Abstract
This work demonstrates a facile fabrication method to produce superhydrophobic coatings on chemically distinct materials using the electrospraying process. Coatings are mechanically robust, three-dimensional, and formed using a single fabrication step.
Interest continues to evolve in superhydrophobic surfaces, where the unique property of having a permanent or semi-permanent air layer at a material surface can lead to improved material performance in specific applications.1–15 Superhydrophobicity is achieved by trapping air at the material-water interface by adding sufficient surface roughness to a low energy material using one of a variety of microfabrication, surface modification, or surface coating techniques.16–25 Fabricating a surface with superhydrophobicity has become a relatively straight-forward procedure, but a number of complexities arise with the usage of such surfaces for practical application.16 Producing a superhydrophobic surface that meets all of the following design criteria represents a significant challenge: 1) use of readily available hydrophobic materials which can be fabricated with sufficient surface roughness to promote superhydrophobicity; 2) selection of a one-step fabrication technique that is easily scalable for industrial use, utilizes relatively mild processing conditions, and can be coated over large areas; and 3) sufficient mechanical integrity of the surface for its intended application.
Our interest in superhydrophobic materials originates from the use of micron-thick superhydrophobic electrospun meshes as drug delivery devices, where entrapped air within a bulk superhydrophobic material promotes long-term controlled release of an active biologic agent, as well as effectiveness in in vitro cytotoxicity assays.26 Specifically, we selected poly(ε-caprolactone) (PCL) as a base polymer for electrospinning, and demonstrated that, with small additions of the hydrophobic polymer poly(glycerol monostearate-co-ε-caprolactone) (PGC-C18), the stability of the superhydrophobic state is controlled. Building on these results, we are now investigating whether the biocompatible electrospun PCL-PGC-C18 system could be adapted to electrospraying, where a superhydrophobic coating is applied to a material surface. Previous work by others has shown that many polymers used for electrospinning can be electrosprayed by modifying solution parameters (i.e., viscosity, molecular weight, solvents) and operating conditions (i.e., voltage, working distance, flow rate), leading to the formation of discrete polymer particles rather than fibers.27–30 Although superhydrophobic surfaces have been prepared using the electrospraying technique, the fabricated surfaces lack mechanical robustness due to ease in removing the electrosprayed particles from the substrate material.31–33 We postulated that a set of electrosprayed particles connected in 3D may be ideal for producing a generic superhydrophobic coating, as this structure may provide longer-term mechanical integrity compared to discrete particles. Electrosprayed particles that have not completely dried will be deposited onto a material surface, allowing them to meld with neighbouring particles and form a porous 3D superhydrophobic coating. Herein we report a single-step fabrication method for creating mechanically robust superhydrophobic coatings on varied material types.
Connected electrosprayed particle coatings were fabricated from PCL and PGC-C18. Specifically, PCL (45 kD, 10 wt%, CHCl3) was doped with varying amounts of PGC-C18 in order to modulate hydrophobicity and achieve the desired superhydrophobic state. The blended polymer solutions were electrosprayed (20 kV, 20 cm working distance (WD), 5 mL/hr, 18 G needle) on aluminium foil. SEM images of the electrosprayed surfaces are shown in Figure 1, where changing the PGC-C18 concentration from 0 to 100% produced varied particle sizes, particle textures, and 3D connectivity. Higher concentrations of PCL resulted in deposits of large, wet particles due to a high solution viscosity, where a larger viscosity promotes low fragmentation of particles during the electrospraying process due to chain entanglement within the formed/drying droplets.27 Relatively flat surfaces for PCL and PCL with 5% PGC-C18 were observed, where deposition of these wet particles allowed almost total integration into one another. Increasing the PGC-C18 content reduced solution viscosity, as the molecular weight of PGC-C18 is lower than that of PCL (20 kD vs. 45 kD), and afforded smaller, less connected particles. Particle sizes ranged from 46±12 µm for PCL without PGC-C18 doping, to as small as 3.3±1.1 µm for 50% PGC-C18 doping. Electrosprayed coatings with >50% PGC-C18 showed a slight increase in particle size even with a continued drop in viscosity, where the waxy, low melting point PGC-C1834 may allow additional plasticity of the droplets to form larger more interconnected features once deposited. Electrospraying the PCL-PGC-C18 system produced a highly porous structure with large apparent contact angles. The addition of 25% or more PGC-C18 to PCL afforded an apparent contact angle of 172° with contact angle hysteresis as low as 5° (See SI; Figure S1).
Figure 1.
SEM images of electrosprayed PCL coating with varying PGC-C18 content (PCL:PGC-C18). Scale bar = 20 µm.
Next, electrospinning solution and processing parameters for the 75:25 PCL:PGC-C18 and 50:50 PCL:PGC-C18 coatings were optimized to produce more robust, connective superhydrophobic coatings by increasing the solvent present when individual sprayed particles were deposited. First, the electrospraying solution concentration was doubled to 20 wt% in addition to decreasing the working distance to 10 cm from the initial parameters (Figure 2). These changes resulted in large individual particles that were not directly connected, but were instead threaded together by thin fibers, forming a beads-on-a-string morphology. Second, reduction of the electrospinning solution concentration back to 10 wt% resulted in loss of the thin fibers, and microspheres were more heavily textured and minimally connected in three dimensions. Third, the PCL molecular weight was decreased from 45 kD to 10 kD, and the applied voltage decreased, resulting in wet particles being deposited and connected in 3D. All electrosprayed surfaces have advancing ACA > 165°, with CA hysteresis <7°.
Figure 2.
SEM images of electrosprayed coatings produced by varying solution parameters and processing parameters for 75:25 and 50:50 PCL:PGC-C18 compositions. All coatings shown have ACA > 165°. Scale bar = 10 µm.
The electrosprayed 75:25 PCL:PGC-C18 and 50:50 PCL:PGC-C18 coatings were then tested for mechanical robustness using ultrasonication and scotch tape delamination treatments. Electrosprayed surfaces were submerged in water, where an ultrasonication treatment was performed for 30 seconds, after which control and ultrasonicated samples were probed. Surfaces identified as not connected in 3D by SEM were easily sheared off from their aluminium substrates (see 75:25 and 50:50 PCL:PGC-C18 coatings in Figure 1), where individual particles were freed from the surface and observed floating in solution during treatment. The materials post-ultrasound treatment wet and the superhydrophobic property was loss. Using less aggressive treatments (scotch tape or rubbing the surface) also resulted in removal of the loosely adhered particles from these coated surfaces. For comparison, a scotch tape delamination test of a similar electrospun mesh composition easily draws fibers away from the material leaving a damaged material. With an identical ultrasonication or scotch tape treatment, the electrosprayed superhydrophobic coatings which formed connected structures (see 75:25 and 50:50 PCL:PGC-C18 coatings in Figure 2) retained their integrity and connectedness. The apparent contact angle remained unchanged before and after treatment. While portions of the top layer of the coating may have been removed with either treatment (not visually observed), the newly exposed underlying layer afforded a superhydrophobic coating. More extreme ultrasonication conditions did not damage the coatings (20 minutes, 3× power), and only with aggressive sheer conditions with forceful scraping were such coatings visually damaged where the majority of the superhydrophobic coating had been removed. Based on the above studies, the 50:50 PCL:PGC-C18 coating (45 kD PCL, 10 wt%, 10 cm WD, 20 kV) was selected for further experiments as a representative coating with favourable particle connectivity and superhydrophobicity.
Different thickness superhydrophobic coatings were then fabricated to investigate the three-dimensional nature of these electrosprayed coatings. By varying the time of deposition the total thickness of the superhydrophobic coating was controlled (47, 73, 110, and 156 µm). The expected linear trend between deposition time and thickness was observed (see SI, Figure S2).
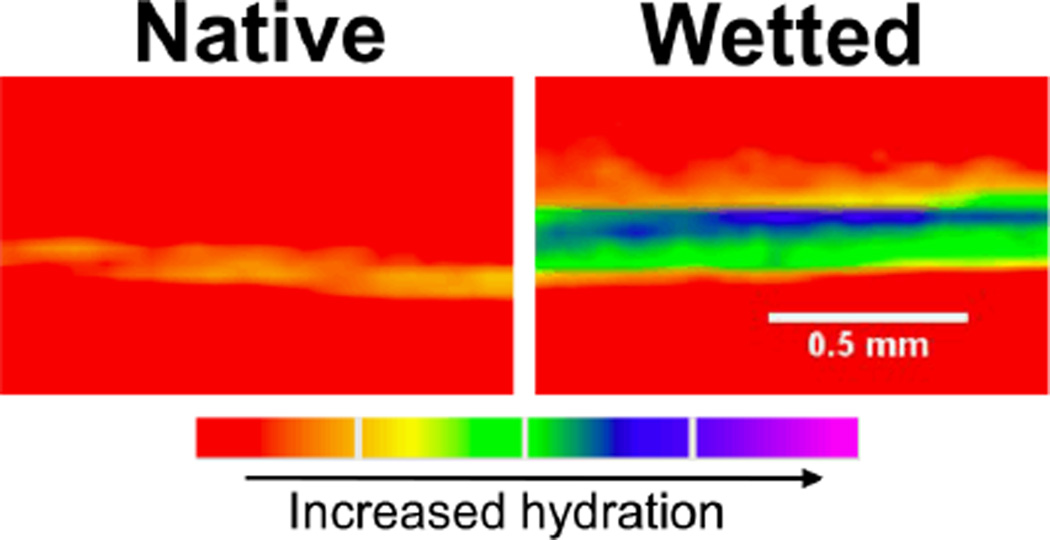
The 3D nature of these coatings was confirmed by imaging a 156 µm thick 50:50 PCL:PGC-C18 coating using X-ray computed tomography. First, the electrosprayed surface was dipped in a saline-ioxaglate (a computed tomography contrast agent) solution for 2 hours to show no contrast agent infiltration and to demonstrate superhydrophobicity over the entirety of the material surface (Figure 3), where only the aluminium foil-and-air interface on the underside of the coatings was observed when imaged. Following an ethanol dip treatment, to forcefully wet the electrosprayed coating, and immersion into the saline-ioxaglate solution, X-ray imaging revealed a wetted 3D thick coating with loss of air. Additionally, ethanol-treated coatings sank after removal of the entrapped air, where untreated coatings needed to be forcefully immersed during incubation.
Figure 3.
µCT cross-sectional images of a 156 µm 50:50 PCL:PGC-C18 electrosprayed native and wetted coatings. Prior to ethanol treatment and wetting, superhydrophobicity is demonstrated over the entire surface coating and there is no water penetration (red coloration indicates the absence of water). After ethanol wetting, water immediately infiltrates into the coating and confirms their 3D nature (green-to-purple coloration indicates increased water content).
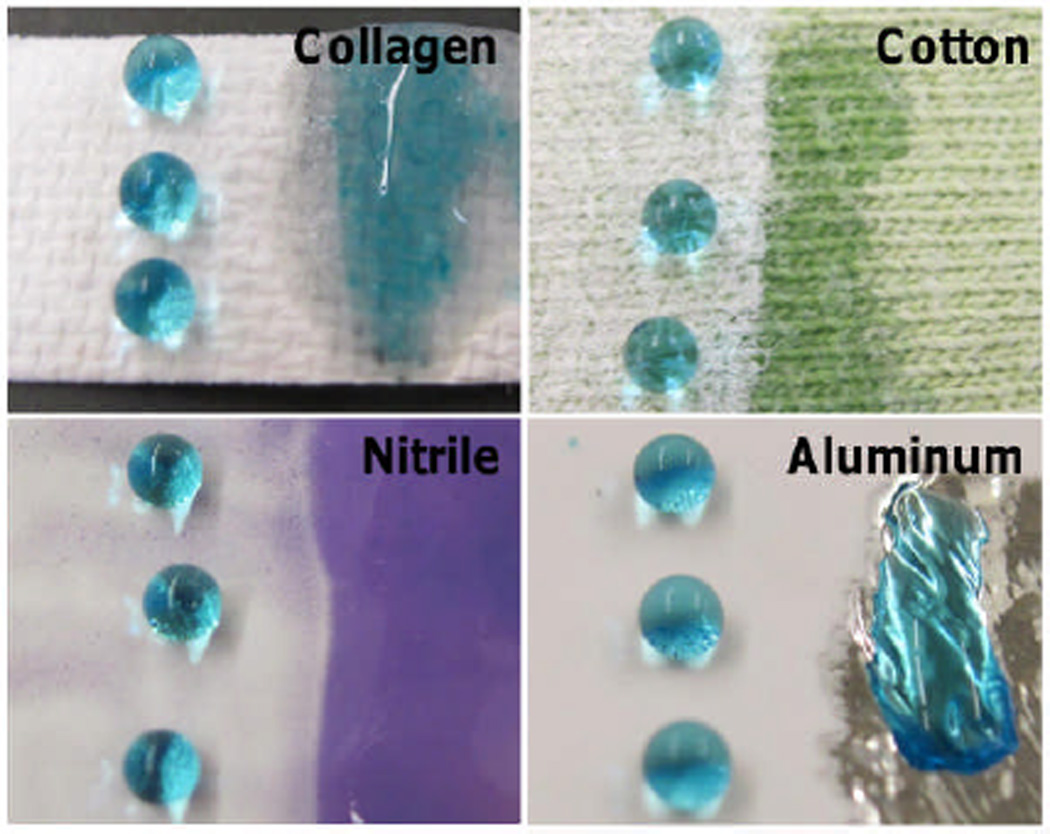
This superhydrophobic electrosprayed coating technique is a substrate generic approach to coat structurally and compositionally different materials such as collagen, cotton fabric, nitrile rubber, and aluminium foil (Figure 4). After electrospraying onto these surfaces, the resultant contact angle of all four surfaces is >167° (hysteresis <7°), whereas the uncoated portions of the material are easily and quickly wetted. Materials which are electrically insulating, such as glass, could be coated with the use of conductive copper tape near the material surface to ground the current used in the electrospraying process.
Figure 4.
Electrosprayed superhydrophobic coating on collagen (top left), cotton fabric (top right), nitrile rubber (bottom left), and aluminium foil (bottom right). Wettability of the electrosprayed portion of the material (left) is compared to the non-electrosprayed portion (right). Contact angle of all surfaces are >167° with consistent morphology. Droplets contain <1 wt% blue food coloring.
By controlling the fabrication step, superhydrophobic coatings are prepared via electrospraying, and both the chemistry and the microstructure can be modified for a specific application. This process is highly customizable, and a number of electrospraying parameters can be quickly and easily tailored for a set of design requirements, as well as the ability to deposit on materials of different composition. In this instance, the use of PCL:PGC-C18 blending adds additional functionality, where changes in the PGC-C18 doping concentration allow a specific superhydrophobic state to be achieved. Electrospraying a hydrophobic blend to fabricate superhydrophobic coatings is a single step, facile procedure to form mechanically robust surfaces. As such, this method complements other fabrication methods to produce superhydrophobic coatings or surfaces on varied substrates, and will facilitate the design and preparation of these materials for evaluation in additional industrial and biomedical applications.
Supplementary Material
Acknowledgments
This work was supported in part by the NIH T32 in Pharmacology and Experimental Therapeutics, BU Nanotheranostics ARC, BU Nanomedicine Program and Cross-Disciplinary Training in Nanotechnology NIH R25 CA153955, and NIH R01CA149561. We also thank Jonathan Freedman for assistance with the CT x-ray imaging studies.
Notes and references
- 1.Zhang X, Shi F, Niu J, Jiang Y, Wang Z. J. Mater. Chem. 2008;18:621–633. [Google Scholar]
- 2.Genzer J, Efimenko K. Biofouling. 2006;22:339–360. doi: 10.1080/08927010600980223. [DOI] [PubMed] [Google Scholar]
- 3.McHale G, Newton MI, Shirtcliffe NJ. Soft Matter. 2010;6:714–719. [Google Scholar]
- 4.Marmur A. Biofouling. 2006;22:107–115. doi: 10.1080/08927010600562328. [DOI] [PubMed] [Google Scholar]
- 5.Yao X, Song Y, Jiang L. Adv. Mat. 2011;23:719–734. doi: 10.1002/adma.201002689. [DOI] [PubMed] [Google Scholar]
- 6.Voronov RS, Papavassiliou DV, Lee LL. Ind. Eng. Chem. 2008;47:2455–2477. [Google Scholar]
- 7.Rothstein JP. Ann. Rev. Fluid Mech. 2010;42:89–109. [Google Scholar]
- 8.Plawsky JL, Ojha M, Chatterjee A, Wayner PC., Jr Chem. Eng. Commun. 2008;196:658–696. [Google Scholar]
- 9.Verplanck N, Coffinier Y, Thomy V, Boukherroub R. Nanoscale Res. Lett. 2007;2:577–596. [Google Scholar]
- 10.Heikenfeld J, Dhindsa M. J. Adhes. Sci. Technol. 2008;3:319–334. [Google Scholar]
- 11.Guo Z, Liu W, Su BL. J. Colloid Interf. Sci. 2011;353:335–355. doi: 10.1016/j.jcis.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 12.Feng L, Li S, Li Y, Li H, Zhang L, Zhai J, Song Y, Liu B, Jiang L, Zhu D. Adv. Mat. 2002;14:1857–1860. [Google Scholar]
- 13.Webb HK, Hasan J, Truong VK, Crawford RJ, Ivanova EP. Curr. Med. Chem. 2011;18:3367–3375. doi: 10.2174/092986711796504673. [DOI] [PubMed] [Google Scholar]
- 14.Nosonovsky M, Bhushan B. Philos. T. R. Soc. A. 2009;367:1511–1539. doi: 10.1098/rsta.2009.0008. [DOI] [PubMed] [Google Scholar]
- 15.Privett BJ, Youn J, Hong SA, Lee J, Han J, Shin JH, Schoenfisch MH. Langmuir. 2011;27:9597–9601. doi: 10.1021/la201801e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X-M, Reinhoudt D, Crego-Calama M. Chem. Soc. Rev. 2007;36:1350–1368. doi: 10.1039/b602486f. [DOI] [PubMed] [Google Scholar]
- 17.Ma M, Hill RM, Rutledge GC. J. of Adhes. Sci. Technol. 2008;22:1799–1817. [Google Scholar]
- 18.Ma M, Hill RM. Current Opin. Colloid In. 2006;11:193–202. [Google Scholar]
- 19.Xue CH, Jia ST, Zhang J, Ma JZ. Sci. Technol. Adv. Mat. 2010;11:033002. doi: 10.1088/1468-6996/11/3/033002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roach P, Shirtcliffe NJ, Newton MI. Soft Matter. 2008;4:224–240. doi: 10.1039/b712575p. [DOI] [PubMed] [Google Scholar]
- 21.Yan Y, Gao N, Barthlott W. Adv. Colloid Interfac. 2011;169:80–105. doi: 10.1016/j.cis.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Nakajima A, Hashimoto K, Watanabe T. Monatsh. Chem. 2001;132:31–41. [Google Scholar]
- 23.Feng X, Jiang L. Adv. Mat. 2006;18:3063–3078. [Google Scholar]
- 24.Cheng Q, Li M, Zheng Y, Su B, Wang S, Jiang L. Soft Matter. 2011;7:5948–5951. [Google Scholar]
- 25.Jiang L, Zhao Y, Zhai J. Angew. Chem. Int. Edit. 2004;116:4438–4441. [Google Scholar]
- 26.Yohe ST, Colson YL, Grinstaff MW. J. Am. Chem. Soc. 2012;134:2016–2019. doi: 10.1021/ja211148a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng J, He A, Li J, Xu J, Han CC. Polymer. 2006;47:7095–7102. [Google Scholar]
- 28.Gupta D, Venugopal J, Mitra S, Giri Dev V, Ramakrishna S. Biomaterials. 2009;30:2085–2094. doi: 10.1016/j.biomaterials.2008.12.079. [DOI] [PubMed] [Google Scholar]
- 29.Kenawy ER, Layman JM, Watkins JR, Bowlin GL, Matthews JA, Simpson DG, Wnek GE. Biomaterials. 2003;24:907–913. doi: 10.1016/s0142-9612(02)00422-2. [DOI] [PubMed] [Google Scholar]
- 30.Rutledge GC, Fridrikh SV. Adv. Drug Deliv. Rev. 2007;59:1384–1391. doi: 10.1016/j.addr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Burkarter E, Saul C, Thomazi F, Cruz N, Roman L, Schreiner W. Surface Coat. Technol. 2007;202:194–198. [Google Scholar]
- 32.Yoon H, Park JH, Kim GH. Macromol. Rapid Comm. 2010;31:1435–1439. doi: 10.1002/marc.201000131. [DOI] [PubMed] [Google Scholar]
- 33.Papadopoulou S, Tsioptsias C, Pavlou A, Kaderides K, Sotiriou S, Panayiotou C. Colloid. Surface. A. 2011;387:71–78. [Google Scholar]
- 34.Wolinsky JB, Ray WC, III, Colson YL, Grinstaff MW. Macromolecules. 2007;40:7065–7068. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.