and tetracycline (25 μg/ml).
Blood specimens.
Rabbit blood samples were collected routinely from the ear veins of New Zealand White rabbits (8 to 12 weeks old) from the animal center of the College of Medicine, National Taiwan University. Human blood samples were collected from volunteers in the laboratory. The statement of informed consent for the use of human blood samples followed the regulations of the NTUH Committee for Regulation of Human Specimens and Volunteers. The blood samples were immediately prepared for the isolation of platelets, and plasma samples were stored frozen at −80°C until used.
Preparation of platelets.
Rabbit platelets were prepared as described previously (12) with modifications. In brief, whole blood was collected from healthy New Zealand White rabbits, mixed with 3.8% buffered citrate solution (0.11 M sodium citrate, 0.02 M citrate acid [pH 5.5]) at a final volume ratio of 1:9, and centrifuged at 225 × g for 20 min at 25°C. After centrifugation, the upper layer was collected as the platelet-rich plasma (PRP) layer. Platelet-poor plasma (PPP) was obtained following centrifugation of the remaining blood sample at 2,000 × g for 10 min at 25°C. The concentrations of platelets in PRP were adjusted to 3 × 108 to 5 × 108 platelets per ml by the addition of PPP and use of a platelet counter (Z1 Coulter Counter; Beckman Coulter). In some experiments, PRP was processed further into a platelet suspension (PS) by centrifugation at 2,000 × g for 10 min at 25°C to pellet the platelets. The platelets were washed twice with Tyrode solution (136.8 mM NaCl, 2.8 mM KCl, 11.9 mM NaHCO3, 1.1 mM MgCl2, 0.33 mM NaH2PO4, 1.0 mM CaCl2, 11.2 mM glucose, and 3.5 mg of bovine serum albumin per ml) and finally resuspended in Tyrode solution at a concentration of 3 × 108 to 5 × 108 platelets per ml.
Human platelets were prepared essentially as described previously (41). Briefly, peripheral whole blood was collected from healthy donors and mixed with 3.8% sodium citrate. PRP was collected following centrifugation at 200 × g for 15 min at 25°C. For the preparation of human PS preparations, 1 μM prostaglandin E1 (PGE1) and 6.4 U of heparin/ml were added into PRP and incubated at 37°C for 10 min. Platelets were then pelleted by centrifugation at 790 × g for 10 min at 25°C and resuspended in Tyrode solution. PGE1(1μM), 6.4 U of heparin/ml, and 0.5 U of apyrase/ml were added to the suspension and incubated at 37°C for 10 min. Platelets were pelleted again by centrifugation at 790 × g for 10 min at 25°C and resuspended in Tyrode solution. Apyrase (0.5 U/ml) was added to the suspension and incubated at 37°C for 10 min. After centrifugation, platelets were suspended in Tyrode solution at concentrations of 3 × 108 to 5 × 108 platelets per ml.
Platelet aggregation.
Platelet aggregation was analyzed by a turbidimetric method (4) with a Lumi-Aggregometer (Payton, Vancouver, Canada). PPP or Tyrode solution was used to adjust the baseline of minimal light transmission. PRP or PS preparations were prewarmed for 3 min prior to the addition of bacteria, and all the procedures were carried out at 37°C, with shaking at 900 rpm. The aggregation lag phase was defined as the time interval between the addition of bacteria to either the PRP or PS preparation and the detection of an increase in light transmission. The reaction was allowed to proceed for at least 6 min, and the degree of aggregation was expressed directly as light transmission units or quantitated as a percentage of aggregation. The percentage of aggregation was calculated by the following formula (where absorbance is measured by optical density [OD]): % aggregation = {[OD before the addition of bacteria − OD after the addition of bacteria]/[OD before the addition of bacteria − OD of Tyrode solution or PPP]} × 100.
Platelets were tested for a normal response to 20 μM ADP. All studies were performed at least twice on two separate occasions in triplicate, and data with standard deviations within 10% of the mean are reported.
Preparation of CWP.
The extraction and preparation of cell wall-associated proteins (CWP) from S. mutans were described previously (8, 9). S. mutans GS-5 was grown in BHI broth. For the extraction of CWP, cells of streptococci from 20 liters of batch culture were washed extensively with 10 mM sodium phosphate buffer and incubated with 8 M urea extraction fluid for 1 h at 25°C. The extract was then dialyzed against 10 mM sodium phosphate buffer (pH 6.5) to remove the urea and subsequently concentrated by 60% (saturation) ammonium sulfate precipitation and dialyzed against the same buffer containing 1 mM phenylmethylsulfonyl fluoride. Protein concentrations were determined with bicinchoninic acid as the colorimetric detection reagent (Pierce).
Preparation of cell wall polysaccharides.
Cell wall polysaccharides were extracted following the methods described previously (19), with modification. Lyophilized bacterial cells with a biomass of 20 mg were resuspended in 5 ml of distilled water, autoclaved at 120°C for 20 min, and centrifuged at 8,000 × g for 20 min, and the supernatants were collected. The cell wall extracts were filtered through a 0.45-μm-pore-size cellulose acetate membrane, and the filtrates were concentrated by lyophilization. Lyophilized cell wall extracts were dissolved in 0.1% phosphate-buffered saline (PBS), and protein or lipid components were removed by repeated cycles of phenol-chloroform extraction. The final cell wall polysaccharide extracts were dialyzed and concentrated by lyophilization. Protein concentrations were measured by using a Bio-Rad protein assay kit. Total hexose and sugar were measured by a colorimetric method as described previously (13). Carbohydrate compositions were confirmed and analyzed by gas chromatography and mass spectrometry (GC-MS).
Analysis of sugar composition.
Cell wall polysaccharide extracts were methanolyzed with 0.5 M methanolic-HCl at 80°C for 16 h, re-N-acetylated with 500 μl of acetic anhydride for 15 min at room temperature, and then trimethylsilylated with 200 μl of the Sylon HTP trimethylsilylating reagent (Supelco) for 20 min at room temperature. Reagents used in each step were removed under a stream of nitrogen, and the final trimethylsilylated products were kept in hexane for GC-MS analysis. GC-MS analysis was carried out on a Hewlett Packard model 6890 gas chromatograph connected to a Hewlett Packard 5973 mass selective detector. Samples were dissolved in hexane prior to splitless injection into an HP-5MS (Hewlett Packard) fused silica capillary column (30 m; 0.25-mm interior diameter). The trimethylsilyl derivatives and the partially methylated alditol acetate were dissolved in hexane prior to on-column injection at 60°C. The column head pressure was maintained at around 8.2 lb/in2 to give a constant flow rate of 1 ml/min with helium used as the carrier gas. For sugar analysis, the oven was held at 60°C for 1 min before being increased to 140°C at 25°C/min and then to 200°C at 5°C/min and, finally, to 300°C at 10°C/min.
Detection of platelet shape change.
Platelet shape change was examined by using a fluorescence microscope and followed immunofluorescence detection procedures described previously (25). Rabbit and human PS preparations (3 × 108 platelets/ml) were incubated with bacterial cell wall polysaccharide (2 mg/ml) at 37°C, with stirring at 900 rpm for 5 min. After incubation, platelets were fixed with paraformaldehyde at a final concentration of 1% at 25°C for 30 min. After fixation, platelets were spun down onto glass slides (Cytospin; Kubota, Tokyo, Japan). Coated glass slides were washed three times with PBS, and fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD9 monoclonal antibody (RDI-CBL 162; Research Diagnostics, Inc., Flanders, N.J.) was added at a concentration of 1:25 and incubated at 37°C for 1 h. Antibody-reacted slides were washed four times in PBS, dried, and covered with fluorescent mounting gel (Biomeda, Vancouver, Canada) before being examined under an immunofluorescence microscope.
Binding of polysaccharides to platelets.
The binding of bacterial cell wall polysaccharides to rabbit or human platelets was detected by flow cytometry. Rabbit or human PS preparations were fixed with paraformaldehyde (1%) at 4°C overnight. Fixed platelets (3 ×108/ml) were washed twice with PBS and incubated with bacterial cell wall polysaccharide extracts of Xc or control strain Xc24R at various concentrations at 37°C for 1 h. After incubation, platelets were washed twice in PBS and incubated with rabbit anti-S. mutans serotype c polysaccharide rabbit IgG (10 μg/ml) on ice for 40 min (7). Platelets were then washed and incubated with FITC-conjugated goat F(ab′)2 anti-rabbit IgG (Roche) on ice for 40 min at a concentration of 1:50. Platelets were washed twice in PBS and analyzed immediately by flow cytometry (fluorescence-activated cell sorting [FACS] Calibur; Becton Dickinson, Paramus, N.J.).
RESULTS
S. mutans-induced aggregation of rabbit platelets requires plasma components.
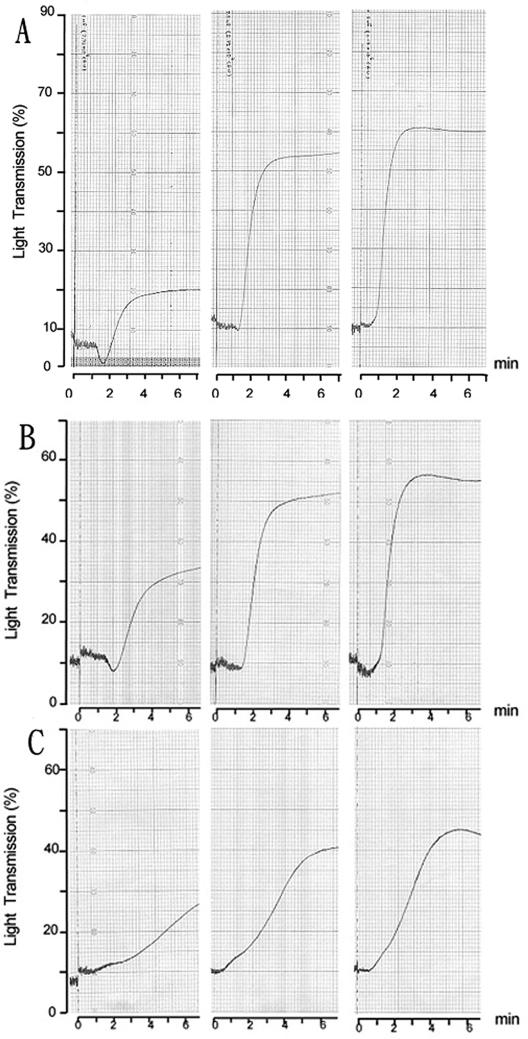
Because the ability of S. mutans to aggregate platelets has not been documented previously, the platelet aggregation assay was validated by testing four serotype c strains, two laboratory strains (GS-5 and Xc), and clinical isolates from the oral cavity (NTU-4312) and serum (NTU-5526) of patients with infective endocarditis, by using rabbit PRP. Typical and representative results for the aggregation of rabbit platelets in PRP by strains GS-5 and Xc are shown in Fig. 1. A mean lag phase of 1.5 min (range, 1 to 2.5 min) was observed initially, followed by brisk aggregation, as indicated by the upward deflection of the tracing, resulting from increased light transmission. Aggregation was complete within 1 min of onset, and the degree of aggregation, as indicated by the percentage of light transmitted, was dependent on the number of bacteria added (Fig. 1A). Optimal aggregation, to a level that was induced by the addition of 2 × 10−5 M ADP in parallel experiments, could be achieved when the bacterial suspension was added to rabbit PRP to give a final ratio of bacteria to platelets of approximately 1:1. The percentage of platelet aggregation induced by S. mutans whole cells could be inhibited, down to 50% of optimal, by PGI2 at a concentration of 130 μM. No aggregation was detected after 20 min of observation when GS-5 was tested with paraformaldehyde-fixed platelets from the same animal. These results confirmed that physiologic aggregation, rather than passive agglutination, was being observed. Similar lag phases and patterns of aggregation were observed when platelets were tested with laboratory strain Xc (Fig. 1B) or the two clinical isolates NTU-4312 and NTU-5526 (data not shown), even though optimal aggregation varied slightly among different PRP preparations. These results indicated that laboratory strains and clinical isolates of S. mutans could induce the aggregation of rabbit platelets in PRP preparations.
FIG. 1.
S. mutans-induced rabbit platelet aggregation and inhibition of aggregation detected by aggregometry. Representative platelet aggre-gation responses to S. mutans strain GS-5, Xc, or Xc24R. The traces show the effects of different doses of bacteria on platelet aggregation. A short lag time (around 1.5 min) was detected in each response. Dose-dependent aggregation response in PRP from a rabbit to S. mutans strain GS-5 (A) at a dose of 2.3 × 107 CFU (left panel), 5.8 × 107 CFU (center panel), or 1.1 × 108 CFU (right panel); to strain Xc (B) at a dose of 3.5 ×107 CFU (left panel), 8.9 × 107 CFU (center panel), or 1.2 × 108 CFU (right panel); and to strain Xc24R (C) at a dose of 3.8 × 107 CFU (left panel), 7.5 × 107 CFU (center panel), or 1.5 × 108 CFU (right panel).
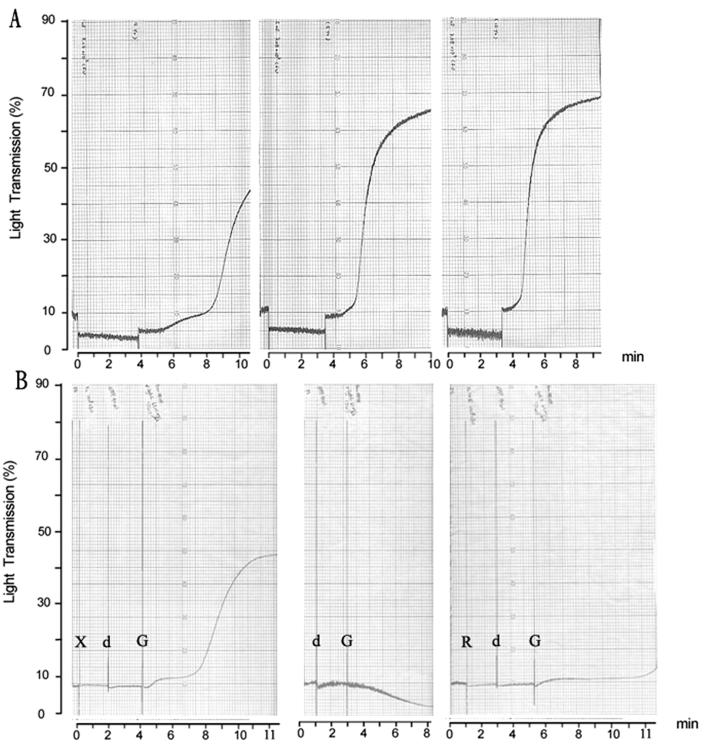
To examine further whether S. mutans could aggregate rabbit platelets directly in the absence of plasma components, the aggregation assays were performed with freshly prepared PS preparations deprived of plasma components. No aggregation could be observed by aggregometry with any strain of S. mutans tested over a period of 25 min. However, aggregation could be restored when PPP was added to the bacteria-PS preparation being tested. As shown in Fig. 2A, aggregation was restored to a level comparable to that found in the PRP preparation when the final PPP reached 10%. An increased lag phase of around 4.5 min was observed when PPP was added at a lower concentration (2%) (Fig. 2A, left panel). No aggregation was detected when PPP was added back to the PS preparation in the absence of bacteria. These results indicated that S. mutans alone cannot induce the aggregation of rabbit platelets to a level detectable by aggregometry and that plasma components are essential for aggregation. Previous results from our laboratory showed that S. mutans could adsorb a significant number of plasma components onto its outer surface (9), and these might serve as a bridge between the platelets and the bacteria. To test this possibility, strain GS-5 was incubated with PPP and washed before addition to the PS preparation. Preadsorption of plasma components to the bacterial cell wall enabled the aggregation of platelets, and the level of aggregation increased, dependent on the amount of bacteria added (Fig. 3A). However, the aggregation achieved by a ratio of bacteria to platelets of 1:1 was only half that for PRP. These results suggested that direct interaction of S. mutans with plasma components is essential for induction of maximal platelet aggregation.
FIG. 2.
(A) Plasma-dependent aggregation response in PPP from a rabbit to S. mutans strain GS-5 at a dose 7 × 107 CFU. No aggregation was observed unless plasma was added (4 min later) to a concentration of 2% (left panel), 5% (middle panel), or 10% (right panel). (B) Essential role of anti-serotype c IgG on aggregation response in PPP from a rabbit to S. mutans strain Xc at a dose 108 CFU. No aggregation was observed in PS after the addition of Xc (left panel) at a dose of 108 CFU (labeled X) and dPPP (labeled d). But aggregation was induced by the addition of anti-serotype c IgG (62.5 μg/ml; labeled G). No aggregation was detected after the addition of dPPP and anti-serotype c IgG (center panel). No aggregation was observed when Xc24R (labeled R) was added in the presence of dPPP and anti-serotype c IgG (right panel).
FIG. 3.
Preadsorption of plasma induced aggregation in PPP. Representative traces of aggregation induced by S. mutans at different doses. (A) Strain Xc was preincubated with plasma, washed, and then added to PPP at a dose of 5.8 × 107 CFU (left panel), 1.2 × 108 CFU (left center panel), 2.4 × 108 CFU (right center panel), or 4.7 × 108 CFU (right panel). (B) Strain Xc24R, defective in the synthesis of RGPs, was preincubated with plasma, washed, and then added to PPP at a dose of 6.2 × 107 CFU (left panel), 2.2 × 108 CFU (center panel), or 4.1 × 108 CFU (right panel).
Role of cell wall polysaccharide extracts in aggregation.
The results of preadsorption with plasma suggested that cell wall components of S. mutans are involved in the induction of platelet aggregation. To investigate the nature of the candidate components on the cell wall, aggregation assays were performed with whole live bacteria or bacteria killed by heat inactivation. When added to PRP from rabbits, strain GS-5, 5526, or 4312 induced platelet aggregation at 6 min with a similar percentage of aggregation (80% versus 75 to 78% for live or heat inactivated bacteria, respectively). Therefore, heat inactivation did not reduce significantly the percentage of aggregation induced by the cell wall extracts from different strains. These results suggested that heat-resistant components of the cell wall of S. mutans could induce platelet aggregation in PRP preparations.
To determine whether cell wall-associated serotype polysaccharides composed of RGPs are components involved in aggregation, platelet aggregation assays were performed with another isogenic mutant, Xc24R, defective in the synthesis of serotype-specific RGPs. Xc24R was still able to induce the rabbit platelet aggregation in PRP but at a lower efficiency than the parental Xc or GS-5 strains (Fig. 1C). The percentage of aggregation of rabbit platelets induced by Xc24R in PRP was significantly lower than that induced by the parental strain Xc at various doses tested. The aggregation was reduced around 40% at the higher doses tested and dose-dependent aggregation of platelets was still detectable when Xc24R was tested (Fig. 1C). However, when tested with PS preparations deprived of plasma components, Xc24R could not induce aggregation, even when preadsorbed with plasma (Fig. 3B). In parallel experiments, the percentages of aggregation induced by the two additional isogenic mutant strains Xc25 (48) and NHS1DD (50) were comparable to the percentage induced by the parental Xc strain. Xc25 carried an insertional mutation at mutX, immediately downstream of the rmlB locus (48). Therefore, the reduced ability of Xc24R to aggregate rabbit platelets was not due to polar effects on the downstream gene. NHS1DD is deficient in the expression of the glucosyltransferase B, C, and D enzymes and carried two antibiotic resistance genes against erythromycin and tetracycline inserted at unrelated loci (50). The results of tests with the NHS1DD strain indicated that the observed difference in Xc24R was not due to changes in the other surface components induced by growth in the presence of antibiotics. Taken together, these results suggested that serotype polysaccharides of S. mutans are among the major components responsible for inducing the aggregation of rabbit platelets and that the interaction of these polysaccharides with plasma components is essential for this aggregation.
Serotype polysaccharides induce aggregation.
To confirm further the role of serotype polysaccharides in platelet aggregation, serotype polysaccharides were extracted from strains Xc or Xc24R with autoclaving and partially purified to remove protein contaminants. The final polysaccharide extracts did not contain detectable proteins, and their sugar compositions were analyzed by GC-MS. The polysaccharide extracts from the wild-type Xc strain were composed primarily of glucose and rhamnose (0.59 and 0.89 nmol/μg, respectively). However, the rhamnose peak was not detected in the polysaccharide extracts from strain Xc24R. The amount of glucose in the Xc24R extracts was markedly reduced (0.29 nmol/μg) compared to the extracts from strain Xc. No significant difference in the amount of galactose was found between the two extracts (concentration in Xc, 0.05 nmol/μg; concentration in Xc24R, 0.04 nmol/μg). Rabbit IgG from a serotype c-specific rabbit antiserum, prepared previously in this laboratory (7), was purified and the serotype specificity was confirmed with polysaccharide extracts from strains GS-5 (serotype c), MT730R (serotype e), and OMZ175 (serotype f) by immunodiffusion analysis (33) in 1% (wt/vol) Noble agar in saline. The serotype c-specific IgG reacted with the polysaccharide extracts from strains Xc and GS-5 but did not react with the extracts from Xc24R. These results confirmed that serotype c-specific RGPs were found in the polysaccharide extracts from Xc but were not detectable in those from Xc24R.
The polysaccharide extracts from both Xc and Xc24R were incubated with PRP of rabbits preimmunized with GS-5 at various concentrations (0.1 to 4 mg/ml), and the aggregation response was monitored over a period of 20 min by aggregometry. The aggregation was detected after 3 to 4 min when polysaccharide extracts from strain Xc were added at a concentration of 1 mg/ml, and a dose-response reaction was observable with a final percentage aggregation of 25%, reached after 6 min of reaction. However, no aggregation was detected over a period of 20 min following the addition of the polysaccharide extracts from strain Xc24R to a final concentration of 4 mg/ml. These results indicated that the serotype-specific RGPs alone could induce aggregation of rabbit platelets in the presence of plasma components.
Interaction of specific IgG in plasma is essential for S. mutans-induced aggregation of rabbit platelets.
Previous results from studies conducted on S. aureus, S. sanguis, and S. salivarius indicated that platelet aggregation induced by this microorganism involves interaction-specific IgG (43, 45, 46). Given the fact that platelet aggregation by S. mutans also requires plasma, it is possible that a similar interaction is also involved. To investigate the role of IgG specific to S. mutans in inducing aggregation, rabbit PS was incubated with PPP depleted of IgG. Adding PPP or depleted PPP (dPPP) alone would not cause any platelet aggregation in PS. When cells of Xc strain at a dose of 108 CFU were added to PS, no aggregation was observed unless PPP was added, which is analogous to the earlier results. Distinct from the earlier results, no aggregation was observed by aggregometry when dPPP was added to PS preparations in the presence of S. mutans strain Xc. But aggregation could be restored by the addition of anti-RGP-specific IgG (Fig. 2B, left panel). The addition of anti-RGP-specific IgG alone did not induce aggregation (Fig. 2B, center panel). In addition, no aggregation was observed when PS was incubated in the presence of Xc24R and dPPP or after replenishment with anti-RGP-specific IgG (Fig. 2B, right panel). These results indicated that interaction of specific IgG in plasma with S. mutans is essential for inducing platelet aggregation.
Binding of serotype polysaccharides to platelets.
To determine whether RGPs could bind directly to platelets, platelets were isolated from PS preparations, and binding was identified by flow cytometry with a FACS Caliber instrument. Platelets were incubated with polysaccharide extracts or left unstimulated. Significant binding of RGPs to platelets was detected in a dose-dependent manner by flow cytometry, and representative results of one experiment are shown in Fig. 4. Unstimulated platelets or platelets treated with polysaccharide dissolving buffer showed very low reactivity with the anti-serotype c polysaccharide-specific rabbit IgG (Fig. 4A and B). The mean fluorescence intensity increased with the concentration of polysaccharide in a dose-dependent manner (Fig. 4B to D). The preadsorption of polysaccharides with anti-serotype polysaccharide IgG by immunoprecipitation abolished the binding of polysaccharides to platelets and also the activation of platelets (data not shown).These results indicated that serotype polysaccharides could bind directly to the platelets.
FIG. 4.
Binding of S. mutans RGPs to rabbit platelets determined by flow cytometry. Cell wall polysaccharide extracts containing serotype c-specific RGPs were incubated with rabbit PPP and subsequently labeled with anti-serotype c-specific rabbit IgG. Mean fluorescence intensities are noted. (A) Unstimulated platelets only. (B) Background fluorescence (rabbit IgG and anti-rabbit FITC). (C through E) Cell wall polysaccharides were added to final concentrations of 0.5, 1.0, and 2.0 mg/ml, respectively.
Platelet shape change induced by serotype polysaccharides.
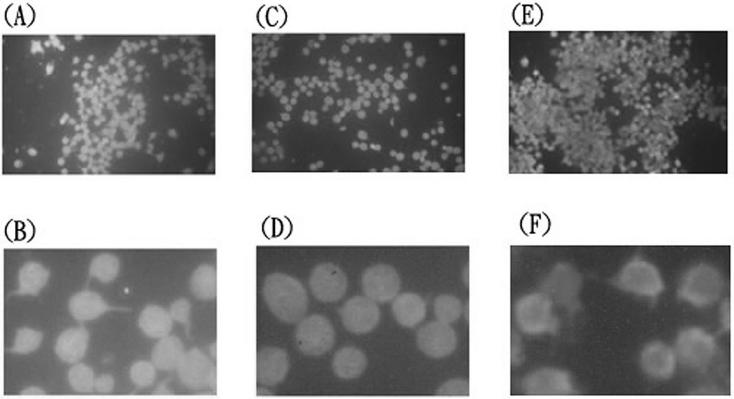
To determine whether RGPs could activate platelets directly after binding and whether this reaction is essential for the subsequent aggregation response, rabbit platelets in PS preparations were examined by fluorescence microscopy after the addition of whole bacteria or RGPs and anti-CD9 monoclonal antibody. Activated platelets are characterized by a change in cell shape from round to irregular, with multiple pseudopod protrusions. Untreated platelets in suspension exhibited a typical round shape and uniform size, but when platelets were treated with ADP, whole bacterium, GS-5, or Xc, pseudopod formation was readily detected. When PS was incubated with serotype polysaccharides extracted from the wild-type Xc strain, a proportion of the platelets (35 to 40%) acquired a shape change characterized by pseudopod protrusion around an altered cell shape (Fig. 5A and B). However, the shape change characteristic of platelet activation was not observed when polysaccharides from the Xc24R strain were added (Fig. 5C and D). To determine whether the activation of human platelets could also be demonstrated by using serotype polysaccharides, PS preparations were isolated from four volunteers and examined by fluorescence microscopy. Platelets from each individual could be activated by the polysaccharide extracts containing RGPs and exhibited pseudopod formation around an altered cell shape. Representative results from one of the tested samples are shown in Fig. 6 (compare panels B and D). However, none of the human platelet samples responded to the polysaccharide extract from the Xc24R strain, which is defective in the expression of RGPs (Fig. 6, compare panels B and D). Direct binding of the RGPs to human platelets also could be demonstrated by flow cytometry. These results indicated that serotype polysaccharides, soluble components of S. mutans, could activate the platelets from both rabbit and human.
FIG. 5.
Rabbit platelet shape changes induced by cell wall polysaccharides with and without RGPs. Rabbit platelets in PS preparations deprived of plasma components were incubated with cell wall polysaccharides extracted from S. mutans strain Xc (panels A and B), strain Xc24R (panels C and D) or ADP (panels E and F). Activated platelets, characterized by a shape change from discoid to irregular with multiple protrusions, were readily detectable in panel C.
FIG. 6.
Human platelet shape change induced by cell wall polysaccharides with and without RGPs. Human platelets in PS preparations deprived of plasma components were incubated with cell wall polysaccharides extracted from S. mutans strain Xc (panels A and B) or strain Xc24R (panels C and D). Unstimulated platelets exhibited a typical discoid shape with a uniform size (panels E and F). Panels B, D, and F show portions of the corresponding top panels at higher magnification.
Human platelet aggregation induced by S. mutans and RGPs.
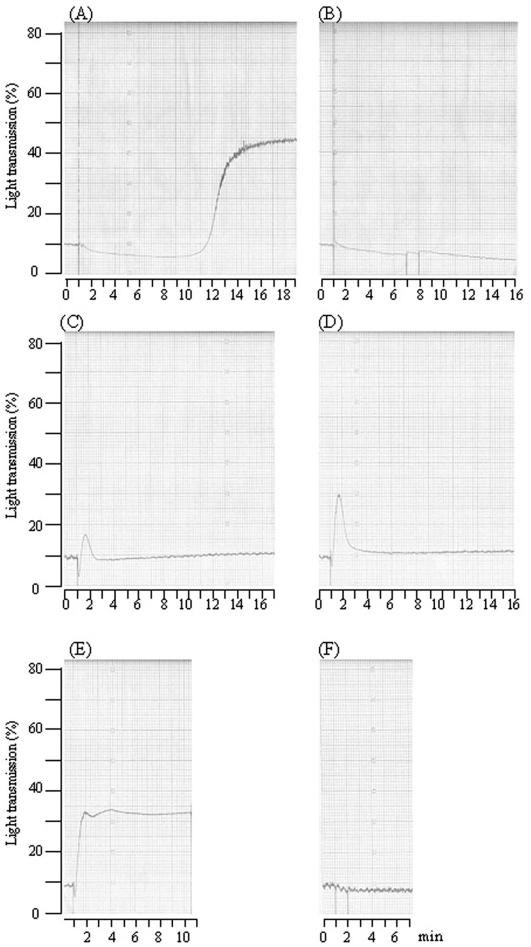
To investigate whether S. mutans and its RGPs could aggregate human platelets in a manner similar to or distinct from that found in the rabbit, human PRP was prepared from six volunteers and examined by aggregometry. Strain Xc, or clinical isolate 5526, aggregated human platelets suspended in PRP from four of six donors tested, and representative results of the aggregation induced by Xc are shown in Fig. 7. Distinct from results with the rabbit platelets, a mean lag phase of 13 min was required before brisk aggregation was started. In addition, the aggregation could be completely blocked by the addition of PGI2 (Fig. 7B). The aggregation of human PRP preparations was not detectable by aggregometry when IgG was depleted, suggesting that anti-S. mutans-specific IgG is also essential for the aggregation of human platelets, as was found for rabbit platelets. Depending on the donors, the percentages of aggregation of human platelets exhibited greater variation than that of rabbit platelets. Similar results were also found when RGP was subsequently examined by the incubation of human PRP preparations in the presence of RPGs at different concentrations. Distinct from the slow response observed when whole bacteria were added, brisk aggregation, as indicated by the upward deflection of the tracing, was noted immediately after the addition of RGPs (Fig. 7C and D). Depending on the donor, the aggregations triggered by RGPs at the same concentration (0.5 mg/ml) exhibited different patterns, from a transient and reversible mild response (Fig. 7C and D) to a typical abrupt and irreversible manner (Fig. 7E) and up to a level similar to that induced by whole bacterium. In addition, the aggregation induced by RGPs could be completely abolished by preincubation for 1 min with PGI2, suggesting that physiological aggregation rather than passive aggregation was being observed (Fig. 7F). These results indicated that S. mutans could also induce the aggregation of human platelets but required a longer lag phase. In addition, RPGs, a soluble product of S. mutans, when added alone could also induce an aggregation response in a donor-dependent manner.
FIG. 7.
Induction and inhibition of human platelet aggregation detected by aggregometry. (A) Representative platelet aggregation response from one donor to S. mutans strain Xc at a dose of 1.2 × 108 CFU. A longer lag time (around 13 min) was detected. (B) The aggregation was inhibited completely by the addition of PGI2. (C through E) Aggregation induced by RGPs at a concentration of 0.5 mg/ml from three donors. (F) Aggregation triggered by RGPs in panel E could be blocked completely by pretreatment with PGI2.
DISCUSSION
In this study, we confirmed by aggregometry that S. mutans could induce the aggregation in vitro of platelets from both rabbits and humans and that S. mutans could bind directly to, and activate, platelets of both rabbits and humans through serotype polysaccharides. The observed shape change, along with the typical aggregation profiles detected by aggregometry and inhibition by PGI2, suggested that the platelet response to the various strains of S. mutans, including laboratory stocks and clinical isolates, was true aggregation. The species-specific difference in the response to S. mutans-induced platelet aggregation was an interesting observation. Similar observations have been demonstrated previously in S. sanguis-induced platelet aggregation. When tested with platelets from rats, rabbits, or humans, S. sanguis strains exhibited different aggregation profiles in a strain-dependent manner (31). The majority of strains tested (76%) could trigger aggregation of both rat and human platelets, but there was no correlation of the ability, in terms of lag times and percentages of aggregation, to aggregate human and rat platelets. In addition, the ability of some representative strains to aggregate rabbit platelets was similar to that for rat platelets. The major difference between animal and human platelets was the lag time to the onset of aggregation, detectable by aggregometry. When human platelets were tested, a longer lag time was required than for rabbit or rat platelets (12 min for human versus 1 min for rabbit or rat platelets). The average lag time for S. mutans is around 1.5 min for the aggregation of rabbit platelets, which is similar to the lag time of S. sanguis, but for human platelets, aggregation was observed after a mean lag time of 13 min and was donor dependent. Distinct from the aggregation of the rabbit platelets, the aggregation of human platelets induced by S. mutans could be blocked completely by PGI2, a potent inhibitor of aggregation. Therefore, species-specific differences in terms of platelet composition, such as platelet receptors, might exist and account for the differences observed in the aggregation patterns or profiles induced by microorganisms such as S. mutans or S. sanguis.
The requirement of cofactors other than specific IgG for inducing human platelet aggregation has also been reported previously when aggregation was tested with S. salivarius and S. sanguis (45, 46). In addition, aggregation by these two streptococci required direct platelet-bacterial interaction and was not mediated exclusively by a soluble bacterial product (46). Distinct from these reports, we found that RGPs alone, soluble products of S. mutans, could activate and induce the aggregation of platelets from both rabbit and human. But the degree of aggregation of human platelets showed greater variation than that of rabbit platelets, especially when RGPs were examined (Fig. 7). In two of the donors included in this study, the aggregation response was transient and reversible. For this reason, alternative methods were developed for the detection of platelet activation, such as detecting changes in ultramorphology by confocal microscopy (25) or electron microscopy (25). We obtained similar results by using fluorescence microscopy and blood from various healthy donors. In the present study, using S. mutans as a model, we successfully demonstrated that detecting platelet shape change by fluorescence microscopy is a direct and simple method for addressing relatively weak platelet activation that is not readily detected by the conventional method of aggregometry coupled with aggregation inhibitors or antagonists.
Platelet aggregates form through sequential stages of adhesion, activation, and aggregation (38). In the presence of high shear stress, adhesion is mediated through the binding of von Willebrand factor (vWF) to glycoprotein Ib (18, 24, 26). After adhesion occurs, shape changes take place in the platelets, allowing alteration of platelet receptor glycoprotein IIb/IIIa, which then binds fibrinogen and vWF, inducing aggregation. Bacteria binding to platelets at the site of cardiac valve lesions may promote infective endocarditis by facilitating the further deposition of platelets and the subsequent development of platelet-fibrin matrices, ultimately leading to enlargement of endocardial vegetations (39). Analysis of S. mutans-platelet binding by quantitative flow cytometry indicated that RGPs of S. mutans can bind to platelets directly (i.e., in the absence of plasma cofactors), and this process was rapid and saturable, suggesting a receptor-ligand interaction. The binding of bacteria to platelets through carbohydrate components has been documented previously in S. aureus, the most common etiological agent of intravascular infections (11). The modification of S. aureus surface carbohydrates susceptible to periodate oxidation resulted in significantly reduced platelet binding in all isolates examined, suggesting that carbohydrate moieties are involved (17). However, the nature or the components of the polysaccharides were not identified. The results of this study indicate that RGPs are one of the major components responsible for the binding of S. mutans to platelets. In addition, the RGPs could activate rabbit and human platelets by inducing shape changes. Interestingly, the RGPs of S. mutans share a common structural relationship with the group-specific polysaccharide antigens of Lancefield group A, C, and E streptococci and the RGP antigen of Streptococcus sobrinus (5, 35).
The backbones of RGPs are polymers of α1,2- and α1-3-linked rhamnose units, and the rhamnose backbone has been identified in many streptococci. Although the genes involved in the synthesis of the RGPs by S. mutans have been well characterized (49), little is known about the structural organization of the RGPs and how these polysaccharides are anchored to the bacterial cell wall. Interestingly, PAAP of S. sanguis is a rhamnose-rich glycoprotein (14). Expression of PAAP is associated with more severe experimental endocarditis than seen when PAAP is not expressed or blocked by monospecific antibodies (22). The binding domain of PAAP for interaction with the platelet was identified as a 7-mer peptide that conformed to the predicted structural motif of the platelet-interactive domains of type I and type III collagen. Although rhamnose was the major carbohydrate moiety in PAAP, it was assumed that carbohydrate polymers associated with PAAP would not participate in platelet interaction, since PAAP peptide fragments devoid of carbohydrate still retain biological activity (15). However, no direct evidence has yet been provided in the structure or function of intact PAAP to exclude a role for rhamnose polymers involved in the binding of the glycoprotein to platelets.
The rhamnose polymers also are found in another glycopeptide, ristocetin, an antimicrobial compound synthesized by the actinomycete Norcardia lurida and discovered in the late 1950s (34). Drug-induced platelet agglutination and thrombocytopenia prompted the discontinuation of the use of the drug as a therapeutic agent. A consequence of its discovery was the development of the ristocetin-induced platelet agglutination assay, a screening test for von Willebrand's disease. The biologic activities of ristocetin are mediated by dimers of the glycopeptide. The mechanism for ristocetin-induced agglutination involves the binding and bridging of the vWF and other plasma proteins to the surfaces of platelets (40). Interestingly, enzymatic cleavage of the rhamnose tetrasaccharide of ristocetin abolished its ability to induce platelet aggregation in plasma, suggesting that rhamnose plays an important structural and/or functional role in the platelet aggregation response (2). When rhamnose-glucose polymers were depleted from the cell wall of S. mutans, the bacterium lost its ability to aggregate platelets significantly, down to 50% of the level of the wild-type strain. Taken together, these results suggested that the rhamnose backbone, existing in different forms as structural units, might contribute directly to the induction of platelet aggregation. The finding that the Xc24R strain, defective for RGPs, was still capable of aggregating platelets suggested that components other than RGPs also are involved in triggering and/or enhancing the aggregation response.
RGPs were extracted from S. mutans serotype c strain Xc by using an autoclaving procedure, as described previously (19). Although the polysaccharide extracts contained no detectable protein components, carbohydrates other than the RGPs, such as galactose, were present. The origins of these carbohydrates might be from lipoteichoic acid or surface components, such as glycoproteins, and these served as internal controls for comparing the carbohydrate contents of extracts from different strains. The results of GC analysis indicated that there is no difference, other than in the rhamnose-glucose content, between the polysaccharide extracts from the Xc and Xc24R strains. Therefore, the observed differences in biological activity were attributable to the effect of RGPs and not to other carbohydrates or unidentified components in the crude extracts. In fact, lipoteichoic acid from S. aureus has been shown to inhibit, rather than activate, platelet aggregation (41).
Structural aspects of RGPs, such as the linkage and configuration of sugar residues, have been analyzed for the S. mutans serotype e strain. Results from methylation analysis and 13C nuclear magnetic resonance spectroscopy provide strong evidence that the RGPs from S. mutans possess chemical structures indistinguishable from the Lancefield group E streptococcal polysaccharides (36). Both polysaccharides were found to consist of a backbone of alternating 2- and 2,3-linked α-l-rhamnose units and side chains of β-d-glucose units linked to position 2 of the branching rhamnose units. A polyrhamnose backbone of such alternating 2- and 3-linked α-l-rhamnose units has also been reported to be present in the group-specific polysaccharides of Lancefield's group A, A-variant, and C streptococci (10). The existence of a common polyrhamnose backbone in streptococcal polysaccharides was proposed long ago (36). It seems highly likely that a similar situation exists for the biological functions examined in this study, which would account for the general observation of platelet aggregation induced by related viridans and other streptococci.
Acknowledgments
We thank H. K. Kuramitsu for NHS1-DD strain and Y. Yamashita for Xc24R and Xc25 strains. We thank Tur Fu Huanz for helpful discussion and instruction in aggregometry. We thank Shu-Wha Lin for instructions in handling blood specimens. We thank Tim J. Harrison, Reader in Molecular Virology, Department of Medicine, Royal Free and University College Medical School, UCL, for his kind review and help in the preparation of this manuscript.
This work was supported in part by the National Science Council (grant NSC-902320-B002-134, NSC-912320-B002-101, NSC-922320-B002-166) and National Health Research Institute (grant NHRI-EX91-9139SI and NHRI-EX92-9139SI).
Editor: V. J. DiRita
REFERENCES
- 1.Baddour, L. M. 1988. Twelve-year review of recurrent native valve infectious endocarditis: a disease of the modern antibiotic era. Rev. Infect. Dis. 10:1163-1170. [DOI] [PubMed] [Google Scholar]
- 2.Bardsley, B., D. H. Williams, and T. P. Bablin. 1998. Cleavage of rhamnose from ristocetin A removes its ability to induce platelet aggregation. Blood Coagul. Fibrinolysis 9:241-244. [DOI] [PubMed] [Google Scholar]
- 3.Bensing, B. A., C. E. Rubens, and P. M. Sullam. 2001. Genetic loci of Streptococcus mitis that mediate binding to human platelets. Infect. Immun. 69:1373-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Born, G. V. R., and M. J. Gross. 1963. The aggregation of blood platelets. J. Physiol. 168:178-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun, D. G. 1983. The use of streptococcal antigens to probe the mechanisms of immunity. Microbiol. Immunol. 27:823-836. [DOI] [PubMed] [Google Scholar]
- 6.Chang, S. C., K. T. Luh, L. J. Deng, and W. C. Hsieh. 1987. Bacteriology of viridans streptococcal bacteremia. Chinese J. Microbiol. Immumol. 20:311-318. [PubMed] [Google Scholar]
- 7.Chia, J. S., T. Y. Hsu, L. J. Teng, J. Y Chen, L. J. Hahn, and C. S. Yang. 1991. Glucosyltransferase gene polymorphism among Streptococcus mutans strains. Infect. Immun. 65:1563-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chia, J. S., Y. Y. Lee, P. T. Huang, and J. Y. Chen. 2001. Identification of stress-responsive genes in Streptococcus mutans by differential display reverse transciption-PCR. Infect. Immun. 69:2493-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chia, J. S., C. Y. Yeh, and J. Y. Chen. 2000. Identification of a fibronectin binding protein from Streptococcus mutans. Infect. Immun. 68:1864-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coligan, J. E., T. J. Kindt, and R. M. Krause. 1978. Structure of the streptococcal groups A, A-variant and C carbohydrates. Immunochemistry 15:755-760. [DOI] [PubMed] [Google Scholar]
- 11.Cunha, B. A., V. Gill, and J. M. Lazar. 1996. Acute infective endocarditis. Diagnostic and therapeutic approach. Infect. Dis. Clin. N. Am. 10:811-834. [DOI] [PubMed] [Google Scholar]
- 12.Dankert, J., J. Krijgsveld, J. van der Werff, W. Joldersma, and S. A. J. Zaat. 2001. Platelet microbicidal activity is an important defense factor against viridans streptococcal endocarditis. J. Infect. Dis. 184:597-605. [DOI] [PubMed] [Google Scholar]
- 13.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 14.Erickson, P. R., and M. C. Herzberg. 1993. The Streptococcus sanguis platelet aggregation-associated protein: identification and characterization of the minimal platelet-interactive domain. J. Biol. Chem. 268:1646-1649. [PubMed] [Google Scholar]
- 15.Erickson, P. R., and M. C. Herzberg. 1993. Evidence for the covalent linkage of carbohydrate polymers to a glycoprotein from Streptococcus sanguis. J. Biol. Chem. 268:23780-23783. [PubMed] [Google Scholar]
- 16.Fekete, T. 1990. Controversies in the prevention of infective endocarditis related to dental procedures. Dent. Clin. N. Am. 34:79-90. [PubMed] [Google Scholar]
- 17.Fournier, J. M., W. F. Vann, and W. W. Karakawa. 1984. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect. Immun. 45:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto, S., D. R. Salomon, Y. Ikeda, and Z. M. Ruggeri. 1995. Characterization of the unique mechanism mediating the shear-dependent binding of soluble von Willebrand factor to platelets. J. Biol. Chem. 270:23352-23361. [DOI] [PubMed] [Google Scholar]
- 19.Hamada, S., K. Gill, and H. D. Slade. 1976. Chemical and immunological properties of the type f polysaccharide antigen of Streptococcus mutans. Infect. Immun. 14:203-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzberg, M. C. 1996. Platelet-streptococcal interactions in endocarditis. Crit. Rev. Oral Biol. Med. 7:222-236. [DOI] [PubMed] [Google Scholar]
- 21.Herzberg, M. C., G. D. MacFarlane, K. Gong, N. N. Armstrong, A. R. Witt, and P. R. Erickson. 1992. Platelet interactivity phenotype of Streptococcus sanguis influences the course of experimental endocarditis. Infect. Immun. 60:4809-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzberg, M. C., K. Gong, G. D. MacFarlane, P. R. Erickson, A. H. Soberay, P. H. Krebsbach, G. Manjula, K. Schilling, and W. H. Bowen. 1990. Phenotypic characterization of Streptococcus sanguis virulence factors associated with bacterial endocarditis. Infect. Immun. 58:515-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horaud, T., and F. Delbos. 1984. Viridans streptococci in infective endocarditis: species distribution and susceptibility to antibiotics. Eur. Heart J. 5(Suppl. C):39-44. [DOI] [PubMed] [Google Scholar]
- 24.Ikeda, Y., M. Handa, and K. Kawano. 1991. The role of von Willebrand factor and fibrinogen in platelet aggregation under varying shear stress. J. Clin. Investig. 87:1234-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karpman, D., D. Papadopoulou, K. Nilsson, A.-C. Sjogren, C. Mikaelsson, and S. Lethan. 2001. Platelet activation by Shiga toxin and circulatory factors as a pathogenic mechanism in hemolytic uremic syndrome. Blood 97:3100-3108. [DOI] [PubMed] [Google Scholar]
- 26.Kerrigan, S. W., I. Douglas, A. Wray, J. Heath, M. F. Byrne, D. Fitzgerald, and D. Cox. 2002. A role for glycoprotein Ib in Streptococcus sanguis-induced platelet aggregation. Blood 100:509-516. [DOI] [PubMed] [Google Scholar]
- 27.Kitada, K., M. Inoue, and M. Kitano. 1997. Experimental endocarditis induction and platelet aggregation by Streptococcus anginosus, Streptococcus constellatus, and Streptococcus intermedius. FEMS Immunol. Microbiol. 19:25-32. [DOI] [PubMed] [Google Scholar]
- 28.Koga, T., H. Asakawa, N. Okahashi, and I. Takahashi. 1989. Effect of subculturing on expression of a cell-surface protein antigen by Streptococcus mutans. J. Gen. Microbiol. 135:3199-3207. [DOI] [PubMed] [Google Scholar]
- 29.Kuramitsu, H. 1973. Characterization of invertase activity from cariogenic Streptococcus mutans. J. Bacteriol. 115:1003-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manning, J. E., A. J. Geyelin, L. M. Ansmits, H. J. Oakey, and K. W. Knox. 1994. A comparative study of the aggregation of human, rat and rabbit platelets by members of the Streptococcus sanguis group. J. Med. Microbiol. 41:10-13. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen, T., B. Ghebreheiwet, and E. I. B. Peerschke. 2000. Staphylococcus aureus protein A recognizes platelet gC1qR/p33: a novel mechanism for staphylococcal interactions with platelets. Infect. Immun. 68:2061-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ouchterlony, O. 1958. Diffusion-in-gel methods for immunological analysis. Prog. Allergy 5:1-78. [PubMed] [Google Scholar]
- 34.Philip, J. E., J. R. Scheneck, and M. P. Hargie. 1975. Ristocetin, p. 699-705. In H. Welch and F. Marti-Ibanez (ed.), Antibiotics Annual: 1956-1957. Medical Encyclopedia, Inc., New York, N.Y.
- 35.Pritchard, D. G. 1985. Structure of the group-specific polysaccharide of group E Streptococcus. Carbohydr. Res. 144:289-296. [DOI] [PubMed] [Google Scholar]
- 36.Pritchard, D. G., R. L. Gregory, S. M. Michalek, and J. R. McGhee. 1986. Biochemical aspects of serotype carbohydrate antigens of Streptococcus mutans, p. 39-49. In S. Hamada, S. M. Michalek, H. Kiyono, L. Menaker, and J. R. McGhee (ed.), Molecular microbiology and immunology of Streptococcus mutans. Elsevier Science Publishers, Amsterdam, The Netherlands.
- 37.Roberts, R. B. 1995. Streptococcal endocarditis, p. 191-208. In D. Kaye (ed.), Infective endocarditis. Raven Press, New York, N.Y.
- 38.Roth, G. J. 1992. Platelets and blood vessels: the adhesion event. Immunol. Today 13:100-105. [DOI] [PubMed] [Google Scholar]
- 39.Scheld, W. M., J. A. Valone, M. A. Sande. 1978. Bacterial adherence in the pathogenesis of endocarditis: interaction of bacterial dextran, platelets, and fibrin. J. Clin. Invest. 61:1394-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott, J. P., R. R. Montgomery, and G. S. Retzinger. 1991. Dimeric ristocetin flocculates proteins, binds to platelets, and mediates von Willebrand factor-dependent agglutination of platelets. J. Biol. Chem. 266:8149-8155. [PubMed] [Google Scholar]
- 41.Sheu, J. R., G. Hsiao, C. R. Lee, W. C. Chang, L. W. Lee, C. H. Su, and C. H. Lin. 2000. Antiplatelet activity of Staphylococcus aureus lipoteichoic acid is mediated through a cyclic AMP pathway. Thromb. Res. 99:249-258. [DOI] [PubMed] [Google Scholar]
- 42.Siboo, I. R., A. L. Cheung, A. S. Bayer, and P. M. Sullam. 2001. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect. Immun. 69:3120-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sjobring, U., U. Ringdahl, and Z. M. Ruggeri. 2002. Induction of platelet thrombi by bacteria and antibodies. Blood 100:4470-4477. [DOI] [PubMed] [Google Scholar]
- 44.Sullam, P. M., A. S. Bayer, W. M. Foss, and A. L. Cheung. 1996. Diminished platelet binding in vitro by Staphylococcus aureus is associated with reduced virulence in a rabbit model of infective endocarditis. Infect. Immun. 64:4915-4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullam, P. M., G. A. Jarvis, and F. H. Valone. 1988. Role of immunoglobulin G in platelet aggregation by viridans streptococci. Infect. Immun. 56:2907-2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullam, P. M., F. H. Valone, and J. Mills. 1987. Mechanisms of platelet aggregation by viridans group streptococci. Infect. Immun. 55:1643-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorpe, C. M., R. Flaumenhaft, B. Hurley, M. Jacewicz, D. W. K. Acheson, G. T. Keusch. 1999. Shiga toxins do not directly stimulate alpha-granule secretion or enhance aggregation of human platelets. Acta Haematol. 102:51-55. [DOI] [PubMed] [Google Scholar]
- 48.Tsuda, H., Y. Yamashita, K. Toyoshima, N. Yamaguchi, T. Oho, Y. Nakano, K. Nagata, and T. Koga. 2000. Role of serotype-specific polysaccharide in the resistance of Streptococcus mutans to phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 68:644-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsukioka, Y., Y. Yamashita, T. Oho, Y. Nakano, and T. Koga. 1997. Biological function of the dTDP-rhmnose synthesis pathway in Streptococcus mutans. J. Bacteriol. 179:1126-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsumori, H., and H. Kuramitsu. 1997. The role of the Streptococcus mutans glucosyltransferases in the sucrose-dependent attachment to smooth surfaces: essential role of the GtfC enzyme. Oral Microbiol. Immunol. 12:274-280. [DOI] [PubMed] [Google Scholar]
- 51.Ullman, R. F., S. J. Miller, M. J. Strampfer, B. A. Cunha. 1988. Streptococcus mutans endocarditis: report of three cases and review of the literature. Heart Lung 17:209-211. [PubMed] [Google Scholar]
- 52.Yeaman, M. R., P. M. Sullam, P. F. Dazin, D. C. Norman, and A. S. Bayer. 1992. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J. Infect. Dis. 166:65-73. [DOI] [PubMed] [Google Scholar]