Abstract
We have previously shown that a hexavalent group A streptococcal M protein-based vaccine evoked bactericidal antibodies after intramuscular injection. In the present study, we show that the hexavalent vaccine formulated with several different mucosal adjuvants and delivered intranasally induced serum and salivary antibodies that protected mice from intranasal challenge infections with virulent group A streptococci. The hexavalent vaccine was formulated with liposomes with or without monophosphorylated lipid A (MPL), cholera toxin B subunit with or without holotoxin, or proteosomes from Neisseria meningitidis outer membrane proteins complexed with lipopolysaccharide from Shigella flexneri. Intranasal immunization with the hexavalent vaccine mixed with these adjuvants resulted in significant levels of antibodies in serum 2 weeks after the final dose. Mean serum antibody titers were equivalent in all groups of mice except those that were immunized with hexavalent protein plus liposomes without MPL, which were significantly lower. Salivary antibodies were also detected in mice that received the vaccine formulated with the four strongest adjuvants. T-cell proliferative assays and cytokine assays using lymphocytes from cervical lymph nodes and spleens from mice immunized with the hexavalent vaccine formulated with proteosomes indicated the presence of hexavalent protein-specific T cells and a Th1-weighted mixed Th1-Th2 cytokine profile. Intranasal immunization with adjuvanted formulations of the hexavalent vaccine resulted in significant levels of protection (80 to 100%) following intranasal challenge infections with type 24 group A streptococci. Our results indicate that intranasal delivery of adjuvanted multivalent M protein vaccines induces protective antibody responses and may provide an alternative to parenteral vaccine formulations.
Streptococcus pyogenes (group A streptococci) is a widespread human pathogen responsible for a variety of clinical syndromes, ranging from uncomplicated pharyngitis and impetigo to pneumonia, sepsis, necrotizing fasciitis, and streptococcal toxic shock syndrome. Untreated streptococcal pharyngitis may lead to acute rheumatic fever and chronic rheumatic heart disease (8). Infections of the skin or throat by certain group A streptococcal strains can also result in acute glomerulonephritis (4).
The search for an effective vaccine to prevent group A streptococcal infections has been ongoing for many decades. One of the major vaccine candidates is the surface M protein, which is a primary virulence determinant of these organisms (18). Conserved C-repeat regions of the M proteins have been shown to evoke protective immune responses after mucosal administration (3, 7, 14). The type-specific N-terminal regions of M proteins contain epitopes that evoke serum bactericidal antibodies after parenteral administration (2). It was previously shown that recombinant multivalent M protein-based vaccines containing N-terminal peptides from 4, 6, 8, and 26 different M serotypes evoke opsonic antibodies to the respective vaccine strains after intramuscular injection (10, 12, 13, 17). In addition, it was shown that intranasal (i.n.) administration of a recombinant fusion protein containing an N-terminal peptide of type 5 M protein and the B subunit of Escherichia coli labile toxin protected mice from intraperitoneal challenge infections with type 5 streptococci (11).
We undertook the present studies to determine the protective efficacy of a multivalent M protein-based vaccine formulated with various mucosal adjuvants and administered via the i.n. route. As a prototype vaccine, we selected the recombinant hexavalent protein that has been the subject of previous reports with animals (10) and a recently completed phase I clinical trial in adult volunteers (unpublished data). The hexavalent protein was formulated with liposomes with and without monophosphorylated lipid A (MPL), cholera toxin (CT) B subunit (CT-B) with and without holotoxin (CT), or proteosomes, which are composed of outer membrane proteins of Neisseria meningitidis complexed with lipopolysaccharide (LPS) from Shigella flexneri (Pr/LPS) (15). The immunogenicity of the i.n. vaccine formulations was evaluated by measuring specific antibody levels in serum and saliva from immunized and control mice. In addition, lymphocyte proliferation and ELISPOT assays were performed using cells from spleen or cervical lymph nodes (CLN) to measure systemic or local immune responses, respectively, and to quantify Th1- or Th2-type responses. Protective efficacy was determined after i.n. challenge with virulent type 24 streptococci.
MATERIALS AND METHODS
Hexavalent vaccine.
Specific 5′ regions of the emm genes from six different serotypes of group A streptococci were amplified using PCR, and the hexavalent fusion gene was constructed as previously described (10). The hexavalent protein contained N-terminal amino acids from M24 (1 to 60), M5 (1 to 58), M6 (1 to 35), M19 (1 to 35), M3 (21 to 70), M1 (1 to 50), and M24 (1 to 80). The recombinant fusion protein was expressed in E. coli strain MC4100 with pQE-30 (Qiagen Inc., Valencia, Calif.) and purified as previously described (10).
Vaccine formulations.
Liposomes were prepared with or without lipid A (Ribi Immunochem, Hamilton, Mont.) as previously described (1, 20). The bulk lipid consisted of dipalmitoyl phosphatidylcholine (Genzyme, Cambridge, Mass.), cholesterol (Sigma, St. Louis, Mo.), and dimyristoyl phosphatidylglycerol (Genzyme) at a molar ratio of 3:1:0.25, respectively. The final product contained approximately 20 μg of bulk lipid/μl. The formulations were suspended in phosphate-buffered saline (PBS), pH 7.4, and consisted of hexavalent protein (30 μg/10-μl dose) encapsulated in liposomes with lipid A (2.8 μg/dose) (hexa/liposomes/MPL) or in liposomes alone (hexa/liposomes). Liposomes were also formulated without hexavalent protein or lipid A for use as a control. Liposome size was 1,700 to 2,100 nm as determined by dynamic light scattering.
Formulations containing CT were prepared by mixing hexavalent protein with either CT-B (Sigma) or CT-B plus holotoxin (CT) (Sigma) in PBS, pH 7.4, to achieve concentrations of 30 μg of hexavalent protein and 33 μg of CT-B with or without 2.5 μg of CT (hexa/CT-B/CT and hexa/CT-B, respectively) per 10-μl dose. Formulations containing only CT-B and CT were prepared at 33 and 2.5 μg, respectively, per dose for use as a control.
Proteosomes were prepared as previously described (15) from outer membrane proteins of group B N. meningitidis complexed with purified LPS from S. flexneri serotype 2a (Pr/LPS). The weight ratio of outer membrane proteins to LPS was approximately 1:1. The final vaccine formulation consisted of 30 μg of hexavalent protein and 7.5 μg each of proteosome protein and LPS (hexa/Pr/LPS) in PBS, pH 7.4, per 10-μl dose. Pr/LPS formulated without hexavalent protein was used at 7.5 μg/dose as a control.
Immunization of mice.
Adult (7- to 8-week-old) female BALB/c mice were obtained from Harlan (Indianapolis, Ind.). After very light anesthesia, mice were immunized i.n. with formulations of hexavalent vaccine (30 μg of hexavalent protein per dose) containing either liposomes (hexa/liposomes), liposomes with lipid A (hexa/liposomes/MPL), CT-B with or without CT (hexa/CT-B/CT or hexa/CT-B, respectively), or Pr/LPS (hexa/Pr/LPS). Control mice were given either liposomes, CT-B/CT, or Pr/LPS alone in PBS i.n. The total volume of vaccine given to each mouse was 10 μl. Ten mice were immunized per group. Additional age-matched mice were immunized i.n. with hexa/Pr/LPS or given Pr/LPS alone for use in lymphocyte proliferation and ELISPOT assays. Mice were immunized at 0, 2, 4, and 8 weeks when vaccines containing liposomes or CT were used or at 0, 2, and 6 weeks when Pr/LPS was used.
Collection of serum and saliva.
Sera and saliva were obtained prior to immunization and at 1 to 3 weeks following the final dose of vaccine. Anesthetized mice were bled by puncturing the retro-orbital plexus by using microhematocrit capillary tubes (Fisher Scientific, Pittsburgh, Pa.). Blood was allowed to clot at room temperature, and the clot was allowed to retract at 4°C overnight. Serum was separated from red blood cells by microcentrifugation and stored at 4°C until tested. Saliva samples were obtained after light anesthesia by rinsing the mouth three to four times with 150 μl of PBS, pH 7.4, containing a complete protease inhibitor cocktail (Roche Diagnostics, Basel, Switzerland). Saliva samples were stored at −20°C until tested.
Detection of antibodies.
An enzyme-linked immunosorbent assay was used to measure specific antibody in preimmune and immune mouse sera and saliva. Purified recombinant hexavalent fusion protein or purified recombinant dimeric amino-terminal M peptides (5 μg/ml) were bound to flat-bottomed microtiter wells (Nunc-Immuno modules; Nalge Nunc International, Roskilde, Denmark) in 0.1 M sodium carbonate, pH 9.8 (100 μl/well), overnight at 37°C. Excess peptide was removed, and wells were washed five times with 0.15 M NaCl containing 0.05% Tween 20 (Sigma).
Mouse sera were serially diluted from 1:100 in PBS, pH 7.4, containing 0.05% Tween 20 (PBS/TW). Diluted sera or undiluted saliva samples were added to wells (100 μl/well) and incubated at 37°C for 2 h. Unbound primary antibody was removed, and the wash step was repeated. Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG), IgA, and IgM (ICN Biomedicals, Inc., Irvine, Calif.) was diluted 1:10,000 or 1:2,000 in PBS/TW and added to wells (100 μl/well) to detect specific serum or salivary antibody, respectively. Incubation followed at 37°C for 2 h.
After removal of unbound secondary antibody and washing, disodium p-nitrophenol phosphate (Sigma) at 1 mg/ml in 1 M Trizma base-0.3 mM MgCl2, pH 9.8, was added (100 μl/well). Substrate was allowed to develop for 20 or 30 min for serum or saliva samples, respectively. Serum antibody titer was defined as the reciprocal of the highest dilution of serum which yielded an absorbance of ≥0.1 at 405 nm. Specific salivary antibody was expressed as fold increase in absorbance at 405 nm between pre- and postimmunization samples.
Preparation of lymphocytes.
Mice were immunized with hexa/Pr/LPS or Pr/LPS, as described above, and were given booster doses 6 months later. Spleens and CLN were harvested 8 days later, and mononuclear cells were pooled from four or five mice per group for use in the blastogenesis and ELISPOT assays. Tissues were minced with sterile forceps in HL-1 tissue culture medium (Cambrex Bio Science Walkersville, Inc., Walkersville, Md.) supplemented with 2 mM l-glutamine (Gibco Invitrogen Corp., Invitrogen Life Technologies, Carlsbad, Calif.), 1% penicillin-streptomycin (Gibco Invitrogen Corp.), 0.05 mM 2-mercaptoethanol (Sigma), and 0.1% bovine serum albumin, low endotoxin (Sigma) (HL-1 complete). Lymphocytes were purified from cell suspensions with the use of Hystopaque-1077 (Sigma), washed, counted, and adjusted to desired concentrations in HL-1 complete medium for use in lymphocyte assays.
Lymphocyte proliferation assays.
Lymphocytes (5 × 106/ml) were stimulated with various concentrations of hexavalent protein (1 to 30 μg/ml) or concanavalin A (0.25 μg/ml) (Sigma) in 96-well tissue culture plates (Corning Inc., Corning, N.Y.) for 72 h at 37°C with 5% CO2 and humidity. Cells were tested in triplicate for each antigen concentration. [3H]thymidine (Perkin-Elmer, Inc., Boston, Mass.) was added (1 μCi/well), and the cells were incubated for an additional 6 h. Cells were harvested onto glass fiber filters (Packard Instrument Co., Meriden, Conn.) with a Packard Filtermate 196 cell harvester (Packard) and allowed to dry overnight at room temperature, and radioactivity was measured using a Matrix 96 direct beta counter (Packard). Counts per minute for each sample tested were divided by counts per minute for respective negative controls (i.e., cells incubated without antigen) to obtain a stimulation index. Lymphocyte proliferation was expressed as a mean stimulation index for each sample tested in triplicate.
ELISPOT assays for hexavalent protein-specific antibodies and cytokines.
Specific antibody-, interleukin-4 (IL-4)- or gamma interferon (IFN-γ)-producing cells were enumerated using modifications of the enzyme-liked immunospot (ELISPOT) assay (9, 16). Sterile MultiScreen 96-well ELISPOT HP plates (Millipore Corp., Bedford, Mass.) were utilized. Hexavalent protein (30 μg/ml), anti-mouse IL-4 antibody, or anti-mouse IFN-γ antibody (5 μg/ml each) (BD Biosciences PharMingen, San Diego, Calif.) was bound to the plates in sterile PBS, pH 7.4 (100 μl/well), for 16 to 18 h at 4°C for the enumeration of antibody-, IL-4-, or IFN-γ-producing cells, respectively. Excess protein was removed, and the plates were washed and blocked with HL-1 complete medium containing 10% sterile-filtered fetal bovine serum (FBS; HyClone Laboratories, Logan, Utah). Lymphocytes from spleen or CLN were cultured in duplicate in HL-1 complete medium at various concentrations (0.63 × 105 to 1 × 106/100 μl) and stimulated with either bound hexavalent protein (for antibody-producing cells) or with 100 μl of hexavalent protein at 30 μg/ml in HL-1 complete medium (for cytokine-producing cells) for 40 h at 37°C with 5% CO2 and humidity. Negative-control wells contained cells with 100 μl of additional medium, while positive-control wells included cells with concanavalin A (cytokine-producing cells) or additional hexavalent protein (antibody-producing cells).
Cell suspensions were aspirated, and wells were washed twice with deionized water (200 μl/well for 3 min/wash) and then three times with PBS/TW (200 μl/well). Horseradish peroxidase (HRP)-conjugated anti-mouse IgG, IgA, and IgM (ICN Biomedicals, Inc.) were diluted 1:2,000 for use as the detection antibody for antibody-producing cells. Biotinylated anti-mouse IL-4 or IFN-γ (BD Biosciences PharMingen) was diluted for use at 2 or 0.5 μg/ml, respectively, as detection antibody for cytokine-producing cells. Detection antibodies were diluted in PBS with 10% FBS (PBS/FBS), added to appropriate wells (100 μl/well), and incubated at room temperature.
After 2 h of incubation, wells containing biotinylated anti-mouse IL-4 and IFN-γ antibodies were aspirated and washed three times with PBS/TW. Avidin-HRP (BD Biosciences PharMingen) was diluted 1:100 in PBS/FBS, added to these wells (100 μl/well), and incubated for 1 h at room temperature. Wells containing HRP-anti-mouse IgG, IgA, and IgM were not disturbed and were allowed to continue incubation.
All wells were aspirated and washed four times with PBS/TW and two times with PBS, pH 7.4. Spots formed by antibody- or cytokine-producing cells were developed using the substrate 3-amino-9-ethylcarbazole provided in a 3-amino-9-ethylcarbazole substrate kit (BD Biosciences PharMingen). Substrate was prepared and filtered just prior to use at 100 μl/well and allowed to react for 50 min. Wells were aspirated, washed once with deionized water, and allowed to air dry overnight at room temperature. Spots were analyzed and enumerated using a Zeiss Axioplan 2 scope and KS ELISPOT software (Carl Zeiss, Inc., Gottingen, Germany). The mean number of spots for each duplicate sample was calculated and expressed as the mean number of antibody-, IL-4-, or IFN-γ-producing cells per lymphocyte population.
i.n. challenge with group A streptococci.
Immunized and control mice were challenged via the i.n. route (6) with 4 × 107 type 24 streptococci (Vaughn strain) and observed for 15 days. Ten mice were challenged from each group unless otherwise noted. Challenge took place 8 weeks after the last immunization was administered.
Statistical analysis.
Statistical comparisons were made using a one-way analysis of variance with JMP Version 4 software (JMP Sales, Cary, N.C.). A P value of ≤0.05 was considered significant.
RESULTS
Hexavalent protein serum antibodies.
Mice were immunized i.n. with the hexavalent protein adjuvanted with liposomes (hexa/liposomes), liposomes containing MPL (hexa/liposomes/MPL), CT-B (hexa/CT-B), CT-B plus holotoxin (hexa/CT-B/CT), or proteosomes containing LPS (hexa/Pr/LPS). Control mice received only the respective adjuvant. Specific antibody levels were measured in sera collected at various times post-primary immunization (data not shown) and were found to be highest in sera collected after two to three immunizing boosts. Therefore, the data presented represent antibody titers in sera collected 2 weeks after the final immunization (Fig. 1). Hexavalent protein-specific serum antibody levels were significantly higher in mice that received the hexa/liposome/MPL vaccine formulation than in mice that received the hexa/liposome formulation or liposomes alone (P < 0.05) (Fig. 1A). Hexavalent protein-specific antibody levels in sera from mice immunized with hexa/CT-B/CT were significantly greater than those in sera from control mice given only CT-B/CT (Fig. 1B). In addition, the mean antibody titer in mice immunized with hexa/CT-B/CT was greater than that in mice given hexa/CT-B. However, differences in antibody titers between these two groups of mice were not significant.
FIG. 1.
Hexavalent protein-specific antibody titers in sera from mice immunized i.n. with formulations of hexavalent protein containing liposomes and lipid A (H/L/MPL) or liposomes only (H/L) (A), CT-B with or without holotoxin (H/CT-B/CT or H/CT-B, respectively) (B), or proteosomes (H/Pr/LPS) (C). Specific antibody titers for control mice that received only liposomes (L), CT-B/CT, or proteosomes (Pr/LPS), respectively, are also indicated (titers < 100). Each bar represents the mean (± standard error of the mean) antibody titer in serum from 10 mice in each group 2 weeks after the final immunization. *, P < 0.05 for H/L/MPL versus H/L and versus L, for H/CT-B/CT versus CT-B/CT, and for H/Pr/LPS versus Pr/LPS.
Mice immunized with the hexa/Pr/LPS formulation produced significantly higher levels of hexavalent protein-specific serum antibodies than did their respective control mice (Fig. 1C). A comparison of hexavalent protein-specific serum antibody titers among mice immunized with hexa/liposomes/MPL, hexa/CT-B/CT, hexa/CT-B, and hexa/Pr/LPS revealed no significant differences among the four groups. This suggests that liposomes with MPL, CT-B with or without CT, and Pr/LPS have similar mucosal adjuvant activities when formulated with the hexavalent protein.
Type-specific serum antibody responses.
When mouse sera were tested against the individual type-specific peptide components of the hexavalent vaccine, antibodies to M24, M6, M5, and M19 peptides were detected in mice immunized with hexa/liposomes/MPL (Table 1). Levels of antibody specific for M24 and M6 in these mice were significantly higher than those in control mice (P < 0.05). For mice given the hexa/liposome formulation, serum antibody reactivity was very low to undetectable with each M peptide. Specific antibodies to M24, M6, and M5 were also detected in immune sera from mice given either hexa/CT-B or hexa/CT-B/CT. Immune sera from mice immunized with hexa/Pr/LPS contained antibodies to M3, M5, M6, and M24. The levels of M24-specific antibody in these mice were significantly greater than those in the respective control animals (P < 0.05). All respective control mice in each group had antibody titers of <100 against each type-specific peptide (Table 1).
TABLE 1.
Type-specific serum antibodies evoked in mice after i.n. administration of hexavalent vaccines
| Vaccine formulation | Mean serum titer (± SEM) against hexavalent vaccine M peptides
|
|||||
|---|---|---|---|---|---|---|
| M1 | M3 | M5 | M6 | M19 | M24 | |
| Hexa/liposomes/MPL | <100 | <100 | 310 (149) | 540 (190)a | 210 (72) | 850 (319)a |
| Hexa/liposomes | <100 | <100 | 110 (10) | 130 (30) | 130 (30) | 120 (13) |
| Liposomes | <100 | <100 | <100 | <100 | <100 | <100 |
| Hexa/CT-B/CT | <100 | <100 | 340 (105)a | 790 (331)a | <100 | 380 (99) |
| Hexa/CT-B | <100 | <100 | 330 (90) | 450 (83) | <100 | 460 (154)a |
| CT-B/CT | <100 | <100 | <100 | <100 | <100 | <100 |
| Hexa/Pr/LPS | <100 | 130 (30) | 120 (13) | 420 (160) | <100 | 210 (43)a |
| Pr/LPS | <100 | <100 | <100 | <100 | <100 | <100 |
P < 0.05 for immune titers versus respective controls.
Hexavalent protein-specific antibody in saliva.
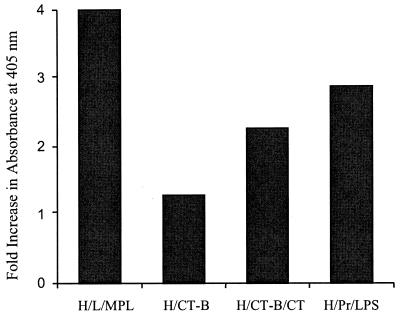
Antibody specific for hexavalent protein was detected in saliva from mice immunized with hexa/liposomes/MPL, hexa/CT-B/CT, hexa/CT-B, or hexa/Pr/LPS (Fig. 2). Hexavalent-specific antibody was not detected in salivary samples from mice given hexa/liposomes or from any of the control mice (data not shown). Salivary sample quantities were not sufficient to assay for type-specific peptide antibodies.
FIG. 2.
Hexavalent protein-specific antibody levels in saliva from mice immunized i.n. with formulations of hexavalent protein containing liposomes and lipid A (H/L/MPL), CT-B with or without CT (H/CT-B/CT or H/CT-B, respectively), or proteosomes (H/Pr/LPS). Each bar represents the fold increase in absorbance at 405 nm between pre- and postimmunization samples for 10 mice in each group.
T-lymphocyte proliferative and cytokine- and antibody-secreting cell (ASC) assays.
In vitro lymphocyte blastogenic responses and cytokine production were assessed using lymphocytes from spleen and CLN that were pooled from several mice that were immunized with hexa/Pr/LPS (Table 2). Lymphocyte stimulation indices for both pooled splenic lymphocytes and pooled CLN lymphocytes in response to the hexavalent protein were significantly greater than those found for lymphocytes from control animals (P < 0.05) (Table 2). The in vitro blastogenic responses of CLN lymphocytes from mice that received hexa/Pr/LPS were significantly higher than those for splenic lymphocytes (P < 0.05), indicating that the i.n. vaccine formulation may have preferentially expanded T-cell populations in the draining cervical nodes.
TABLE 2.
Lymphocyte proliferation and ELISPOT assays with hexavalent protein at 30 μg/ml and spleen or CLN lymphocytes from mice immunized i.n. with hexa/Pr/LPS or Pr/LPS only
| Vaccine formulation (na) | Lymphocyte proliferation
|
ELISPOT
|
|||
|---|---|---|---|---|---|
| Lymphocyte source | Stimulation index (per 5 × 105 cells) | Hexavalent specific antibody-producing cells (per 106 cells) | IL-4-producing cells (per 5 × 105 cells) | IFN-γ-producing cells (per 5 × 105 cells) | |
| Hexa/Pr/LPS (4) | Spleen | 8.65b | 26 | 74b | 240b |
| CLN | 17.59b | 168c | 88b | 408b | |
| Pr/LPS (5) | Spleen | 2.15 | 0d | 1 | 12 |
| CLN | 1.82 | 0d | 8 | 8 | |
n = number of mice used to pool lymphocytes.
P < 0.05 for hexa/Pr/LPS spleen or hexa/Pr/LPS CLN versus Pr/LPS spleen or Pr/LPS CLN and for hexa/Pr/LPS spleen versus hexa/Pr/LPS CLN.
P < 0.05 for hexa/Pr/LPS CLN versus Pr/LPS CLN.
Results were subtracted from those obtained from experimental animals and assumed to represent background signal in the ELISPOT assay.
The ELISPOT assay revealed that there were significantly greater numbers of IL-4- and IFN-γ-producing lymphocytes from both spleen and CLN in mice given hexa/Pr/LPS than from control mice (P < 0.05) (Table 2). Also, the numbers of cytokine-producing cells from CLN were significantly greater than the numbers of those from the spleen (P < 0.05). These data indicate that both Th1 and Th2 cytokines were produced in both the spleen and CLN in response to i.n. immunization with Pr/LPS-adjuvanted hexavalent protein. The ratios of IFN-γ to IL-4 of 3.2 and 4.6 in the spleen and CLN, respectively, suggest that the responses in both compartments were weighted toward the Th1 phenotype. Hexavalent protein-specific ASCs detected by ELISPOT assays were more numerous in animals immunized with hexa/Pr/LPS than in control mice. However, only specific ASCs from CLN of immunized mice were found to be significantly higher in number (P < 0.05). These data indicate that both cellular and humoral immune responses were stimulated both locally and systemically by the hexa/Pr/LPS vaccine formulation given i.n. and that locally responding cells in CLN are greater in number than are cells involved in a systemic response.
Protection against i.n. challenge with group A streptococci.
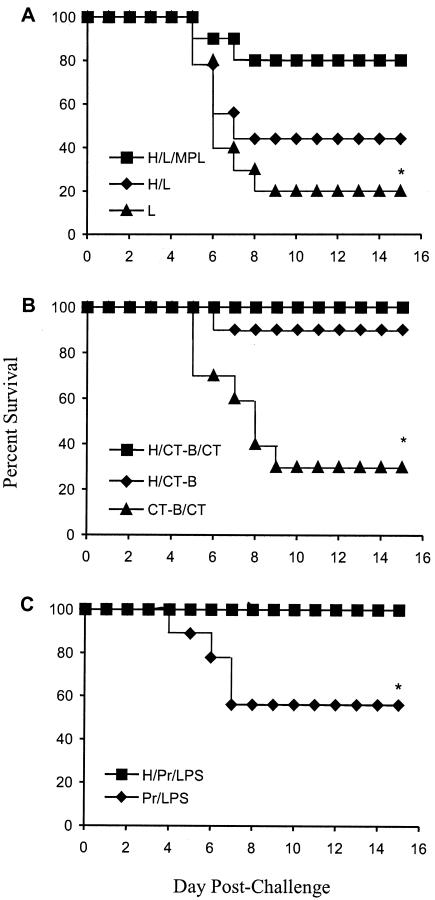
Mice were challenged i.n. with 4 × 107 virulent type 24 group A streptococci. A protection rate of 80% was observed for mice immunized with the hexa/liposome/MPL formulation (Fig. 3A). The survival rate for mice immunized with the hexa/liposome vaccine was 44% (one mouse from this group died prior to challenge). Only 20% of the control mice that received liposomes alone survived the challenge infections.
FIG. 3.
Survival of mice immunized i.n. with formulations of hexavalent group A streptococcal protein containing liposomes and lipid A (H/L/MPL) or liposomes only (H/L) (A), CT-B with or without CT (H/CT-B/CT or H/CT-B, respectively) (B), or proteosomes (H/Pr/LPS) (C) and challenged via the i.n. route with type 24 group A streptococci. Percent survival is also indicated for control mice that received only liposomes (L), CT-B/CT, or proteosomes (Pr/LPS), respectively. Each data set represents the percent survival for 10 mice challenged in each group (with the exception that only nine mice were challenged in each of the H/L and Pr/LPS groups, due to deaths prior to challenge). *, P < 0.05 for H/L/MPL versus L, for H/CT-B/CT and H/CT-B versus CT-B/CT, and for H/Pr/LPS versus Pr/LPS.
Immunization with hexa/CT-B/CT or hexa/Pr/LPS protected 100% of mice challenged with type 24 streptococci (Fig. 3B and C, respectively). The survival rate for mice immunized with hexa/CT-B was 90%, while only 30% of control mice given CT-B/CT survived the challenge. Of nine mice given only Pr/LPS (one mouse died prior to challenge), five survived challenge (56%). Thus, all of the hexavalent vaccine formulations, with the exception of hexa/liposomes, evoked significant protective immune responses compared to those of the respective controls (P < 0.05).
DISCUSSION
It was previously shown that multivalent M protein-based streptococcal vaccines evoke serum opsonic antibodies after intramuscular administration (10, 17). The strategy has been to incorporate small N-terminal peptides of different M proteins into multivalent vaccines that evoke opsonic antibodies in the absence of tissue cross-reactive antibodies. Since many group A streptococcal infections begin on the mucosal surface of the tonsils and pharynx, the concept of stimulating both secretory and systemic immunity in response to vaccines administered via the i.n. route is appealing. Mucosal antibodies capable of blocking attachment of the organisms to epithelial cells would provide the first level of defense by preventing subsequent colonization. Serum bactericidal antibodies would protect against infection by streptococci that manage to penetrate the mucosal barrier.
Previous studies from our laboratory have shown that pretreatment of group A streptococci with type-specific M protein antibodies prevented infection of mice challenged via the i.n. route (6). In addition, i.n. immunization with peptide M24, which represents the type-specific amino-terminal half of the M24 protein, also protected mice against i.n. challenge infections (6). Common epitopes of M proteins located in the C-repeat region of the molecules are exposed on the surface of the organisms and evoke mucosal antibodies in mice that block colonization and infection (3, 7, 14). In some studies, serum antibodies to C-repeat epitopes promoted moderate levels of opsonization (5), whereas in others they had no opsonic activity (3, 7). Nonetheless, it appears that mucosal antibodies that bind to exposed M protein epitopes in sufficient quantity can prevent infection in mice independent of serum bactericidal antibodies.
In the present study, the hexavalent vaccine formulated with several different mucosal adjuvants and delivered via the i.n. route evoked protective immune responses against i.n. challenge infections with type 24 streptococci. The vaccines evoked serum antibodies and low levels of salivary antibodies to the hexavalent protein. An analysis of serum antibody responses against the type-specific component peptides of the vaccine revealed that not all were equally immunogenic. The hexavalent vaccine is a fusion protein that contains six different N-terminal M protein peptides ranging in size from 35 to 80 amino acids (10). The N-terminal peptide is M24, followed by M5, M6, M19, M1, and M3. The M24 peptide is reiterated in the C-terminal location. Previous studies have shown that the C-terminal epitopes of multivalent fusion proteins might be preferentially degraded in vivo, thus becoming nonimmunogenic haptens (12, 13). The M24 peptide was repeated on the C-terminal end of the protein to function as a sacrificial peptide in order to protect the M1 and M3 peptides (10), which were the least immunogenic in the present study. We speculate that mucosal administration may result in greater proteolytic degradation of the protein, resulting in low levels of immunogenicity of the M1 and M3 peptides. Future studies will determine if administration of higher doses of the vaccine protein might overcome this problem.
The present studies were designed to compare the protective immunogenicities of the hexavalent protein formulated with several different mucosal adjuvants. CT, which is known to have potent adjuvant activity, was used as a standard to which the other adjuvants were compared. Levels of protection following i.n. challenge infections ranged from 44% in mice that received hexa/liposomes to 100% in mice that were immunized with hexa/CT-B/CT or hexa/Pr/LPS. Apart from the group that received hexa/liposomes, there were no statistical differences in titers of serum antibodies to the hexavalent protein or survival among the mice that were immunized with vaccines containing the four remaining adjuvants. Our goal is to identify adjuvants that may be suitable for eventual clinical trials with more complex multivalent M protein-based vaccines. Although CT is a potent adjuvant, its safety profile has been less than optimal (21). In the present studies, liposomes containing MPL and proteosomes complexed with LPS from S. flexneri had adjuvant activity equivalent to that of each other and to that of CT-B/CT. Furthermore, the hexavalent protein formulated with Pr/LPS stimulated a significant number of hexavalent protein-specific ASCs in CLN and a cytokine profile that indicated a Th1-weighted mixed Th1-Th2 response. The successful protective, antibody, and cellular responses elicited by the Pr/LPS adjuvanted hexavalent protein were particularly encouraging for development of a nasal streptococcal vaccine for humans, since this adjuvant preparation already has a good safety profile for i.n. human use as shown by preclinical toxicity studies and in human phase I and phase II clinical trials (15, 19).
Taken together, our results indicate that mucosal administration of multivalent streptococcal vaccines evokes protective immune responses in mice. Because the hexavalent protein contains opsonic M protein epitopes and we did not perform experiments to assess levels of mucosal colonization following challenge infections, we were unable to determine if protection was primarily mediated by the mucosal or by the systemic immune responses. Nonetheless, these results serve as a basis for further studies to identify optimal mucosal vaccine formulations that may be suitable candidates for clinical evaluation.
Acknowledgments
This work was supported by Public Health Service grant AI-10085 from the National Institute of Allergy and Infectious Diseases, research funds from the Department of Veterans Affairs, and ID Biomedical Corporation.
We thank Scott Brown, Department of Immunology, St. Jude Children's Research Hospital, Memphis, Tenn., for his assistance with the ELISPOT imaging system and software.
Editor: D. L. Burns
REFERENCES
- 1.Alving, C. R. 1991. Liposomes as carriers of antigens and adjuvants. J. Immunol. Methods 140:1-13. [DOI] [PubMed] [Google Scholar]
- 2.Beachey, E. H., M. Bronze, J. B. Dale, W. Kraus, T. Poirier, and S. Sargent. 1988. Protective and autoimmune epitopes of streptococcal M proteins. Vaccine 6:192-196. [DOI] [PubMed] [Google Scholar]
- 3.Bessen, D., and V. A. Fischetti. 1988. Influence of intranasal immunization with synthetic peptides corresponding to conserved epitopes of M protein on mucosal colonization by group A streptococci. Infect. Immun. 56:2666-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisno, A., I. Pearce, H. Wall, M. Moody, and G. Stollerman. 1970. Contrasting epidemiology of acute rheumatic fever and acute glomerulonephritis: nature of the antecedent streptococcal infection. N. Engl. J. Med. 283:561-565. [DOI] [PubMed] [Google Scholar]
- 5.Brandt, E. R., W. A. Hayman, B. Currie, S. Pruksakorn, and M. F. Good. 1997. Human antibodies to the conserved region of the M protein: opsonization of heterologous strains of group A streptococci. Vaccine 15:1805-1812. [DOI] [PubMed] [Google Scholar]
- 6.Bronze, M., D. McKinsey, C. Corbett, E. H. Beachey, and J. B. Dale. 1988. Protective immunity evoked by locally administered group A streptococcal vaccines in mice. J. Immunol. 141:2767-2770. [PubMed] [Google Scholar]
- 7.Bronze, M. S., H. S. Courtney, and J. B. Dale. 1992. Epitopes of group A streptococcal M protein that evoke cross-protective local immune responses. J. Immunol. 148:888-893. [PubMed] [Google Scholar]
- 8.Bronze, M. S., and J. B. Dale. 1996. The reemergence of serious group A streptococcal infections and acute rheumatic fever. Am. J. Med. Sci. 311:41-54. [DOI] [PubMed] [Google Scholar]
- 9.Czerkinsky, C. C., L. A. Nilsson, H. Nygren, O. Ouchterlony, and A. Tarkowski. 1983. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 65:109-121. [DOI] [PubMed] [Google Scholar]
- 10.Dale, J. B. 1999. Multivalent group A streptococcal vaccine designed to optimize the immunogenicity of six tandem M protein fragments. Vaccine 17:193-200. [DOI] [PubMed] [Google Scholar]
- 11.Dale, J. B., and E. C. Chiang. 1995. Intranasal immunization with recombinant group a streptococcal M protein fragment fused to the B subunit of Escherichia coli labile toxin protects mice against systemic challenge infections. J. Infect. Dis. 171:1038-1041. [DOI] [PubMed] [Google Scholar]
- 12.Dale, J. B., E. Y. Chiang, and J. W. Lederer. 1993. Recombinant tetravalent group A streptococcal M protein vaccine. J. Immunol. 151:2188-2194. [PubMed] [Google Scholar]
- 13.Dale, J. B., M. Simmons, E. C. Chiang, and E. Y. Chiang. 1996. Recombinant, octavalent group A streptococcal M protein vaccine. Vaccine 14:944-948. [DOI] [PubMed] [Google Scholar]
- 14.Fischetti, V. A., W. M. Hodges, and D. E. Hruby. 1989. Protection against streptococcal pharyngeal colonization with a vaccinia: M protein recombinant. Science 244:1487-1490. [DOI] [PubMed] [Google Scholar]
- 15.Fries, L. F., A. D. Montemarano, C. P. Mallett, D. N. Taylor, T. L. Hale, and G. H. Lowell. 2001. Safety and immunogenicity of a proteosome-Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect. Immun. 69:4545-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujihashi, K., J. R. McGhee, K. W. Beagley, D. T. McPherson, S. A. McPherson, C. M. Huang, and H. Kiyono. 1993. Cytokine-specific ELISPOT assay. Single cell analysis of IL-2, IL-4 and IL-6 producing cells. J. Immunol. Methods 160:181-189. [DOI] [PubMed] [Google Scholar]
- 17.Hu, M., M. Walls, S. Stroop, M. Reddish, B. Beall, and J. Dale. 2002. Immunogenicity of a 26-valent group A streptococcal vaccine. Infect. Immun. 70:2171-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lancefield, R. C. 1962. Current knowledge of the type specific M antigens of group A streptococci. J. Immunol. 89:307-313. [PubMed] [Google Scholar]
- 19.Lowell, G. H., D. Burt, G. White, and L. Fries. 2003. Proteosome technology for vaccines and adjuvants, p. 271-282. In M. M. Levine, J. B. Kaper, R. Rappuoli, M. Liu, and M. F. Good (ed.), New generation vaccines, 3rd ed. Marcel Dekker, New York, N.Y.
- 20.Samuel, J., W. A. Budzynski, M. A. Reddish, L. Ding, G. L. Zimmermann, M. J. Krantz, R. R. Koganty, and B. M. Longenecker. 1998. Immunogenicity and antitumor activity of a liposomal MUC1 peptide-based vaccine. Int. J. Cancer 75:295-302. [DOI] [PubMed] [Google Scholar]
- 21.Tamura, S. I., and T. Kurata. 2000. A proposal for safety standards for human use of cholera toxin (or Escherichia coli heat-labile enterotoxin) derivatives as an adjuvant of nasal inactivated influenza vaccine. Jpn. J. Infect. Dis. 53:98-106. [PubMed] [Google Scholar]