Abstract
The involvement of the choroid plexus in host defense during bacterial meningitis is unclear. Aiming to elucidate possible antibacterial mechanisms, we stimulated primary porcine choroid plexus epithelial cells (pCPEC) with proinflammatory cytokines and challenged them with various Streptococcus suis strains. In the supernatant of gamma interferon (IFN-γ)-stimulated pCPEC, streptococcal growth was markedly suppressed. Costimulation with tumor necrosis factor alpha enhanced this bacteriostatic effect, while supplementation of l-tryptophan completely eliminated it. We also demonstrate that an activation of indoleamine 2,3-dioxygenase in the pCPEC seems to be responsible for the IFN-γ-induced bacteriostasis. This supports the hypothesis of an active role of the choroid plexus in host defense against bacterial meningitis.
The pathogenesis of streptococcal meningitis is poorly understood, but the replication of bacteria within the cerebrospinal fluid (CSF) with the subsequent release of proinflammatory and toxic compounds is thought to be a crucial step (10). Due to its outfoldings and brush border extensions, the choroid plexus shares a large surface with the CSF volume and is highly metabolically active. This makes it uniquely suited for a defensive role once microorganisms have entered the ventricular space. However, potential antibacterial mechanisms of the choroid plexus have not been studied, so the objective of our research was to clarify its role in host defense.
We cultivated primary porcine choroid plexus epithelial cells (pCPEC) for our investigations. They were prepared as described previously with minor modifications (6). Briefly, brains from freshly slaughtered pigs were dissected, and the choroid plexus tissue from the lateral and fourth ventricles was removed and treated with mixed cold and warm trypsinization (0.2% solution [Biochrom, Berlin, Germany] for 45 min at 4°C and 20 min at 37°C). Proteolysis was stopped by addition of fetal calf serum (FCS; Biochrom). The cells were centrifuged at 20 × g and resuspended in a 1:1 mixture of Dulbecco's modified Eagle medium and Ham's F-12 medium supplemented with 4 mM l-glutamine, 5% heat-inactivated FCS, 5 μg of insulin/ml, and 100 μg of penicillin-streptomycin/ml. In order to suppress the growth of contaminating fibroblast-like cells, 20 μM cytosine-arabinoside was added. The cells were seeded onto 96- or 12-well culture plates at ∼105 cells/cm2. Upon confluence, pCPEC were cultivated in FCS-free medium and were used for the experiments 3 days later.
For our experiments, we selected Streptococcus suis because it is pathogenic for humans on the one hand and species specific for the pCPEC on the other. It can cause bacterial meningitis in people that are frequently exposed to pigs or their derivatives (4), and it is an important opportunistic pathogen in pigs, causing meningitis, arthritis, and septicemia (16). The following strains were used: 99-734723 (serotype 2; M. Gottschalk, Faculté de Médecine Vétérinaire, Quebec, Canada), A386/94 (a nontypeable clinical isolate from a pig), and T15 (serotype 2; H. Smith, DLO-Institute for Animal Science and Health, Lelystad, The Netherlands). The last two strains have been described previously (1). In addition, a Staphylococcus aureus strain (a blood culture isolate characterized by standard laboratory procedures; Institute for Medical Microbiology, Düsseldorf, Germany) was used as a control (15). Bacteria were maintained as stock cultures at −80°C in Todd-Hewitt broth (Oxoid, Wesel, Germany) with 20% glycerol. Fifty microliters of the stock was grown for 6 h (overnight) in 10 ml of Todd-Hewitt broth at 37°C with mild agitation to mid-log phase to an optical density at 600 nm (OD600) of 0.65 (∼1 × 108 to 5 × 108 CFU/ml).
In order to activate the pCPEC, we chose gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), as they are key proinflammatory mediators found in the CSF of patients with meningitis (7, 11). We stimulated the pCPEC with 0 to 500 U of IFN-γ/ml and/or 100 U of TNF-α/ml for 3 days and then added the bacteria (∼100 CFU, 10 μl to each well). After incubating for another 12 to 24 h, we assessed the effect of pCPEC cytokine stimulation on bacterial growth by measuring the OD620 of the supernatant with a microplate reader (Titertek Multiscan; ICN/Flow, Meckenheim, Germany). All assays were performed in triplicate and repeated at least three times. In addition, bacteria were enumerated directly by plating out 10 μl of serial dilutions of supernatants from identically stimulated cells grown on 12-well plates.
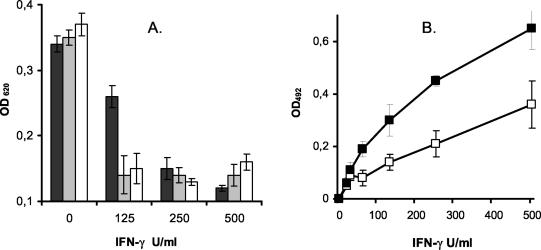
The stimulation of pCPEC with IFN-γ resulted in a significant reduction of bacterial growth compared to that of nonstimulated cells during 24 h of incubation. This effect could be demonstrated for all streptococcal strains and the staphylococcus in a dose-dependent manner. Costimulation with TNF-α enhanced the bacteriostatic effect in pCPEC stimulated with suboptimal doses of IFN-γ, whereas 100 U of TNF-α/ml alone had no effect on the bacterial growth as determined photometrically by measuring the OD620 (Fig. 1A). TNF-α or IFN-γ had no direct effect on S. suis growth in a cell-free assay (data not shown).
FIG. 1.
(A) Bacteriostatic effect of IFN-γ-stimulated pCPEC and increase in kynurenine in the supernatant. Stimulation of the pCPEC with increasing doses of IFN-γ (0 to 500 U/ml) led to a significant reduction in bacterial growth (S. suis strains 99-734723 [black bar], A386/94 [grey bar], and T15 [white bar]) as determined by measuring the OD620. (B) Under the same stimulation with IFN-γ, kynurenine production increased dose dependently (□) with a marked synergistic effect of TNF-α (▪). The data in both panels represent means ± standard errors of at least three independent experiments.
In order to identify the mechanism responsible for the observed bacteriostasis, we assessed the generation of nitric oxide (NO), the superoxide anion production, and the degradation of tryptophan. NO production was determined by the Griess reaction as previously described (3). Sodium nitrite was used as a standard, the detection limit was 1 μM nitrite. In the IFN-γ-stimulated pCPEC, no NO production could be detected (data not shown). Furthermore, we coincubated the pCPEC with 100 μmol of NG-monomethyl-l-arginine or NG-nitro-l-arginine methyl ester (Sigma, Deisenhofen, Germany) per liter, two nonselective inhibitors of the inducible nitric oxide synthase, and found no effect on the bacteriostasis induced by IFN-γ stimulation. In addition, we tried to identify reactive oxygen metabolites as a cause of bacterial growth inhibition. Even maximal stimulation of the pCPEC with phorbol myristate acetate concentrations up to 500 ng/ml did not lead to a measurable activity of superoxide dismutase-sensitive reduction of cytochrome c (data not shown).
To identify whether an activation of indoleamine 2,3-dioxygenase (IDO) accounted for the observed bacteriostasis, we measured kynurenine, the metabolic product of l-tryptophan degradation, as described previously (2). Briefly, 160 μl of cell culture supernatant protein was deproteinized by trichloracetic acid. The samples were centrifuged at 300 × g for 10 min, and Ehrlich reagent was added 1:1 to determine the kynurenine concentration by photometric analysis of the OD492. In addition, l-tryptophan and kynurenine concentrations were determined by high-pressure liquid chromatography (HPLC) (Alliance separations module 2690; Waters, Milford, Mass.). For separation, reversed-phase C18 columns (Supelcosil; Supelco, Bellefonte, Pa.) were used. l-Tryptophan was detected by fluorescence at 285-nm excitation and 365-nm emission wavelengths. l-Kynurenine was detected by measuring the UV absorption at 360 nm (scanning fluorescence detector 474; Waters). Peaks were compared to calibrated l-kynurenine and l-tryptophan controls.
Detection of kynurenine by photometric analysis revealed a dose-dependent accumulation in the pCPEC supernatant, suggesting an activation of IDO. As observed before in other cells (15), the addition of TNF-α further increased the kynurenine production (Fig. 1B) and caused an earlier drop in bacterial growth rates (see Fig. 3).
FIG. 3.
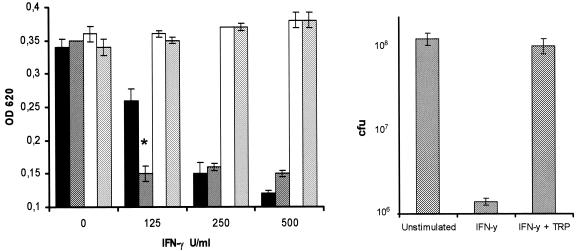
Antagonistic effect of l-tryptophan repletion on the IFN-γ-induced bacteriostasis (S. suis strain A386/94). (A) The bacteriostatic effect of pCPEC stimulation with IFN-γ (black bars) and significant enhancement with TNF-α costimulation (grey bars; *, P < 0.01, as determined by analysis of variance) could be completely eliminated with the repletion of l-tryptophan (white and light grey bars). Data represent means ± standard errors of three independent experiments. (B) Direct counting of CFU in representative experiments demonstrated an IFN-γ-related reduction of bacterial growth by 2 orders of magnitude. Repletion of l-tryptophan showed a return of growth rates to normal levels without l-tryptophan starvation (data represent means ± standard errors of three experiments).
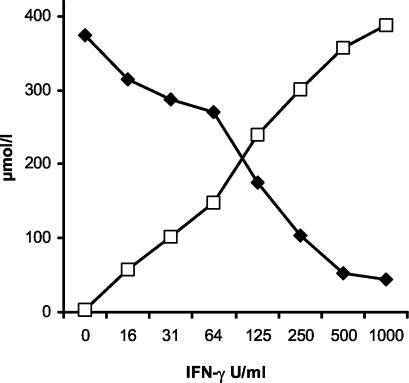
The complete metabolization of l-tryptophan could be verified by HPLC. The concentration of l-tryptophan, added in excess to almost 400 μmol/liter, steadily decreased with an inversely increasing amount of kynurenine, always giving an equimolar total concentration of the two metabolites (Fig. 2). To demonstrate the causal relationship between bacteriostasis and IDO-driven l-tryptophan depletion, we incubated IFN-γ-activated cells with excess l-tryptophan (100 μg/ml) either at the start of cytokine stimulation or at the start of bacterial inoculation. Both methods of l-tryptophan repletion completely inhibited the bacteriostatic effect of the pCPEC supernatant, leading to the same growth rates as in unstimulated controls (Fig. 3).
FIG. 2.
IDO activity in pCPEC stimulated with IFN-γ for 3 days. HPLC measurements show a constant and dose-dependent depletion of l-tryptophan (▪) in the supernatant of IFN-γ (0 to 1,000 U/ml)-stimulated pCPEC with an equimolar increase in kynurenine (□).
Thus, IDO activation with subsequent depletion of the essential amino acid l-tryptophan seems to be responsible for the observed bacteriostasis. l-Tryptophan starvation resulting from IFN-γ activation is a well-characterized mechanism against intracellular pathogens such as toxoplasma or chlamydia (13, 14). In contrast, only a few studies have shown a direct impact on extracellular bacteria so far. Studies on glioblastoma and uroepithelial cell lines demonstrated IDO-induced growth inhibition of group B streptococci by l-tryptophan degradation (2, 12). Related to central nervous system (CNS) infections, we were able to demonstrate a similar effect of S. aureus growth reduction by human brain microvascular endothelial cells (15).
The in vivo relevance of our data is emphasized by studies of patients with inflammatory CNS diseases, where highly elevated levels of kynurenine could be detected in the CSF (8). In a study of children with bacterial meningitis, CSF samples were shown to contain more than 40-times-higher levels of kynurenine than did healthy controls (9). The choroid plexus as a source of tryptophan degradation has been demonstrated in a study of the rabbit brain, where the highest IDO activity could be demonstrated in the choroid plexus (5).
Taken together, our data have shown that pCPEC are capable of restricting growth of S. suis upon activation with proinflammatory cytokines by creating an unfavorable microenvironment for the bacteria. The choroid plexus not only seems to play a role as a blood-CSF barrier against CSF colonization in the early stages of bacterial meningitis but also once invasion into the CNS has occurred.
Editor: J. N. Weiser
REFERENCES
- 1.Allgaier, A., R. Goethe, H. J. Wisselink, H. E. Smith, and P. Valentin-Weigand. 2000. Relatedness of Streptococcus suis isolates of various serotypes and clinical backgrounds as evaluated by macrorestriction analysis and expression of potential virulence traits. J. Clin. Microbiol. 39:445-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Däubener, W., C. Hucke, K. Seidel, U. Hadding, and C. MacKenzie. 1999. Interleukin-1 inhibits gamma interferon-induced bacteriostasis in human uroepithelial cells. Infect. Immun. 67:5615-5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 4.Dupas, D., M. Vignon, and C. Geraut. 1992. Streptococcus suis meningitis. A severe noncompensated occupational disease. J. Occup. Med. 34:1102-1105. [PubMed]
- 5.Fujiwara, M., M. Shibata, Y. Watanabe, T. Nukiwa, F. Hirata, N. Mizuno, and O. Hayaishi. 1978. Indoleamine 2,3-dioxygenase. Formation of L-kynurenine from L-tryptophan in cultured rabbit pineal gland. J. Biol. Chem. 10:6081-6085. [PubMed] [Google Scholar]
- 6.Gath, U., A. Hakvoort, J. Wegener, S. Decker, and H.-J. Galla. 1997. Porcine choroid plexus cells in culture: expression of polarized phenotype, maintenance of barrier properties and apical secretion of CSF-components. Eur. J. Cell Biol. 74:68-78. [PubMed] [Google Scholar]
- 7.Glinmaker, M., P. Olsen, and B. Andersson. 1994. Interferon-gamma in cerebrospinal fluid from patients with viral and bacterial meningitis. Scand. J. Infect. Dis. 26:141-147. [DOI] [PubMed] [Google Scholar]
- 8.Heyes, M. P., K. Saito, J. S. Crowley, L. E. Davis, M. A. Demitrack, M. Der, L. A. Dilling, J. Elia, M. J. Kruesi, A. Lackner, S. A. Larsen, K. Lee, H. L. Leonard, S. P. Markey, A. Martin, S. Milstein, M. M. Mouradian, M. R. Pranzatelli, B. J. Quearry, A. Salazar, M. Smith, S. E. Strauss, T. Sunderland, S. W. Swedo, and W. W. Tourtellotte. 1992. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain 115:1249-1273. [DOI] [PubMed] [Google Scholar]
- 9.Heyes, M. P., K. Saito, S. Milstien, and S. J. Schiff. 1995. Quinolinic acid in tumors, hemorrhage and bacterial infections of the central nervous system in children. J. Neurol. Sci. 133:112-118. [DOI] [PubMed] [Google Scholar]
- 10.Kim, K. S. 2003. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nat. Rev. Neurosci. 4:376-385. [DOI] [PubMed] [Google Scholar]
- 11.Kornelisse, E. F., C. E. Hack, H. F. J. Savelkoul, T. C. van der Pouw Kraan, W. C. J. Hop, G. van Mierlo, M. H. Suur, H. J. Nejens, and R. de Groot. 1997. Intrathecal production of interleukin-12 and gamma interferon in patients with bacterial meningitis. Infect. Immun. 65:877-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacKenzie, C. R., C. Willberg, and W. Däubener. 1998. Inhibition of group B streptococcal growth by IFN-γ-activated human glioblastoma cells. J. Neuroimmunol. 89:191-197. [DOI] [PubMed] [Google Scholar]
- 13.Paguirigan, A. M., G. I. Byrne, S. Becht, and J. M. Carlin. 1994. Cytokine-mediated indoleamine 2,3-dioxygenase induction in response to Chlamydia infection in human macrophage cultures. Infect. Immun. 62:1131-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfefferkorn, E. R. 1984. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc. Natl. Acad. Sci. USA 81:908-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroten, H., B. Spors, C. Hucke, M. Stins, K. S. Kim, R. Adam, and W. Däubener. 2001. Potential role of human brain microvascular endothelial cells in the pathogenesis of brain abscess: inhibition of Staphylococcus aureus by activation of indoleamine 2,3 dioxygenase. Neuropediatrics 32:206-210. [DOI] [PubMed] [Google Scholar]
- 16.Staats, J. J., I. Feder, O. Okwumabua, and M. M. Chengappa. 1997. Streptococcus suis: past and present. Vet. Res. Commun. 21:381-407. [DOI] [PubMed] [Google Scholar]