Abstract
The potential of recombinant lactic acid bacteria (LAB) to deliver heterologous antigens to the immune system and to induce protective immunity has been best demonstrated by using the C subunit of tetanus toxin (TTFC) as a model antigen. Two types of LAB carriers have mainly been used, Lactobacillus plantarum and Lactococcus lactis, which differ substantially in their abilities to resist passage through the stomach and to persist in the mouse gastrointestinal tract. Here we analyzed the effect of a deficiency in alanine racemase, an enzyme that participates in cell wall synthesis, in each of these bacterial carriers. Recombinant wild-type and mutant strains of L. plantarum NCIMB8826 and L. lactis MG1363 producing TTFC intracellularly were constructed and used in mouse immunization experiments. Remarkably, we observed that the two cell wall mutant strains were far more immunogenic than their wild-type counterparts when the intragastric route was used. However, intestinal TTFC-specific immunoglobulin A was induced only after immunization with the recombinant L. plantarum mutant strain. Moreover, the alanine racemase mutant of either LAB strain allowed induction of a much stronger serum TTFC-specific immune response after immunization via the vagina, which is a quite different ecosystem than the gastrointestinal tract. The design and use of these mutants thus resulted in a major improvement in the mucosal delivery of antigens exhibiting vaccine properties.
One of the current goals in vaccine development is induction of mucosal and systemic responses against protective antigens delivered by mucosal routes. Live vaccines represent a promising approach in this area. Most of the systems currently under development involve pathogenic microorganisms for which attenuated variants have to be isolated or constructed (1, 5, 13, 15, 19, 27). Nonpathogenic food-grade gram-positive bacteria (i.e., lactic acid bacteria [LAB]) represent an attractive alternative to this end. The potential of these organisms to deliver heterologous antigens to the mucosal immune system has been investigated during the last decade, and the most complete studies have been performed with tetanus toxin fragment C (TTFC) as a model antigen (7, 8, 17, 18, 21, 25, 26, 28). We and other workers have previously shown that TTFC can be efficiently produced in a variety of LAB strains, including Lactococcus lactis (21, 26), Streptococcus gordonii, and Lactobacillus spp. (7, 8, 17, 18, 25, 28). The best recombinant strains resulted in induction of local and protective systemic antibody responses, as well as cellular immune responses, after parenteral or intranasal administration to mice (7, 8, 21). In the case of lactobacilli, the amount of cytoplasmic antigen was found to be critical for induction of a significant immune response by the oral route (7). Indeed, a protective immunoglobulin G (IgG)-mediated response was obtained when mice were immunized with the Lactobacillus plantarum NCIMB8826 strain producing large amounts of TTFC intracellularly, whereas the equivalent strain producing only moderate amounts of antigen proved to be inefficient (7). However, both strains exhibited elevated immunogenicity when they were administered by the nasal route (8). High levels of intracellular expression might be an important bottleneck depending on the nature of the heterologous polypeptide. We therefore examined whether developing mutant bacterial carriers could enhance the potential of LAB as a delivery system. The approach which we decided to pursue was to try to increase the in vivo release of the cytoplasmic antigen by interfering with cell wall biosynthesis. By analogy to the work described for Bacillus subtilis (9), we exploited alanine racemase (alr) mutants. This enzyme interconverts l-alanine and d-alanine and provides the main biosynthesis pathway for d-alanine, which is an essential molecule in the construction of the two major cell wall polymers of gram-positive bacteria, peptidoglycan and teichoic acids (22). Alr-deficient (Alr−) mutants have been constructed for both L. plantarum NCIMB8826 (10) and L. lactis MG1363 (11). These mutants are unable to grow in the absence of d-alanine, and a lack of this amino acid in the growth medium leads to cells that have a severely altered cell wall structure (22a). L. plantarum and L. lactis differ substantially with respect to the ability to survive in the gastrointestinal tracts of rodents (4) and humans (14, 30), and L. plantarum is much more resistant to the harsh conditions encountered in the stomach and the upper part of the intestine. We hypothesized that the greater and longer persistence of L. plantarum, while conferring interesting pharmacokinetic properties to this carrier (30), may also lead to sequestration of intracellular bioactive molecules. In this case, cell wall mutants should perform better as antigen carriers when the oral route is used. We therefore constructed recombinant strains producing TTFC intracellularly in either an L. plantarum or L. lactis Alr− background and compared their immunogenicities to those of the corresponding wild-type (WT) strains after intragastric administration to mice. The potential of the Alr− recombinant LAB strains was further investigated by immunizing mice intravaginally, taking into consideration the fact that the vagina of mice is recognized as a poor immune inductive site (29) and is an ecosystem that is much different than the intestine.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and preparation of bacterial inocula.
All LAB strains and plasmids used in this study are listed in Table 1. The recombinant wild-type (rWT) L. plantarum and L. lactis strains have been described previously (7, 8). The plasmid constructs were introduced by electroporation into the appropriate Alr− mutants (MD007, MD007Int6, and PH3960) (10, 11). WT lactobacilli were grown at 37°C in MRS broth (Difco, Detroit, Mich.) containing erythromycin (5 μg/ml) for NCIMB8826/pMEC4 and NCIMB8826/pMEC127 and chloramphenicol (10 μg/ml) for NCIMB8826/pTG2247, while the Alr− mutants (MD007 and MD007Int6) required addition of d-alanine (200 μg/ml) for growth. L. lactis WT strains were grown at 30°C in M17 supplemented with 0.5% glucose containing chloramphenicol (5 μg/ml) for NZ3900/pMEC46 and erythromycin (5 μg/ml) for MG1363/pTX. The Alr− L. lactis PH3960 strain required addition of d-alanine (400 μg/ml) for growth. An overnight culture was used to inoculate fresh medium at a 1:20 dilution, and cells were grown to the exponential phase (optical density at 600 nm [OD600], 1 to 2). For the L. plantarum and L. lactis Alr− mutants, alanine starvation was achieved when the cultures reached an OD600 of 0.7 by washing the bacteria and incubating them in medium without d-alanine for 3 and 2 h, respectively.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| L. plantarum strains | ||
| NCIMB8826 | Isolated from human saliva | NCIMBa |
| NCIMB8826Int1 | Emr, carrying a chromosomal copy of nisRK | 24 |
| MD007 (Alr−) | L. plantarum NCIMB882 Δalr, d-alanine auxotroph | 10 |
| MD007Int6 (Alr−) | Emr, carrying a chromosomal copy of nisRK | S. Pavan, unpublished data |
| L. lactis strains | ||
| MG1363 | 6 | |
| NZ3900 | MG1363 carrying a chromosomal copy of nisRK | 3 |
| PH3960 (Alr−) | NZ3900 Δalr, d-alanine auxotroph | 11 |
| Plasmids | ||
| PTG2247 | Cmr, control plasmid | 12 |
| pMEC10 | Integration plasmid carrying nisRK | 24 |
| pMEC46 | TTFC gene fused to nisA promoter | 24 |
| pMEC127 | TTFC gene fused to L-ldh promoter | 25 |
| pTX | Eryr, control plasmid | 21 |
NCIMB, National Collection of Industrial and Marine Bacteria, Terry Research Station, Aberdeen, Scotland.
The bacterial membrane integrity of the MD007 and PH3960 Alr− mutants was measured at different times during d-alanine starvation by using a LIVE/DEAD Baclight bacterial viability kit (Molecular Probes, Leiden, The Netherlands). This kit contains a mixture of two nucleic acid stains, the green fluorescent stain SYTO 9 and the red fluorescent stain propidium iodide (PI). The SYTO 9 stain labels all bacteria in a population, while PI penetrates only bacteria with damaged membranes, causing a reduction in the SYTO 9 stain fluorescence when both dyes are present. Cell samples were diluted to a concentration of approximately 106 cells/ml in phosphate-buffered saline (PBS). Equal volumes of component A (3.34 mM SYTO 9) and component B (20 mM PI) were prepared extemporaneously, and 3 μl was mixed with each bacterial suspension and incubated at room temperature in the dark for 15 min. Flow cytometry analyses were performed with a FACSCalibur flow cytometer by using the CellQuest software (Beckton Dickinson, Mountain View, Calif.).
Antigen (inducible promoter) synthesis by the NCIMB8826/pMEC46 strain was induced by addition of 20 ng of nisin (Sigma, St. Louis, Mo.) per ml 1 h after 1:20 dilution of an overnight culture in fresh medium, followed by 4 h of incubation at 37°C. For the mutant MD007Int6/pMEC46 strain, induction was performed for 1 h, and this was followed by 3 h of starvation (as described above) in the presence of nisin (20 ng per ml). For strain NZ3900/pMEC46, antigen production was induced by addition of 5 ng of nisin per ml when the culture reached an OD600 of 0.5 and incubation at 30°C for 3 h. For the mutant PH3960 strain, a 1-h period of induction was followed by 2 h of starvation in the presence of nisin (5 ng per ml). The cells were then pelleted, washed twice in sterile PBS (pH 7.2), and resuspended at a concentration of 1010 CFU per ml in a solution containing 0.2 M sodium bicarbonate, 5% casein hydrolysate, and 0.5% glucose (gavage buffer) for intragastric administration and at a concentration of 1011 CFU per ml in PBS for intravaginal immunization. Production of heterologous antigen (TTFC) was verified by Western blotting after alkaline lysis, as previously described (8).
Immunization and analysis of immune responses.
Animal experiments were performed at an accredited establishment (no. A59107) according to governmental guidelines (no. 86/609/CEE). Eight-week-old female C57BL/6 N Crl BR mice (Charles River Laboratories, St. Aubin-les-Elbeuf, France) were immunized with TTFC-producing WT or mutant L. plantarum or L. lactis strains and control nonexpressing strains. An additional control group received buffer alone. In order to facilitate establishment of the LAB in the vaginas of mice and to synchronize the menstrual cycle of the animals, a hormonal treatment was performed before each administration of bacteria (23). For intravaginal immunization, intraperitoneal injection of medroxyprogesterone acetate (2 mg per mouse; Dépo-provéra; Pharmacia & Upjohn, St. Quentin-Yvelines, France) was performed 5 days before each administration of bacteria. Three consecutive daily doses of 1 × 109 CFU or buffer (100 and 10 μl for intragastric and intravaginal administration, respectively) were administered at 3-week intervals (priming and two or three boosts). Ten days after each administration, serum samples were collected and stored at −20°C until they were used. Mice were sacrificed 10 days after the last boost. For the intragastric experiment, the small intestine of each mouse was removed, opened, and washed with 1 ml of PBS containing a protease inhibitor cocktail (Complete; Boehringer, Mannheim, Germany). Intestinal lavages were clarified by centrifugation at 4,000 × g followed by centrifugation for 10 min at 12,000 × g and 4°C, and the supernatants were collected and stored at −20°C until analysis. The immunoglobulin titers of sera and intestinal fluids were determined by an enzyme-linked immunosorbent assay ELISA as previously described (8). The titer corresponded to the reciprocal of the serum dilution that gave an optical density that was three times the background value. Normalization of the ratio of the TTFC-specific local IgA concentration to the total IgA concentration in intestinal lavages was carried out as previously reported (8). Briefly, the results are expressed as specific activities, which were calculated by dividing the endpoint titer by the total IgA concentration (expressed in micrograms per milliliter) for each lavage.
Statistical analysis.
The results are expressed as means ± standard errors of the means. Statistical significance was evaluated by the Mann-Whitney U test. Differences were considered significant at a P value of <0.05.
Protection assay: determination of TT neutralizing antibody titers in mouse sera.
The toxin neutralization test was performed with the pooled sera of each group of immunized and control mice by using the specifications of the European Pharmacopoeia, with slight modifications. Briefly, mixtures containing serial twofold dilutions of a pool and a definite amount of tetanus toxin (TT) (L+/4,000 level) were injected subcutaneously into two OF1 mice (obtained from the animal facility of the Institut Pasteur de Bruxelles) per mixture. A series of dilutions of a standard antitoxin solution mixed with the same amount of toxin were included in each experiment. The neutralizing antibody content was expressed in international units per milliliter as a range that included the actual value. Due to the small volumes of sera that were available, the detection limit was 0.0025 IU/ml of pooled sera. The protective level of tetanus antitoxin is usually considered to be 0.01 IU/ml (16).
RESULTS
TTFC production by the recombinant strains.
Cell wall mutants with a defective alanine racemase gene (Alr− mutants) have been described for both L. plantarum NCIMB8826 (12) and L. lactis MG1363 (11). When we compared the WT and Alr− strains, we observed that the growth of both the MD007 and PH3960 mutant strains was impaired after d-alanine starvation, as expected (Fig. 1A). The bacterial membrane integrity was further evaluated by flow cytometry by using PI and SYTO 9 staining. Membrane permeability was induced in both mutants as a function of the d-alanine starvation time, which resulted in a maximum number of injured cells after 24 h. In contrast, the membrane of the WT strains remained largely unaffected, and less than 10 to 20% of the cells were injured at the end of the analysis (Fig. 1B). The increased membrane permeability of the Alr− mutants seemed not to affect their in vivo persistence in mice, since the viable counts of mutants in feces were similar to those obtained for their WT counterparts (data not shown). The plasmid constructs used in this study (Table 1) were introduced by electroporation into the Alr− mutants of L. plantarum (MD007, MD007Int6) and L. lactis (PH3960). The intracellular levels of TTFC produced by the different recombinant strains are shown in Fig. 2. Low, medium, and high levels of antigen (i.e., ≤1, ≤5, and ≤10% of the total protein content, respectively) (7, 25) were produced by L. plantarum recombinant strains harboring the expression plasmids pMEC4, pMEC46 (after nisin induction), and pMEC127, respectively (Fig. 2, lanes 2 to 7). No striking difference was observed in TTFC production between the corresponding WT and Alr− mutant strains. The recombinant L. lactis strains harboring pMEC46 (lanes 9 and 10) produced levels of TTFC similar to those produced by the L. plantarum strains carrying pMEC127 (lanes 6 and 7). These four strains were thus chosen for comparative immunization with the two LAB vectors. No specific signal was detected in the control extracts of strains NCIMB8826/pTG2247 and MG1363/pTX (lanes 1 and 8).
FIG. 1.
Effect of d-alanine starvation on bacterial growth and cell membrane integrity. Culture growth was measured by monitoring the OD600 (A), and bacterial viability and membrane permeability were examined after 3 h (solid bars), 6 h (striped bars), and 24 h (cross-hatched bars) of d-alanine starvation for the WT and Alr− mutant strains (B). The proportion of injured cells was determined by determining the proportion of the PI-labeled cell population.
FIG. 2.
Levels of antigen production by recombinant L. plantarum and L. lactis strains: immunoblotting of cell extracts (equivalent to 108 CFU) obtained from the L. plantarum NCIMB8826/pTG2247 (lane 1) and L. lactis MG1363/pTX (lane 8) control strains and from the TTFC-producing strains L. plantarum NCIMB8826/pMEC4 (lane 2), MD007/pMEC4 (lane 3), NCIMB8826Int1/pMEC46 (lane 4), MD007Int6/pMEC46 (lane 5), NCIMB8826/pMEC127 (lane 6), and MD007/pMEC127 (lane 7) and L. lactis NZ3900/pMEC46 (lane 9) and PH3960/pMEC46 (lane 10) and immunoblotting of 50 ng of purified TTFC (lane 11).
Induction of anti-TTFC antibody responses after intragastric immunization.
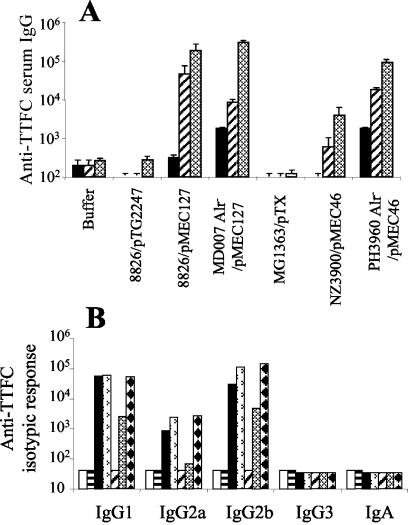
No significant IgG responses were elicited when mice were immunized intragastrically with the rWT L. plantarum strains producing small (NCIMB8826/pMEC4) or moderate (NCIMB8826Int1/pMEC46) amounts of TTFC compared to the IgG levels produced by the control groups (P < 0.05) (Fig. 3A). In contrast, successful intragastric immunization was obtained in mice which received the strain producing higher levels of antigen (NCIMB8826/pMEC127), which resulted in anti-TTFC IgG mean endpoint titers of 9 × 103 and 2.1 × 104 after the first and second boosts, respectively. The response triggered by Alr− mutant MD007/pMEC127 was significantly greater (P < 0.05) than that induced by the corresponding rWT strain, NCIMB8826/pMEC127, and the endpoint titers were 1.3 × 105, 1.9 × 105, and 4.2 × 105 after the first, second, and third boosts, respectively. A significant response was also obtained with the mutant MD007Int6/pMEC46 strain, and the mean endpoint titers were 7.1 × 103 and 3.2 × 104 after the first and second boosts, respectively. However, no specific response was generated by the Alr− MD007/pMEC4 strain. In contrast to previous experiments conducted with WT strains, including NCIMB8826/pMEC127, that never allowed induction of detectable mucosal IgA responses (7), significant local IgA responses were observed in the intestinal lavages of mice immunized with the Alr− mutant strain MD007/pMEC127 compared to the responses of the control groups (P < 0.05) (Fig. 3B). The cell wall defect of the L. plantarum Alr− mutant thus clearly improved its efficacy as an oral delivery system. If this resulted from reduced sequestration of the cytoplasmic antigen TTFC, a similar mutation in L. lactis should have led to no improvement as this strain is known to extensively lyse when it passes through the gastrointestinal tract (4). We therefore analyzed the effect of the alr mutation in the L. lactis background. Surprisingly, as in the case of L. plantarum, intragastric administration of the mutant strain of L. lactis (Alr− PH3960/pMEC46) led to a significantly enhanced (20- to 30-fold) anti-TTFC IgG response (mean endpoint titers, 1.8 × 104 and 8.9 × 104 after the first and second boosts, respectively) compared to the response obtained with the recombinant parental strain, NZ3900/pMEC46 (mean endpoint titers, 6.2 × 102 and 3.9 × 103 after the first and second boosts, respectively) (Fig. 4A). The response elicited by the rWT L. lactis NZ3900/pMEC46 strain was significantly (P < 0.05) less than the response elicited by the rWT L. plantarum NCIMB8826/pMEC127 strain even though both strains produced equivalent amounts of antigen in vitro (Fig. 2, lanes 6 and 9). No difference in the isotypic profiles was observed for the WT and mutant strains. The dominant anti-TTFC antibody isotypes induced by all bacterial carriers were IgG1, IgG2b, and, to a lesser extent, IgG2a (Fig. 4B), which corroborates previous observations (7, 8). No serum IgA was detected.
FIG. 3.
Immune responses following intragastric immunization with recombinant L. plantarum NCIMB8826 strains. (A) Anti-TTFC serum IgG titers. Individual sera were collected 10 days after priming (solid bars) and after the first boost (diagonally striped bars), the second boost (cross-hatched bars), and the third boost (horizontally striped bars) from groups of eight mice that were immunized with buffer alone, with 109 CFU of the control nonexpressing strain NCIMB8826/pTG2247, or with 109 CFU of a TTFC-producing strain (WT or MD007 Alr− mutant harboring an expression plasmid). The bars indicate the mean ELISA IgG titer ± standard error of the mean for each group. (B) Anti-TTFC IgA levels in intestinal lavages from groups of mice immunized intragastrically with buffer, with the control NCIMB8826/pTG2247 strain, or with the TTFC-producing MD007/pMEC127 recombinant strain. Individual lavages were performed 10 days after the last feeding. The specific IgA response was normalized by using the level of total IgA and was expressed as the specific activity (S.A.). The bars indicate the mean IgA level ± standard error of the mean for each group.
FIG. 4.
Comparative immune responses following intragastric immunization with recombinant L. plantarum and L. lactis strains. (A) Anti-TTFC serum IgG titers. Individual sera were collected 10 days after priming (solid bars) and after the first boost (striped bars) or the second boost (cross-hatched bars) from groups of eight mice immunized either with buffer alone, with 109 CFU of the control nonexpressing strain NCIMB8826/pTG2247 or MG1363/pTX, or with 109 CFU of the TTFC-producing strain NCIMB8826/pMEC127, MD007/pMEC127, NZ3900/pMEC46, or PH3960/pMEC46. The bars indicate the mean ELISA IgG titer ± standard error of the mean for each group. (B) Serum anti-TTFC antibody IgG1, IgG2a, IgG2b, IgG3, and IgA titers after intragastric immunization (second boost) of mice with buffer (open bars) or with 109 CFU of L. plantarum NCIMB8826/pTG2247 (horizontally striped bars), NCIMB8826/pMEC127 (solid bars), or MD007/pMEC127 (bars with arrowheads) or L. lactis MG1363/pTX (diagonally striped bars), NZ3900/pMEC46 (cross-hatched bars), or PH3960/pMEC46 (bars with diamonds). The isotypic response was analyzed by using pooled sera of each group collected after the last boost.
Protective capacity of the anti-TTFC serum antibodies of intragastrically immunized mice.
Protection was evaluated by measuring the ability of elicited serum antibodies to neutralize TT, an assay whose results have been proven to correlate well with the results obtained after a direct lethal challenge with TT (8). Notably, protective levels of anti-TT antibodies were induced by both recombinant Alr− mutant strains, MD007/pMEC127 and PH3960/pMEC46. In the case of the WT strains, only L. plantarum strain NCIMB8826/pMEC127 was found to induce neutralizing antibodies at levels above the protective limit, in contrast to L. lactis strain NZ3900/pMEC46 (Table 2).
TABLE 2.
Neutralizing activities of serum antibodies elicited by recombinant LAB strains
| Immunization protocol | Neutralizing tetanus antibody level in pooled sera (IU/ml)a | Protectionb |
|---|---|---|
| Buffer | <<0.0025 | − |
| 8826/pTG2247 | <<0.0025 | − |
| 8826/pMEC127 | 0.02-0.04 | + |
| Alr− MD007/pMEC127 | 0.01-0.02 | + |
| MG1363/pTX | <<0.0025 | − |
| NZ3900/pMEC46 | <<0.0025 | − |
| Alr−PH3960/pMEC46 | 0.04-0.08 | + |
Sera from intragastrically immunized mice were collected and pooled 10 days after the second boost and were used in the TT neutralization assay.
Protection against tetanus toxin requires a minimum neutralizing antibody titer of 0.01 IU/ml.
Induction of anti-TTFC antibody responses after intravaginal immunization.
To evaluate if the improved performance of the mutant strains as antigen delivery systems was restricted to the gastrointestinal tract, we compared the immunogenicities of the four recombinant strains using the vaginal route of delivery. We first optimized the mouse hormonal treatment by comparing the effects of administration of progesterone and estrogen to the effect of no hormone administration. The two latter regimens did not allow induction of a detectable and reproducible immune response after vaginal administration of the rWT L. plantarum NCIMB8826/pMEC127 strain, and the estrogen treatment led to a notable thickening of the vaginal epithelium (data not shown). In contrast, after progesterone treatment a remarkably high anti-TTFC humoral IgG response was elicited by intravaginal administration of the L. plantarum NCIMB8826/pMEC127 strain, and the mean endpoint titer was 8.9 × 103 after the second boost (Fig. 5). Moreover, as observed for the oral route, the use of the Alr− strain MD007/pMEC127 significantly enhanced the IgG response, particularly at the priming level. The response elicited by the rWT L. lactis NZ3900/pMEC46 strain was significantly less than the response obtained with the rWT lactobacilli. Administration of the Alr− mutant PH3960/pMEC46 strain led to a significantly enhanced response; however, the IgG level remained close to the IgG level induced with the rWT lactobacilli.
FIG. 5.
Anti-TTFC serum IgG titers following intravaginal immunization with recombinant L. plantarum NCIMB8826 and L. lactis MG1363 strains. Individual sera were collected 10 days after priming (solid bars) and after the first boost (striped bars) and the second boost (cross-hatched bars) from groups of eight mice immunized with buffer alone, with 109 CFU of the control nonexpressing strain NCIMB8826/pTG2247 or MG1363/pTX, or with 109 CFU of the TTFC-producing strain NCIMB8826/pMEC127, MD007/pMEC127, NZ3900/pMEC46, or PH3960/pMEC46. The bars indicate the mean ELISA IgG titer ± standard error of the mean for each group.
DISCUSSION
The ability of recombinant LAB to act as efficient live vaccines has been best demonstrated by systemic and intranasal routes using TTFC as a model antigen (8, 21, 28). In our hands, comparative studies conducted with L. plantarum and L. lactis producing TTFC intracellularly demonstrated that the former organism was more immunogenic than the latter when mice were immunized by the intragastric route. The greater resistance of L. plantarum to the gastric environment and its better survival in the gastrointestinal tract might explain this observation, yet this property could also lead to limited in vivo release of bioactive molecules. Therefore, we investigated whether the potential of L. plantarum as a live delivery system could be improved by using a mutant having a more permeable cell envelope. A similar mutant constructed in a L. lactis background, which is intrinsically more susceptible to lysis, was expected to be a less effective vaccine vehicle. Surprisingly, both L. plantarum and L. lactis Alr− mutants producing TTFC intracellularly turned out to be far more immunogenic by the intragastric route than their WT counterparts. As mentioned above, successful intragastric immunization is highly dependent on the antigen dose delivered by the recombinant LAB. When the Alr− mutants were used as carriers, the anti-TTFC immune responses induced were significantly higher than those induced by the WT vectors (Fig. 3A and 4A). In our hands, although both the WT and mutant strains of L. plantarum elicited protective immune responses, only the recombinant Alr− mutant of L. lactis led to protective neutralizing antibody titers. Even when the antigen dose did not seem to be limiting, as in the case of the rWT strain L. plantarum NCIMB8826/pMEC127, the specific serum IgG levels were higher after immunization with the Alr− mutant MD007/pMEC127. Notably, while no serum IgA was detected, significant levels of intestinal TTFC-specific IgA were observed only after intragastric administration of the mutant L. plantarum carrier. As shown by flow cytometry with bacterial viability dyes, both the MD007 and PH3960 Alr− mutants were much more permeable than their rWT counterparts. It is thus tempting to propose that increased in vivo delivery of the antigen plays a major role in the effect observed, even though the possibility of better antigen presentation to immune cells cannot be excluded. The mutant Alr− strains were indeed shown to release larger amounts of cytoplasmic enzymes, at least in vitro (22a). We also compared the immunogenicities of the four recombinant strains producing TTFC by the vaginal route. The vaginal and gastrointestinal mucosae differ substantially in terms of the nature of the associated antigen-presenting cells and in structure; the former is a stratified epithelium, while the latter is a single-cell layer (20). Both rWT LAB strains were able to induce significant humoral IgG responses by the vaginal route after an optimized hormonal treatment, and the immunogenicity of the rWT lactobacilli was greater than that of the rWT lactococci in this case. These results are quite remarkable, as the vagina is generally not considered a potent immune inductive site in mice. As observed for intragastric immunization, the use of Alr− mutants led to dramatic enhancement of the serum antibody titers for both LAB strains. The IgG titers (endpoint titers, >104) obtained with the MD007 and PH3960 Alr− mutants reached levels similar to those shown to be protective after nasal administration (8) and oral administration (7). We thus speculate that vaginal immunization with these vectors could confer protection against TT. We recently confirmed the performance of the Alr− mutant of L. plantarum as a mucosal antigen carrier in a Helicobacter felis infection mouse model. Notably, significant protection was induced after intragastric administration of the recombinant mutant strain producing the weakly immunogenic urease B subunit, an effect not exhibited by the corresponding recombinant WT strain (B. Corthesy et al., unpublished data). Altogether, our results demonstrate that the Alr− mutants allow substantially improved antigen presentation to the immune system. This might not be solely linked to increased leakage of TTFC in vivo but could be a consequence of better antigen presentation by recombinant LAB. Soluble antigens are known to be less immunogenic than particulate or replicative antigens, especially when mucosal routes are used. Moreover, Corinti et al. (2) demonstrated that dendritic cells pulsed with recombinant LAB engineered for cell surface exposure of TTFC were more efficient at stimulating specific CD4+ T cells than dendritic cells incubated with soluble TTFC mixed with nonrecombinant bacterial cells. Finally, we cannot rule out the possibility that the cell wall modifications induced by inactivation of the alr gene also affect the cross talk between the bacterial strain and the host. Taken together, our results demonstrate that the Alr− mutants described here constitute a substantially improved antigen delivery system for different mucosal routes. In addition, these and other cell wall mutant strains might help unravel the complex mechanisms underlying commensal bacterium-host interactions.
Acknowledgments
This work was supported by grants EU BIO4-CT96-0542 and EU QLK3-2000-00340 and by the Institut Pasteur de Lille and the Institut Pasteur de Bruxelles.
We are grateful to E. Van Nerom and F. Tweepenninckx for their skillful help with protection experiments. We warmly thank S. Pavan for providing strain MD007Int6 and B. Corthesy for critical reading of the manuscript. E. Sablon, Innogenetics N.V., Zwijnaarde, Belgium, kindly supplied rabbit anti-TTFC antibodies.
Editor: J. N. Weiser
REFERENCES
- 1.Chatfield, S. N., I. G. Charles, A. J. Makoff, M. D. Oxer, G. Dougan, D. Pickard, D. Slater, and N. F. Fairweather. 1992. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus toxin vaccine. Bio/Technology 10:888-892. [DOI] [PubMed] [Google Scholar]
- 2.Corinti, S., D. Medaglini, A. Cavani, M. Rescigno, G. Pozzi, P. Ricciardi-Castagnoli, and G. Girolomoni. 1999. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 163:3029-3036. [PubMed] [Google Scholar]
- 3.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drouault, S., G. Corthier, S. D. Ehrlich, and P. Renault. 1999. Survival, physiology, and lysis of Lactococcus lactis in the digestive tract. Appl. Environ. Microbiol. 65:4881-4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunstan, S. J., C. P. Simmons, and R. A. Strugnell. 1998. Comparison of the abilities of different attenuated Salmonella typhimurium strains to elicit humoral immune responses against a heterologous antigen. Behring Inst. Mitt. 98:732-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast inducing curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grangette, C., H. Muller-Alouf, M. Geoffroy, D. Goudercourt, M. Turneer, and A. Mercenier. 2002. Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria: impact of strain viability and in vivo persistence. Vaccine 20:3304-3309. [DOI] [PubMed] [Google Scholar]
- 8.Grangette, C., H. Müller-Alouf, D. Goudercourt, M.-C. Geoffroy, M. Turneer, and A. Mercenier. 2001. Mucosal immune responses and protection against tetanus toxin after intranasal immunization with recombinant Lactobacillus plantarum. Infect. Immun. 69:1547-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heaton, M. P., R. B. Johnston, and T. L. Thompson. 1988. Controlled lysis of bacterial cells utilizing mutants with defective synthesis of d-alanine. Can. J. Microbiol. 34:256-261. [DOI] [PubMed] [Google Scholar]
- 10.Hols, P., C. Defrenne, T. Ferain, S. Derzelle, B. Delplace, and J. Delcour. 1997. The alanine racemase gene is essential for growth of Lactobacillus plantarum. J. Bacteriol. 179:3804-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hols, P., M. Kleerebezem, A. N. Schanck, T. Ferain, J. Hugenholz, J. Delcour, and W. M. de Vos. 1999. Conversion of Lactococcus lactis from homolactic to homoalanine fermentation through metabolic engineering. Nat. Biotechnol. 17:588-592. [DOI] [PubMed] [Google Scholar]
- 12.Hols, P., P. Slos, P. Dutot, J. Reymund, P. Chabot, B. Delplace, J. Delcour, and A. Mercenier. 1997. Efficient secretion of the model antigen M6-gp41E in Lactobacillus plantarum NCIMB 8826. Microbiology 143:2733-2741. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs, W. R., S. B. Snapper, L. Lugosi, and B. R. Bloom. 1990. Development of BCG as recombinant vaccine delivery vehicle. Curr. Top. Microbiol. Immunol. 155:153-160. [DOI] [PubMed] [Google Scholar]
- 14.Klijn, N., A. H. Weerkamp, and W. M. de Vos. 1995. Genetic marking of Lactococcus lactis shows its survival in the human gastrointestinal tract. Appl. Environ. Microbiol. 61:2771-2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kremer, L., L. Dupré, G. Riveau, A. Capron, and C. Locht. 1998. Systemic and mucosal immune responses after intranasal administration of recombinant Mycobacterium bovis Bacillus Calmette-Guérin expressing gluthathione S-transferase from Schistosoma haematobium. Infect. Immun. 66:5669-5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McComb, J. A. 1964. The prophylactic dose of homologous tetanus antitoxin. N. Engl. J. Med. 270:175-178. [DOI] [PubMed] [Google Scholar]
- 17.Medaglini, D., A. Ciabattini, M. R. Spinosa, T. Maggi, H. Marcotte, M. R. Oggioni, and G. Pozzi. 2001. Immunization with recombinant. Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine 19:1931-1939. [DOI] [PubMed] [Google Scholar]
- 18.Medaglini, D., C. M. Rush, P. Sestini, and G. Pozzi. 1997. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases: vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 15:1330-1337. [DOI] [PubMed] [Google Scholar]
- 19.Mielcarek, N., J. Cornette, A. M. Schacht, R. J. Pierce, C. Locht, A. Capron, and G. Riveau. 1997. Intranasal priming with recombinant Bordetella pertussis for the induction of a systemic immune response against a heterologous antigen. Infect. Immun. 65:544-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neutra, M. R., E. Pringault, and J. P. Kraehenbuhl. 1996. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu. Rev. Immunol. 14:275-300. [DOI] [PubMed] [Google Scholar]
- 21.Norton, P. M., J. M. Wells, H. W. G. Brown, A. M. Macpherson, and R. W. F. Le Page. 1997. Protection against tetanus toxin in mice nasally immunized with recombinant Lactococcus lactis expressing tetanus toxin fragment C. Vaccine 15:616-619. [DOI] [PubMed] [Google Scholar]
- 22.Ntamere, A. S., D. J. Taron, and F. C. Neuhaus. 1987. Assembly of d-alanyl-lipoteichoic acid in Lactobacillus casei: mutants deficient in the d-alanyl ester content of this amphiphile. J. Bacteriol. 169:1702-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Palumbo, E., C. Favier, M. Deghorain, P. S. Cocconcelli, C. Grangette, A. Mercenier, E. Vaughan, and P. Hols. 2004. Knockout of the alanine racemase gene in Lactobacillus plantarum results in septation defects and cell wall perforation. FEMS Microbiol. Lett. 233:131-138. [DOI] [PubMed] [Google Scholar]
- 23.Parr, M. B., and E. L. Parr. 1990. Antigen recognition in the female reproductive tract. I. Uptake of intraluminal protein tracers in the mouse vagina. J. Reprod. Immunol. 17:101-114. [DOI] [PubMed] [Google Scholar]
- 24.Pavan, S., P. Hols, J. Delcour, M. C. Geoffroy, C. Grangette, M. Kleerebezem, and A. Mercenier. 2000. Adaptation of the nisin-controlled expression system in Lactobacillus plantarum: a tool to study in vivo biological effects. Appl. Environ. Microbiol. 66:4427-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reveneau, N., M.-C. Geoffroy, C. Locht, P. Chagnaud, and A. Mercenier. 2001. Comparison of the immune responses induced by local immunizations with recombinant Lactobacillus plantarum producing tetanus toxin fragment C in different cellular locations. Vaccine 20:1769-1777. [DOI] [PubMed] [Google Scholar]
- 26.Robinson, K., L. M. Chamberlain, K. M. Schofield, J. M. Wells, and R. W. F. Le Page. 1997. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 15:653-657. [DOI] [PubMed] [Google Scholar]
- 27.Ryan, E. T., J. R. Butterton, R. Neal Smith, P. A. Carroll, T. I. Crean, and S. B. Calderwood. 1997. Protective immunity against Clostridium difficile toxin a induced by oral immunization with a live, attenuated Vibrio cholerae vector strain. Infect. Immun. 65:2941-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw, D. M., B. Gaerthé, R. J. Leer, J. G. M. M. Van Der Stap, C. Smittenaar, M.-J. Heijne den Bak-Glashouwer, J. E. R. Thole, F. J. Tielen, P. H. Pouwels, and C. E. G. Havenith. 2000. Engineering the microflora to vaccinate the mucosa: serum immunoglobulin G responses and activated draining cervical lymph nodes following mucosal application of tetanus toxin fragment C-expressing lactobacilli. Immunology 100:510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thapar, M. A., E. L. Parr, and M. B. Parr. 1990. The effect of adjuvants on antibody titers in mouse vaginal fluid after intravaginal immunization. J. Reprod. Immunol. 17:207-216. [DOI] [PubMed] [Google Scholar]
- 30.Vesa, T., P. Pochart, and P. Marteau. 2000. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment. Pharmacol. Ther. 14:823-828. [DOI] [PubMed] [Google Scholar]