Abstract
Schistosoma mansoni, an intravascular parasite, has evolved a number of immune evasion mechanisms to establish itself in the host, such as antioxidant enzymes. Our laboratory has demonstrated that the highest levels of certain antioxidant enzymes are found in adult worms, which are the least susceptible to immune killing. Vaccination of mice with naked DNA constructs containing the gene encoding Cu/Zn cytosolic superoxide dismutase (SmCT-SOD) showed significant levels of protection compared to a control group, and our data demonstrate that the adult worms are a target of the immune response that confers resistance in SmCT-SOD DNA-vaccinated mice. Because SmCT-SOD shows significant identity with the human homologue, we evaluated the reactivity of anti-SmCT-SOD antibodies derived from SmCT-SOD-immunized mice and rabbits and from S. mansoni-infected individuals to human superoxide dismutase (hSOD) and SmCT-SOD parasite-specific peptides to assess the potential for autoimmune responses from immunization with the recombinant molecule. In addition, we evaluated the ability of various SmCT-SOD adjuvant-delivered immunizations to induce cross-reactive antibodies. Both mouse and rabbit antibodies generated against SmCT-SOD recognized the denatured form of hSOD. The same antibodies did not recognize nondenatured hSOD. Sera from infected individuals with different clinical forms of schistosomiasis recognized SmCT-SOD but not hSOD. Antibodies from mice immunized with different SmCT-SOD-containing formulations of both DNA and protein were able to recognize SmCT-SOD-derived peptides but not soluble hSOD. All together, these findings serve as a basis for developing a subunit vaccine against schistosomiasis.
Schistosomiasis remains an endemic problem in over 76 countries, although approaches to reduce transmission and morbidity as well as to improve sanitation, mollusciciding and mass chemotherapy, have been attempted (3, 13). Vaccine development to control schistosomiasis is a high priority, as it would offer the potential of cost-efficient, long-lasting prophylaxis (2, 65). Schistosoma mansoni, an intravascular parasite, lives in a hostile environment in close contact with host humoral and cellular cytotoxic factors. Larval parasite killing mechanisms include the release of oxidants by eosinophils, neutrophils, and macrophages potentiated by antibodies and/or cytokines (8, 12, 16, 31, 32). However, to establish itself in the host, the parasite has evolved a number of immune evasion mechanisms (17, 39, 51, 60), such as antioxidant enzymes (7, 11, 30, 37). These are essential enzymes involved in the prevention of reactive oxygen species-derived damage. Evidence for the involvement of schistosome antioxidant enzymes, such as cytosolic superoxide dismutase (SmCT-SOD) and glutathione peroxidase (SmGPX), in immune evasion has been presented elsewhere (39). SmCT-SOD and GPX localize to the host-parasite interface (worm tegument and gut epithelium of the adult but not the larval schistosome) (41) and show developmental regulation (29, 41, 42) with the highest levels present in the adult worm, the stage least susceptible to immune elimination (43, 44), and the lowest levels in the larval stages, the stage most susceptible to immune elimination (43). Consistent with these data, studies in our laboratory demonstrated that vaccination of mice with naked DNA constructs that contained the genes encoding SmCT-SOD and SmGPX showed significant levels of protection compared to a control group (58). Moreover, we have demonstrated that DNA encoding SmCT-SOD as a vaccine is able to significantly and consistently reduce worm burden by targeting 21-day and older adult S. mansoni worms for immune elimination (R. Cook, C. Carvalho-Queiroz, and P. T. LoVerde, submitted for publication). The ubiquitous distribution and essential activity of antioxidant enzymes suggest that there is relatively high homology between species. Autoimmune responses elicited by cross-reactive antibodies could lead to tissue damage, such as that seen in individuals that develop antibodies to streptococcal M antigens (67). To assess the potential for autoimmune responses from immunizing humans with the S. mansoni intact molecule (20), we evaluated the potential of anti-SmCT-SOD antibodies derived from SmCT-SOD-immunized mice and rabbits and from S. mansoni-infected individuals with different clinical forms of the disease to react with human SOD (hSOD)- and to SmCT-SOD-derived peptides. Analyses of the reactivity of specific antibody isotypes against these peptides were aimed to identify relevant B-cell epitopes that could be used in a subunit vaccine (33, 62). Since the efficacy of a given vaccine depends on proper antigen processing and presentation within the host cells as well as on the need and/or type of vaccine adjuvant required, as reviewed in reference 47, we also evaluated the reactivity of antibody isotypes from mice immunized with different formulations of SmCT-SOD.
MATERIALS AND METHODS
PILEUP analysis.
Amino acid sequences were aligned using the GCG Wisconsin Package PILEUP program in order to analyze the percentage of identity and similarity between SmCT-SOD and host homologues.
Human subjects.
Patients for this study reside in endemic areas of Minas Gerais, Brazil, and were included after giving informed consent. All individuals were subjected to clinical and physical examination, and active infection was assessed by Kato-Katz thick stool smears (34). All diagnosed individuals were treated with oxamniquine regardless of their participation in the study. Chronically infected individuals were classified as having the asymptomatic intestinal form or presenting with the severe hepatosplenic compensated form of the disease, the latter characterized by enlargement of the liver and spleen (4). Individuals presenting with the acute phase were those not living in the endemic areas but who acquired infection with S. mansoni (24). The uninfected putatively resistant endemic normal (EN) individuals (15) are those who reside in areas endemic for schistosomiasis, frequent sites of active transmission, have never been treated for schistosomiasis, and have multiple egg-negative stools after a follow up of 5 years and no circulating parasite antigens, as reviewed in reference 14. The exposed but noninfected (area-negative) individuals were those from the endemic area that had been treated previously and were not harboring eggs in their feces at the time of the study. Noninfected nonexposed individuals were volunteers living in Buffalo, N.Y., with no previous history of contact with the parasite. Sera were obtained from heparinized venous blood and stored at −20°C until needed. This study was approved by The State University of New York at Buffalo Institutional Review Board, the Ethical Committee of the Centro de Pesquisas René Rachou of the Oswaldo Cruz Foundation in Belo Horizonte, and the National Council for Ethics in Research—COBNEP.
Antigens.
The entire open reading frame of SmCT-SOD was cloned from the pcDNAI/Amp vector (58) into the pGEX-4T-1 or pGEX-3X (Amersham Biosciences, Piscataway, N.J.), pMALc2x (New England Biolabs, Beverly, Mass.), and pET14b (Novagen, Madison, Wis.) expression vectors, while SmGPXm was cloned into the pGEX-4T-1 vector (58). Recombinant protein was expressed in prokaryotic expression systems and purified by column elution. hSOD was obtained from Sigma (St. Louis, Mo.), and bovine serum albumin (BSA) was from Pierce and Sigma. Glutathione S-transferase (GST; Schistosoma japonicum Sj26), His-SmCT-SOD, maltose binding protein (MBP)-SmCT-SOD, GST-SmCT-SOD, and hSOD were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting prior to use in enzyme-linked immunosorbent assays (ELISAs). The peptides were generated based on the full 153-amino-acid sequence of SmCT-SOD (29) and obtained from Molecular Genetics Instrumentation Facility (University of Georgia). Lyophilized peptides were suspended in 2 ml of sterile phosphate-buffered saline (PBS), and aliquots were frozen at −20°C. The protein concentration of all samples was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, Ill.).
Preparation of the antioxidant protein-based vaccine.
The antioxidant recombinant SmCT-SOD-GST was used in an immunization protocol in two different formulations: encapsulated in polylactic acid (PLA) microspheres, or diluted in alum (Inject alum; Pierce). A phase-inversion nanoencapsulation technique was used for encapsulation of protein into PLA microspheres as described previously (40). Briefly, BSA (radioimmunoassay grade; Sigma Chemical Co., St. Louis, Mo.) and PLA (molecular weights, 24,000 and 2,000 [1:1, wt/wt], Birmingham Polymers, Inc., Birmingham, Ala.) in methylene chloride (Fisher, Pittsburgh, Pa.) were rapidly poured into petroleum ether (Fisher) for formation of microspheres. Microspheres were filtered and lyophilized overnight for complete removal of solvent. Lyophilized microspheres (0.1 to 10 μm) were stored at −20°C until needed. Before vaccination, the microspheres were suspended in 1% hydroxypropylmethylcellulose (Dow Chemical Co., Midland, Mich.) and 1% Pluronic F127 (Sigma Chemical Co.) in PBS (pH 7.2) (15% of the final volume), vortexed for 10 s, and sonicated for 1 min. The volume was completed with PBS (with or without 0.5% mouse serum), vortexed for 10 s, and sonicated for 1 min. The same amount of recombinant antigen was emulsified 1:2 in alum-based adjuvant according to the manufacturer's specifications at a final concentration of 50 μg/100 μl of suspension, just prior to use. The fusion partner Sj26-GST was included as a control in all experiments.
Determination of encapsulation efficiency.
To determine protein encapsulation efficiency, 4 mg of microspheres was weighed in a 1.5-ml centrifuge tube (two aliquots for each sample). Then, 700 μl of ethyl acetate was added to each tube and vortexed for 10 s. A 700-μl aliquot of methylene chloride was added, and tubes were vortexed (until no more solid particles were present) and centrifuged for 10 min (13,000 × g). After 1.3 ml of supernatant was removed, the samples were centrifuged again. As much fluid as possible was decanted without any solid, and the remaining fluid was left to evaporate. In each tube, 1.0 ml of distilled water was added, and the samples were vortexed until as much as possible was dissolved. Protein concentration of all samples was determined using a bicinchoninic acid protein assay kit. The results were expressed as micrograms of protein per milligram of microspheres.
Immunization of mice with protein-based vaccines.
Female BALB/c mice 5 to 6 weeks old were used in all vaccination protocols, with four mice per antigenic preparation. The first experimental group was given an injection intramuscularly with 50 μl of suspended microspheres in each hind leg (one to two sites per leg), with a total of 5 mg of polymer/100 μl/mouse. The second experimental group was given an injection intramuscularly with 50 μl of suspended microspheres in each hind leg (one to two sites per leg), but with an adjusted amount of encapsulated microspheres capable of releasing 50 μg/100 μl/mouse (ranging from 1.47 to 1.56 mg of polymer/100 μl/mouse), according to the extraction and quantification protocol. Blood samples were collected retroorbitally from individual mice before and after immunization or boost, and each serum sample was stored at −20°C.
ELISA.
The titers of the pre- and postimmunization immunoglobulin G (IgG) antibodies in the sera against recombinant antigens derived from S. mansoni and hSOD were determined by ELISA (38) with minor modifications. Flat-bottom 96-well polystyrene plates (Maxisorp Nunc; GIBCO, Scotland) were coated overnight at 4°C with 50 to 100 μl of antigens diluted in PBS (0.15 M, pH 7.2) at a concentration of 5 μg/ml. After washing (automatic ELISA washer MR 5000; Dynatech) and blocking, 50 to 100 μl of doubling dilution of mouse anti-hSOD (Pharmingen)/well and pooled sera samples from each experimental or human infected group were diluted in PBS-0.05% Tween 20 (PBS-T) added to each plate, and the plates were incubated overnight at 4°C. After washing, 100 μl of alkaline phosphatase-conjugated mouse anti-human IgG antibodies (Sigma), rabbit anti-mouse IgG antibodies (Sigma), or unconjugated rabbit anti-mouse IgM, IgG1, IgG2a, IgG2b, IgG3, and IgA (Bio-Rad) and IgE (Pharmingen) antibodies were added per well, and the plates were incubated for 1 h at 37°C. After the incubation, the plates were washed, 50 to 100 μl of alkaline phosphatase-conjugated goat anti-rabbit IgG antibodies (Sigma) was added to those plates with unconjugated antibody, and the plates were incubated for another 1 h at 37°C. Detection of reactivity was performed by using 100 μl of pNPP in diethanolamine buffer (pNPP microwell substrate system; Kirkegaard and Perry Laboratories)/well, and the absorbance was measured at intervals at 405 nm by using an automatic ELISA reader (Bio-Rad). All the assay conditions were previously set up for optimal concentrations through checkerboard and titration curves, and all the reagents for antibody detection used in this assay were shown to be clear of nonspecific reactions. The cutoff value was calculated using the mean optical density (OD) plus 3 times the SD of normal or preimmune mouse and rabbit sera and of noninfected human sera (NI) at the first dilution (1:80), for each assay. Where appropriate, the endpoint serum titer was calculated as the last dilution with a given reactivity above the cutoff line.
Western blotting.
Antigens (0.8 to 1.0 μg/well) were size fractionated by electrophoresis on sodium dodecyl sulfate-polyacrylamide gel electrophoresis using either denaturing or nondenaturing conditions (35), and stained with Coomassie brilliant blue R (Sigma) or blotted to polyvinylidene difluoride membranes (Immobilon; Millipore) in a semidry Trans-Blot system (Bio-Rad) as described elsewhere (66). After blocking overnight at 4°C, the membranes were incubated with primary antibody diluted in 2% milk in PBS-T for 1 h at room temperature, washed, incubated with the appropriate secondary antibody (rabbit anti-mouse IgG or goat anti-rabbit IgG conjugated to alkaline phosphatase or unconjugated rabbit anti-mouse IgG1, IgG2a, IgG2b, and IgG3), and diluted in 2% milk in PBS-T for 1 h at room temperature. After washing, alkaline phosphatase-conjugated goat anti-rabbit IgG antibodies diluted in 2% milk in PBS-T were added for 1 h at room temperature to those membranes previously incubated with unconjugated antibody. Detection of reactivity was achieved using a substrate detection kit (Vector II; Vector, Burlingame, Calif.).
RESULTS
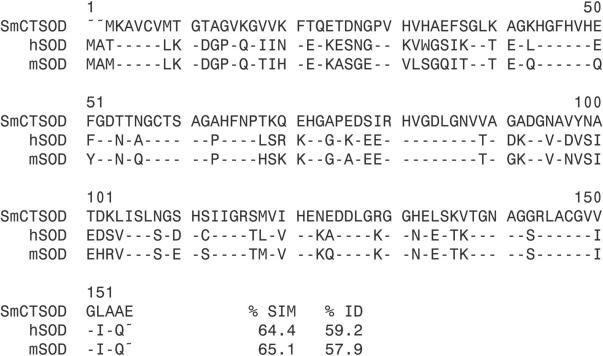
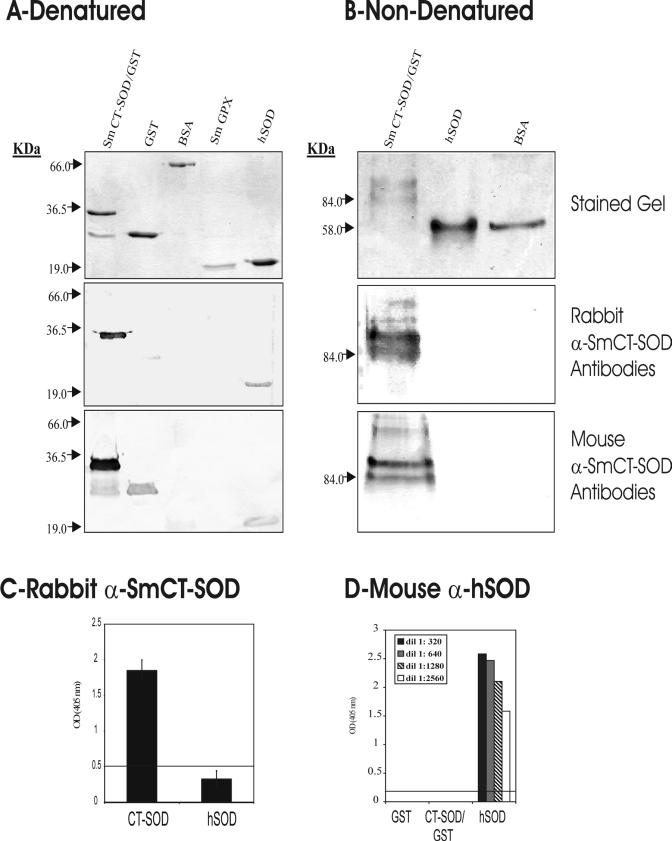
The amino acid sequences of SmCT-SOD, mouse SOD, and hSOD were analyzed in order to verify the degree of similarity and identity between them. The PILEUP diagram (Fig. 1) showed 57 to 59% identity and 64 to 65% similarity shared between the three cytosolic forms of the enzyme. To investigate whether antibodies produced against SmCT-SOD would cross-react with human enzymes, antibodies to SmCT-SOD-GST and GST were generated by immunization with the recombinant protein emulsified in Freund's adjuvant (data not shown) or encapsulated in PLA microspheres (see Materials and Methods) in rabbits and mice, respectively. Western blots of SmCT-SOD-GST, GST, BSA, SmGPX (the latter two used as irrelevant antigen controls), and hSOD proteins that were size separated under denaturing (Fig. 2A, top panel) and nondenaturing (Fig. 2B, top panel) conditions were probed with whole sera or the affinity-purified IgG fraction. Both rabbit anti-SmCT-SOD-GST (Fig. 2A, middle panel) and mouse anti-SmCT-SOD-GST (Fig. 2A, lower panel) antibodies recognized the denatured form of hSOD. However, when nondenaturing conditions were used, neither rabbit anti-SmCT-SOD-GST (Fig. 2B, middle panel) nor mouse anti-SmCT-SOD-GST (Fig. 2B, lower panel) antibodies reacted with hSOD. The antibodies did recognize SmCT-SOD (Fig. 2A and B). In addition, Western blotting with MBP- and His-tagged SmCT-SOD demonstrated that the antibody recognized the SmCT-SOD and not the fusion partners (data not shown). Likewise, antibodies from mice immunized with SmCT-SOD emulsified in alum as an adjuvant did not recognize native hSOD (data not shown). Further, evaluation of the isotype subclass responsible for the reactivity to hSOD revealed that, under denaturing conditions, sera from mice immunized with SmCT-SOD-GST encapsulated in PLA microspheres developed IgG1, IgG2a, and IgG2b but not IgG3 antibodies to hSOD, while mice immunized with SmCT-SOD-GST emulsified in alum developed IgG1 and IgG2b, but not IgG2a or IgG3, reactivity to hSOD (data not shown). The intensity of the reaction to hSOD was weak, but present, when compared to the intense reactivity of all isotypes to SmCT-SOD, in both groups. Figure 2C shows that in an ELISA, rabbit anti-SmCT-SOD-GST antibodies (1:500) strongly reacted with SmCT-SOD but not with hSOD, since the reactivity to hSOD was below the cutoff line. The rabbit immunized with SmCT-SOD serum titer against SmCT-SOD was 1:16,000. When mouse anti-hSOD antibodies were used, reactivity was detected against hSOD but not SmCT-SOD-GST or GST (Fig. 2D).
FIG. 1.
Comparison of Cu/Zn SOD amino acid sequences: SmCT-SOD, accession number L12159; hSOD, accession number NP 000445; mSOD (mouse SOD), accession number XP 128337. The percentages of identity (ID) and similarity (SIM) are in comparison to SmCT-SOD, and identical amino acids are represented as dashes.
FIG. 2.
(A and B) Comparison of anti-SmCT-SOD antibody responses to hSOD under denaturing (A) and nondenaturing (B) conditions. The top gels are acrylamide gels of SmCT-SOD-GST, GST, BSA, SmGPX, and hSOD antigens stained with Coomassie blue. The middle panels are Western blots after incubation with rabbit anti-SmCT-SOD-GST IgG antibodies (1:2,500). Bottom gels are Western blots after incubation with mouse anti-SmCT-SOD-GST IgG antibodies (1:10,000). (C) ELISA results showing the mean reactivity (± standard error of the mean) of rabbit anti-SmCT-SOD-GST sera (1:500) to SmCT-SOD and hSOD antigens from three experiments. (D) ELISA results showing reactivities of mouse anti-hSOD sera against SmCT-SOD-GST, GST, and hSOD proteins. The line represents the cutoff value (mean OD value + 3 times the SD) of preimmune sera.
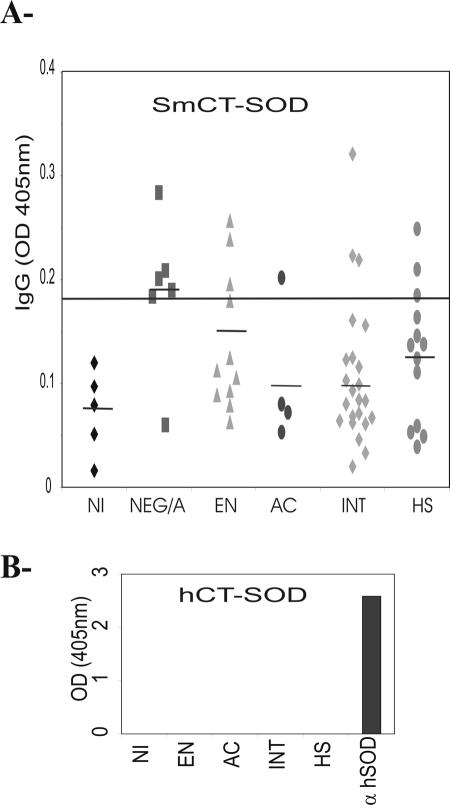
The next step was to determine if sera from individuals naturally infected with S. mansoni contained antibodies against SmCT-SOD and/or antibodies that could react with hSOD. Figure 3 shows the antibody reactivity of samples from acutely and chronically infected (asymptomatic intestinal and severe hepatosplenic), uninfected (putatively resistant EN and endemic area egg negative) but exposed to S. mansoni, and noninfected nonexposed individuals to recombinant SmCT-SOD at a 1:800 dilution. Our results showed that all groups of individuals naturally exposed to the parasite infection exhibited some reactivity of IgG antibodies against SmCT-SOD. Interestingly, the group of individuals exposed to S. mansoni infection but that did not harbor eggs in their stools showed high reactivity to SmCT-SOD (above the cutoff line) when compared to the infected individuals exhibiting acute or chronic schistosomiasis (Fig. 3A), although the variance analyses failed to show a statistical difference between them. Importantly, we were not able to detect IgG antibodies against hSOD from pooled sera from individuals naturally exposed to parasite infection (Fig. 3B).
FIG. 3.
Reactivity to SmCT-SOD of sera from individuals naturally infected with S. mansoni. Pooled sera were from acutely infected (AC; n = 4), chronically infected (asymptomatic intestinal [INT], n = 23; severe hepatosplenic [HS], n = 13), or uninfected (putatively resistant EN, n = 13; endemic area, egg negative [NEG/A], n = 6) individuals exposed to S. mansoni infection, as well as noninfected (NI; n = 5) individuals not exposed to S. mansoni infection. Sera were diluted 1:800, and ELISAs were performed using 5 μg of recombinant antigen (A) or hSOD (B) per ml. SmCT-SOD-MBP and MBP were analyzed separately, and the background was subtracted (SmCT-SOD-MBP response − MBP response = SmCT-SOD response). Dashes in panel A indicate the mean absorbance (OD) in each group. The cutoff value (line in panel A) was calculated using the mean + 3 SD of noninfected NI sera. Mouse anti-hSOD antibody was included as a positive control (B). The results represent the individual reactivity to SmCT-SOD. For the anti-hSOD reactivity, human sera were pooled. Analysis of variance showed that P was 0.08.
In order to determine the immunogenicity of a prime-boost regimen with PLA microspheres, BALB/c mice were immunized with recombinant SmCT-SOD encapsulated in PLA microspheres and the titer of specific antibodies was determined from pooled sera. The reactivities of IgG antibodies against SmCT-SOD-GST from mice immunized with 5 mg of PLA microspheres alone, encapsulated with GST (GST microspheres), or encapsulated with SmCT-SOD-GST at three time points, before immunization (preimmune), 4 weeks after immunization (immune), and 4 weeks after boost (postboost), are shown in Fig. 4. After the first immunization, as expected the group vaccinated with SmCT-SOD-GST microspheres showed increased titers of IgG anti-SmCT-SOD-GST of over 1:10,240 (Fig. 4A), and the GST-microspheres-vaccinated group titers to GST were 1:640 (Fig. 4B). The titers of the SmCT-SOD-GST microspheres and the GST microspheres groups to CT-SOD and GST, respectively, increased significantly after the boost, with the anti-CT-SOD IgG antibodies exhibiting a titer of over 1:327,680 and the anti-GST antibodies with an end point of 1:163,840 (data not shown).
FIG. 4.
Reactivity of antisera against SmCT-SOD from immunization with different adjuvants. (A and B) Reactivity against SmCT-SOD-GST (A) and GST (B) recombinant antigens of IgG antibodies from pooled sera of mice preimmune, immunized, and postboost with 5 mg of PLA microspheres encapsulated with or without GST and SmCT-SOD-GST antigens/100 μl/mouse. (C and D) Reactivity of IgG antibodies from pooled sera of mice immunized twice with an equivalent amount of the PLA microspheres adjusted to release 50 μg of recombinant antigen/100 μl/mouse or prime-boosted with the same antigens mixed in alum (50 μg/100 μl) against SmCT-SOD (C) and GST (D). The background reactivities of control mice vaccinated with microspheres alone or alum alone were subtracted from the respective other group. A group of mice were prime-boosted with 50 μg of naked pcDNA encoding SmCT-SOD. Rabbit anti-SmCT-SOD was included as a positive control. The line represents the cutoff value that was calculated using the mean + 3 SD of preimmune mouse sera absorbance at the lowest dilution (1:80).
Next, we compared the immunogenicity with 50 μg of recombinant antigen released by encapsulated PLA microspheres with that of the same amount of soluble antigen emulsified with alum, an adjuvant approved for use in humans. We analyzed the reactivity of IgG antibodies against SmCT-SOD-GST (Fig. 4C) and GST (Fig. 4D) from pooled sera from mice prime-boosted with equivalent amounts of the PLA microspheres adjusted to release 50 μg of recombinant antigen/100 μl/mouse (ranging from 1.47 to 1.56 mg of microspheres/100 μl/mouse) in PBS or prime-boosted with the same antigen emulsified in alum (50 μg/100 μl), as well as control groups vaccinated with microspheres alone or alum alone. The background reactivities in mice vaccinated with microspheres alone and alum alone were subtracted from the other respective groups (Fig. 4C and D). The reactivity of pooled sera from another group of mice that was prime-boosted with 50 μg of naked pcDNA encoding SmCT-SOD (SmCT-SOD DNA) was also analyzed. The titers of IgG anti-SmCT-SOD-GST and GST antibodies were low in mice vaccinated with CT-SOD DNA, at 1:80 (Fig. 4C and D). On the other hand, the titers of IgG anti-SmCT-SOD-GST in both groups vaccinated with SmCT-SOD-GST microspheres or SmCT-SOD-GST alum (Fig. 4C) were very high, 1:20,480 and over 1:40,960, respectively, when compared to the reactivity to GST (Fig. 4D), although the background reactivity to GST was much higher among the groups (both SmCT-SOD-GST and GST) that used alum as adjuvant.
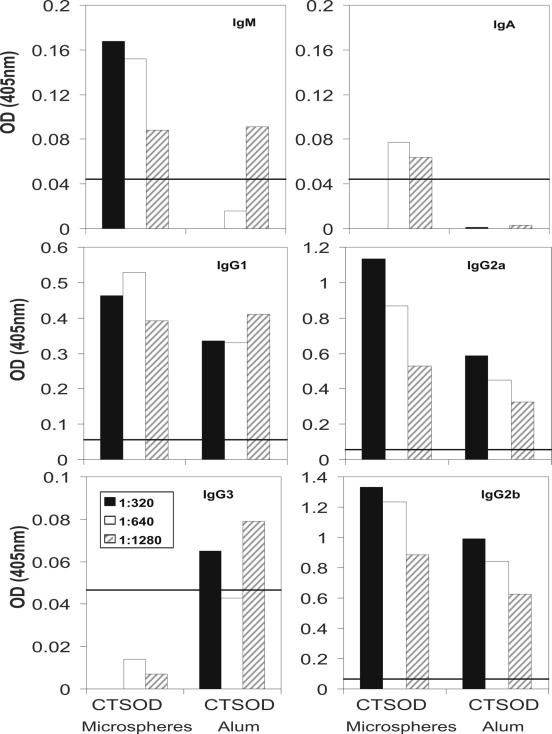
We next evaluated Ig isotypes in an attempt to correlate the different antibody isotypes that were stimulated by these vaccine preparations with a Th1- or Th2-like response. The response to SmCT-SOD was evaluated by subtracting the reactivity of GST microspheres from SmCT-SOD-GST microspheres and GST alum from SmCT-SOD-GST alum (Fig. 5). Immunization with SmCT-SOD-GST microspheres induced higher specific levels of IgG2a anti-SmCT-SOD and IgG2b anti-SmCT-SOD than the group vaccinated with SmCT-SOD mixed in alum. On the other hand, SmCT-SOD alum stimulated similar levels of IgG1 anti-SmCT-SOD when compared to SmCT-SOD microspheres, although the overall reactivity of IgG1 antibodies from mice immunized with alum was higher against both SmCT-SOD and GST proteins (data not shown). The anti-SmCT-SOD IgM, IgA, and IgG3 antibody levels were low but slightly above the cutoff value for IgM and IgA in the SmCT-SOD-microspheres-vaccinated group and for IgG3 in the SmCT-SOD alum group. Anti-SmCT-SOD IgE antibodies (neither anti CT-SOD-GST nor GST) were not detectable in our assay (data not shown).
FIG. 5.
Reactivity of antibody isotype against SmCT-SOD. Reactivity of IgM, IgA, IgG1, IgG2a, IgG2b, and IgG3 antibodies from pooled sera of mice immunized with equivalent amounts of SmCT-SOD-GST microspheres capable of releasing 50 μg/100 μl/mouse (CT-SOD microspheres), 50 μg of SmCT-SOD-GST mixed in alum/100 μl/mouse (CT-SOD Alum), and controls (GST microspheres only and GST alum only). The background reactivities to SmCT-SOD were subtracted (SmCT-SOD-GST − GST microspheres or GST alum). The line represents the cutoff value that was calculated using the mean + 3 SD of preimmune mouse sera absorbance at the lowest dilution (1:80).
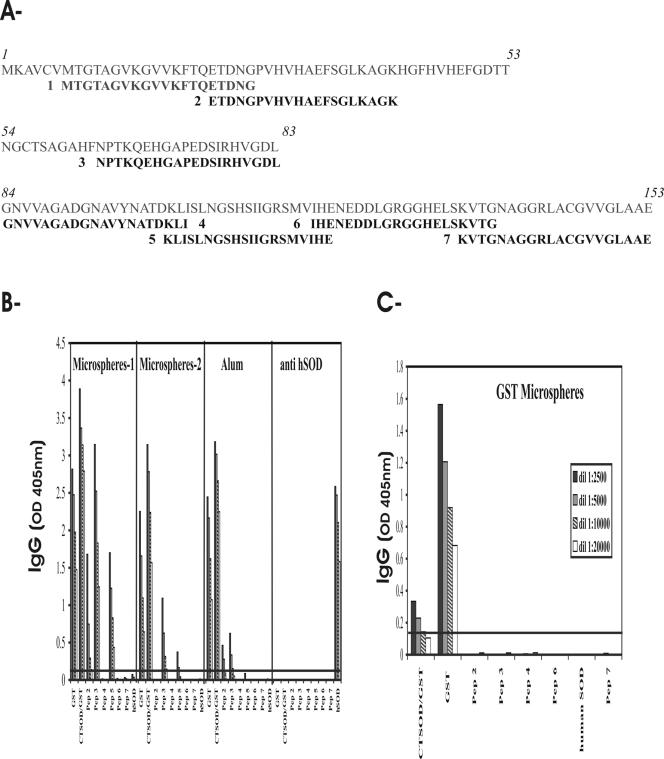
To identify parasite-specific B epitopes, we analyzed the antigenicity of linear synthetic peptides delineated from SmCT-SOD. Seven peptide constructs corresponding to the N-terminal, middle, and C-terminal regions of SmCT-SOD were synthesized (Fig. 6A). The N-terminal peptide 1 (Pep1) failed to be synthesized in four independent attempts. The reactivities of IgG antibodies from mice immunized with different formulations of SmCT-SOD-GST, GST or the mouse anti-hSOD to the various antigens, including SmCT-SOD-derived peptides, are shown in Fig. 6B and C. The microspheres 1 group was immunized with 5 mg of encapsulated microspheres/100 μl/mouse, while the microspheres 2 group was immunized with microspheres capable of releasing 50 μg of proteins/100 μl/mouse, in comparison with antigens adsorbed in alum (50 μg of proteins/100 μl/mouse; see Materials and Methods and Fig. 4). The IgG antibodies from the two groups of mice immunized with SmCT-SOD-GST-encapsulated PLA microspheres reacted strongly to SmCT-SOD (and GST), but not with hSOD (where the reactivity was below the cutoff line). From the six peptides tested, both microspheres groups showed high IgG reactivity against Pep3, followed by Pep2 and Pep5 (Fig. 6B). The antibodies from the group vaccinated with SmCT-SOD adsorbed in alum strongly recognized the soluble form of SmCT-SOD and to some extent Pep2 and Pep3, but not hSOD. The anti-hSOD antibodies failed to react against GST, SmCT-SOD, and SmCT-SOD-derived peptides. To demonstrate the specificity of antibody reactivity against derived peptides after immunization with SmCT-SOD-GST, sera from mice immunized with GST-encapsulated PLA microspheres were shown to react with GST, poorly to SmCT-SOD-GST, and not with any SmCT-SOD-derived peptides or hSOD (Fig. 6C).
FIG. 6.
B-epitope map of SmCT-SOD. (A) SmCT-SOD sequence (153 amino acids; in italics) and derived peptides. Overlapping and nonoverlapping peptides of the 20-mer (in bold) were synthesized. The inclusive amino acids (aa) of the peptides were as follows: aa 7 to 26 (Pep 1); aa 22 to 41 (Pep 2); aa 64 to 83 (Pep 3); aa 84 to 103 (Pep 4); aa 101 to 120 (Pep 5); aa 118 to 137 (Pep 6); aa 134 to 153 (Pep 7). Pep 1 could not be synthesized in four attempts. (B and C) Reactivity of IgG antibodies from pooled sera of mice immunized with 5 mg of SmCT-SOD-GST-encapsulated microspheres/mouse (Microspheres-1); equivalent amount of SmCT-SOD-GST microspheres capable of releasing 50 μg/100 μl/mouse (Microspheres-2); 50 μg of CT-SOD-GST emulsified in alum/100 μl/mouse (Alum); mouse anti-hSOD antibodies (anti-hSOD); and 5 mg of GST-encapsulated microspheres (GST Microspheres) against GST and SmCT-SOD antigens (5 μg/ml) and SmCT-SOD-derived peptides (2 μg/ml). The sera of group Microspheres-1 were diluted starting from 1:2,500. The line represents the cutoff value (mean OD + 3 SD) of preimmune sera.
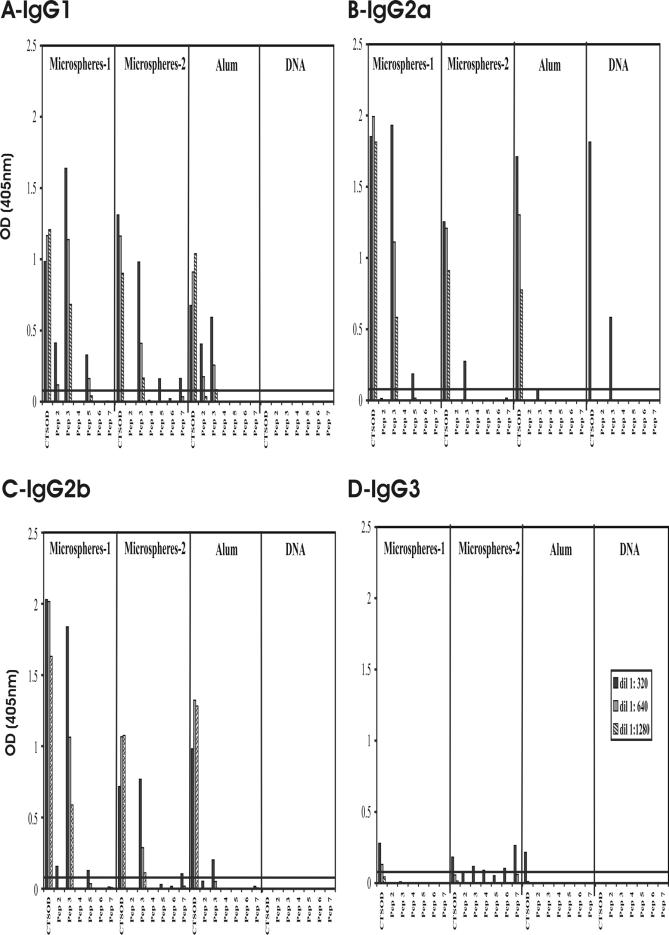
We also evaluated the isotypic response to the SmCT-SOD-derived peptides in mice immunized with different formulations of SmCT-SOD in an attempt to better characterize the immune response stimulated after immunization with the protective antioxidant. The response to SmCT-SOD was calculated by subtracting the reactivity of GST microspheres from that of SmCT-SOD-GST microspheres and the reactivity of GST alum from SmCT-SOD-GST alum (Fig. 7). Vaccination of mice with SmCT-SOD-GST induced high levels of IgG2a anti-SmCT-SOD (Fig. 7B) in pcDNA SmCT-SOD-vaccinated group; however, higher levels of IgG2a anti-SmCT-SOD antibodies were induced by immunization with SmCT-SOD proteins, regardless of the formulation of the antigenic preparation (if encapsulated in PLA microspheres or adsorbed in alum). The reactivity was mainly directed to the SmCT-SOD-derived Pep3, followed by Pep5 (Fig. 7B). Immunization with SmCT-SOD DNA did not induce specific IgG1, IgG2b, or IgG3 antibodies (Fig. 7A, C, and D). However, immunization of mice with SmCT-SOD protein induced high levels of IgG1 and IgG2b isotypes against SmCT-SOD and Pep3 and some reactivity to Pep2 and Pep5 (Fig. 7A and C). In general, immunization with SmCT-SOD adsorbed on alum did not induce levels of antibodies against Pep3 as high as those in groups immunized with SmCT-SOD-encapsulated PLA microspheres. The levels of specific IgG3 were low after immunization with SmCT-SOD proteins (Fig. 7D). The IgA and IgE anti-SmCT-SOD antibodies were not detectable in our assays (data not shown).
FIG. 7.
Isotype responses to SmCT-SOD-derived peptides. Reactivity of IgG1 (A), IgG2a (B), IgG2b (C), and IgG3 (D) antibodies from pooled sera of mice immunized with 5 mg of SmCT-SOD-GST-encapsulated microspheres/mouse (Microspheres-1); equivalent amount of SmCT-SOD-GST microspheres capable of releasing 50 μg/100 μl/mouse (Microspheres-2); 50 μg of CT-SOD-GST mixed in alum/100 μl/mouse (Alum); 100 μg of SmCT-SOD DNA/100 μl/mouse (DNA) against SmCT-SOD antigens (2 μg/ml) and SmCT-SOD derived-peptides (2 μg/ml). The sera of the Microspheres-1 group were diluted starting from 1:2,500. The line represents the cutoff value (mean OD + 3 SD) of preimmune sera.
DISCUSSION
Schistosomiasis is a major cause of morbidity in the world, afflicting over 200 million people, and over 600 million people are at risk of contracting the disease (3, 13). Although drug treatment of the mild forms is available (10), often there are very high reinfection rates that make the treatment very costly, especially in developing countries. Once drug treatment ceases, with time infection rates reach pretreatment levels (26). This, coupled with the risk of drug resistance, supports the need for the development of a vaccination program that would result in control of transmission and permanent reduction of morbidity (63). The ultimate goal of our research is the development of an efficacious vaccine against schistosomiasis that contains parasite-specific epitopes.
First, it was crucial to ensure that the vaccine candidate would not be potentially harmful to the host. Because of the shared identity between S. mansoni and host antioxidant enzymes, the possibility of generating antibodies to human epitopes was addressed. Autoimmune responses elicited by cross-reactive antibodies could lead to tissue damage, such as that seen in individuals that develop antibody to streptococcal M antigens (67). Our results showed that there was no cross-reactivity between antibodies from SmCT-SOD-immunized animals (mouse and rabbit) or humans naturally exposed to S. mansoni infection and nondenatured hSOD. However, both mouse and rabbit antibodies generated against SmCT-SOD recognized the denatured form of hSOD. The recognition of denatured but not native forms of myosin and S. mansoni phosphoglycerate kinase following immunization has also been described (36, 67). These cross-reactive antibodies only recognized denatured forms of host antigens. To circumvent the concern for epitopes that are formed from denatured host proteins inducing an autoimmune response, we analyzed the immunogenicity of linear synthetic peptides derived from SmCT-SOD. Synthetic peptides have been widely used for the evaluation of both B- and T-cell epitopes responsible for (or that could lead to) protection against other pathogens, including Schistosoma (20, 33, 54, 55). In the present study, we demonstrate that BALB/c mice vaccinated with SmCT-SOD developed high levels of antibodies directed mainly to the SmCT-SOD-derived Pep 3, which included amino acid residues 64 to 83. We are currently investigating if such reactivity is major histocompatibility complex restricted.
Among other factors, the dose and nature of the antigen as well as the route or kind of adjuvant used can ultimately dictate the outcome of an immunization. It may be possible that the simple alteration of antigen introduction and formulation can prevent an autoimmune response. New-generation vaccines, such as synthetic peptides, protein polysaccharide conjugates, and plasmid DNA, are likely to be less toxic but also less immunogenic, which has prompted the development of potent and safe adjuvants, both as vaccine delivery systems and as immunostimulatory adjuvants (47). The only adjuvant currently approved by the U.S. Food and Drug Administration is aluminum-based mineral salts (generically called alum). However, the induction of a T helper (Th2)-dominant immune response remains a major limitation to the application of alum to several vaccines (52). The mechanism(s) which allows alum to initiate Th2 responses against adsorbed antigens remains unclear, although the responses have been shown to be independent of the key Th2-associated cytokines, interleukin-4 or interleukin-13, and signaling via the signal transducer and activation of transcription-6 (STAT-6) (5). The biodegradable and biocompatible polyester polymers, such as PLA-co-glycolides, poly-d,l-lactide-co-glycolic acid, and PLA, although used for many years as suture material and as controlled-release drug delivery systems (49, 53), had their adjuvant effects achieved through the encapsulation of antigens (46, 48). Applications from cancer therapy (21, 22) to infectious diseases (57, 59) have been demonstrated. The enhanced adjuvant effect of microparticles appears to be effective as a consequence of efficient delivery of the adsorbed proteins into dendritic cells and macrophages at the injection site and local lymph nodes (61).
Another feature of microparticles is their ability to control the rate of release of entrapped antigens (45). In addition, the polymers may protect the polypeptides from denaturing conditions, such as water and proteolytic enzymes. Controlled release of antigen allows the development of single-dose vaccines, which would result in improved vaccine compliance, particularly in the countries where the disease is endemic (47). High levels of protective antibodies were observed recently using a single immunization of both Plasmodium falciparum (57) and hepatitis B virus (59) antigens that were encapsulated in microspheres, in comparison with alum-based formulations. Concurrent with these findings, our results showed that, along with double immunization, one single dose of SmCT-SOD proteins encapsulated in PLA microspheres was able to induce high titers of specific antibodies (over 1:10,240) in immunized mice (Fig. 4A), raising the possibility of the SmCT-SOD vaccine candidate given as a single dose.
Clearance of parasitic infections often depends on specific classes and subclasses of antibodies, since each isotype has a distinct biological function (23). The identification of antigens that elicit strong, but not detrimental, antibody responses, that is, ones that will lead to a subclass most likely to produce the desired effect, are fundamental to vaccine strategies. Surprisingly, the levels of anti-SmCT-SOD IgG1 antibodies stimulated by vaccination with SmCT-SOD absorbed on alum and with PLA encapsulated were somewhat similar. One possible explanation is that SmCT-SOD protein is a Th1 inducer by itself, but when administered with alum (a Th2 stimulant) it was not strong enough to induce a complete switch from Th2 to Th1 response and rather induced a diminution of the Th2 response or increase of Th1 responses, which made them relatively equivalent. Interestingly, our results showed that although both adjuvant formulations used for delivery of SmCT-SOD proteins (encapsulated in microspheres or absorbed on alum) elicited overall high levels of IgG antibodies, the mice vaccinated with SmCT-SOD-encapsulated PLA microspheres developed higher levels of IgG2a anti-SmCT-SOD and anti-Pep 3 antibodies than those in the alum adjuvant group. The subclass IgG2a (and the human homologue IgG1) is a Th1 cytokine-derived isotype that has been described as protective against larval stages (schistosomules) in a murine model, possibly through enhanced phagocytosis and antibody-dependent cell cytotoxic mechanisms. We have evidence that in vaccination with SmCT-SOD DNA (Cook et al., submitted) followed by challenge with 21-day-old and older worms, protected mice developed high levels of specific IgG2a antibodies, raising the possibility of their participation in the protection against adult worms in this experimental model. However, the significance of the administration of SmCT-SOD with different adjuvant formulations (including DNA and protein) as well as the role of specific antibodies or cellular responses and the development of protection against challenge with S. mansoni are unknown and are currently under investigation. Mice vaccinated with DNA that contained the SmCT-SOD gene seemed to react with GST (Fig. 4D), although previous reports showed that there was no cross-reactivity between the S. japonicum (Sj26) GST and the S. mansoni counterpart in mice (28, 64). However, as this reactivity occurred only at a very low titer (1:80), we considered such reactivity irrelevant in our study.
Numerous studies on individuals in areas endemic for Schistosoma have shown that parasite-specific humoral and cellular responses vary in their correlation with the development of resistance and/or susceptibility and morbidity to infection and reinfection (9, 50). Furthermore, there is still controversy around the role of Th1 versus Th2 in human schistosomiasis. For instance, a Th1 profile has been associated with protection against infection in naturally resistant individuals, where high levels of gamma interferon (1, 6, 68) and tumor necrosis factor alpha cytokines (6) were found. However, a correlation between high levels of specific IgE (a Th2 type) and resistance to both infection (in putative resistant EN individuals) and reinfection (in posttreatment resistant individuals) has also been described (18, 19, 27, 56). On the other hand, elevated production of specific IgG4 antibody (also a Th2 type) was associated with increased susceptibility to infection (18, 19, 25, 27). Consistent with the findings on acquired resistance, increasing evidence has shown that a balance between both Th1 and Th2 responses rather than a polarization could be beneficial in the development of protection against S. mansoni infections, both in humans (14) and in experimental models (69). In our study, the reactivity of IgG anti-SmCT-SOD was not statistically distinct among individuals presenting different clinical forms, perhaps due to the number of individuals assayed or simply because the natural infection itself does not stimulate a differential response to SmCT-SOD. However, this IgG reactivity seemed to be higher (above the cutoff) among the group that was in contact with the parasite but did not harbor eggs in their feces, those cured after treatment. It is not known yet if higher anti-SmCT-SOD reactivity of any particular subclass(es) of IgG or any other antibodies following immunization with SmCT-SOD could be related to the development of resistance to infection and reinfection in humans. Since there has been concern recently that potent adjuvants might activate immunity to such an extent that an autoimmune condition might be triggered (47), it was crucial to verify in our study that immunization of mice with different formulations of SmCT-SOD (encapsulated in PLA microspheres, adsorbed on alum, or as naked DNA) did not stimulate a humoral immune response to the native hSOD homologue. Since DNA vaccination allows for the expression of antigens in their native form, the next step is to evaluate if the immunization protocol of priming with naked SmCT-SOD DNA and boosting with SmCT-SOD proteins and/or derived peptides in different adjuvant formulations can meet or even enhance the protection levels achieved by prime-boost immunization with DNA only.
In summary, our results show that there is the potential for immunization with the whole SmCT-SOD molecule to induce antibodies that react with denatured hSOD. However, there was no cross-reactivity between antibodies from SmCT-SOD-immunized animals or humans naturally exposed to S. mansoni infection and the nondenatured hSOD. We identified parasite-specific epitopes in CT-SOD and demonstrated the utility of various adjuvants, especially microspheres, to induce high-titer responses. Future experiments will evaluate the potential of a parasite-specific subunit vaccine to target S. mansoni larvae as well as adult worms without stimulating a potentially harmful autoreactive response.
Acknowledgments
This research was supported by National Institute of Allergy and Infectious Diseases grants AII8867 and AI45451.
We thank Sharon Willard for expert technical assistance.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Bahia-Oliveira, L. M., A. J. Simpson, L. F. Alves-Oliveira, C. Carvalho-Queiroz, A. M. Silveira, I. R. Viana, J. R. Cunha-Melo, P. Hagan, G. Gazzinelli, and R. Correa-Oliveira. 1996. Evidence that cellular immune responses to soluble and membrane associated antigens are independently regulated during human schistosomiasis mansoni. Parasite Immunol. 18:53-63. [DOI] [PubMed] [Google Scholar]
- 2.Bergquist, N. R. 1998. Schistosomiasis vaccine development: progress and prospects. Mem. Inst. Oswaldo Cruz. 93(Suppl. 1):95-101. [DOI] [PubMed] [Google Scholar]
- 3.Bergquist, N. R. 2002. Schistosomiasis: from risk assessment to control. Trends Parasitol. 18:309-314. [DOI] [PubMed] [Google Scholar]
- 4.Boros, D. L. 1989. Immunopathology of Schistosoma mansoni infection. Clin. Microbiol. Rev. 2:250-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brewer, J. M., M. Conacher, C. A. Hunter, M. Mohrs, F. Brombacher, and J. Alexander. 1999. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J. Immunol. 163:6448-6454. [PubMed] [Google Scholar]
- 6.Brito, C. F., I. R. Caldas, P. Coura Filho, R. Correa-Oliveira, and S. C. Oliveira. 2000. CD4+ T cells of schistosomiasis naturally resistant individuals living in an endemic area produce interferon-gamma and tumour necrosis factor-alpha in response to the recombinant 14KDA Schistosoma mansoni fatty acid-binding protein. Scand. J. Immunol. 51:595-601. [DOI] [PubMed] [Google Scholar]
- 7.Brophy, P. M., and D. I. Pritchard. 1992. Immunity to helminths: ready to tip the biochemical balance? Parasitol. Today 8:419. [DOI] [PubMed] [Google Scholar]
- 8.Butterworth, A. E. 1984. Cell-mediated damage to helminths. Adv. Parasitol. 23:143-235. [DOI] [PubMed] [Google Scholar]
- 9.Butterworth, A. E. 1998. Immunological aspects of human schistosomiasis. Br. Med. Bull. 54:357-368. [DOI] [PubMed] [Google Scholar]
- 10.Butterworth, A. E., D. W. Dunne, A. J. Fulford, K. J. Thorne, K. Gachuhi, J. H. Ouma, and R. F. Sturrock. 1992. Human immunity to Schistosoma mansoni: observations on mechanisms, and implications for control. Immunol. Investig. 21:391-407. [DOI] [PubMed] [Google Scholar]
- 11.Callahan, H. L., R. K. Crouch, and E. R. James. 1988. Helminth anti-oxidant enzymes: a protective mechanism against host oxidants? Parasitol. Today 4:218-225. [DOI] [PubMed] [Google Scholar]
- 12.Capron, M., and A. Capron. 1992. Effector functions of eosinophils in schistosomiasis. Mem. Inst. Oswaldo Cruz. 87(Suppl. 4):167-170. [DOI] [PubMed] [Google Scholar]
- 13.Chitsulo, L., D. Engels, A. Montresor, and L. Savioli. 2000. The global status of schistosomiasis and its control. Acta Trop. 77:41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Correa-Oliveira, R., I. R. Caldas, and G. Gazzinelli. 2000. Natural versus drug-induced resistance in Schistosoma mansoni infection. Parasitol. Today 16:397-399. [DOI] [PubMed] [Google Scholar]
- 15.Correa-Oliveira, R., E. J. Pearce, G. C. Oliveira, D. B. Golgher, N. Katz, L. G. Bahia, O. S. Carvalho, G. Gazzinelli, and A. Sher. 1989. The human immune response to defined immunogens of Schistosoma mansoni: elevated antibody levels to paramyosin in stool-negative individuals from two endemic areas in Brazil. Trans. R. Soc. Trop. Med. Hyg. 83:798-804. [DOI] [PubMed] [Google Scholar]
- 16.Coulson, P. S., L. E. Smythies, and R. A. Wilson. 1993. Pulmonary granulomatous hypersensitivity: cell-mediated responses to embolized schistosome larvae and eggs. Regul. Immunol. 5:165-173. [PubMed] [Google Scholar]
- 17.Damian, R. T. 1989. Molecular mimicry: parasite evasion and host defense. Curr. Top. Microbiol. Immunol. 145:101-115. [DOI] [PubMed] [Google Scholar]
- 18.Demeure, C. E., P. Rihet, L. Abel, M. Ouattara, A. Bourgois, and A. J. Dessein. 1993. Resistance to Schistosoma mansoni in humans: influence of the IgE/IgG4 balance and IgG2 in immunity to reinfection after chemotherapy. J. Infect. Dis. 168:1000-1008. [DOI] [PubMed] [Google Scholar]
- 19.Dunne, D. W., A. E. Butterworth, A. J. Fulford, H. C. Kariuki, J. G. Langley, J. H. Ouma, A. Capron, R. J. Pierce, and R. F. Sturrock. 1992. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur. J. Immunol. 22:1483-1494. [DOI] [PubMed] [Google Scholar]
- 20.Eggleton, P., F. J. Ward, S. Johnson, M. A. Khamashta, G. R. Hughes, V. A. Hajela, M. Michalak, E. F. Corbett, N. A. Staines, and K. B. Reid. 2000. Fine specificity of autoantibodies to calreticulin: epitope mapping and characterization. Clin. Exp. Immunol. 120:384-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egilmez, N. K., Y. S. Jong, Y. Iwanuma, J. S. Jacob, C. A. Santos, F. A. Chen, E. Mathiowitz, and R. B. Bankert. 1998. Cytokine immunotherapy of cancer with controlled release biodegradable microspheres in a human tumor xenograft/SCID mouse model. Cancer Immunol. Immunother. 46:21-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egilmez, N. K., Y. S. Jong, M. S. Sabel, J. S. Jacob, E. Mathiowitz, and R. B. Bankert. 2000. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 60:3832-3837. [PubMed] [Google Scholar]
- 23.Garraud, O., R. Perraut, G. Riveau, and T. B. Nutman. 2003. Class and subclass selection in parasite-specific antibody responses. Trends Parasitol. 19:300-304. [DOI] [PubMed] [Google Scholar]
- 24.Gazzinelli, G., J. R. Lambertucci, N. Katz, R. S. Rocha, M. S. Lima, and D. G. Colley. 1985. Immune responses during human Schistosomiasis mansoni. XI. Immunologic status of patients with acute infections and after treatment. J. Immunol. 135:2121-2127. [PubMed] [Google Scholar]
- 25.Grogan, J. L., P. G. Kremsner, G. J. van Dam, A. M. Deelder, and M. Yazdanbakhsh. 1997. Anti-schistosome IgG4 and IgE at 2 years after chemotherapy: infected versus uninfected individuals. J. Infect. Dis. 176:1344-1350. [DOI] [PubMed] [Google Scholar]
- 26.Gryseels, B., L. Nkulikyinka, and D. Engels. 1994. Impact of repeated community-based selective chemotherapy on morbidity due to schistosomiasis mansoni. Am. J. Trop. Med. Hyg. 51:634-641. [DOI] [PubMed] [Google Scholar]
- 27.Hagan, P., U. J. Blumenthal, D. Dunn, A. J. Simpson, and H. A. Wilkins. 1991. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 349:243-245. [DOI] [PubMed] [Google Scholar]
- 28.Henkle, K. J., K. M. Davern, M. D. Wright, A. J. Ramos, and G. F. Mitchell. 1990. Comparison of the cloned genes of the 26- and 28-kilodalton glutathione S-transferases of Schistosoma japonicum and Schistosoma mansoni. Mol. Biochem. Parasitol. 40:23-34. [DOI] [PubMed] [Google Scholar]
- 29.Hong, Z., P. T. LoVerde, M. L. Hammarskjold, and D. Rekosh. 1992. Schistosoma mansoni: cloning of a complementary DNA encoding a cytosolic Cu/Zn superoxide dismutase and high-yield expression of the enzymatically active gene product in Escherichia coli. Exp. Parasitol. 75:308-322. [DOI] [PubMed] [Google Scholar]
- 30.James, E. R. 1994. Superoxide dismutase. Parasitol. Today 10:481-484. [DOI] [PubMed] [Google Scholar]
- 31.James, S. L., and D. L. Boros. 1994. Immune effector role of macrophages in experimental schistosomiasis mansoni. Immunol. Ser. 60:461-473. [PubMed] [Google Scholar]
- 32.James, S. L., and A. Sher. 1983. Mechanisms of protective immunity against Schistosoma mansoni infection in mice vaccinated with irradiated cercariae. III. Identification of a mouse strain, P/N, that fails to respond to vaccination. Parasite Immunol. 5:567-575. [DOI] [PubMed] [Google Scholar]
- 33.Joshi, S. K., A. Bharadwaj, S. Chatterjee, and V. S. Chauhan. 2000. Analysis of immune responses against T- and B-cell epitopes from Plasmodium falciparum liver-stage antigen 1 in rodent malaria models and malaria-exposed human subjects in India. Infect. Immun. 68:141-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz, N., A. Chaves, and J. Pellegrino. 1972. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev. Inst. Med. Trop. Sao Paulo 14:397-400. [PubMed] [Google Scholar]
- 35.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 36.Lee, K. W., A. Thakur, A. M. Karim, and P. T. LoVerde. 1995. Immune response to Schistosoma mansoni phosphoglycerate kinase during natural and experimental infection: identification of a schistosome-specific B-cell epitope. Infect. Immun. 63:4307-4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LoVerde, P. T. 1998. Do antioxidants play a role in schistosome host-parasite interactions? Parasitol. Today 14:284-289. [DOI] [PubMed] [Google Scholar]
- 38.Lunde, M. N., E. A. Ottesen, and A. W. Cheever. 1979. Serological differences between acute and chronic schistosomiasis mansoni detected by enzyme-linked immunosorbent assay (ELISA). Am. J. Trop. Med. Hyg. 28:87-91. [DOI] [PubMed] [Google Scholar]
- 39.Maizels, R. M., D. A. Bundy, M. E. Selkirk, D. F. Smith, and R. M. Anderson. 1993. Immunological modulation and evasion by helminth parasites in human populations. Nature 365:797-805. [DOI] [PubMed] [Google Scholar]
- 40.Mathiowitz, E., J. S. Jacob, Y. S. Jong, G. P. Carino, D. E. Chickering, P. Chaturvedi, C. A. Santos, K. Vijayaraghavan, S. Montgomery, M. Bassett, and C. Morrell. 1997. Biologically erodable microspheres as potential oral drug delivery systems. Nature 386:410-414. [DOI] [PubMed] [Google Scholar]
- 41.Mei, H., and P. T. LoVerde. 1997. Schistosoma mansoni: the developmental regulation and immunolocalization of antioxidant enzymes. Exp. Parasitol. 86:69-78. [DOI] [PubMed] [Google Scholar]
- 42.Mei, H., A. Thakur, J. Schwartz, and P. T. Lo Verde. 1996. Expression and characterization of glutathione peroxidase activity in the human blood fluke Schistosoma mansoni. Infect. Immun. 64:4299-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mkoji, G. M., J. M. Smith, and R. K. Prichard. 1988. Antioxidant systems in Schistosoma mansoni: evidence for their role in protection of the adult worms against oxidant killing. Int. J. Parasitol. 18:667-673. [DOI] [PubMed] [Google Scholar]
- 44.Nare, B., J. M. Smith, and R. K. Prichard. 1990. Schistosoma mansoni: levels of antioxidants and resistance to oxidants increase during development. Exp. Parasitol. 70:389-397. [DOI] [PubMed] [Google Scholar]
- 45.O'Hagan, D. T. 1998. Microparticles and polymers for the mucosal delivery of vaccines. Adv. Drug Deliv. Rev. 34:305-320. [DOI] [PubMed] [Google Scholar]
- 46.O'Hagan, D. T., H. Jeffery, M. J. Roberts, J. P. McGee, and S. S. Davis. 1991. Controlled release microparticles for vaccine development. Vaccine 9:768-771. [DOI] [PubMed] [Google Scholar]
- 47.O'Hagan, D. T., M. L. MacKichan, and M. Singh. 2001. Recent developments in adjuvants for vaccines against infectious diseases. Biomol. Eng. 18:69-85. [DOI] [PubMed] [Google Scholar]
- 48.O'Hagan, D. T., D. Rahman, J. P. McGee, H. Jeffery, M. C. Davies, P. Williams, S. S. Davis, and S. J. Challacombe. 1991. Biodegradable microparticles as controlled release antigen delivery systems. Immunology 73:239-242. [PMC free article] [PubMed] [Google Scholar]
- 49.Okada, H., and H. Toguchi. 1995. Biodegradable microspheres in drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 12:1-99. [DOI] [PubMed] [Google Scholar]
- 50.Pearce, E. J., and A. S. MacDonald. 2002. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2:499-511. [DOI] [PubMed] [Google Scholar]
- 51.Pearce, E. J., and A. Sher. 1987. Mechanisms of immune evasion in schistosomiasis. Contrib. Microbiol. Immunol. 8:219-232. [PubMed] [Google Scholar]
- 52.Pollock, K. G., M. Conacher, X. Q. Wei, J. Alexander, and J. M. Brewer. 2003. Interleukin-18 plays a role in both the alum-induced T helper 2 response and the T helper 1 response induced by alum-adsorbed interleukin-12. Immunology 108:137-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Putney, S. D., and P. A. Burke. 1998. Improving protein therapeutics with sustained-release formulations. Nat. Biotechnol. 16:153-157. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds, S. R., C. E. Dahl, and D. A. Harn. 1994. T and B epitope determination and analysis of multiple antigenic peptides for the Schistosoma mansoni experimental vaccine triose-phosphate isomerase. J. Immunol. 152:193-200. [PubMed] [Google Scholar]
- 55.Reynolds, S. R., C. B. Shoemaker, and D. A. Harn. 1992. T and B cell epitope mapping of SM23, an integral membrane protein of Schistosoma mansoni. J. Immunol. 149:3995-4001. [PubMed] [Google Scholar]
- 56.Rihet, P., C. E. Demeure, A. Bourgois, A. Prata, and A. J. Dessein. 1991. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur. J. Immunol. 21:2679-2686. [DOI] [PubMed] [Google Scholar]
- 57.Rosas, J. E., J. L. Pedraz, R. M. Hernandez, A. R. Gascon, M. Igartua, F. Guzman, R. Rodriguez, J. Cortes, and M. E. Patarroyo. 2002. Remarkably high antibody levels and protection against P. falciparum malaria in Aotus monkeys after a single immunisation of SPf66 encapsulated in PLGA microspheres. Vaccine 20:1707-1710. [DOI] [PubMed] [Google Scholar]
- 58.Shalaby, K. A., L. Yin, A. Thakur, L. Christen, E. G. Niles, and P. T. LoVerde. 2003. Protection against Schistosoma mansoni utilizing DNA vaccination with genes encoding Cu/Zn cytosolic superoxide dismutase, signal peptide-containing superoxide dimutase, and glutathione peroxidase enzymes. Vaccine 22:130-136. [DOI] [PubMed] [Google Scholar]
- 59.Shi, L., M. J. Caulfield, R. T. Chern, R. A. Wilson, G. Sanyal, and D. B. Volkin. 2002. Pharmaceutical and immunological evaluation of a single-shot hepatitis B vaccine formulated with PLGA microspheres. J. Pharm. Sci. 91:1019-1035. [DOI] [PubMed] [Google Scholar]
- 60.Smithers, S. R., and M. J. Doenhoff. 1982. Schistosomiasis, p. 527-607. In S. A. W. Cohen, Immunology of Parasitic Diseases. Blackwell Scientific Publications, Oxford, England.
- 61.Sun, H., K. G. Pollock, and J. M. Brewer. 2003. Analysis of the role of vaccine adjuvants in modulating dendritic cell activation and antigen presentation in vitro. Vaccine 21:849-855. [DOI] [PubMed] [Google Scholar]
- 62.Tallima, H., M. Montash, P. Veprek, J. Velek, J. Jezek, and R. El Ridi. 2003. Differences in immunogenicity and vaccine potential of peptides from Schistosoma mansoni glyceraldehyde 3-phosphate dehydrogenase. Vaccine 21:3290-3300. [DOI] [PubMed] [Google Scholar]
- 63.Taylor, D. W. 1991. Schistosome vaccines. Experientia 47:152-157. [DOI] [PubMed] [Google Scholar]
- 64.Tiu, W. U., K. M. Davern, M. D. Wright, P. G. Board, and G. F. Mitchell. 1988. Molecular and serological characteristics of the glutathione S-transferases of Schistosoma japonicum and Schistosoma mansoni. Parasite Immunol. 10:693-706. [DOI] [PubMed] [Google Scholar]
- 65.Todd, C. W., and D. G. Colley. 2002. Practical and ethical issues in the development of a vaccine against schistosomiasis mansoni. Am. J. Trop. Med. Hyg. 66:348-358. [DOI] [PubMed] [Google Scholar]
- 66.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vashishtha, A., and V. A. Fischetti. 1993. Surface-exposed conserved region of the streptococcal M protein induces antibodies cross-reactive with denatured forms of myosin. J. Immunol. 150:4693-4701. [PubMed] [Google Scholar]
- 68.Viana, I. R., A. Sher, O. S. Carvalho, C. L. Massara, S. M. Eloi-Santos, E. J. Pearce, D. G. Colley, G. Gazzinelli, and R. Correa-Oliveira. 1994. Interferon-gamma production by peripheral blood mononuclear cells from residents of an area endemic for Schistosoma mansoni. Trans. R Soc. Trop. Med. Hyg. 88:466-470. [DOI] [PubMed] [Google Scholar]
- 69.Wynn, T. A., and K. F. Hoffmann. 2000. Defining a schistosomiasis vaccination strategy—is it really Th1 versus Th2? Parasitol. Today 16:497-501. [DOI] [PubMed] [Google Scholar]