Abstract
Several studies have shown that conflict processing improves from childhood to adulthood and declines from adulthood to old age. However the neural mechanisms underlying this lifespan asymmetry were previously unexplored. We combined event-related potentials (ERPs) and electromyography (EMG) to examine lifespan changes in stimulus and response conflict processing using a modified Stroop task. We used a Stroop task that a priori dissociated stimulus and response conflict. Delayed P3b latency and increased amplitude revealed that middle age adults have a deficit in stimulus processing. Additionally a sustained P3a across frontal and central electrodes occurred only in middle age adults indicating the recruitment of frontal activity. Conversely, decreased lateralized readiness potential (LRP) amplitude and increased EMG activity in the incorrect hand in adolescents reveal protracted development of response processing into late adolescence. The N450, a measure of conflict processing, was found to be sensitive to both stimulus and response conflict. Altogether these results provide evidence for asymmetrical differences in stimulus and response conflict processing across adolescence, young adulthood and middle age.
Keywords: Ageing, Middle age, Adolescence, Colour word Stroop task, N450, P3a, Lateralized readiness potential
1. Introduction
Asymmetries in cognitive maturation throughout the lifespan demonstrate that ageing does not simply reflect development in reverse (Craik & Bialystok, 2006). As we transition through different phases of life external changes to our bodies follow a relatively symmetrical pattern; weakness in infancy is followed by strength in adolescence and middle age and finally frailty again in old age. This growth and decline appears to be mirrored in our mental capabilities. However a more fine-grained analysis reveals subtle yet critical asymmetries in development and ageing. For example, even though very young children and elderly adults have difficulty with vocabulary, the process underlying this difficulty is very different. Children are still acquiring knowledge and are in the process building their vocabulary whereas older adults have a strong vocabulary base but may have difficulty accessing or remembering the words. Cognitive change during development and ageing seems dissimilar and asymmetrical (Craik & Bialystok, 2006; Sander, Lindenberger, & Werkle-Bergner, 2012).
In line with the above notion, cognitive neuroscientists and psychologists are beginning to argue that there is no period of optimal performance during young adulthood. Instead throughout the lifespan we may experience shifts in our ability to perform certain cognitive functions (Craik & Bialystok, 2006; Crone & Dahl, 2012). At different points in the lifespan cognitive abilities may come online or go offline. For example, children and adolescents are creative and flexible yet impulsive (Crone & Dahl, 2012), young adults are efficient and resourceful yet more regimented, finally older adults have strong crystallized intelligence (i.e., experience, comprehension, judgement and wisdom) but difficulties with fluid intelligence (i.e., cognitive control and access to knowledge) (Craik & Bialystok, 2006). Now the challenge is to document strengths and weaknesses across the lifespan so that cognitive strengths can be enhanced and weaknesses can be moderated. In order to get a more complete picture of the asymmetrical nature of cognitive change research should focus on more detailed analysis and investigations to identify the specific changes (Craik & Bialystok, 2006).
Here we focused on specific mechanisms of change that underlie conflict processing during adolescence and middle age. Two key transitional periods in the adult lifespan, the end of adolescence and the end of middle age, show asymmetrical patterns of difficulties in conflict processing (Hämmerer, Li, Müller, & Lindenberger, 2010). Behaviourally it is often found that adolescents and children commit more errors on conflict tasks (Segalowitz & Davies, 2004) whereas older adults are generally slower (Falkenstein, Yordanova, & Kolev, 2006). One of the most prolific conflict tasks is the Stroop task. In the original Stroop paradigm participants name the ink colour of colour words. It is more difficult to name the ink colour when it is incongruent with word meaning (i.e., RED in green ink) than when ink colour and meaning are congruent (i.e., RED in red ink) (Stroop, 1935). Two different types of conflict contribute to poor performance on conflict tasks such as the Stroop task (de Houwer, 2003; Milham et al., 2001; Zhang & Kornblum, 1998). Stimulus conflict can be generated early in the processing stream for example during semantic processing (Hock & Egeth, 1970). Response conflict occurs later during motor response activation whereby task relevant and task irrelevant information are processed in parallel and trigger competing motor responses (Morton & Chambers, 1973). Both adolescents and middle age adults show marked decrements in performance in Stroop tasks [i.e., increased errors and slow reaction time (RT), Leon-Carrion, García-Orza, & Pérez-Santamaría, 2004; Zysset, Schroeter, Neumann, & Yves von Cramon, 2006]. Some neuroimaging research suggests that these decrements may in fact be related to asymmetrical developmental patterns (Yordanova, Kolev, Hohnsbein, & Falkenstein, 2004). Brain areas supporting response conflict continue to develop into adolescence (Adleman et al., 2002; Hämmerer et al., 2010; Velanova, Wheeler, & Luna, 2009) whereas neural activity involved in stimulus processing declines early during ageing (Mager et al., 2007; Vallesi, Stuss, McIntosh, & Picton, 2009; Wiegand, Finke, Müller, & Töllner, 2013).
Two approaches are commonly used to examine the neural correlates of age-related change in conflict processing. First, we can examine group differences in how information is processed at different stages. For example we can examine whether age-related neural change occurs at the stimulus identification stage or response selection and execution stages (Bryce, Szũcs, Soltész, & Whitebread, 2011; Szucs, Soltész, Bryce, & Whitebread, 2009; Szucs, Soltész, & White, 2009). The second approach uses a paradigm to evoke stimulus and response conflict in separable conditions e.g., stimuli that evoke stimulus conflict in one condition and response conflict in another condition (Chen, Bailey, Tiernan, & West, 2011; de Houwer, 2003; Jongen & Jonkman, 2008). Neural change associated with these two types of conflict can then be compared across the lifespan.
The first approach asserts that stimulus and response processing stages are marked by separable stimulus and response related event-related potentials (ERPs) components. For example several studies have used the P3a and P3b components as markers of stimulus level processing (Duncan-Johnson & Kopell, 1981; Ilan & Polich, 1999; Szucs & Soltész, 2010b) while LRP and EMG activities are thought to measure response level processing (Falkenstein et al., 2006; Roggeveen, Prime, & Ward, 2007; Van der Lubbe & Verleger, 2002; Wiegand et al., 2013). The P3b is commonly used to separate developmental change at the stimulus level from change at the response level as the P3b is thought to represent stimulus processing independently of response level processing (Szucs, Soltész, Bryce, et al., 2009; Szucs, Soltész, & White, 2009; however see Verleger, 1997). This can mark if developmental and age-related change occurs during the stimulus processing stage. Further, one of the most reliable findings in the ageing literature is increased frontal positivity at 300 msec in ageing adults (Fjell & Walhovd, 2004; O'Connell et al., 2012; Polich & Criado, 2006). Currently the functional significance of the frontal P3a shift with ageing remains ambiguous (Dien, Spencer, & Donchin, 2004; Szucs & Soltész, 2010a, 2010b). Some accounts suggest that the attention of older adults is more easily captured by irrelevant stimuli (Tays, Dywan, Mathewson, & Segalowitz, 2008) or that the P3a is representative of an early reflexive response in ageing (Jacoby, Bishara, Hessels, & Toth, 2005). If middle age adults experience a specific deficit during stimulus processing perhaps the P3a will be predominantly recruited during stimulus conflict.
In terms of later response related components the lateralized readiness potential (LRP) is an increased negative potential over the primary motor cortex contralateral to the responding hand that occurs prior to motor response execution. This is thought to represent differential left/right motor cortex activation (Coles, 1989; Coles, Gratton, Bashore, Eriksen, & Donchin, 1985; Gratton, Coles, Sirevaag, Eriksen, & Donchin, 1988). The stimulus locked LRP can therefore be used to demarcate differences in the initiation or onset of motor preparation across the lifespan. In this study the LRP is used to mark development and age-related change in response selection.
Finally, electromyography (EMG) can be used to study response processing during peripheral motor execution. Because it is applied to both the left and right hands in parallel EMG can examine correct and incorrect hand activity simultaneously. Szucs, Soltesz, and White (2009) detected increased incorrect hand EMG activity prior to a correct hand response during the incongruent condition of a Stroop task. This confirms that response conflict extends down the stream of information processing just prior to response execution (Szucs, Soltész, Bryce, et al., 2009; Szucs, Soltész, & White, 2009). In combination, stimulus locked LRP and EMG measurements enable the continuous tracking of motor cortex activation (response selection and response execution) to determine whether response stages are differentially affected throughout the lifespan.
The second common approach to examine conflict processing seeks to isolate change in specific types of conflict by using a paradigm that evokes separable stimulus (SC), response (RC), and general conflict conditions. For example the de Houwer (2003) colour word Stroop paradigm in principle evokes stimulus and response conflict in different conditions. The task has three conditions, four colour words and four colours, and two response options. Two colours are mapped to the same response option (e.g., RED and GREEN should be responded by a button on the left while BLUE and YELLOW should be responded by a button on the right). The congruent condition contains no stimulus or response conflict; the written meaning and the printed ink colour are the same (e.g., RED in red ink). In the stimulus conflict condition, there is conflict at the stimulus but not at the response level. That is, the ink colour and the word meaning are different however they are mapped to the same response hand (i.e., RED in green ink). In the response conflict condition the written word and the ink colour are different and they are also mapped to different response hands, therefore evoking both stimulus and response conflict (i.e., RED in blue ink). An advantage of this task over the original Stroop task is that it allows the two types of conflict to be examined separately during development and ageing.
The difference waves of key ERP components can then be analyzed to isolate specific change during stimulus or response conflict processing. Stimulus conflict can be measured by analyzing SC minus congruent conditions; response conflict can be measured by analyzing RC minus SC, finally general conflict (or combined stimulus and response level conflict) can be measured by analyzing RC minus congruent condition. For example the most established ERP measure of Stroop conflict is usually called the N450. The N450 is an enhanced negativity with a latency of 300–500 msec in the incongruent condition relative to the neutral/congruent conditions over midline electrodes (Eppinger, Kray, Mecklinger, & John, 2007; Hanslmayr et al., 2008; Rebai, Bernard, & Lannou, 1997; West & Alain, 2000b). Recent evidence suggests it represents general conflict detection (Szucs & Soltesz, 2012; Szucs, Soltész, Bryce, et al., 2009; West, Bowry, & McConville, 2004; West & Schwarb, 2006). Across the lifespan the N450 shows distinct maturational patterns in terms of topography, amplitude, and latency; however the functional significance of these changes has not been determined. Jongen and Jonkman (2008) documented the developmental emergence of the N450 around 10–12 years of age. Unlike in adults who had left frontal activity they found that the topography of the N450 was focused over left and right parietal sites in children. The developmental hemispheric shift over parietal sites may be representative of either reduced ability (e.g., to inhibit responses) or compensatory processes (e.g., the engagement of higher levels of attention) (Jongen & Jonkman, 2008). Some ageing literature suggests the latency and amplitude of the N450 decline with age (West & Alain, 2000a; West et al., 2004). However, others found increased N450 amplitude (Mager et al., 2007). These inconsistent findings could be due to the different age range of participants and slight differences in task manipulations. Here we examined this question and related the modulations of the N450 to the manipulation of stimulus and response conflict.
Here our overall objective was to identify developmental asymmetries in conflict processing across the lifespan. First we identified any age-related differences in stages of information processing by examining neural activity representative of stimulus processing (P3a, P3b) as well as response levels of processing (LRP, EMG). Secondly we isolated differences in stimulus (SC minus CON), response (RC minus SC) and general (RC minus CON) conflict processing by examining the main effects of congruency effects and the difference waves of key components during the de Houwer colour word Stroop task.
In terms of developmental predictions first, we expected that adolescents will show immature response conflict processing (Hämmerer et al., 2010). In terms of the information processing approach we expected response related stages to be delayed in adolescence. This would be reflected in P3b duration, amplitude and latency similar to young adults followed by delayed onset and increased incorrect hand activity in LRP and EMG activation respectively. This would confirm response activation and execution stages to be the loci of immaturity in late adolescence. In terms of the conflict-related congruency effects we expected to see increased RT and decreased accuracy for the RC condition as well as increased amplitude and latency for response conflict related components (N450, LRP and EMG) during the RC condition overall indicative of increased sensitivity to response conflict.
Second, we expected middle age adults to display difficulties in stimulus conflict processing (Hämmerer et al., 2010; Mager et al., 2007). In terms of the information stages of processing this would manifest in an increased latency, duration and decreased amplitude of the P3b (related to stimulus categorization) and increased amplitude of the P3a (related to stimulus selection). However later response level activation and execution in terms of the LRP latency and amplitude and lack of EMG incorrect hand activity would be similar to young adults. In terms of congruency effects we expected to see a delay in RT during the SC condition and increased neural processing of stimulus components (i.e., increased amplitude and latency of P3a, P3b) during this condition.
In order to characterize the time course of cognitive processes besides typical peak and mean amplitude and latency measures we also assessed the onset/offset and durations of the P3a/P3b waves in each participant. With regard to the N450 effect we examined the functional significance of the topographic change in relation to stimulus, response or general conflict processing. Finally in terms of behavioural performance we expected that the congruent condition would yield the fastest RT followed by the SC and finally RC condition. Accuracy would follow a similar pattern with highest accuracy in the congruent condition followed by the SC and finally RC condition. In terms of group differences we expected that middle age adults would be slower than young adults, whereas adolescents would be faster than young adults but commit more errors.
2. Method
2.1. Participants
Initially 64 participants were examined. Due to electroencephalography (EEG) artefacts 10 participants were rejected from the analysis. Participants were excluded during preprocessing, before any data analysis had occurred. Three age groups were examined: 18 adolescents (16–17-year olds, mean age 16.55 years, one left handed, 10 females), 18 young adults (23–30-year olds, mean age 25.83, four left handed, 11 females) and 18 middle age adults (45–62-year olds, mean age 56.6, three left handed, nine females). Notably the middle age adults' age range used in this current study spans 17 years. We used a similar age range used by other studies examining ‘middle age’ adults (Zysset et al., 2006, range: 45–75 years). Most importantly we used an age range similar to previous ERP studies of middle age so that the results would be comparable (Falkenstein et al., 2006, mean age 58.3, range not given; Mager et al., 2007, 41–61 years). All participants were fluent in English, had normal or corrected to normal vision and had no history of psychiatric or neurological disorders. Informed written consent was obtained from each participant and from the parent or guardian of the adolescent participants. The adults were graduate students and staff at the University of Cambridge, UK. Middle age adults were staff at the University of Cambridge or employed in the Cambridge area and had completed at least 14 years of formal schooling (A Levels UK). Adolescents were students at the Hills Road 6th Form College, Cambridge, UK. The study received ethical approval from the Psychology Research Ethics Committee of the University of Cambridge.
Although no measure of general intelligence was administered in this study, an indication of memory ability was derived by comparing group differences in raw scores on the digit span (forward and backward) subtest of the Wechsler Adult Intelligence Scale (WAIS) III (UK). Scores on the combined digit span forwards and backwards were not significantly different between groups [F(2,42) = 3.199, p > .05].
2.2. Task and stimuli
Stimuli were the following English words: BLUE, RED, GREEN, YELLOW. Words could be presented in each of the following colours; blue, red, green or yellow. Stimulus presentation was pseudo-randomized whereby each subject had a different random order of stimuli presented. Participants were seated in a small room facing a 19 inch computer screen and they watched the computer screen and held a video game controller. Participants responded to the ink colour of the word by using their left and right thumbs. According to one response assignment participants pressed the left button if the ink colour was red or green. They pressed the right button if the ink colour was yellow or blue. Response assignments were counter-balanced between participants.
In the congruent condition there is no stimulus or response conflict. The semantic meaning and the correct response engage the same hand (e.g., ‘RED’ printed in red ink). In the stimulus conflict (SC) condition even though the semantic meaning and correct response are incongruent they are mapped to the same response hand thereby eliminating response conflict (e.g., the word RED printed in green ink). In the response conflict (RC) condition the printed colour is incongruent with the semantic meaning of the word (e.g., the word RED printed in blue ink) and additionally the associated responses are mapped to different response hands. This condition is considered to produce both stimulus and response conflict. Therefore to isolate response conflict the SC condition is subtracted from the RC condition. Colour word stimuli were presented on a black background. Trials started with a fixation sign (picture of an eye) shown for 300 msec. This was followed by a black screen for 1000 msec (followed by +− 100 msec random jittering). Stimuli appeared for 800 msec followed by a response period of 500 msec. Participants were instructed to blink when they saw the fixation sign. There were five experimental blocks with 128 trials in each block. In each block 50% of trials were RC while 25% were SC and 25% were congruent. Before the experimental blocks one practice block was completed with 16 stimuli. Stimuli were presented using the Neurobehavioral Systems Presentation 11 program.
2.3. EEG recording and ERP analyses
EEG data were recorded in an electrically and acoustically shielded booth using 129-channel Hydro-Cell Net from an Electrical Geodesics system. A sampling rate of 500 Hz was used. An online band-pass filter of .01–70 Hz was used. Offline the data were band-pass filtered between .01 and 30 Hz and recomputed to an average reference. Epochs extended from −100 to 1000 msec relative to stimulus presentation. Data were baseline corrected from −100 to 0 msec before stimulus presentation. Spline interpolation was conducted on noisy electrodes for no more than 10% of electrodes following the recommendation of Electrical Geodesics (EGI, Oregon, USA). Epochs were excluded from the analysis if the following artefact rejection criteria were violated; voltage deviations exceeding +− 120 μV relative to baseline, maximum gradient exceeding 50 μV, and the lowest activity below .5 μV. After artefact rejection at least 50% of trials had to be included for each participant and for each condition. The minimum number of trials included for each participant in the congruent and SC conditions was 80 and in the RC condition 160 (at least 50% of the total number of trials in each condition). In adolescents 76.7% of all trials were accepted, in young adults 74.15% of all trials were accepted and in middle age adults 69.85% of all trials were accepted after artefact rejection.
2.4. Statistical analysis
2.4.1. Behavioural data
Mixed between/within participants analysis of variance (ANOVA) examined RT and accuracy. Group (adolescents, young adults, middle age adults) was the between participants factor while congruency (congruent, SC, RC) was the within participants factor. Difference values between the three different conditions were also calculated (RC − congruent, SC − congruent, RC − SC) to examine the proportion of conflict between each condition. In both behavioural and physiological analyses post hoc Tukey-honestly significant difference (HSD) tests were used to examine the contrasts unless stated otherwise. Where the assumption of sphericity has been violated the Greenhouse–Geisser epsilon (ε) correction was used. The epsilon value is indicated along with the adjusted p value and original degrees of freedom. The EEG analysis and behavioural analysis included only correctly responded trials.
2.4.2. ERP data
First the major ERP components (P1, P3a, P3b, N450 and LRP) were identified in the original (raw) ERP waveforms to examine differences in the early stimulus and later response stages of processing. Second, group × congruency ANOVA's were examined to isolate congruency effects. If significant congruency effects were identified, stimulus and response conflict effects in the difference waves were analyzed (RC − CON, SC − CON, RC − SC). In order to determine time points and electrodes for analyses the following information was considered. A point-by-point ANOVA (electrodes × time points) was run to isolate and identify significant time points and electrode locations (Szucs and Soltész, 2007; Szucs & Soltesz, 2010a, 2010b). Effects were considered significant if p < .01 over 20 consecutive time points. Additionally previous literature was consulted to further refine electrode locations and time points of interest.
2.4.3. P3a and P3b
Based on the point-by-point ANOVA the peak latencies and amplitudes of the major ERP components were measured in the time intervals displayed in Table 1. Electrode locations are shown in Fig. 1(A). The P3a was identified as the most positive peaks in frontal electrodes during the specified time periods for each age group (frontal electrodes 21, 22, 17, 15, 14, 9) based on previous frontal electrode examinations (Fallgatter, Mueller, & Strik, 1999; Fjell & Walhovd, 2003). The P3b was identified as the maximum amplitude at centro-parietal electrodes (54, 61, 67, 55, 62, 72, 79, 78, 77) during the specified time period and in accordance with previous studies (Dien et al., 2004; Szucs & Soltész, 2010a, 2010b). P3a and P3b peak amplitudes and latencies were entered into a congruency (3) × group (3) ANOVA.
Table 1.
Time intervals (msec) of peak amplitude and latency ERP measurements.
| ERP | Adolescents |
Young adults |
Middle age adults |
|||
|---|---|---|---|---|---|---|
| Start | End | Start | End | Start | End | |
| P3a | 180 | 200 | 180 | 230 | 250 | 335 |
| P3b | 200 | 600 | 250 | 600 | 250 | 600 |
Fig. 1.
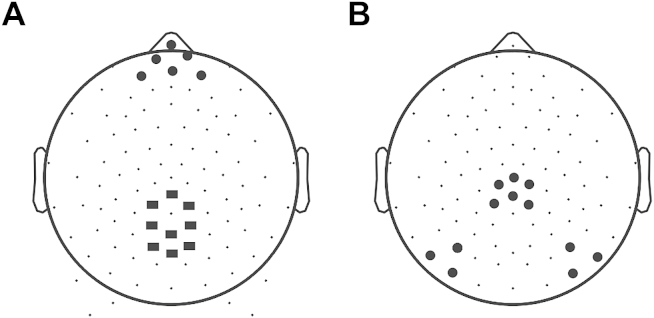
Maps of electrode locations (A) poolings for ERPs; squares represent P3b pool, circles represent P3a pool (B) N450 three electrode pools (left, central, right) used to compare the mean amplitude of the N450 across group.
The duration of the P3a and P3b ERP waves was determined in each individual. First, the peak amplitude and peak latency of the P3a/P3b waves were identified individually. Second, we determined the latencies of the sampling points preceding (onset latency) and following (offset latency) peak amplitude latency where the amplitude level crossed 60% of the peak amplitude level. Duration was defined as the time difference between the onset and offset latencies.
We also examined the P1 occipital ERP component to dissociate P3a activity from P1 perceptual encoding. The P1 occipital component was examined between 80 and 150 msec (electrodes for P1 left 65, 66, 68, 69, 70; electrodes for P1 right 84, 90, 83, 89, 94) in accordance with previous findings (Folstein & Van Petten, 2008; Luck, 2005).
2.4.4. N450
The mean amplitude of the raw N450 was firstly examined between 300 and 550 msec at a pooling of 16 central electrodes that showed the maximum amplitude in the topography of the N450 effect across the three groups of participants (cento-parietal electrodes 129, 55, 54, 42, 53, 52, 51, 59, 60, 61, 79, 62, 67, 66, 72, 85) (Jongen & Jonkman, 2008; Szucs & Soltesz, 2012; West & Schwarb, 2006). The mean amplitude of the raw N450 was entered into a group × congruency ANOVA.
2.4.5. Congruency analysis: difference waves of N450
The difference waves were calculated for RC − congruent, SC − congruent and RC − SC. A repeated measures ANOVA was performed for group (3) × difference wave (3). A topographical analysis was also performed to further quantify the differences between the three groups. Based on observations of the topographical differences between the three groups a series of electrode pools were used to capture this difference. The mean amplitude of the difference waves between 400 and 500 msec was examined at three central and parietal pools (central: 129, 31, 54, 55, 80, 79; left parietal: 58, 59, 65; right parietal: 91, 90, 96) (see Fig. 1B). A repeated measures ANOVA was performed for group (3) × congruency (3) × pool (3).
2.4.6. LRP
The LRP was calculated according to convention (Coles, 1989): [(ER − EL) left hand response + (EL − ER) right hand response]/2. ER represents the amplitude of the ERP at the electrode over the right motor cortex, whereas EL represents the amplitude of the ERP at the electrode over the left motor cortex. The left and right motor cortex electrodes were electrodes 36 and 104 respectively. These have the equivalent of positions C3 and C4 in the traditional 10–20 electrode system. The raw LRP waveforms were smoothed using a 50 msec moving average window to improve signal to noise ratio. The peak latency and peak amplitude of stimulus locked LRPs were calculated from 250 to 600 msec. Response locked LRPs' peak amplitude, latency and mean amplitude were examined between −300 msec and 0 msec relative to response. A repeated measures ANOVA of congruency (3) × group (3) was done on the peak latencies and peak amplitudes. As the peak amplitude of the LRP can be variable particularly in developmental studies (Bryce et al., 2011) the mean amplitude of the LRP was also calculated with separate time periods for each group based on group differences in the mean RT data. In order to accurately detect the initiation of response selection the stimulus locked LRP peak latency was matched with the proportional change in RT in the three age groups. As the stimulus locked LRP tends to peak between 400 and 600 msec after stimulus onset young adults were examined between this time point. Adolescents RT was 45 msec faster than young adults therefore the LRP was examined 45 sec earlier than in young adults (355–555 msec). Middle age adults were 34 sec slower than young adults therefore the LRP was examined from 434 to 634 msec relative to stimulus onset.
EEG data were processed using Brain Vision Analyzer (Brain Products, Munich), Matlab 7.9, SPSS 17.0 and Statistica 9.
2.4.7. LRP jackknifing
LRP jackknifing is a procedure commonly used in the LRP literature as it is thought to provide a more accurate estimate of LRP onset latencies (Lansbergen, van Hell, & Kenemans, 2007; Miller, Patterson, & Ulrich, 1998; Wild-Wall, Falkenstein, & Hohnsbein, 2008). This is a statistical method that combines the data from different participants into a subaverage instead of determining the average for each individual participant. This subaverage for each data entry is calculated as the grand average (with one participant removed). Therefore when N = 18 participants, each data entry is the mean of 17 participants instead of one (Bryce et al., 2011; Miller, Patterson, & Ulrich, 1998; Ulrich & Miller, 2001). This method is found to reduce variation and increase signal to noise ratio. In order to compensate for the artificial reduction of variance a correction is used to adjust the critical F value. Onset latencies of the smoothed LRP waveform were determined at 70% of the relevant peak's amplitude.
2.5. EMG data
Muscle activity was recorded using EMG. Using an MP150 data acquisition unit (Biopac Inc.) EMG was measured by EMG110C amplifiers. EMG110S shielded touch-proof leads where connected to two disposable cloth-based hypoallergenic Ag-AgCl EL504 recording disc electrodes. The electrodes were placed along the left and right flexors of the thumb (flexor pollicis brevis). An electrode on the left elbow was used as a ground. Before the electrodes were applied the skin was washed with soap and cleaned with alcohol wipes. The electrodes were attached by adhesive solid gel. EMG was sampled at 2000 Hz and band-pass filtered between 10 and 500 Hz. The data were then rectified and scaled relative to the maximum amplitude in each individual as measured from continuous data. EMG was baseline corrected between −100 and 0 msec relative to stimulus presentation and is displayed as a percentage of the maximum value measured. Epochs extended from −100 to 1000 msec relative to stimulus presentation. Grand average EMG waves were calculated for each condition and smoothed with a 50 msec moving average window. Point-by-point group (3) × congruency (3) ANOVAs were performed on the mean amplitudes of correct hand activity and incorrect hand activity between 200 and 600 msec. In order for effects to be considered significant they had to be longer than 20 sampling points at an alpha level of p < .01 (Szucs & Soltész, 2010a; Szucs, Soltész, & White, 2009).
3. Results
As stated previously, first the major ERP components (P3a, P3b, N450 and LRP) were identified in the original (raw) ERP waveforms to examine differences in the early stimulus and later response stages of processing. Second, group × congruency ANOVA's were examined to isolate congruency effects. If significant congruency effects were identified, stimulus and response conflict effects in the difference waves were analyzed (RC − CON, SC − CON, RC − SC).
3.1. Behavioural results
Accuracy and RT values are presented in Table 2. A repeated measures ANOVA of group (adolescents, young adults, middle-aged adults) × condition was performed on RT and accuracy data. In terms of accuracy there was a significant congruency effect [F(2,102) = 8.63, ɛ = .536, p = .0040]. Post hoc Tukey contrasts revealed that there were more incorrect responses in the RC condition compared to SC condition (p = .0012, 88.9 vs 93.8%) and compared to the congruent condition (p = .0020, 88.9 vs 93.6%). Error rates between the three groups did not significantly differ [F(2,51) = .632, p = .5358] and there were no interactions [F(4,102) = 2.205, p = .0736]. In RT there was a significant effect of group [F(2,51) = 3.74, p = .0305]. Post hoc Tukey contrasts revealed that adolescents were 79 msec faster than middle-aged adults (p = .0235, 571 vs 650 msec). There were no other significant group interactions [F(4,102) = 1.888, p = .1181]. RT showed a significant congruency effect [F(2,102) = 101.41, ɛ = .950, p < .0001]. Post hoc Tukey contrasts revealed the congruent condition was 20 msec faster than the SC condition (p = .0001, 592 vs 612) and 41 msec faster than the RC condition (p = .0001, 592 vs 633 msec). The SC condition was 21 msec faster than the RC condition (p = .0001, 612 vs 633).
Table 2.
Accuracy (% correct) and mean RT (milliseconds) for each group as a function of condition ± standard error (SE) con=congruent, SC= stimulus conflict, RC=response conflict.
| Reaction time ± SE | CON | SC | RC |
|---|---|---|---|
| Adolescents | 552.67 ± 18.45 | 574.50 ± 18.39 | 587.33 ± 19.15 |
| Young | 599.00 ± 17.92 | 614.63 ± 19.20 | 636.06 ± 19.40 |
| Midde age adults | 625.50 ± 24.20 | 649.54 ± 24.20 | 675.97 ± 23.52 |
| Accuracy ± SE | CON | SC | RC |
|---|---|---|---|
| Adolescents | 91.8 ± 1.69 | 92.5 ± 1.68 | 89.9 ± 1.56 |
| Young adults | 94.2 ± 1.37 | 95.1 ± 1.23 | 92.1 ± 1.82 |
| Middle age adults | 94.6 ± 1.32 | 93.7 ± 1.37 | 84.6 ± 5.48 |
3.1.1. Congruency effects: difference values
The RT difference values that indicate specific types of conflict e.g., general conflict (RC − CON), stimulus conflict (SC − CON) and response conflict (RC − SC) were also examined. Combined stimulus and response conflict [or general conflict (RC − CON)] yielded the greatest increase in RT compared to the congruent condition and this was significant across all groups [F(2,102) = 24.209, ε = .6603, p < .0001]. Interestingly RT isolated during stimulus (SC − CON) and response (RC − SC) conflict did not significantly differ (p = .9965).
Overall there were three important findings from the behavioural results. First the task was validated, as there was a significant difference in RT between the three conditions across all age groups. Second in terms of group differences, there were no significant group differences in accuracy that is unexpected as we predicted that adolescents would perform less accurately than older adults. Also the RT of middle age adults and adolescents did not differ from young adults. We expected that adolescents would be faster than young adults, however this is not the case, although they were significantly faster than middle age adults. Third, in terms of congruency effects, the two types of conflict did not differentially affect RT. This indicates that at the final overt level, RT is not differentially sensitive to stimulus or response conflict in this task.
3.2. ERPs
Fig. 2 depicts the grand-averaged ERPs from a pool of centro-parietal electrodes (129, 55, 54, 42, 53, 52, 51, 59, 60, 61, 79, 62, 67, 66, 72, 85). For an overview of significant results refer to Tables 3 and 4. These results outline the two approaches described in the Introduction; first, group differences in the stimulus and response stages of information processing are presented. This is followed by any specific changes in either stimulus or response conflict processing as evident from significant congruency effects or from an analysis of the difference waves.
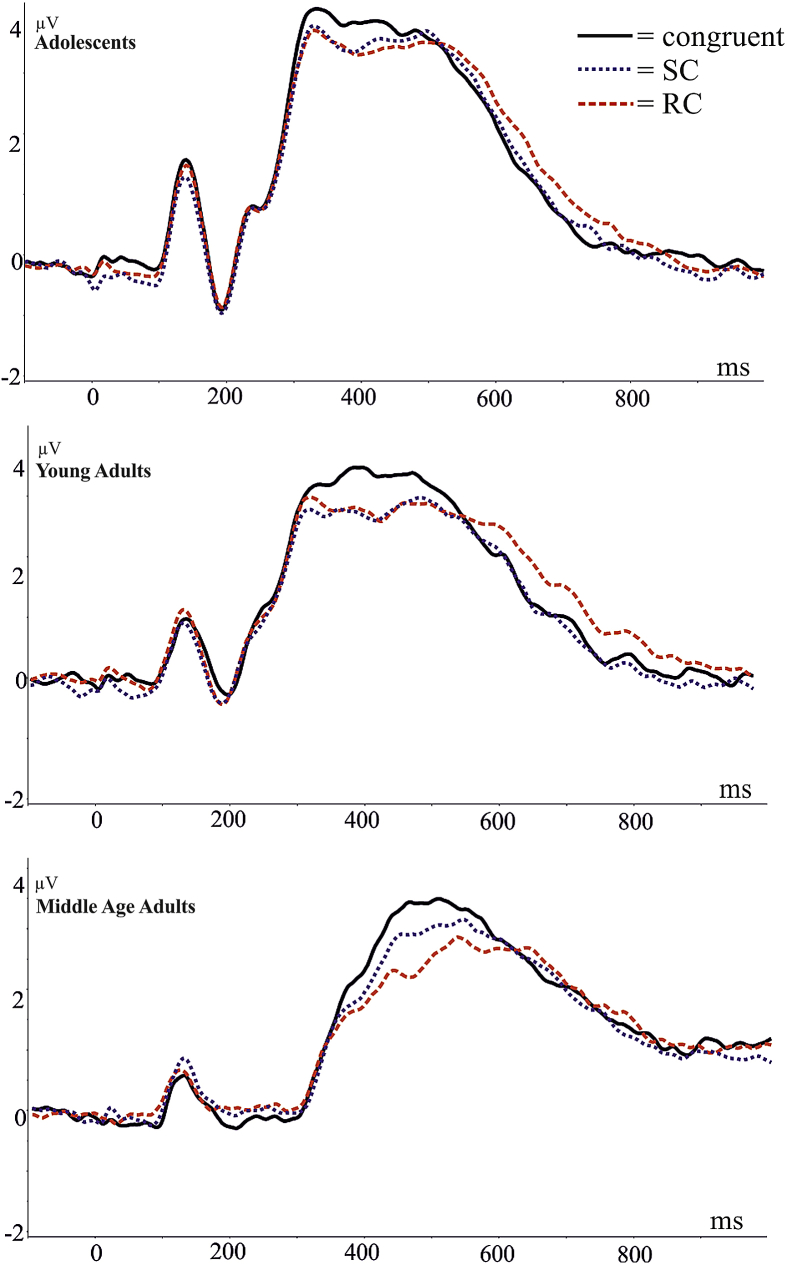
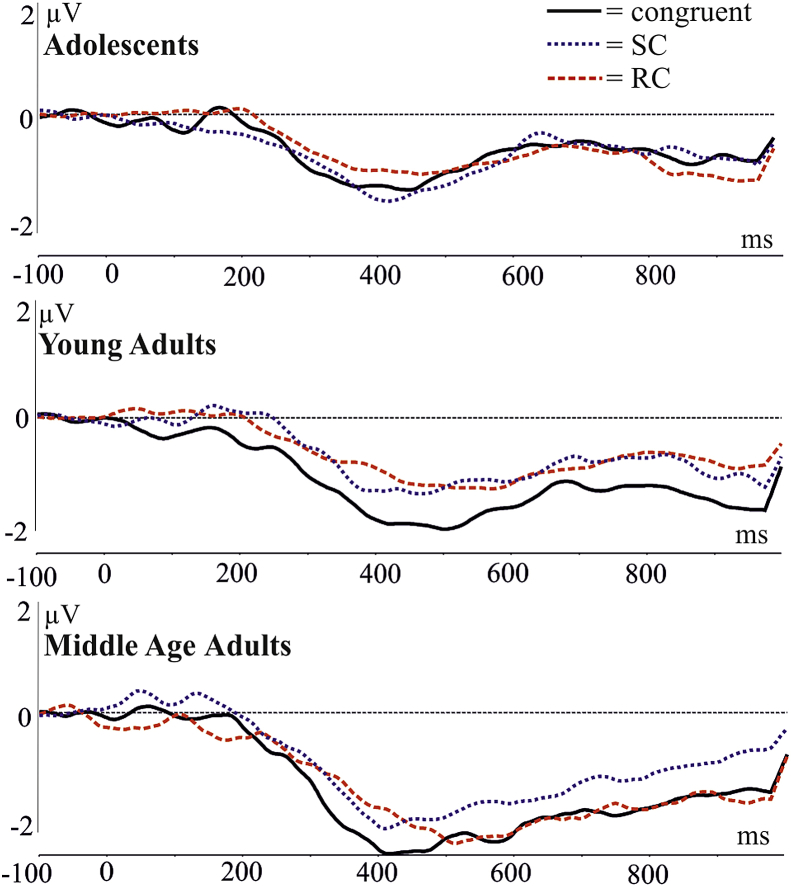
Fig. 2.
Grand average ERP of centro-parietal electrode pool (129, 55, 54, 42, 53, 52, 51, 59, 60, 61, 79, 62, 67, 66, 72, 85) showing the N450 between 300 and 550 msec. The congruent condition has a significantly greater amplitude [F(2,102) = 12.81, ɛ = .947, p < .0001].
Table 3.
Summary of significant group main effects.
| Adolescents | Young adults | Middle age adults | |
|---|---|---|---|
| P3a duration (msec) | Absent | 78a | 139 |
| P3b peak latency (msec) | 406 | 408a | 501 |
| N450 RC mean amplitude (μV) | −.31 | .32a | −.76 |
| LRP peak amplitude (μV) | −1.76a | −2.04 | −2.66 |
| EMG incorrect hand mean amplitude (μV) | .12a | .03 | .04 |
Pairwise differences between two groups, p < .03.
Table 4.
Summary of significant congruency main effects.
| Congruent | Stimulus conflict | Response conflict | |
|---|---|---|---|
| RT (msec) | 592a | 612a | 633 |
| LRP peak lat (μV) | 471 | 466a | 494 |
| EMG incorrect hand mean amplitude (μV) | .04 | .04a | .07 |
| EMG correct hand mean amplitude (μV) | 1.79a | 1.65a | 1.50 |
Pairwise differences between conditions p < .03.
3.2.1. Stimulus level change: P1, P3a and P3b
3.2.1.1. P1
The repeated measures ANOVA of congruency (3) × hemisphere (2) × group (3) revealed that the P1 (occipital) peak latency significantly differed between the three groups [F(2,51) = 5.607, p = .0062]. Tukey post hocs revealed that adolescents' peak latency (116.38 msec) was significantly longer than young adults (103.33 msec) (p = .0179) and middle-aged adults (102.72 msec) (p = .0127). There was no significant main effect of congruency [F(2,102) = 1.500, p = .2280] or hemisphere [F(1,5) = 1.388, p = .2442], and no group × congruency interaction [F(4,102) = 1.155, p = .3353] or group × hemisphere interaction [F(2,51) = .253, p = .777] or group × hemisphere × congruency interaction [F(4,102) = .637, p = .6370]. No significant main effects or interactions were found in the P1 amplitude (all p > .05). The P1 was examined to separate P1 activity from P3a activity.
3.2.1.2. P3a
Fig. 3 displays the topography of the P3a. The P3a peak latency significantly differed across groups [F(2,51) = 146.88, p < .0001]. Tukey post hocs revealed that the peak latency in middle-aged adults was significantly longer than young adults (p < .0001, 298 vs 199 msec), and adolescents (p < .0001, 298 vs 190 msec). There was no significant main effect of congruency [F(2,102) = .926, p = .3993] and no interactions [F(4,102) = 1.923, p = .1123]. Additionally the P3a peak amplitude increased across groups [F(2,51) = 5.82, p = .0052]. Tukey post hocs revealed that the peak amplitude in the middle-aged adults was larger when compared with young adults (p = .0237, 4.83 vs 2.31 μV) and adolescents (p = .0078, 4.83 vs 1.85 μV). There was no significant main effect of congruency [F(2,102) = .041, p = .9595] and no interactions [F(4,102) = .258, p = .9038].
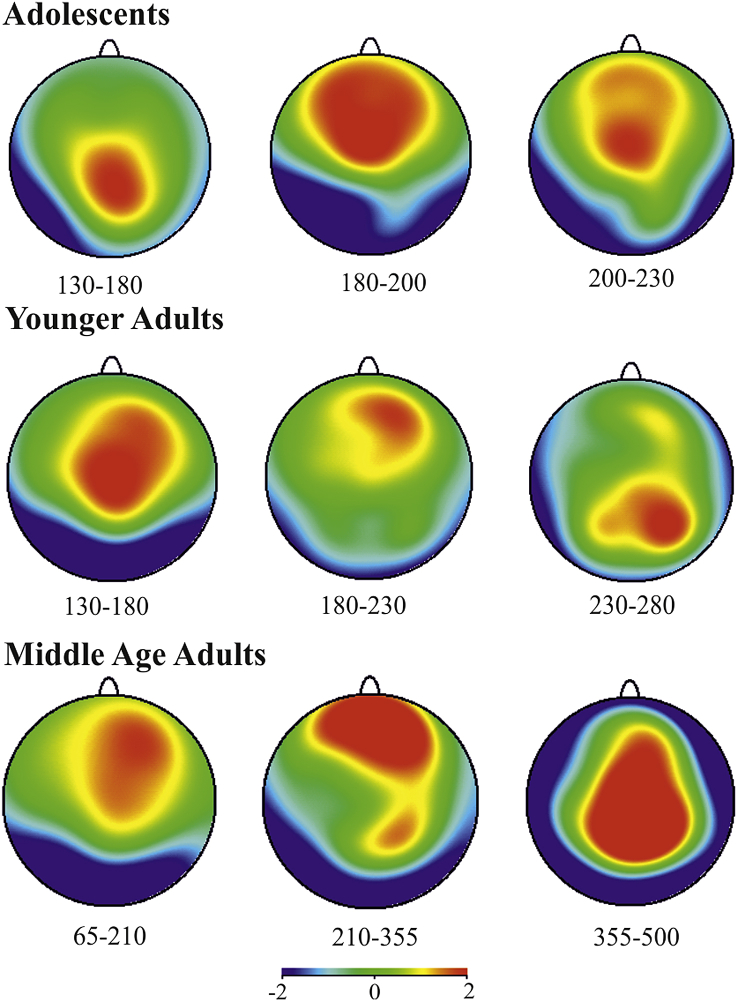
Fig. 3.
P3a topographies depicting the duration of the P3a in adolescents 20 msec; young adults 78 msec; middle age adults 139 msec. P3a peak amplitude increased across groups [F(2,51) = 5.82, p < .0052].
The ANOVA on the duration of the P3a from onset to offset revealed a significant main effect of group [F(1,34) = 7.16, p = .0113]. The duration of the P3a in the young adults was significantly shorter by 61 msec than middle-aged adults (p = .0115, 78 vs 139). There was no significant main effect of congruency [F(2,68) = .383, p = .6830] and no significant interaction [F(2,68) = 1.589, p = .2114]. Overall the P3a in young and middle-aged adults had the same onset but a longer duration in the middle age group. The duration of the P3a was not examined in adolescents because the P3a either did not appear at all or it was completely suppressed by the P1 wave.
3.2.1.3. P3b
Regarding the P3b peak latency there was a significant group effect [F(2,51) = 11.55, p < .0001]. Post hoc Tukey contrasts revealed that the peak latency of the P3b was significantly longer in middle-aged adults compared to younger adults (p = .0005, 501 vs 408 msec) and adolescents (p < .0004, 501 vs 406 msec). There was no significant congruency effect [F(2,102) = 1.864, p = .1602] or interaction [F(4,102) = .690, p = .6002] in the P3b peak latency. There were no group differences in the peak amplitude of the P3b [F(2,51) = 1.900, p = .1598] or interactions [F(4,102) = .987, p = .4178]. However there was a significant main effect of congruency [F(2,102) = 16.82, ɛ = .928, p < .0001]. Tukey post hoc contrasts revealed that the peak amplitude of the P3b in the congruent condition was significantly larger than the SC (p = .0003, 5.97 vs 5.48 μV) and RC (p < .0001, 5.97 vs 5.30 μV) conditions. There was no difference between the SC and RC conditions (p = .3035). There was a significant group effect for the onset [F(1,34) = 11.43, p = .0018] and offset [F(1,34) = 4.84, p = .0348] of the P3b peak latency. Tukey post hoc contrasts revealed an earlier onset by 76 msec in younger adults when compared to middle-aged adults (p = .0019, 298 vs 374 msec). In terms of offset, Tukey Post hoc contrasts revealed that younger adults had an earlier offset by 67 msec compared to middle-aged adults (p = .0348, 601 vs 668 msec). Additionally there was a significant congruency effect in the offset of the P3b peak latency [F(2,68) = 4.76, ɛ = .938, p = .0133]. Tukey post hocs revealed that offset in condition RC was significantly later than congruent offset (p = .0082, 665 vs 602). There was no significant interaction of group × congruency in the P3b offset [F(2,68) = 1.452, p = .2412]. In terms of onset there was no significant main effect of congruency [F(2,68) = .3711, p = .6913] and no interaction [F(2,68) = .3711, p = .6913].
3.2.2. N450
Fig. 2 depicts the grand average raw ERP of a pool of centro-parietal electrodes showing the N450 between 300 and 550 msec. There was a significant congruency effect in the mean amplitude of the raw ERPs at this time range [F(2,102) = 12.81, ɛ = .947, p < .0001]. Tukey post hocs revealed that the amplitude of the congruent condition was significantly more positive than the SC (p = .0013, 3.37 vs 3.02 μV) and RC (p < .0001, 3.37 vs 2.91 μV) conditions. There was no significant main effect of group [F(2,51) = 2.496, p = .0923] and no interaction [F(4,102) = .943, p = .4420].
3.2.3. Congruency effects: differences waves
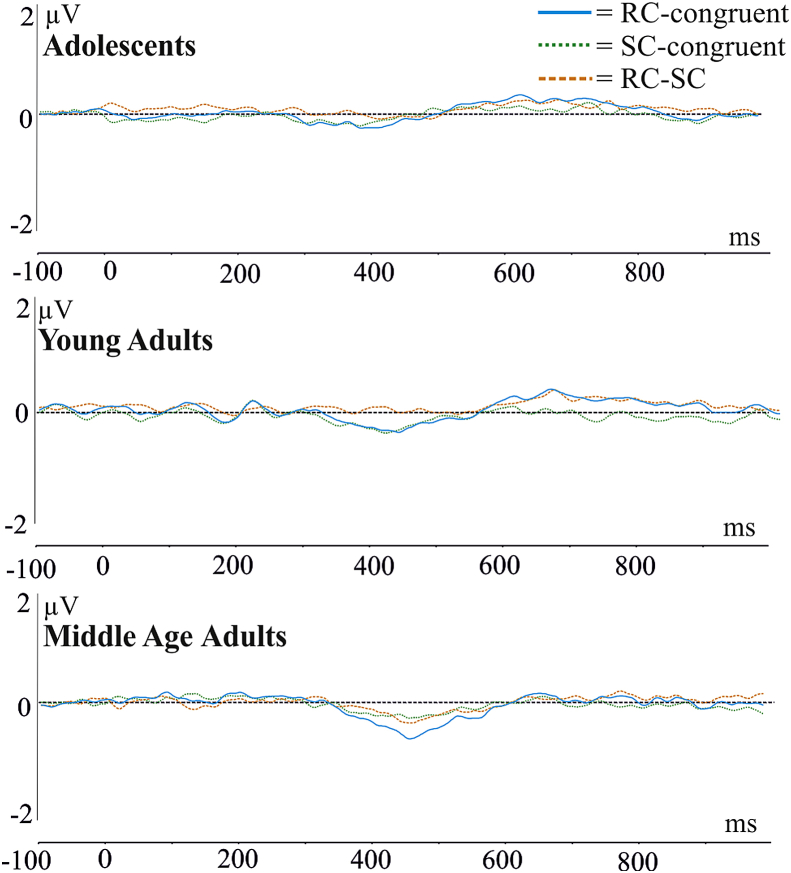
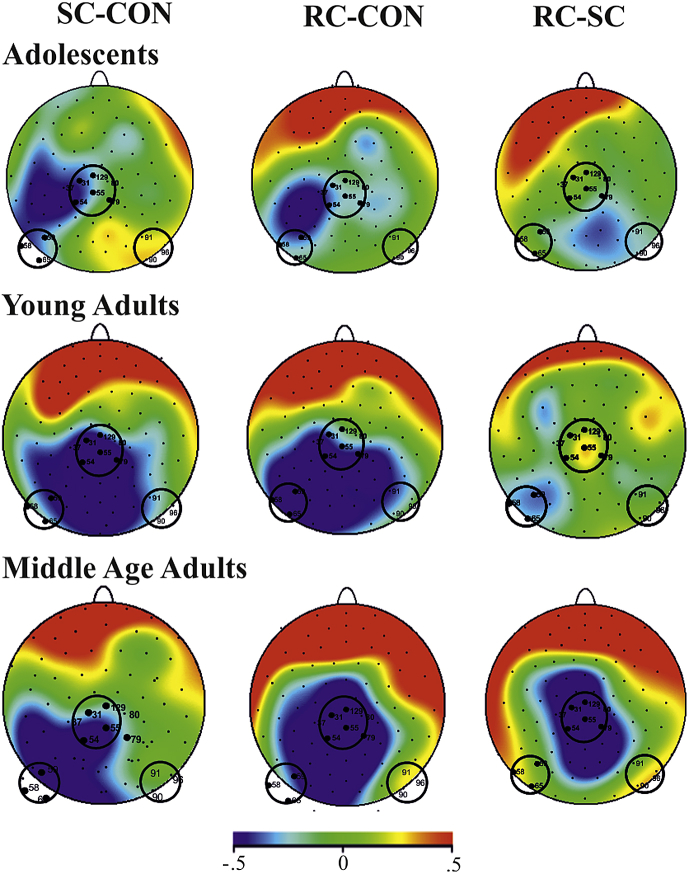
Fig. 4 shows the N450 difference waves. ANOVA compared the mean amplitude of the N450 difference waves [i.e., RC − congruent (general conflict), SC − congruent, RC − SC]. A significant main effect of congruency was found [F(2,102) = 3.73, ɛ = .580, p < .05]. Post hoc Tukey contrasts revealed that the mean amplitude of RC − CON (general conflict) difference wave was significantly more negative than the RC − SC difference wave (p = .0232, −.46 vs −.11 μV). The SC − CON difference wave was also examined however there were no significant differences with RC − CON or RC − SC difference waves (p > .05). Additionally there was no main effect of group [F(2,51) = 1.118, p = .3347] and no interaction [F(4,102) = .378, p = .5057]. Fig. 5 shows the topographies of the N450 difference waves in each group. A topographical analysis of three different representative electrode pools was performed. There were no significant group × pool differences in stimulus conflict detection (in SC − congruent difference waves) [F(4,102) = .237, e = .9201, p = .9040]. However in the RC − SC difference wave there was a significant group × pool interaction [F(4,102) = 4.97, ɛ = .949, p = .0013]. The left, central and right pools significantly differed between the three groups. Post hoc Tukey tests revealed that the mean amplitude of the central pool was significantly more positive in the young adult group when compared with the middle age group (p = .0004, .32 vs −.76 μv). The mean amplitude of the right hemisphere pool was significantly more negative in the adolescent group when compared to the adult group (p = .0370, −.31 vs .32 μV). The mean amplitude of the central pool in adolescents was significantly less negative than the middle age group (p = .0404, −.14 vs −.76 μV). There was no main effect of group [F(2,51) = .3566, p = .7017] or pool [F(2,102) = .1387, p = .8711].
Fig. 4.
Difference waves of the N450.
Fig. 5.
N450 topography of mean amplitude difference waves from 400 to 500 msec. Circles represent the three different pools. Statistical comparison of the three pools revealed that the three groups have significantly different topographies during RC; a significant group × pool interaction [F(4,102) = 4.97, ɛ = .949, p < .0013].
3.2.4. Summary of stimulus level change
Overall measures of stimulus level processing revealed five main findings. First the P3a amplitude and latency is larger and delayed in middle age adults. This P3a activity is absent in adolescents. Second the P3b latency is later in middle age adults. This is in line with our prediction of stimulus level change in middle age adults and absence of stimulus level effects in adolescents. Third in terms of congruency effects, there were no significant differences between the SC and RC conditions in the P3a, P3b peak amplitude or latency and the N450 mean amplitude, which suggests that differences in conflict processing occur at later stages. Fourth the topographic analysis of the N450 revealed potential differences in RC − SC processing between the three groups. Fifth, additionally, the N450 differences waves showed that processing of combined SC and RC (general conflict) increased the N450 amplitude greater amount than just RC − SC (response conflict alone), which supports the prediction that the N450 amplitude is more sensitive to general conflict processing.
3.2.5. Response level change: LRP
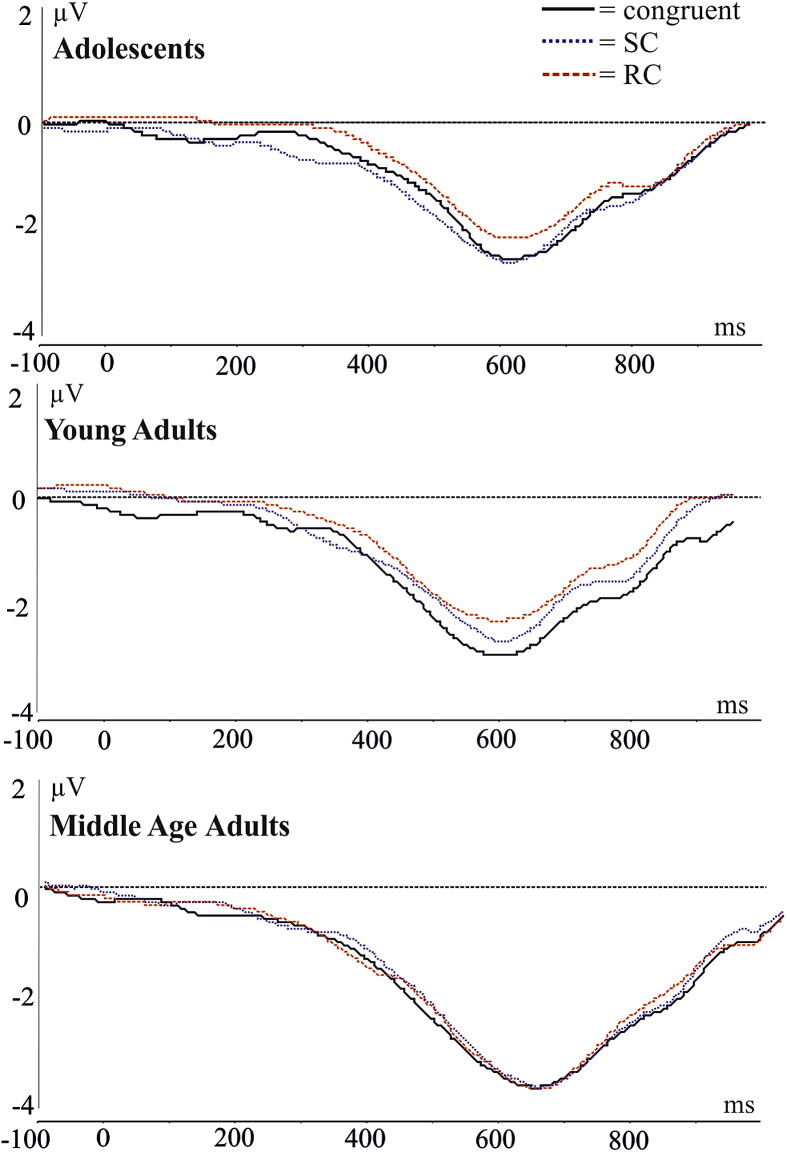
3.2.5.1. Stimulus locked LRP
Fig. 6 depicts the stimulus locked grand-averaged LRP waveforms. Analysis of the stimulus locked LRP peak amplitude revealed a significant effect of group [F(2,51) = 3.64, p = .0333]. Tukey post hocs revealed that adolescents had smaller peak amplitude than the middle age group (p = .0295, −1.76 vs −2.66 μV). LRP peak amplitude also had a significant congruency effect [F(2,102) = 4.26, ɛ = .926, p = .0192]. Tukey post hocs revealed that the amplitude of the RC condition was significantly smaller than the amplitude of the congruent condition (p = .0120, −1.92 vs −2.40 μV). The peak amplitude of the RC condition was also smaller than the SC condition (−2.15 μV) however this was not statistically significant (p = .2435). There were no significant group × congruency interactions in the peak amplitude [F(2,102) = 1.387, p = .2453].
Fig. 6.
Stimulus locked LRP. Adolescents had significantly smaller peak amplitude between 250 and 600 msec [F(2,51) = 3.62, p < .0338].
The peak latency of the stimulus locked LRP showed a significant congruency effect [F(2,102) = 4.40, ɛ = .971, p = .0156]. Tukey post hocs revealed that the RC condition peak latency was significantly later than the SC condition (p = .0169, 494 vs 466 msec).
There was no main effect of group in the peak latency [F(2,51) = 2.127, p = .1296] and there were no group × congruency interactions in the peak latency [F(4,102) = 1.242, p = .2979].
A significant main effect of group was found in the mean amplitude of the stimulus locked LRP [F(2,51) = 3.62, p = .0338]. Tukey post hocs revealed that middle-aged adults had increased mean amplitude compared to adolescents (p = .0322, −1.9 vs −1.1 μV). There was no congruency main effect in the mean amplitude of the LRP [F(2,102) = 2.767, p = .0670] and no group × congruency interactions [F(2,102) = 1.727, p = .1496].
3.2.5.2. Response locked lrp
Fig. 7 depicts the response locked grand-averaged LRP waveforms. The peak amplitude of the middle age adults' response locked LRP was significantly greater (−3.87 μV) than adolescents (−2.62 μV) and young adults (−2.88 μV) [F(2,51) = 4.54, p = .015]. Tukey-HSD post hocs revealed that the peak amplitude significantly differed (p = .0169) between adolescents and middle age adults. There were no other significant effects in peak amplitude (group × congruency interaction, p = .5455), latency (group × congruency interaction, p = .9411), or mean amplitude (group × congruency interaction, p = .7973).
Fig. 7.
Response locked LRP. Zero represents the onset of the response.
As peak analysis in LRP is sometimes variable particularly across development (Bryce et al., 2011), this data is further analyzed using jackknifing to clarify and elucidate these findings.
3.2.5.3. lrp jackknifing
After jackknifing onset latencies were entered into a group (3) × congruency (3) repeated measures ANOVA. All of the results were non-significant [F(4,102) = .334, p = .8545]. The original degrees of freedom and adjusted F value were used as suggested by Ulrich and Miller (2001).
3.2.6. Summary of response level change
Overall ERP measures of response level processing revealed two main findings. First, in terms of the LRP analysis group differences were found in the mean and peak stimulus locked LRP. There was decreased amplitude in the adolescent group when compared to the middle age group. This is in line with our prediction that adolescents would show differences in response level processing. This was also found for the peak amplitude of the response locked LRP. Second, in terms of congruency effects the latency in the RC condition was significantly later than the SC condition. This fits with the hypothesized predictions and the RT data; RC is expected to yield the slowest responses.
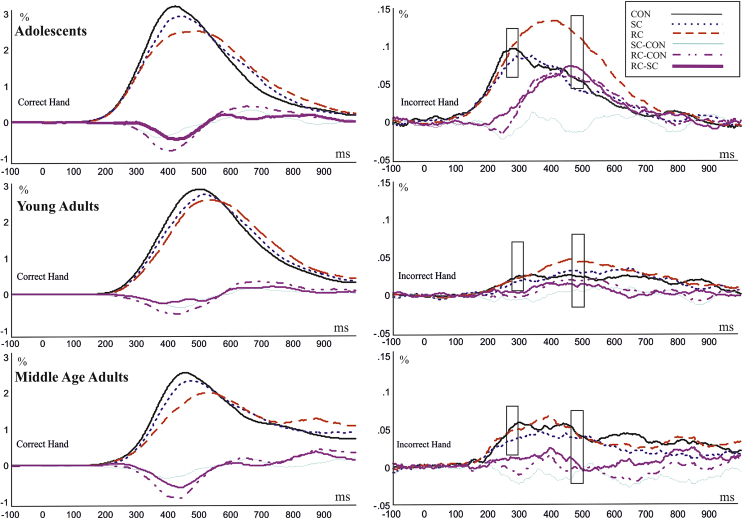
3.3. EMG
The grand-averaged EMG signal for correct and incorrect response hands is shown in Fig. 8. Correct response hand activity: One sample t-tests indicated that EMG activations in the correct hand robustly deviated from baseline across all the conditions (all .007 < p < .05). Mean EMG amplitude between 200 and 600 msec was entered into a group (3) × congruency (3) ANOVA. A significant main effect of congruency was found [F(2,102) = 24.71719, ɛ = .6772] and all congruency conditions significantly differed (p < .0001). There was no group difference [F(2,51) = 1.448, p = 9.2445] and no group × congruency interaction [F(2,102) = .358, p = .8375].
Fig. 8.
Average of correct and incorrect EMG hand activity for the three groups. Scaled as a percentage. Boxes mark two time points where incorrect hand activity had statistically significant effects. (1) 280–300 msec. Group effect approached significance [F(2,51) = 2.48, p = .093]. (2) 460–480 msec. Congruency effect [F(2,102) = 7.24, ɛ = .769, p = .0031] and group × congruency interaction [F(4,102) = 3.06, ɛ = .769, p = .0317].
Incorrect response hand activity: One sample t-tests confirmed that incorrect EMG hand activation was significantly larger than zero (all .004 < p < .04) at two time points; an early activation between 280 and 300 msec and later between 460 and 480 msec. The EMG activation was not different from zero in the SC condition in middle age group. Mean EMG amplitude between 280 and 300 msec was entered into a group (3) × congruency (3) ANOVA. In this early time window there were no significant congruency effects [F(2,102) = 1.664, p = .1943] or interactions [F(4,102) = .3713, p = .8286] but a group effect approached significance [F(2,51) = 2.48, p = .093]. Mean EMG amplitude between 460 and 480 msec was entered into a group (3) × congruency (3) ANOVA. In the mean amplitude of the 460–480 msec time interval there was a congruency effect [F(2,102) = 7.24, ɛ = .769, p = .0031]. Post hoc Tukey contrasts on the incorrect hand mean amplitude revealed that the congruent condition had significantly less amplitude than the RC condition (p = .0011, .045 vs .07 μV) and SC had significantly less amplitude than RC (p = .0011, .04 vs .07 μV). However there was no difference between congruent and SC in incorrect hand activation. Additionally there was a group × congruency interaction [F(4,102) = 3.06, ɛ = .769, p = .0317]. Tukey post hoc tests showed that in the adolescent group the amplitude in the RC condition (.120 μV) was significantly larger than the congruent (.06 μV, p = .0198) and SC (.05 μV, p = .0198) conditions. There was no similar difference in the adult and middle age groups. There was no main effect of group [F(2,51) = 1.014, p = .3698].
Overall in terms of correct hand activity there were no significant group differences however in terms of incorrect hand activity, at the time point between 460 and 480 msec, the adolescent group showed significantly increased incorrect hand activity during the RC condition. This is in line with our prediction of response level change during adolescence.
4. Discussion
Following Craik and Bialystoke's (2006) call to identify the specific nature of age-related change here we systematically tracked neuro-cognitive asymmetries in stimulus and response conflict processing throughout the lifespan within the framework of a single study. We measured ERPs, the LRP, and EMG in an adaptation of the colour word Stroop task that a priori separates stimulus and response level conflict. Behavioural effects, in terms of RT and accuracy, revealed that the congruency manipulations were successful. The RC manipulation yielded the slowest RTs. This replicates previous studies (de Houwer, 2003; Melcher & Gruber, 2009). However, unexpectedly there were no differences between groups in terms of the congruency effects. We predicted that adolescents would be more susceptible to response conflict whereas middle age adults would be sensitive to stimulus conflict however no differences were found behaviourally. At the neural level we found age-related and developmental asymmetries in stimulus and response stages of processing. Adolescents showed protracted development specifically related to response level processing (LRP and EMG), whereas middle age adults showed neural changes related to stimulus level processing (P3a and P3b).
4.1. Origins of mature conflict processing: adolescence to adulthood
In terms of adolescent development, differences in performance on the Stroop task predominantly lie with late response level processing. Despite RT and P3b latency and amplitude being similar to adults, adolescents showed decreased LRP amplitude and increased incorrect EMG hand activity. Although no previous studies have examined the LRP in adolescents, studies with children have also found P3b amplitude and latency similar to adults followed by developmental change in the LRP. Bryce et al. (2011), Ridderinkhof and van der Molen (1995), Szucs, Soltész, Bryce, et al. (2009) and Szucs, Soltész, and White (2009) examined the LRP in 5–12-year-old children and found that P3b latency did not change with age whereas LRP latency onset was faster with age. This indicates that the locus of developmental change lies in response level as opposed to stimulus level improvement. Bryce et al. (2011) found that during correct response preparation the fastest responded trials were preceded by higher LRP amplitude whereas the slowly responded trials had smaller amplitude. This indicates that the amplitude of the LRP is potentially representative of response certainty. Hence, the smaller LRP amplitude in adolescents may represent hesitancy or uncertainty in response preparation (Bryce et al., 2011; Gratton et al., 1988; Leuthold, Sommer, & Ulrich, 1996). An alternative explanation that also fits the behavioural data is that the absence of the P3a in adolescents (see discussion below for more details) could indicate a lack of inhibition or reduced control. This would lead to faster RT and increased errors that is suggested by the behavioural data.1 Smaller LRP amplitude in this case could represent fewer resources allocated to response selection. Whether the functional explanation for decreased LRP activity during adolescence is response uncertainty or an inhibitory deficit, it is evident that the LRP response selection stage undergoes protracted developmental change during adolescence. Further investigation is warranted to explore the functional significance of response level LRP change during adolescence.
EMG results confirm the protracted development of response level processing in adolescents. Between 460 and 480 msec adolescents had increased incorrect hand activity during the RC condition relative to the SC and congruent conditions. This increased incorrect hand activity in the RC condition was not present in adults or in middle age adults. This indicates that adolescents were more susceptible to response conflict between the correct and incorrect response hands. No previous studies used EMG measures in adolescents. However Ridderinkhof and van der Molen (1995) examined 5–12-year-old children and found faster EMG onset latency with age. This provides evidence for continued development at the peripheral response level until adolescence.
Notably although young adults and older adults showed a frontal P3a wave; this was almost completely absent in adolescents. To our knowledge our study is the first to document such effects in adolescents in a Stroop task. When children aged 9–10 performed a continuous performance task, where they must respond to the letter X only when preceded by the letter A, the frontal P3 during No-Go trials was absent and this was associated with higher false alarm and impulsivity scores (Dien et al., 2004; Jonkman, Lansbergen, & Stauder, 2003). We interpret developmental P3a changes considering both the adolescent and middle age adult data discussed below.
4.2. Conflict processing during ageing: adulthood to middle age
Middle age adults differed from young adults in stimulus level processing. Although several previous studies have tracked adult lifespan changes in the P3a during oddball tasks [(Fjell & Walhovd, 2004), 20–92-year olds; (Stige, Fjell, & Smith, 2007), 6–90-year olds; (Walhovd & Fjell, 2001), 22–95-year olds] to our knowledge ours is the first study to have examined and documented the P3a in a Stroop task with middle age participants. In our data the enlarged P3a in middle age adults was of much lesser amplitude and shorter duration in young adults and could not be detected at all in adolescents. In young adults the P3a is commonly related to operations at the stimulus selection stage or more specifically attention shifting as part of an attention orienting reflex (Dien et al., 2004; Gaeta, Friedman, Ritter, & Cheng, 2001). One common conclusion in the ageing literature is that middle age adults must rely on additional frontal mechanisms to maintain task performance (Cabeza, 2002; Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008; Eppinger et al., 2007). Fabiani and Friedman (1995) found that when older adults were presented with a repeated stimulus they maintained P3a frontal activity throughout the task whereas in young adults this response waned after the first few tones. They concluded that older adults have greater susceptibility to distraction and interference and may have difficulty holding information in their working memory. Older adults may therefore engage frontal orienting attention mechanisms to a greater degree (Fabiani, 2012). Hence, we conclude that the increased P3a in middle age adults reflects increased use of frontal resources to focus on task-relevant stimulus properties.
Even though middle age adults also showed a significant delay in P3b onset latency compared to young adults their RT was not significantly different. Additionally the amplitudes of the stimulus locked LRPs were significantly larger in the middle age group when compared with adolescents and young adults. As noted above this increase in LRP amplitude could represent increased certainty in responding. This has been found in previous studies listed below; although they did not test the significance of the deviation directly an increased amplitude is visible (Falkenstein et al., 2006, Fig. 2; Wild-Wall et al., 2008, Fig. 2). Additionally correct and incorrect hand EMG amplitude did not significantly differ between the young and middle age adult groups. This indicates that middle age adults demonstrated similar peripheral level response control to young adults (Falkenstein et al., 2006).
4.3. N450 and conflict detection throughout the lifespan
We have confirmed that the N450 is most representative of general conflict detection (Szucs & Soltész, 2010a, 2010b; West et al., 2004; West & Schwarb, 2006). Previously the N450 had been ambiguously related to both response conflict (Liotti, Woldorff, Perez, & Mayberg, 2000) and semantic conflict (Rebai et al., 1997). As we found no significant differences in the mean amplitude of the N450 in the SC and RC conditions we conclude that the N450 is most sensitive to general conflict (Szucs & Soltesz, 2012; Szucs, Soltész, & White, 2009; West et al., 2004).
Our second objective was to map maturational changes in the N450. There were no differences between the adolescent and young adult groups in the topography of the N450 during congruent and SC conditions. However during the RC condition the topography of the N450 was focused on the right scalp in adolescents and on the left scalp in young adults. In adults a similar left hemisphere effect during the N450 has been found in previous Stroop studies (Chen et al., 2011; Jongen & Jonkman, 2008; Lansbergen, van Hell, & Kenemans, 2007). Adleman et al. (2002) found increased left hemisphere activation in adults when compared to adolescents specifically in the left middle frontal gyrus during colour word Stroop conflict. The left middle frontal gyrus has been associated with both word generation (Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997) and generating colour names (Martin, Haxby, Lalonde, Wiggs, & Ungerleider, 1995). The left scalp activation found in adults could represent the increased use of a verbal strategy to resolve conflict.
In adolescents the topography of the N450 was focused on the right scalp. Right scalp activity has also been observed in adolescence during a Stop task and a Go-No/go task (Rubia et al., 2000; Stevens, Kiehl, Pearlson, & Calhoun, 2007) as well as during a Stroop task in children (previously unrecorded in adolescence) (Jongen & Jonkman, 2008). These authors have concluded that this right scalp activity is indicative of improved performance strategy. For example Stevens et al. (2007) found that in adolescents increased frontal–parietal circuit activity was related to good performance however this was not found in adults. Therefore increased right scalp activation may recruit frontal–parietal circuitry and allow for improved performance.
In terms of middle age adults a stimulus conflict deficit was expected. However there were no differences in the topography of the N450 during stimulus conflict detection for young adults and middle age adults. Nevertheless topographical examination of the N450 during the RC condition reveals dispersed and increased negative amplitude with a right scalp shift. In a middle age group (41–61-year olds) Mager et al. (2007) similarly found increased amplitude of the N450. Mathis et al. (2009) using an fMRI study of the colour word Stroop task concluded that middle age adults use an intermediate level of neural recruitment that is more than younger adults but less than older adults. Therefore, in middle age adults the increased negative amplitude of the right scalp shift of the N450 in the RC condition could represent intermediary level of processing, more than young adults but less than older adults, required for response conflict resolution.
4.4. Response conflict and the Stroop effect
By using a combined ERP and EMG methodology we have tracked in real-time the course of stimulus and response conflict processing during the Stroop task. Our study confirms previous findings that both stimulus and response conflict contribute to the Stroop effect (slower RT during incongruent trials) (Chen et al., 2011; de Houwer, 2003). However by using multiple response related measures we have delineated important markers of the Stroop effect at the response level of processing. The current findings support the idea that Stroop conflict, during this manual colour word Stroop task, may be more robust at the response level of processing. In this study we found that there were no differences in the behavioural and neural processing of the two types of conflict (SC compared to RC) when examining accuracy, P3a, P3b and N450 activity. However the LRP peak latency was significantly later in the RC condition than the SC condition and the EMG activity in the correct responding hand was significantly less in the RC when compared to the SC condition, indicating stronger correct responses during SC. This perhaps indicates that during this manual colour word Stroop task the Stroop effect may be more robust during the period of processing between response selection and response execution. Interestingly this occurred across all age groups. We predicted that adolescents would show increased response conflict, for example in poorer behavioural performance during RC and differences in neural activity during RC. We also predicted that middle age adults would show increased stimulus conflict, in terms of increased resources and poorer behavioural performance during the SC condition. Although we found age-related differences in information processing stages, the conflict manipulations in this task were not sensitive to age differences. Perhaps this task did not evoke age differences because the conflict conditions were of a similar level of difficulty. Indeed, the similar neural markers (P3a, P3b, N450) and accuracy performance in the SC and RC conditions indicate that these conditions were not very different in terms of level of difficulty. This could explain why we could not detect any age differences in the task manipulations. This warrants further examination.
5. Conclusion
We combined ERP and EMG to examine lifespan changes in stimulus and response conflict processing using a modified Stroop task. Asymmetries in conflict processing across the lifespan were determined. A protracted developmental course for response conflict processing that extends into late adolescence is followed by early decline in stimulus level processing in middle age adults. These neural asymmetries highlight the complex progression of lifespan cognition.
Acknowledgement
The authors thank Fruzsina Soltész for programming the colour word Stroop task.
Reviewed 13 September 2012. Action editor Paolo Bartolomeo
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
The authors would like to thank an anonymous reviewer for noting this important point.
Contributor Information
Clare Killikelly, Email: ck349@cam.ac.uk, clare.killikelly@gmail.com.
Dénes Szűcs, Email: ds377@cam.ac.uk.
References
- Adleman N.E., Menon V., Blasey C.M., White C.D., Warsofsky I.S., Glover G.H. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16(1):61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Bryce D., Szũcs D., Soltész F., Whitebread D. The development of inhibitory control: an averaged and single-trial Lateralized Readiness Potential study. NeuroImage. 2011;57(3):671–685. doi: 10.1016/j.neuroimage.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. http://www.ncbi.nlm.nih.gov/pubmed/11931290 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Chen A., Bailey K., Tiernan B.N., West R. Neural correlates of stimulus and response interference in a 2-1 mapping Stroop task. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2011;80(2):129–138. doi: 10.1016/j.ijpsycho.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Coles M.G. Modern mind-brain reading: psychophysiology, physiology, and cognition. Psychophysiology. 1989;26(3):251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. http://www.ncbi.nlm.nih.gov/pubmed/2667018 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Coles M.G., Gratton G., Bashore T.R., Eriksen C.W., Donchin E. A psychophysiological investigation of the continuous flow model of human information processing. Journal of Experimental Psychology: Human Perception and Performance. 1985;11(5):529–553. doi: 10.1037//0096-1523.11.5.529. http://www.ncbi.nlm.nih.gov/pubmed/2932529 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Craik F.I.M., Bialystok E. Cognition through the lifespan: mechanisms of change. Trends in Cognitive Sciences. 2006;10(3):131–138. doi: 10.1016/j.tics.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Crone E.A., Dahl R.E. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews. Neuroscience. 2012;13(9):636–650. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Davis S.W., Dennis N.A., Daselaar S.M., Fleck M.S., Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex (New York, N.Y.: 1991) 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J., Spencer K.M., Donchin E. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41(5):665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson C., Kopell B. The Stroop effect: brain potentials localize the source of interference. Science. 1981;214(4523):938–940. doi: 10.1126/science.7302571. [DOI] [PubMed] [Google Scholar]
- Eppinger B., Kray J., Mecklinger A., John O. Age differences in task switching and response monitoring: evidence from ERPs. Biological Psychology. 2007;75(1):52–67. doi: 10.1016/j.biopsycho.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Fabiani M. It was the best of times, it was the worst of times: a psychophysiologist’s view of cognitive aging. Psychophysiology. 2012;49(3):283–304. doi: 10.1111/j.1469-8986.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- Fabiani M., Friedman D. Changes in brain activity patterns in aging: the novelty oddball. Psychophysiology. 1995;32(6):579–594. doi: 10.1111/j.1469-8986.1995.tb01234.x. http://www.ncbi.nlm.nih.gov/pubmed/8524992 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Falkenstein M., Yordanova J., Kolev V. Effects of aging on slowing of motor-response generation. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2006;59(1):22–29. doi: 10.1016/j.ijpsycho.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Fallgatter A.J., Mueller T.J., Strik W.K. Age-related changes in the brain electrical correlates of response control. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 1999;110(5):833–838. doi: 10.1016/s1388-2457(99)00022-x. http://www.ncbi.nlm.nih.gov/pubmed/10400196 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B. On the topography of P3a and P3b across the adult lifespan – a factor-analytic study using orthogonal procrustes rotation. Brain Topography. 2003;15(3):153–164. doi: 10.1023/a:1022654116566. http://www.ncbi.nlm.nih.gov/pubmed/12705811 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Fjell A.M., Walhovd K.B. Life-span changes in P3a. Psychophysiology. 2004;41(4):575–583. doi: 10.1111/j.1469-8986.2004.00177.x. [DOI] [PubMed] [Google Scholar]
- Folstein J.R., Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45(1):152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaeta H., Friedman D., Ritter W., Cheng J. An event-related potential evaluation of involuntary attentional shifts in young and older adults. Psychology and Aging. 2001;16(1):55–68. doi: 10.1037/0882-7974.16.1.55. http://www.ncbi.nlm.nih.gov/pubmed/11302368 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M.G., Sirevaag E.J., Eriksen C.W., Donchin E. Pre- and poststimulus activation of response channels: a psychophysiological analysis. Journal of Experimental Psychology: Human Perception and Performance. 1988;14(3):331–344. doi: 10.1037//0096-1523.14.3.331. http://www.ncbi.nlm.nih.gov/pubmed/2971764 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Hämmerer D., Li S.-C., Müller V., Lindenberger U. An electrophysiological study of response conflict processing across the lifespan: assessing the roles of conflict monitoring, cue utilization, response anticipation, and response suppression. Neuropsychologia. 2010;48(11):3305–3316. doi: 10.1016/j.neuropsychologia.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S., Pastötter B., Bäuml K.-H., Gruber S., Wimber M., Klimesch W. The electrophysiological dynamics of interference during the Stroop task. Journal of Cognitive Neuroscience. 2008;20(2):215–225. doi: 10.1162/jocn.2008.20020. [DOI] [PubMed] [Google Scholar]
- Hock H.S., Egeth H. Verbal interference with encoding in a perceptual classification task. Journal of Experimental Psychology. 1970;83(2):299–303. doi: 10.1037/h0028512. http://www.ncbi.nlm.nih.gov/pubmed/5480902 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Houwer J. de. On the role of stimulus – response and stimulus – stimulus compatibility in the Stroop effect. 2003;31(3):353–359. doi: 10.3758/bf03194393. [DOI] [PubMed] [Google Scholar]
- Ilan A.B., Polich J. P300 and response time from a manual Stroop task. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 1999;110(2):367–373. doi: 10.1016/s0168-5597(98)00053-7. http://www.ncbi.nlm.nih.gov/pubmed/10210626 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Jacoby L.L., Bishara A.J., Hessels S., Toth J.P. Aging, subjective experience, and cognitive control: dramatic false remembering by older adults. Journal of Experimental Psychology. General. 2005;134(2):131–148. doi: 10.1037/0096-3445.134.2.131. [DOI] [PubMed] [Google Scholar]
- Jongen E.M.M., Jonkman L.M. The developmental pattern of stimulus and response interference in a color-object Stroop task: an ERP study. BMC Neuroscience. 2008;9:82. doi: 10.1186/1471-2202-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman L.M., Lansbergen M., Stauder J.E. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology. 2003;40(5):752–761. doi: 10.1111/1469-8986.00075. http://www.ncbi.nlm.nih.gov/pubmed/14696728 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Lansbergen M.M., van Hell E., Kenemans J.L. Impulsivity and conflict in the Stroop task. Journal of Psychophysiology. 2007;21(1):33–50. [Google Scholar]
- Leon-Carrion J., García-Orza J., Pérez-Santamaría F.J. Development of the inhibitory component of the executive functions in children and adolescents. The International Journal of Neuroscience. 2004;114(10):1291–1311. doi: 10.1080/00207450490476066. [DOI] [PubMed] [Google Scholar]
- Leuthold H., Sommer W., Ulrich R. Partial advance information and response preparation: inferences from the lateralized readiness potential. Journal of Experimental Psychology. General. 1996;125(3):307–323. doi: 10.1037//0096-3445.125.3.307. http://www.ncbi.nlm.nih.gov/pubmed/8830109 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Liotti M., Woldorff M.G., Perez R., Mayberg H.S. An ERP study of the temporal course of the Stroop color-word interference effect. Neuropsychologia. 2000;38(5):701–711. doi: 10.1016/s0028-3932(99)00106-2. http://www.ncbi.nlm.nih.gov/pubmed/10689046 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Luck S.J. Massachusetts Institute of Technology; Cambridge, MA: 2005. An Introduction to the Event Related Potential Technique. [Google Scholar]
- Mager R., Bullinger A.H., Brand S., Schmidlin M., Schärli H., Müller-Spahn F. Age-related changes in cognitive conflict processing: an event-related potential study. Neurobiology of Aging. 2007;28(12):1925–1935. doi: 10.1016/j.neurobiolaging.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Martin A., Haxby J.V., Lalonde F.M., Wiggs C.L., Ungerleider L.G. Discrete cortical regions associated with knowledge of color and knowledge of action. Science (New York, N.Y.) 1995;270(5233):102–105. doi: 10.1126/science.270.5233.102. http://www.ncbi.nlm.nih.gov/pubmed/7569934 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Mathis A., Schunck T., Erb G., Namer I.J., Luthinger R. The effect of aging on the inhibitory function in middle-aged subjects: a functional MRI study coupled with a color-matched Stroop task. International Journal of Geriatric Psychiatry. 2009;24:1062–1071. doi: 10.1002/gps.2222. [DOI] [PubMed] [Google Scholar]
- Melcher T., Gruber O. Decomposing interference during Stroop performance into different conflict factors: an event-related fMRI study. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 2009;45(2):189–200. doi: 10.1016/j.cortex.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Milham M., Banich M., Webb A., Barad V., Cohen N., Wszalek T. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cognitive Brain Research. 2001;12(3):467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Miller J., Patterson T., Ulrich R. Jackknife-based method for measuring LRP onset latency differences. Psychophysiology. 1998;35(1):99–115. http://www.ncbi.nlm.nih.gov/pubmed/9499711 Retrieved from. [PubMed] [Google Scholar]
- Morton J., Chambers S.M. Selective attention to words and colours. Quarterly Journal of Experimental Psychology. 1973;25:387–397. [Google Scholar]
- O'Connell R.G., Balsters J.H., Kilcullen S.M., Campbell W., Bokde A.W., Lai R. A simultaneous ERP/fMRI investigation of the P300 aging effect. Neurobiology of Aging. 2012;33(10):2448–2461. doi: 10.1016/j.neurobiolaging.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Polich J., Criado J.R. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2006;60(2):172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Rebai M., Bernard C., Lannou J. The Stroop's test evokes a negative brain potential, the N400. The International Journal of Neuroscience. 1997;91(1–2):85–94. doi: 10.3109/00207459708986367. http://www.ncbi.nlm.nih.gov/pubmed/9394217 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof K., van der Molen M.W. A psychophysiological analysis of developmental differences in the ability to resist interference. Child Development. 1995;66(4):1040–1056. http://onlinelibrary.wiley.com/doi/10.1111/j.1467-8624.1995.tb00921.x/abstract Retrieved from. [Google Scholar]
- Roggeveen A.B., Prime D.J., Ward L.M. Lateralized readiness potentials reveal motor slowing in the aging brain. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2007;62(2):P78–P84. doi: 10.1093/geronb/62.2.p78. http://www.ncbi.nlm.nih.gov/pubmed/17379675 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C., Simmons A. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neuroscience and Biobehavioral Reviews. 2000;24(1):13–19. doi: 10.1016/s0149-7634(99)00055-x. http://www.ncbi.nlm.nih.gov/pubmed/10654655 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Sander M.C., Lindenberger U., Werkle-Bergner M. Lifespan age differences in working memory: a two-component framework. Neuroscience and Biobehavioral Reviews. 2012;36(9):2007–2033. doi: 10.1016/j.neubiorev.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Segalowitz S.J., Davies P.L. Charting the maturation of the frontal lobe: an electrophysiological strategy. Brain and Cognition. 2004;55(1):116–133. doi: 10.1016/S0278-2626(03)00283-5. http://www.ncbi.nlm.nih.gov/pubmed/15134847 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Stevens M.C., Kiehl K.A., Pearlson G.D., Calhoun V.D. Functional neural networks underlying response inhibition in adolescents and adults. Behavioural Brain Research. 2007;181(1):12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stige S., Fjell A.M., Smith L. The development of visual P3a and P3b. Developmental Neuropsychology. 2007;32(1):563–584. doi: 10.1080/87565640701361096. [DOI] [PubMed] [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18(6):643–662. [Google Scholar]
- Szucs D., Soltész F. Event-related potentials dissociate facilitation and interference effects in the numerical Stroop paradigm. Neuropsychologia. 2007;45(14):3190–3202. doi: 10.1016/j.neuropsychologia.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Szucs D., Soltész F. Event-related brain potentials to violations of arithmetic syntax represented by place value structure. Biological Psychology. 2010;84(2):354–367. doi: 10.1016/j.biopsycho.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Szucs D., Soltész F. Stimulus and response conflict in the color-word Stroop task: a combined electro-myography and event-related potential study. Brain Research. 2010;1325:63–76. doi: 10.1016/j.brainres.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Szucs D., Soltesz F. Functional definition of the N450 event-related brain potential marker of conflict processing: a numerical Stroop study. BMC Neuroscience. 2012;13(1):35. doi: 10.1186/1471-2202-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs D., Soltész F., Bryce D., Whitebread D. Real-time tracking of motor response activation and response competition in a Stroop task in young children: a lateralized readiness potential study. Journal of Cognitive Neuroscience. 2009;21(11):2195–2206. doi: 10.1162/jocn.2009.21220. [DOI] [PubMed] [Google Scholar]
- Szucs D., Soltész F., White S. Motor conflict in Stroop tasks: direct evidence from single-trial electro-myography and electro-encephalography. NeuroImage. 2009;47(4):1960–1973. doi: 10.1016/j.neuroimage.2009.05.048. [DOI] [PubMed] [Google Scholar]
- Tays W.J., Dywan J., Mathewson K.J., Segalowitz S.J. Age differences in target detection and interference resolution in working memory: an event-related potential study. Journal of Cognitive Neuroscience. 2008;20(12):2250–2262. doi: 10.1162/jocn.2008.20158. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill S.L., D'Esposito M., Aguirre G.K., Farah M.J. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: a reevaluation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=25116&tool=pmcentrez&rendertype=abstract Retrieved from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich R., Miller J. Using the jackknife-based scoring method for measuring LRP onset effects in factorial designs. Psychophysiology. 2001;38(5):816–827. http://www.ncbi.nlm.nih.gov/pubmed/11577905 Retrieved from. [PubMed] [Google Scholar]
- Vallesi A., Stuss D.T., McIntosh A.R., Picton T.W. Age-related differences in processing irrelevant information: evidence from event-related potentials. Neuropsychologia. 2009;47(2):577–586. doi: 10.1016/j.neuropsychologia.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Van der Lubbe R.H.J., Verleger R. Aging and the Simon task. Psychophysiology. 2002;39(1):100–110. doi: 10.1017/S0048577202001221. [DOI] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(40):12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verleger R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology. 1997;34(2):131–156. doi: 10.1111/j.1469-8986.1997.tb02125.x. http://www.ncbi.nlm.nih.gov/pubmed/9090263 Retrieved from. [DOI] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M. Two- and three-stimuli auditory oddball ERP tasks and neuropsychological measures in aging. NeuroReport. 2001;12(14):3149–3153. doi: 10.1097/00001756-200110080-00033. http://www.ncbi.nlm.nih.gov/pubmed/11568654 Retrieved from. [DOI] [PubMed] [Google Scholar]
- West R., Alain C. Effects of task context and fluctuations of attention on neural activity supporting performance of the Stroop task. Brain Research. 2000;873(1):102–111. doi: 10.1016/s0006-8993(00)02530-0. http://www.ncbi.nlm.nih.gov/pubmed/10915815 Retrieved from. [DOI] [PubMed] [Google Scholar]
- West R., Alain C. Age-related decline in inhibitory control contributes to the increased Stroop effect observed in older adults. Psychophysiology. 2000;37(2):179–189. http://www.ncbi.nlm.nih.gov/pubmed/10731768 Retrieved from. [PubMed] [Google Scholar]
- West R., Bowry R., McConville C. Sensitivity of medial frontal cortex to response and nonresponse conflict. Psychophysiology. 2004;41(5):739–748. doi: 10.1111/j.1469-8986.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- West R., Schwarb H. The influence of aging and frontal function on the neural correlates of regulative and evaluative aspects of cognitive control. Neuropsychology. 2006;20(4):468–481. doi: 10.1037/0894-4105.20.4.468. [DOI] [PubMed] [Google Scholar]
- Wiegand I., Finke K., Müller H.J., Töllner T. Event-related potentials dissociate perceptual from response-related age effects in visual search. Neurobiology of Aging. 2013;34(3):973–985. doi: 10.1016/j.neurobiolaging.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Wild-Wall N., Falkenstein M., Hohnsbein J. Flanker interference in young and older participants as reflected in event-related potentials. Brain Research. 2008;1211:72–84. doi: 10.1016/j.brainres.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Yordanova J., Kolev V., Hohnsbein J., Falkenstein M. Sensorimotor slowing with ageing is mediated by a functional dysregulation of motor-generation processes: evidence from high-resolution event-related potentials. Brain: A Journal of Neurology. 2004;127(Pt 2):351–362. doi: 10.1093/brain/awh042. [DOI] [PubMed] [Google Scholar]
- Zhang H., Kornblum S. The effects of stimulus-response mapping and irrelevant stimulus-response and stimulus-stimulus overlap in four-choice Stroop tasks with single-carrier stimuli. Journal of Experimental Psychology: Human Perception and Performance. 1998;24(1):3–19. doi: 10.1037//0096-1523.24.1.3. [DOI] [PubMed] [Google Scholar]
- Zysset S., Schroeter M.L., Neumann J., Yves von Cramon D. Stroop interference, hemodynamic response and aging: an event-related fMRI study. Neurobiology of Aging. 2006;28:937–946. doi: 10.1016/j.neurobiolaging.2006.05.008. [DOI] [PubMed] [Google Scholar]