Abstract
Background
During female reproductive cycles, a rapid fall in circulating progesterone (P4) levels is one of the earliest events that occur during induced luteolysis in mammals. In rodents, it is well recognized that during luteolysis, P4 is catabolized to its inactive metabolite, 20alpha-hydroxyprogesterone (20alpha-OHP) by the action of 20alpha-hydroxysteroid dehydrogenase (20alpha-HSD) enzyme and involves transcription factor, Nur77. Studies have been carried out to examine expression of 20alpha-HSD and its activity in the corpus luteum (CL) of buffalo cow.
Methods
The expression of 20alpha-HSD across different bovine tissues along with CL was examined by qPCR analysis. Circulating P4 levels were monitored before and during PGF2alpha treatment. Expression of 20alpha-HSD and Nur77 mRNA was determined in CL at different time points post PGF2alpha treatment in buffalo cows. The chromatographic separation of P4 and its metabolite, 20alpha-OHP, in rat and buffalo cow serum samples were performed on reverse phase HPLC system. To further support the findings, 20alpha-HSD enzyme activity was quantitated in cytosolic fraction of CL of both rat and buffalo cow.
Results
Circulating P4 concentration declined rapidly in response to PGF2alpha treatment. HPLC analysis of serum samples did not reveal changes in circulating 20alpha-OHP levels in buffalo cows but serum from pseudo pregnant rats receiving PGF2alpha treatment showed an increased 20alpha-OHP level at 24 h post treatment with accompanying decrease in P4 concentration. qPCR expression of 20alpha-HSD in CL from control and PGF2alpha-treated buffalo cows showed higher expression at 3 and 18 h post treatment, but its specific activity was not altered at different time points post PGF2alpha treatment. The Nur77 expression increased several fold 3 h post PGF2alpha treatment similar to the increased expression observed in the PGF2alpha-treated pseudo pregnant rats which perhaps suggest initiation of activation of apoptotic pathways in response to PGF2alpha treatment.
Conclusions
The results taken together suggest that synthesis of P4 appears to be primarily affected by PGF2alpha treatment in buffalo cows in contrast to increased metabolism of P4 in rodents.
Keywords: Buffalo cow, Corpus luteum, PGF2α, P4, 20α-HSD, 20α-OHP, Nur77
Background
In rats during pregnancy, catabolism of progesterone (P4) to its inactive metabolite, 4-Pregnen-20α-ol-3-one i.e. 20α-hydroxyprogesterone (20α-OHP) has been suggested to be one of the key mechanisms for regulation of circulating P4 concentration both in maternal and fetal compartments [1-3]. The enzyme, 20α-hydroxysteroid dehydrogenase (20α-HSD), classified as one of the members of aldo-keto reductase superfamily is responsible for conversion of P4 into 20α-OHP [1]. In mice null for 20α-HSD gene, the length of estrous cycle and the duration of pseudo pregnancy and pregnancy periods were significantly prolonged although serum P4 levels decreased low enough for delivery of pups at term of pregnancy [4,5]. In pregnant goats, low concentration of P4 and high concentration of 20α-OHP in the fetal blood, while high concentration of P4 and low concentration of 20α-OHP in maternal blood have been reported [2]. In the baboon, the activity of 20α-HSD in placenta was observed to be higher with a corresponding increase in the concentration of 20α-OHP in the fetal compartment during late pregnancy [3]. In many of these species, the observation of increased 20α-OHP levels in the placenta is suggestive of regulation of P4 concentration by the feto-placental unit and/or parturition process. Since 20α-HSD is essential for conversion of P4 into 20α-OHP, it can be suggested that 20α-HSD expression in placenta plays an important role during fetal development and/or parturition process. However, induction of 20α-HSD expression in the corpus luteum (CL) is one of the striking features of luteolysis that occurs immediately prior to parturition and lactogenesis in pregnant rats [6,7].
During PGF2α-induced luteolysis, concomitant with the decreased P4 concentration, an increased concentration of 20α-OHP has been reported in pregnant rats [8]. Rat cDNA expression array analysis findings have provided evidence for convergence of opposing actions of prolactin and PGF2α on 20α-HSD expression in the CL [9]. Furthermore, during PGF2α treatment, an early association of increased expression of nerve growth factor-induced clone-B (NGFIB, also known as Nur77, NR4A1, among other designations) and 20α-HSD has been observed, that suggests participation of Nur77 in the induction of expression of 20α-HSD gene [8]. Nur77 which functions as transcription factor is a nuclear receptor protein belonging to steroid receptor superfamily and is suggested to play an important role in cell fate decisions [10]. Nur77 was originally characterized as immediate early response gene and has been shown to regulate expression of a number of steroidogenic genes in the ovary [11,12]. Also, Nur77 has been implicated as mediator of thymocyte and T-cell apoptosis [13,14]. Studies suggest that Nur77 induces apoptosis by activation of genes involving both extrinsic and intrinsic apoptotic pathways [15,16].
Despite extensive research, the cellular and molecular mechanisms involved in the PGF2α-induced luteal regression remains poorly understood. At present, with the exception of studies in rodents, reports of examination of 20α-HSD expression in CL of other species are sparse [17,18]. Moreover, whether P4 undergoes catabolism in the CL during spontaneous and PGF2α-induced luteolysis has not been reported in other species. It should be pointed out that the function of CL in bovine species unlike species such as primates is largely under the control of luteolytic factor, PGF2α. With a view to further gain insights into the PGF2α-induced luteolysis, several experiments were carried out in the buffalo cows with the following objectives: 1) To study 20α-HSD expression in various tissues including the CL of the buffalo cow, 2) To examine expression of Nur77, expression and activity of 20α-HSD during the PGF2α-induced luteolysis in the buffalo cow, and 3) To determine the concentration of 20α-OHP during PGF2α-induced luteolysis. The experiments involving well established rat model for PGF2α-induced 20α-HSD expression and activity were included for purposes of comparison with buffalo cow experiments.
Methods
Reagents
Juramate® (Cloprostenol sodium, the synthetic analogue of PGF2α) was purchased from Jurox, Australia. P4 (GDN#337) antisera was kindly provided by Prof. G.D. Niswender, Colorado State University, Fort Collins, CO. DyNAzyme™II DNA polymerase was obtained from Finnzymes, Espoo, Finland. Moloney murine lukemia virus (MMuLV) reverse transcriptase (Revert Aid™), RNase inhibitor (RNasein), 10 mM dNTP mix and 100 bp ladder were obtained from MBI Fermentas, Germany. NADP (disodium salt) and NADPH (tetra sodium salt) was obtained from HiMedia Laboratories Pvt. Ltd., Mumbai, India. Reference standards for 4-Pregnen-20α-ol-3-one (20α-OHP) and P4 were obtained from Sigma-Aldrich, Bangalore, India. Oligo dT and oligonucleotide primers were synthesized by Sigma-Genosys, Bangalore, India. The high performance liquid chromatography (HPLC) grade acetonitrile was obtained from Qualigens, Mumbai, India. All other reagents were purchased from Sigma-Aldrich, Bangalore, India or sourced through local suppliers.
Animals, experimental protocol, blood and CL collection schedule
Experiments in buffalo cows
a. Collection of different organs for assessment of 20α-HSD mRNA expression
Non lactating adult buffalo cows (Bubalus bubalis; Surthi breed) aged 5–6 years with a known history of normal cyclicity were recruited for the study. Tissues such as spleen, brain, skeletal muscle, kidney, mammary gland, lung, heart, liver, myometrium and CL (n = 3/tissue) were collected to analyse the expression of 20α-HSD across different tissues.
b. Characterization of PGF2α effects on CL function
The day of onset of estrus was designated as day 0 of estrous cycle. To verify the presence of functional CL, blood samples were collected on days 3 to 7 of the cycle for monitoring circulating P4 concentration. In this experiment, Juramate® (PGF2α) was administered 500 μg i.m., on day 11 of estrous cycle and CL was collected immediately before (0 h), 3, 6, 18 and 36 and 60 h post PGF2α injection. Blood samples were collected immediately before (n = 9 animals) and at different time intervals (n = 6 animals/ time point) post PGF2α injection for determining serum P4 levels. Ovaries containing CL (n = 3/time point) were collected post slaughter and washed in sterile ice cold PBS and transferred into Dulbecco’s Modified Eagles Medium supplemented with penicillin (500 U/ml) and streptomycin (50 μg/ml) and transported to the laboratory on ice within 30 min of collection. Under sterile conditions, CL was extirpated, cut into eight to twelve pieces, transferred to labelled cryovials, snap frozen in liquid nitrogen and stored at −70°C until analysis.
Experiment in rats
Effect of PGF2α treatment on luteal function in rats
It is well documented that PGF2α treatment increases 20α-HSD expression in the CL and circulating 20α-OHP in pseudo pregnant rats [8,19]. We utilized pseudo pregnant rat model system to serve as reference (with regard to post PGF2α treatment related rise in 20α-HSD expression and 20α-OHP concentration) for PGF2α studies in buffalo cows. Three month old adult female rats (Wistar strain) were housed in a controlled environment and kept under a photoperiod of 12 h light and 12 h of darkness cycle with ad libitum access to food and water. Pseudo pregnancy was induced in female rats by cohabitation with vasectomised male rats on the afternoon of proestrus. Following cohabitation, female rats were examined for the presence of vaginal plug and/or subjected to screening of vaginal smears daily for the extension of the diestrus period. The presence of vaginal plug and/or upon confirmation of day 1 of continuous diestrus (observed for 3 consecutive days) following cohabitation with vasectomised male rats was designated as day 1 of pseudo pregnancy. The status of pseudo pregnancy was further confirmed by determining the presence of higher (>50 ng/ml) circulating serum P4 concentration on day 5 of pseudo pregnancy. On day 8 of pseudo pregnancy, rats were injected i.p. with PBS (control) or 10 μg/100 μl of Juramate® (PGF2α). Blood (n = 5 animals/time point) and CL (n = 5 animals/time point) were collected before and 24 h post treatments.
All procedures in animals were approved by the Institutional Animal Ethics Committee, Indian Institute of Science, Bangalore, India.
Hormone assays
Serum P4 concentrations were determined by specific radioimmunoassay as reported previously [20]. The sensitivity of the assay was 0.1 ng/ml and the inter- and intra- assay coefficients of variation were <10%.
RNA isolation
Total RNA was extracted from control and PGF2α treated samples using Tri® Reagent according to the manufacturer’s recommendations, as reported previously [20]. RNA was quantitated spectrophotometrically using ND-1000 (NanoDrop, Thermo Scientific, Wilmington, DE, USA). The quality and quantity of RNA were determined by electrophoresis on a 2% (w/v) formaldehyde agarose gel along with RNA samples of known concentration and A260: A280 ratio was >1.8.
Semi quantitative RT-PCR
Semi quantitative RT-PCR analysis for 20α-HSD was carried out as described previously from the laboratory [20]. L19 expression was used to check for the efficiency of RT-PCR. The primers used for 20α-HSD gene were F:5′-CTGTAACCAGGTCGAATGTCAC-3′ and R:5′-GGGTAGTTCGGGTTCACCC-3′; and for L19 were F:5′-CCACATGTATCACAGCCTGTAC-3′ and R:5′-CTTGGTCTTAGACCTGCGG-3′. Primers were designed from recently reported cattle sequences submitted by Naidansuren et al., 2011 [17] [GenBank: GU064907] using Primer Express™ version 2.0 (Applied Biosystems, Foster City, CA, USA) spanning the exon-exon junctions. PCR products were resolved on 2% Tris- acetate-EDTA agarose gels containing ethidium bromide (0.5 μg/ml), and photographed under UV light and analysed using GBox chemi-HR16, gel documentation system (Synoptics Ltd, Cambridge, UK). The amplified PCR product was eluted and cloned into pGEM-T easy vector system I, sequenced and the nucleotide analysis revealed 71% homology with bovine placental and ovary 20α-HSD sequence [17].
Quantitative real time PCR (qPCR)
The analysis was carried out as described previously from the laboratory [21]. The cDNA samples equivalent to 10 ng of total RNA were subjected to validation analysis on Applied Biosystems 7500 Fast Real Time PCR system with SDS v 1.4 program employing Power SYBR green 2X PCR master mix. The following primers were used for analysis, for 20α-HSD gene, F:5′-CTGTAACCAGGTCGAATGTCAC-3′ and R:5′-GGGTAGTTCGGGTTCACCC-3′; for Nur77 gene, F:5′-CTTCTTCAAGCGCACAGTGCAG-3′ and R: 5′-CTGTCTGTCCGGACAACTTCCTTC-3′ and for L19 gene, F:5′-CCACATGTATCACAGCCTGTAC-3′ and R:5′-CTTGGTCTTAGACCTGCGG-3′. Primers were designed using cattle sequences submitted at NCBI and ENSEMBL using Primer Express™ version 2.0 (Applied Biosystems, Foster City, CA, USA). The primers were designed to cover the exon- exon junctions. Real time PCR efficiencies were acquired by amplification of a standard dilution series (with 10 fold differences) in the Applied Biosystems 7500 Fast Real time PCR system with SDS v 1.4 program employing Power SYBR Green 2X PCR mix. The corresponding efficiencies (E) for 20α-HSD and Nur77 were calculated according to the equation: E = 10[−1/slope] -1 [22] and an efficiency of >90% was obtained for both. Analysis of expression of each gene included a no template control (NTC) and generation of a dissociation curve. Expression levels of the genes validated were normalized by using L19 expression levels as calibrator (internal control) for each cDNA sample. The relative expression and fold change in gene expression was determined using ΔCt and ΔΔCt method, respectively.
Relative expression = 2-ΔCt and fold change = 2-ΔΔCt, where Ct = Threshold cycle i.e. the cycle number at which the relative fluorescence of test samples increases above the background fluorescence, ΔCt = [Ct gene of interest (unknown sample) - Ct of L19 (unknown sample)] and ΔΔCt = [Ct gene of interest (unknown sample) - Ct of L19 (unknown sample)] - [Ct gene of interest (calibrator sample) - Ct of L19 (calibrator sample)]. PCR for each sample was set up in duplicates and the average Ct value was used in the ΔΔCt equation.
HPLC analysis
HPLC unit
The chromatographic separation of P4 and its metabolite, 20α-OHP was performed on reverse phase HPLC system (Agilent 1200). Samples were injected via thermostated autosampler. The stationary phase was a Zorbax Eclipse Plus C18 5 μm column (4.6 X 250 mm) comprising of dense monolayer of dimethyl-n-octadecylsilane stationary phase with improved ultrahigh purity Zorbax Rx-SIL porous silica support. The thermostatted column compartment was used at an ambient temperature of 25°C. The readings at 245 nm were taken using variable UV wavelength detector. The mobile phase was a mixture of water (pH 3.4) and acetonitrile with gradient elution from 20 to 66% acetonitrile in 9 min (held for 3 min), then from 66 to 100% acetonitrile in 22 min. Standards for P4 and 20α-OHP were run on HPLC to determine the elution time separately, as well as, together.
Standard and sample preparation and extraction
For HPLC analysis, known concentration of P4 and 20α-OHP standards were diluted in steroid free serum. To remove steroids, 10 ml of bullock serum was treated with 0.5 g of activated charcoal and stirred for 2 h at 4°C. The slurry was centrifuged at 1750 X g for 10 min. The clear supernatant was collected and stored as 1–2 ml aliquots at −20°C.
The lipid extraction from serum samples was carried out by addition of methanol-diethyl ether mixture. For rat serum extraction (n = 5/time point), 500 μl of serum was mixed with 50 μl methanol and 5 ml diethyl ether, vortexed manually for 2 min and solvents containing lipids were separated after precipitating aqueous phase in liquid nitrogen and evaporating the solvent on a 37°C water bath. After repeating the procedure two more times, the extracted lipid was reconstituted in 10% acetonitrile. For bovine serum (n = 5/time point) lipid extraction, same procedure as used for rat serum was followed but with 2.5 ml serum volume. The samples were run on the HPLC column as mentioned earlier. The run was analysed drawing chromatograms using the Agilent Chemstation software and the runs were compared with P4 and 20α-OHP standards.
Preparation of CL tissue cytosolic fraction
All procedures were performed at 4oC. Frozen CL tissues (10–15 mg wet weight) from rat and buffalo cows were homogenized in 500 μl of potassium phosphate buffer (5 mM, pH 7.0) containing 1 mM EDTA, 1 mM dithiothreitol and 10% glycerol. Protease inhibitors, 1 mM phenylmethanesulfonyl fluoride and 20 μg of leupeptin/ml and 40 μg of aprotenin/ml were used. The homogenate was centrifuged at 10,500 X g for 90 min. The supernatant was used as the cytosolic fraction.
Measurement of luteal 20α-HSD activity
The activity of 20α-HSD was determined by the method of Wiest et al., 1968 [6] with a few modifications. The assay medium was Tris–HCl buffer solution (0.1 mM, pH 8.0) containing 30 μM 20α-OHP, 300 μM NADP, 1 mM EDTA, 5 mM dithiothreitol and 3% ethanol for sterol solubilisation; dithiothreitol and NADP were added immediately before use. The enzyme reaction was initiated at 37°C by adding 12.5 μl sample into the assay medium with rapid mixing. The OD values were recorded spectrophotometrically at 340 nm for 3 min. For sample blank, the cytosolic fraction was mixed with reaction buffer and OD values were recorded. The change in the concentration of NADPH formed in samples was calculated from the NADPH standard graphs. The enzyme activity was defined as the amount of enzyme that could induce 1 nmol NADPH min-1 mg-1 protein at 37°C.
Statistical analysis
Where applicable, data were expressed as mean ± SEM. The arbitrary densitometric units were represented as relative mRNA expression after dividing the band intensity for L19 of the corresponding sample. Comparisons between mean of two groups were carried out using a non-parametric test, Mann–Whitney test, without assuming the Gaussian distribution. For multiple comparisons, the data were analyzed by one way ANOVA, followed by the Newman-Keuls multiple comparison test (PRISM Graph Pad, version 5; Graph Pad Software, Inc., San Diego, CA). A p-value of <0.05 was considered to be significant.
Results
Expression of 20α-HSD in various tissues
The qPCR expression of 20α-HSD mRNA was determined in various tissues of the buffalo cow and the results are presented in Figure 1. The mRNA expression was high in the CL and the expression was also detectable in spleen, brain and liver. However, the expression was low in mammary gland, kidney, heart and myometrium (Figure 1). In lung and skeletal muscle tissues, expression was undetectable (data not shown).
Figure 1.
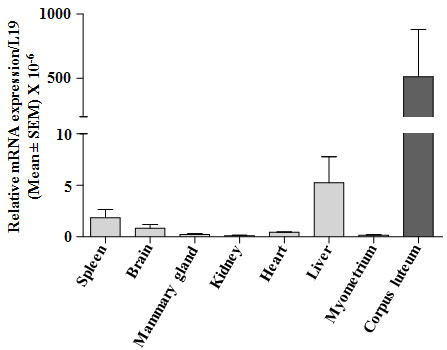
Quantitative real time PCR expression of 20α-HSD mRNA in different tissues of buffalo cows. 20α-HSD expression was normalized to L19 and the 20α-HSD mRNA expression is represented as relative expression level.
Effects of PGF2α treatment on circulating P4 levels, luteal expression of 20α-HSD and Nur77 in the buffalo cow
Circulating P4 concentration in buffalo cows on day 11 of estrous cycle immediately before PGF2α injection was 4.0 ± 0.34 ng/ml, and the concentrations were 1.23 ± 0.12, 1.09 ± 0.18 and 0.76 ± 0.09 ng/ml at 3, 6 and 18 h post treatment, respectively (Figure 2A). A significant (p < 0.001) decrease in P4 concentration was observed within 3 h post treatment and the concentrations further declined at 6 and 18 h time points. The fold change in expression of 20α-HSD mRNA in CL collected from control and PGF2α treated animals are presented in Figure 2B. The 20α-HSD mRNA expression was 4–7 fold higher after PGF2α treatment (Figure 2B). qPCR expression of Nur77 was >15 fold higher at 3 h post PGF2α injection, however, the expression at other time points post PGF2α injection was not significantly different from CL of PGF2α untreated buffalo cows i.e. time 0 time point (Figure 2C).
Figure 2.
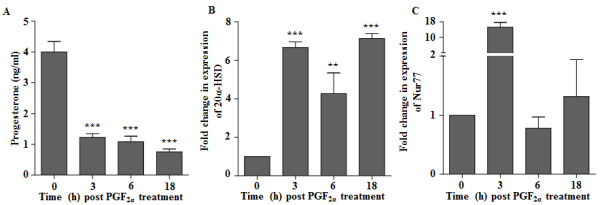
Effect of PGF2α treatment on serum progesterone, luteal 20α-HSD and Nur77 mRNA expressions. Buffalo cows received intramuscular injection of 500 μg of PGF2α on day 11 of estrous cycle and blood and luteal tissue samples were collected at 0, 3, 6 and 18 h post PGF2α treatment. (A) Mean (±SEM) circulating serum progesterone (P4) concentration immediately before (0 h) and after (3, 6 and 18 h) the PGF2α treatment in buffalo cows, (***p < 0.001). (B) qPCR expression of 20α-HSD mRNA levels in the CL from buffalo cows collected at 0, 3, 6 and 18 h post PGF2α treatment with the expression normalized with L19 mRNA. (C) qPCR expression of Nur77 mRNA levels in the CL from buffalo cows collected at 0, 3, 6 and 18 h post PGF2α treatment and the expression was normalized with L19 mRNA. The results are shown as fold changes of mRNA expression compared with that at 0 h PGF2α. Bar represents mean ± SEM, n = 3, ***p < 0.001 and **p < 0.01 versus control (0 h).
Analysis of P4 and 20α-OHP concentrations in biological samples by HPLC
After performing standardization of various parameters including standardization of the appropriate injection volume (10 μl) for determining the minimum detectable steroid concentration (2 ng/10 μl) and retention time (19.1 min for 20α-OHP and 22.8 min for P4, chromatograms not shown), known standards of varied concentrations of P4 and 20α-OHP either alone or after mixing both of them were run on a Zorbax eclipse Plus C18 column. The chromatogram patterns for a range of concentrations of mixture of P4 and 20α-OHP standards are shown in Figure 3. The area under peak (AUP) for each steroid was calculated and the data is presented in Table 1. The chromatogram patterns for fixed concentration of each steroid was also generated in order to rule out that the chromatogram pattern generated in mixture of two steroids was not different compared to pattern when fixed concentration of steroid was run (Figure 3). The representative chromatogram shown in Figure 3, shows an AUP of 120.44, 28.27, 8.73 and 1.96 units for 33, 10, 3.33 and 1 ng/10 μl (Figure 3A-D) of 20α-OHP, respectively. Further, an AUP of 95.72, 23.05, 6.89 and 1.67 units for 33, 10, 3.33 and 1 ng/10 μl (Figure 3A-D) is observed for P4, respectively.
Figure 3.

Chromatograms of 20α-OHP and P4 standards. Different concentrations of 20α-OHP and P4, 33, 10, 3.33 and 1 ng/10 μl (A-D, respectively); P4, 10 ng/10 μl (E) and 20α-OHP, 10 ng/10 μl (F) were prepared and run on a Zorbax eclipse C18 column. The mobile phase comprised of acidified water: acetonitrile and sample injection volume of 10 μl was used. The retention time for 20α-OHP (19.1 min) and P4 (22.8 min) is indicated on each peak. Chromatograms of standards used for each 20α-OHP and P4 are boxed separately. The area under each peak corresponds to respective standard concentration.
Table 1.
HPLC analysis for different concentrations of 20α-OHP and P 4 expressed as area under peak
| Standards (ng/10 μl) | 33 | 10 | 3.3 | 1 |
|---|---|---|---|---|
|
20α-OHP |
119.15 ± 5.22 |
35.71 ± 4.68 |
15.64 ± 6.99 |
4.45 ± 1.99 |
| P 4 | 102.48 ± 4.72 | 33.69 ± 4.49 | 14.51 ± 6.49 | 5.03 ± 2.25 |
Value represents mean ± SEM, in units.
The profile for each steroid was determined on HPLC column for serum samples collected from rats 24 h after PBS (vehicle) or PGF2α injection (Figure 4B and C) and the aggregate values for AUP is represented in Table 2.The AUP for 20α-OHP in serum was significantly increased (p < 0.05) in PGF2α treated rats compared to PBS treated rats. On the other hand, the AUP for P4 peak was significantly decreased (p < 0.01) in serum from PGF2α treated rats compared to serum from PBS treated rats.
Figure 4.
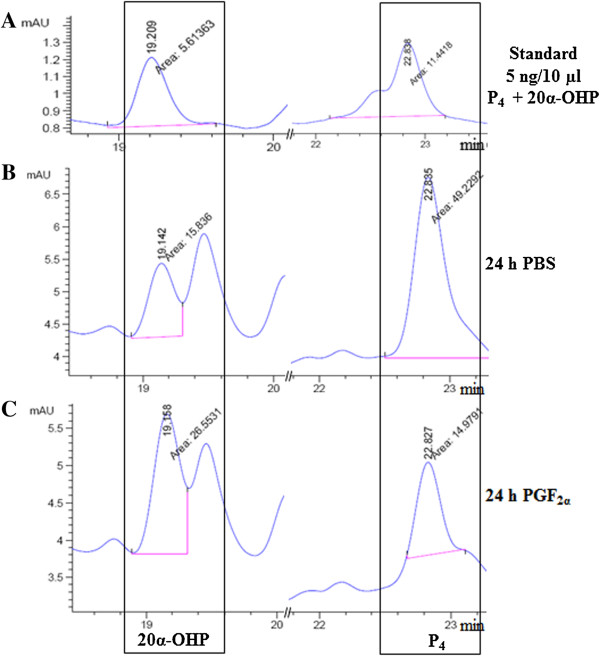
Chromatograms of 20α-OHP and P4 in serum samples from pseudo pregnant rats. (A) A concentration of 5 ng each of 20α-OHP and P4 was used during HPLC run. The peak for 20α-OHP and P4 were noted down at 19.1 and 22.8 min, respectively. (B) and (C) Chromatogram profiles of extracted serum samples collected from pseudo pregnant rats, 24 h treatment with PBS (control) or PGF2α (treatment) are shown. The peaks of 20α-OHP and P4 were indicated at the respective run times for each steroid.
Table 2.
HPLC analysis of 20α-OHP and P 4 expressed as area under peak in rats and buffalo cows
|
Treatments |
Rat |
Buffalo cow |
||
|---|---|---|---|---|
| 24 h PBS | 24 h PGF 2α | 0 h PGF 2α | 18 h PGF 2α | |
|
20α-OHP |
22.79 ± 3.58a |
33.59 ± 2.99b |
ND |
ND |
| P 4 | 55.89 ± 3.58c | 23.09 ± 3.37d | 22.02 ± 3.14x | 13.18 ± 1.25y |
Value represents mean ± SEM; ND = Non-detectable; p < 0.05 (a vs b, x vs y) and p < 0.01 (c vs d).
Similar to HPLC analysis of samples from rats, serum samples from buffalo cows receiving no treatment (0 h) and from animals receiving PGF2α injection at 18 h time point were subjected to chromatographic analysis and a representative chromatogram pattern is presented in Figure 5. The sum total result of AUP values is represented in Table 2. The mixture of steroids at a concentration of 5 ng/10 μl for each HPLC run was analysed under identical HPLC conditions as shown in Figures 4A and 5A. The AUP for P4 peak significantly decreased (p < 0.05) in serum from 18 h post PGF2α injected buffalo cows compared to serum of untreated buffalo cows (time 0 h) on day 11 of the estrous cycle (Figure 5B and C).
Figure 5.
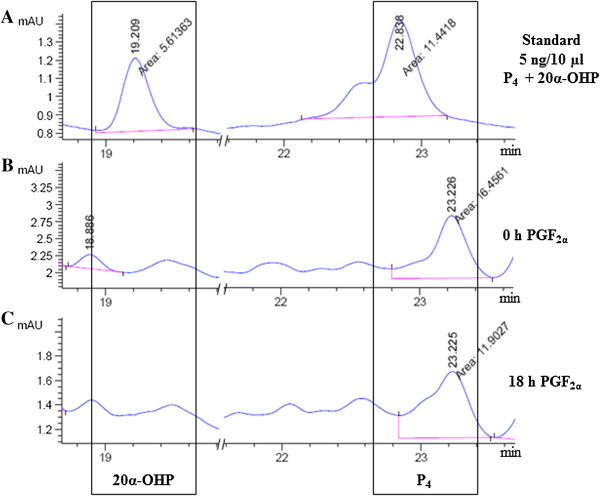
Chromatograms of 20α-OHP and P4 in buffalo cow serum samples before and after PGF2α treatment. (A) The standard mixture of steroids comprising of 5 ng each of 20α-OHP and P4 was used during HPLC run. The peak for 20α-OHP and P4 were noted down at 19.1 and 22.8 min, respectively. Chromatogram showing steroids extracted from serum collected at 0 h (B) and 18 h (C) post PGF2α treatment in the buffalo cow are shown.
Determination of luteal 20α-HSD activity in CL of pseudo pregnant rats and buffalo cows after PGF2α treatment
Figure 6 shows the 20α-HSD activity both in rat and buffalo cow CL cytosolic fractions. The 20α-HSD specific activity (Figure 6A) was significantly higher (p < 0.01) in luteal tissue from PGF2α treated rats compared to PBS treated rats (0.412 ± 0.02 vs 0.171 ± 0.03 nmoles min-1 mg-1 for CL from animals receiving PGF2α and PBS treatment, respectively). On the other hand, examination of specific activity of 20α-HSD in cytosolic fractions of CL from PGF2α-treated buffalo cows at various time points did not change and tended to be lower from 0 h time point (Figure 6B).
Figure 6.
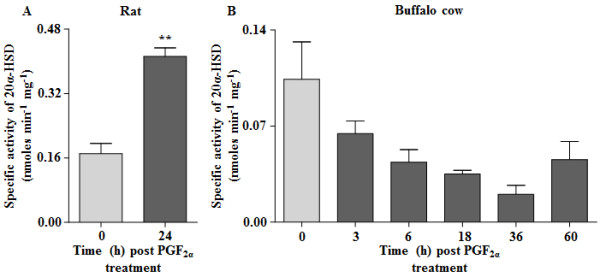
Activity of cytosolic 20α-HSD in luteal tissue of pseudo pregnant rats and buffalo cows. The enzyme activity is expressed as nmoles NADPH min-1 mg-1 with 1 unit of activity defined as the amount of enzyme that can induce formation of 1 nmole NADP min-1 mg-1 extract used at 37 oC. (A) Specific activity of 20α-HSD at 0 and 24 h post PGF2α treated pseudo pregnant rat CL cytosolic extract. (B) Specific activity of 20α-HSD at 0, 3, 6, 18, 36 and 60 h post PGF2α treated buffalo CL cytosolic extract. The cytosolic extract from the luteal tissue collected without PGF2α treatment (0 h) was designated as control. Each bar represents mean ± SEM, n = 5/time point for pseudo pregnant rats and n = 3/time point for buffalo cows, **p < 0.01, 0 h versus different time points.
Discussion
Corpus luteum is a transient endocrine structure formed from the ovarian follicle after ovulation. Through biosynthesis and secretion of P4, it plays a pivotal role in the control of reproduction in mammals. The precise timing of expression of various enzymes/proteins required for synthesis and metabolism of P4 constitutes an important process in the regulation of CL function. In several species including the buffalo cow, PGF2α functions as a physiological luteolysin that curtails CL function at the end of non-pregnant cycle and prior to parturition [23-26]. Despite its central role in luteolysis, PGF2α actions on CL leading to decrease in P4 secretion and subsequent apoptotic changes have not been clearly elucidated. In rats, it is well documented that the initial decrease in luteal function that occurs post PGF2α treatment is precipitated by an increase in P4 metabolism i.e. P4 gets converted to its inactive metabolite 20α-OHP rather than a decrease in its synthesis [9]. The stimulatory effect of PGF2α on 20α-HSD expression in the CL tissue is well recognised in rodents [27-30]. In ruminants including the buffalo cow, PGF2α causes marked rapid decline in circulating concentration of P4 (unpublished data from the laboratory, Davis et al., 2010). As the initial actions of PGF2α on the CL are not well defined, it became of interest to examine whether PGF2α treatment in buffalo cows during luteal phase leads to formation of inactive metabolite such as 20α-OHP. Since the CL of ruminants unlike rodents express P4 receptors, it can be argued that perhaps initial decline in P4 that occurs in response to PGF2α treatment leads to changes in expression of genes associated with control of luteal function [23,31-33].
In order to determine whether rapid decline in circulating P4 was due to its conversion to inactive metabolites, present studies were carried out to examine the activity of 20α-HSD during induced luteolysis in buffalo cows. The results of the present studies demonstrate expression of 20α-HSD in CL and other tissues of the buffalo cow. The importance of 20α-HSD expression in tissues such as spleen, brain and liver is unclear but may be associated with steroid metabolism [18]. Furthermore, despite the increased expression of 20α-HSD post PGF2α treatment, its enzyme activity remained low in the CL during PGF2α treatment. Also, circulating concentration of 20α-OHP did not increase post PGF2α treatment. It is not clear why an increased expression of 20α-HSD was not associated with its increased translation and activity post PGF2α treatment. One explanation could be that PGF2α treatment was detrimental to translational machinery. None the less, the results taken together indicate that decreased circulating P4 concentration seen in response to the luteolytic dose of PGF2α treatment does not appear to be the result of metabolism of P4 in buffalo cows. The present observation of lack of change in 20α-OHP concentration in response to PGF2α treatment in buffalo cows is in contrast to results reported in rodents by others [3,7,8] and as observed in the present rat studies.
In species such as rodents that do not express classical P4 receptors in CL, it becomes of interest to examine whether fall in P4 concentration that occurs due to catabolism is sufficient and necessary for initiation of process of luteolysis. Also, the regulation of 20α-HSD expression has to be taken into consideration during PGF2α-mediated actions on the luteal tissue. It has been shown that prolactin regulates 20α-HSD expression and inhibition of prolactin secretion results in rapid rise in 20α-HSD expression [34-37]. Whether prolactin has a role in the regulation of 20α-HSD expression and whether PGF2α influences prolactin signaling or other factors in the regulation of 20α-HSD need to be investigated. However, it should be pointed out that few studies carried out employing targeted deletion of 20α-HSD in mice model seems to suggest a minor role for catabolism of P4 in the CL [5]. Further, it has been suggested that 20α-HSD may have an important role in the regulation of P4 levels in the placenta for growth and development of foetus rather than regulating P4 levels systemically [1,2,5].
Several studies have suggested participation of Nur77 during parturition process as well as after exogenous PGF2α treatment [3,7,8]. In the present study, a rapid induction of Nur77 expression in CL in response to PGF2α treatment in buffalo cows was also observed. In mice, studies have been carried out extensively to demonstrate that Nur77 binds to the promoter region of 20α-HSD leading to increased transcription [8]. The participation of Nur77 in the regulation of expression of other steroidogenic genes such as adrenal 21-hydroxylase [38], ovarian 3β-HSD [39], 20α-HSD and aromatase as well as StAR, CYP11A1 and CYP17 genes have been reported [11,12]. In addition to transcriptional activation of 20α-HSD expression, Nur77 has been implicated in thymocytic apoptosis following activation of MAP kinases particularly JNK, p38, and possibly ERK5 [40]. The PGF2α-induced luteolysis appears to be initiated through activation of phospholipase C. Earlier reports have suggested a lack of direct participation of PKC during the luteolytic process, but increased intracellular Ca+2 and activation of ERK pathway by Nur77 have been suggested to be involved in the PGF2α-mediated actions in the rat CL [41,42]. Incidentally, it should be pointed out that several MAP kinases are activated during PGF2α-induced luteolysis in the CL of buffalo cows [23] and involvement of MAP kinase pathways have been implicated in the induction of Nur77 expression [40,43]. The above observations point to a critical role of Nur77 in the activation of apoptotic pathway. In the present study, the observation of increased expression of Nur77 suggests that it may be associated with activation of apoptotic pathway, and this is further supported by the observation of increased JAK and p38 activity in CL from buffalo cows treated with PGF2α[23]. However, it remains to be determined what role, if any, Nur77 has in pathways/molecules associated with rapid fall in P4. Also, whether Nur77 is responsible for increased expression of 20α-HSD remains to be determined.
Conclusions
In conclusion, studies carried out to examine 20α-HSD expression and circulating 20α-OHP levels in the buffalo cow indicated expression of 20α-HSD in the CL and it transiently increased at 3 and 18 h post PGF2α treatment, but this was not accompanied by increased activity of 20α-HSD. The results also indicated that Nur77, the transcription factor that has been implicated in transcriptional increase of 20α-HSD expression in rodents was also transiently increased in the buffalo cow CL post PGF2α treatment. The results taken together suggest that catabolism of P4 does not occur in cattle post PGF2α treatment.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
TS and RM participated in designing, conducting experiments, analysis of results and preparation of manuscript. KA participated in the preparation of manuscript. All authors have read and approved the final manuscript.
Contributor Information
Tripathy Sudeshna, Email: sudeshnat@mrdg.iisc.ernet.in.
Kumarasamy Anand, Email: anandk@mrdg.iisc.ernet.in.
Rudraiah Medhamurthy, Email: rmm@mrdg.iisc.ernet.in.
Acknowledgements
Financial support by ICAR (NFBSRA & NAIP) and DBT, India, to conduct these studies is gratefully acknowledged.
References
- Jayasekara WS, Yonezawa T, Ishida M, Yamanouchi K, Nishihara M. Molecular cloning of goat 20alpha-hydroxysteroid dehydrogenase cDNA. J Reprod Dev. 2004;11:323–331. doi: 10.1262/jrd.50.323. [DOI] [PubMed] [Google Scholar]
- Jayasekara WS, Yonezawa T, Ishida M, Yamanouchi K, Nishihara M. Expression and possible role of 20alpha-hydroxysteroid dehydrogenase in the placenta of the goat. J Reprod Dev. 2005;11:265–272. doi: 10.1262/jrd.16074. [DOI] [PubMed] [Google Scholar]
- Waddell BJ, Benediktsson R, Seckl JR. 11beta-Hydroxysteroid dehydrogenase type 2 in the rat corpus luteum: induction of messenger ribonucleic acid expression and bioactivity coincident with luteal regression. Endocrinology. 1996;11:5386–5391. doi: 10.1210/en.137.12.5386. [DOI] [PubMed] [Google Scholar]
- Ishida M, Choi JH, Hirabayashi K, Matsuwaki T, Suzuki M, Yamanouchi K, Horai R, Sudo K, Iwakura Y, Nishihara M. Reproductive phenotypes in mice with targeted disruption of the 20alpha-hydroxysteroid dehydrogenase gene. J Reprod Dev. 2007;11:499–508. doi: 10.1262/jrd.18125. [DOI] [PubMed] [Google Scholar]
- Choi JH, Ishida M, Matsuwaki T, Yamanouchi K, Nishihara M. Involvement of 20alpha-hydroxysteroid dehydrogenase in the maintenance of pregnancy in mice. J Reprod Dev. 2008;11:408–412. doi: 10.1262/jrd.20045. [DOI] [PubMed] [Google Scholar]
- Wiest WG, Kidwell WR, Balogh K Jr. Progesterone catabolism in the rat ovary: a regulatory mechanism for progestational potency during pregnancy. Endocrinology. 1968;11:844–859. doi: 10.1210/endo-82-4-844. [DOI] [PubMed] [Google Scholar]
- Kuhn NJ, Briley MS. The roles of pregn-5-ene-3 beta, 20 alpha-diol and 20 alpha-hydroxy steroid dehydrogenase in the control of progesterone synthesis preceding parturition and lactogenesis in the rat. Biochem J. 1970;11:193–201. doi: 10.1042/bj1170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocco CO, Zhong L, Sugimoto Y, Ichikawa A, Lau LF, Gibori G. Prostaglandin F2alpha-induced expression of 20alpha-hydroxysteroid dehydrogenase involves the transcription factor NUR77. J Biol Chem. 2000;11:37202–37211. doi: 10.1074/jbc.M006016200. [DOI] [PubMed] [Google Scholar]
- Stocco C, Callegari E, Gibori G. Opposite effect of prolactin and prostaglandin F(2 alpha) on the expression of luteal genes as revealed by rat cDNA expression array. Endocrinology. 2001;11:4158–4161. doi: 10.1210/en.142.9.4158. [DOI] [PubMed] [Google Scholar]
- Wingate AD, Arthur JS. Post-translational control of Nur77. Biochem Soc Trans. 2006;11:1107–1109. doi: 10.1042/BST0341107. [DOI] [PubMed] [Google Scholar]
- Li M, Xue K, Ling J, Diao FY, Cui YG, Liu JY. The orphan nuclear receptor NR4A1 regulates transcription of key steroidogenic enzymes in ovarian theca cells. Mol Cell Endocrinol. 2010;11:39–46. doi: 10.1016/j.mce.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Wu Y, Ghosh S, Nishi Y, Yanase T, Nawata H, Hu Y. The orphan nuclear receptors NURR1 and NGFI-B modulate aromatase gene expression in ovarian granulosa cells: a possible mechanism for repression of aromatase expression upon luteinizing hormone surge. Endocrinology. 2005;11:237–246. doi: 10.1210/en.2004-0889. [DOI] [PubMed] [Google Scholar]
- Liu ZG, Smith SW, McLaughlin KA, Schwartz LM, Osborne BA. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature. 1994;11:281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;11:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;11:527–540. doi: 10.1016/S0092-8674(04)00162-X. [DOI] [PubMed] [Google Scholar]
- Weih F, Ryseck RP, Chen L, Bravo R. Apoptosis of nur77/N10-transgenic thymocytes involves the Fas/Fas ligand pathway. Proc Natl Acad Sci. 1996;11:5533–5538. doi: 10.1073/pnas.93.11.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidansuren P, Park CW, Kim SH, Nanjidsuren T, Park JJ, Yun SJ, Sim BW, Hwang S, Kang MH, Ryu BY, Hwang SY, Yoon JT, Yamanouchi K, Min KS. Molecular characterization of bovine placental and ovarian 20α-hydroxysteroid dehydrogenase. Reproduction. 2011;11:723–731. doi: 10.1530/REP-11-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselin E, Fortier MA. Detection and regulation of the messenger for a putative bovine endometrial 9-keto-prostaglandin E(2) reductase: effect of oxytocin and interferon-tau. Biol Reprod. 2000;11:125–131. doi: 10.1095/biolreprod62.1.125. [DOI] [PubMed] [Google Scholar]
- Pharriss BB, Wyngarden LJ. The effect of prostaglandin F 2alpha on the progestogen content of ovaries from pseudopregnant rats. Proc Soc Exp Biol Med. 1969;11:92–94. doi: 10.3181/00379727-130-33495. [DOI] [PubMed] [Google Scholar]
- Jyotsna UR, Medhamurthy R. Standardization and validation of an induced ovulation model system in buffalo cows: characterization of gene expression changes in the periovulatory follicle. Anim Reprod Sci. 2009;11:71–81. doi: 10.1016/j.anireprosci.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Priyanka S, Jayaram P, Sridaran R, Medhamurthy R. Genome-wide gene expression analysis reveals a dynamic interplay between luteotropic and luteolytic factors in the regulation of corpus luteum function in the bonnet monkey (Macaca radiata) Endocrinology. 2009;11:1473–1484. doi: 10.1210/en.2008-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZL, Palmquist DE, Ma M, Liu J, Alexander NJ. Application of a master equation for quantitative mRNA analysis using qRT-PCR. J Biotechnol. 2009;11:10–16. doi: 10.1016/j.jbiotec.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Sudhagar RR, Medhamurthy R. Apoptosis during spontaneous and prostaglandin F2α-induced luteal regression in the buffalo cow (Bubalus bubalis): involvement of mitogen-activated protein kinases. Biol Reprod. 2002;11:752–759. doi: 10.1095/biolreprod.102.004077. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Lakshmi G, Medhamurthy R. Prostaglandin F2alpha-mediated activation of apoptotic signaling cascades in the corpus luteum during apoptosis: involvement of caspase-activated DNase. J Biol Chem. 2005;11:10357–10367. doi: 10.1074/jbc.M409596200. [DOI] [PubMed] [Google Scholar]
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;11:1–29. doi: 10.1152/physrev.2000.80.1.1. [DOI] [PubMed] [Google Scholar]
- Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;11:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- Strauss JF, Stambaugh RL. Induction of 20 alpha-hydroxysteroid dehydrogenase in rat corpora lutea of pregnancy by prostaglandin F-2 alpha. Prostaglandins. 1974;11:73–85. doi: 10.1016/s0090-6980(74)80134-6. [DOI] [PubMed] [Google Scholar]
- Bussmann LE, Deis RP. Studies concerning the hormonal induction of lactogenesis by prostaglandin F2 alpha in pregnant rats. J Steroid Biochem. 1979;11:1485–1489. doi: 10.1016/0022-4731(79)90125-0. [DOI] [PubMed] [Google Scholar]
- Kawano T, Okamura H, Tajima C, Fukuma K, Katabuchi H. Effect of RU 486 on lutealfunction in the early pregnant rat. J Reprod Fertil. 1988;11:279–285. doi: 10.1530/jrf.0.0830279. [DOI] [PubMed] [Google Scholar]
- Telleria CM, Stocco CO, Deis RP. Luteolytic action of RU486: modulation of luteal 3 beta-hydroxysteroid dehydrogenase and 20 alpha-hydroxysteroid dehydrogenase activities in late pregnant rats. J Steroid Biochem Mol Biol. 1995;11:567–573. doi: 10.1016/0960-0760(95)00013-P. [DOI] [PubMed] [Google Scholar]
- Shaw DW, Britt JH. Concentrations of tumor necrosis factor alpha and progesterone within the bovine corpus luteum sampled by continuous-flow microdialysis during luteolysis in vivo. Biol Reprod. 1995;11:847–854. doi: 10.1095/biolreprod53.4.847. [DOI] [PubMed] [Google Scholar]
- Ginther OJ, Araujo RR, Palhão MP, Rodrigues BL, Beg MA. Necessity of sequential pulses of prostaglandin F2alpha for complete physiologic luteolysis in cattle. Biol Reprod. 2009;11:641–648. doi: 10.1095/biolreprod.108.072769. [DOI] [PubMed] [Google Scholar]
- Arvisais E, Hou X, Wyatt TA, Shirasuna K, Bollwein H, Miyamoto A, Hansen TR, Rueda BR, Davis JS. Prostaglandin F2alpha represses IGF-I stimulated IRS1/phosphatidylinositol-3-kinase/AKT signaling in the corpus luteum: role of ERK and P70 ribosomal S6 kinase. Mol Endocrinol. 2010;11:632–643. doi: 10.1210/me.2009-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albarracin CT, Parmer TG, Duan WR, Nelson SE, Gibori G. Identification of a major prolactin-regulated protein as 20 alpha-hydroxysteroid dehydrogenase: coordinate regulation of its activity, protein content, and messenger ribonucleic acid expression. Endocrinology. 1994;11:2453–2460. doi: 10.1210/en.134.6.2453. [DOI] [PubMed] [Google Scholar]
- Piekorz RP, Gingras S, Hoffmeyer A, Ihle JN, Weinstein Y, Weinstein Y. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20alpha-hydroxysteroid dehydrogenase. Mol Endocrinol. 2005;11:431–440. doi: 10.1210/me.2004-0302. [DOI] [PubMed] [Google Scholar]
- Bao L, Tessier C, Prigent-Tessier A, Li F, Buzzio OL, Callegari EA, Horseman ND, Gibori G. Decidual prolactin silences the expression of genes detrimental to pregnancy. Endocrinology. 2007;11:2326–2334. doi: 10.1210/en.2006-1643. [DOI] [PubMed] [Google Scholar]
- Clementi MA, Deis RP, Telleria CM. Luteal 3beta-hydroxysteroid dehydrogenase and 20alpha-hydroxysteroid dehydrogenase activities in the rat corpus luteum of pseudopregnancy: effect of the deciduoma reaction. Reprod Biol Endocrinol. 2004;11:22. doi: 10.1186/1477-7827-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Mouw AR, Weaver CA, Milbrandt J, Parker KL. The orphan Nuclear receptor NGFI-B regulates expression of the gene encoding steroid 21- hydroxylase. Mol Cell Biol. 1993;11:861–868. doi: 10.1128/mcb.13.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelock JC, Smith AL, Seely JB, Dooley CA, Rodgers RJ, Rainey WE, Carr BR. The NGFI-B family of transcription factors regulates expression of 3beta- hydroxysteroid dehydrogenase type 2 in the human ovary. Mol Hum Reprod. 2005;11:79–85. doi: 10.1093/molehr/gah139. [DOI] [PubMed] [Google Scholar]
- Sohn SJ, Thompson J, Winoto A. Apoptosis during negative selection of autoreactivethymocytes. Curr Opin Immunol. 2007;11:510–515. doi: 10.1016/j.coi.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Mizukami Y, Kobayashi S, Uberall F, Hellbert K, Kobayashi N, Yoshida K. Nuclear mitogen-activated protein kinase activation Cζ during reoxygenation after ischemic hypoxia. J Biol Chem. 2000;11:19921–19927. doi: 10.1074/jbc.M907901199. [DOI] [PubMed] [Google Scholar]
- Rojnukarin P, Miyakawa Y, Fox NE, Deou J, Daum G, Kaushansky K. The roles of phosphatidylinositol 3-kinase and protein kinase Cζ for thrombopoietin-induced mitogen-activated protein kinase activation in primary murine megakaryocytes. J Biol Chem. 2001;11:41014–41022. doi: 10.1074/jbc.M106508200. [DOI] [PubMed] [Google Scholar]
- Kovalovsky D, Refojo D, Liberman AC, Hochbaum D, Pereda MP, Coso OA, Stalla GK, Holsboer F, Arzt E. Activation and induction of Nur77/ Nurr1 in corticotrophs by CRH/cAMP: involvement of calcium, protein kinase A, and MAPK pathways. Mol Endocrinol. 2002;11:1638–1651. doi: 10.1210/me.16.7.1638. [DOI] [PubMed] [Google Scholar]


