Abstract
Our previous data demonstrated that live Candida albicans inhibits interleukin-12 (IL-12) production by human monocytes. Here we explored whether C. albicans inhibits IL-12 via a released factor and whether the inhibition is mediated via mitogen-activated protein kinase (MAPK) regulation. Supernatant fluids were obtained from cultured C. albicans (SC5314) as well as cultured Saccharomyces cerevisiae after 20 h of incubation. At 2 h postincubation of monocytes with heat-killed C. albicans (HKCA) (2:1) to stimulate IL-12, concentrated fungal supernatant fluids were added and incubated for an additional 20 h. The present data show that, unlike supernatant fluids obtained from S. cerevisiae, the C. albicans supernatant fluids significantly suppressed IL-12 production induced by HKCA. This suggested that the inhibition is Candida specific. A preliminary biochemical analysis revealed that this secretory IL-12 inhibitory factor is glycoprotein in nature. The inhibitory activity had no effect on the phagocytosis of yeasts. Supernatant fluids from C. albicans markedly induced the phosphorylation of ERK44/42 MAPK, but not p38 and SAPK, 1 min after they were added to monocytes. To test if the induction of ERK44/42 MAPK was central to the IL-12 inhibition, we used gamma interferon (IFN-γ) (1 ng/ml) plus lipopolysaccharide (LPS) (100 ng/ml) to stimulate IL-12 production by monocytes. The inhibition of ERK MAPK by the specific inhibitor PD 98059 significantly reduced phospho-ERK44/42 MAPK levels induced by C. albicans supernatant fluids in the IFN-γ-plus-LPS-driven monocytes. Concomitantly, PD 98059 reversed the IL-12 inhibitory activity of the C. albicans supernatant (P < 0.01). These data indicate that C. albicans can inhibit IL-12 production by secreting an ERK44/42 MAPK-stimulating factor and thus can attenuate effective immune responses.
Monocytes/macrophages are involved in both cellular and humoral immune responses and serve as important effector cells of innate and specific immunity during infectious diseases, including candidiasis. In addition to their involvement in the elimination of pathogens from the bloodstream and tissues, monocytes/macrophages can produce chemokines and cytokines (2) which serve to further promote specific immune responses. Cytokines, especially immunoregulatory cytokines interleukin-10 (IL-10) and IL-12 (3, 23), are released from human monocytes/macrophages interacting with Candida organisms. These cytokines may regulate the acquired immune response through T-cell development. IL-12 is essential for inducing type I immune responses through the development of gamma interferon (IFN-γ)-producing T cells, which in turn are associated with resistance to candidal infections (19, 20).
Our previous studies demonstrated that pathogenic Candida albicans strains inhibit IL-12 produced by monocytes (14) in response to an action that may be responsible for the observed lack of T-cell IFN-γ and may restrain an effective type I immune response to Candida (26). However, the mechanisms of the IL-12 inhibition are not clear. Direct cell-to-cell contact (9, 13) and/or interactions through a C. albicans secreted soluble factor may affect C. albicans inhibition of monocytic IL-12 production. For this study, we hypothesized that a soluble factor(s) released from C. albicans plays a role in the inhibition of IL-12 production by monocytes.
Mitogen-activated protein kinases (MAPKs) are a family of serine/threonine kinases that participate in signal transduction events associated with a number of stimuli, such as mitogens, growth factors, and pathogen-derived products. The three members of this family are extracellular signal-regulated kinases (ERK44/42), p38 MAPK, and c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK). All three MAPKs undergo phosphorylation as a result of the action of upstream phosphokinases (4). Phosphorylation, therefore, is a determinant for activation of MAPKs. Furthermore, phosphorylated specific substrates activate downstream signaling pathways that ultimately regulate the transcription of certain genes. It has been clear that cytokine production by immune cells is regulated primarily at the level of transcription and that transcription factors are major targets for MAPKs. Recent studies have implicated MAPKs in the stimulus-induced activation of macrophages. Additionally, activation of the ERK pathway acts to suppress IL-12 secretion (25). In contrast, the p38 pathway plays a specific role in the activation of IL-12 production in macrophages (24). Furthermore, preincubation of the murine macrophage cell line J774 with a p38 inhibitor leads to an increase in both basal and stimulated ERK activation (5), implying that reciprocal regulation exists in this MAPK family.
Our data indicate that virulent C. albicans inhibits monocyte IL-12 production by secreting a soluble factor with a molecular mass of >30 kDa. We also show that the activity has no effect on the phagocytosis of yeasts. This soluble IL-12 inhibitory factor activates ERK MAPK, and the inhibition of ERK MAPK results in the restoration of IL-12 production by monocytes. Thus, C. albicans-mediated activation of ERK MAPK is biologically relevant to IL-12 modulation.
MATERIALS AND METHODS
Purification of human monocytes from heparinized peripheral blood.
Human blood was obtained from healthy volunteers by a previously described method (27). Briefly, heparinized blood was centrifuged with Ficoll gradient 1077. Peripheral blood mononuclear cells (PBMC) were collected. After the incubation of PBMC in tissue culture dishes for 1 h at 37°C, adherent cells were harvested and treated with an antibody cocktail (StemCell Technologies, Vancouver, Canada) against T cells, B cells, and NK cells. The mixture of cells was then passed through a MACS separation column against a MACS magnet (Miltenyi Biotec, Auburn, Calif.) to obtain the negatively selected monocytes. The purity of the monocytes was >90% (determined by flow cytometry).
Yeast organisms.
The pathogenic fungus C. albicans (SC5314) was used to obtain culture supernatants. A nonpathogenic yeast, Saccharomyces cerevisiae, was used for comparison.
Preparation of supernatant fluids from yeast cells.
The yeast strain was cultured at 3 × 105 cells/well in 1 ml of RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) in 5% CO2 at 37°C for 20 h in six-well plates (14). One unit of C. albicans or S. cerevisiae activity was defined as the activity derived from 1 ml of starting culture. Next, the supernatants (or RPMI plus FBS control medium) were collected, transferred to a Centricon filter with different molecular weight cutoffs as indicated (Amicon Inc., Bedford, Mass.), and centrifuged (6,000 × g at 4°C for 30 min) to concentrate the supernatant fluids. Each 1 ml of supernatant fluid was concentrated to 30 to 50 μl. The concentrate was normalized on a unit basis, i.e., 20 ml of starting supernatant yielded 600 μl of final concentrate, which was then divided by 20 U to determine that 30 μl of concentrated supernatant equaled 1 U of activity by C. albicans. Subsequent experiments were treated with 1 U per experiment. In addition, C. albicans was incubated overnight with shaking in FBS-free RPMI medium. The supernatant fluids were collected and the amount of protein was measured.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis was also performed to determine the molecular weights of the proteins present in the supernatants. Finally, the concentrated supernatant fluids were filtered through a 0.22-μm-pore-size filter unit (Millipore, Molsheim, France), divided into aliquots, and stored at −80°C until use.
Biochemical analysis of C. albicans supernatant fluids.
For characterization of the C. albicans secretory IL-12 inhibitory factor (SIIF), a culture supernatant was obtained as described above and was subjected to proteinase K digestion or mild alkaline oxidation as described previously (10). Proteinase digestion was carried out by incubating 25 μl of supernatant fluid with 10 U of proteinase K (Promega) for 2 h at 37°C, followed by the inactivation of proteinase K at 80°C for 30 min. As a control, in a separate reaction, proteinase K was first heat inactivated, followed by the addition of the C. albicans supernatant, and then treated as described above. The incubation mixture was centrifuged (10,000 × g, 5 min) to collect the supernatant fluid, which was then added to monocytes activated with IFN-γ and lipopolysaccharide (LPS). Next, to determine whether the IL-12 inhibitory activity of the C. albicans supernatant was due to a glycoprotein component, we incubated the supernatant concentrate with 0.1 N NaOH overnight at 37°C. The resulting incubation mixture was dialyzed for 4 h, with two changes, against 1 liter of PBS at 4°C, and the effect of the dialysate on IL-12 production by monocytes was determined.
Quantitation of IL-12 p70.
The levels of IL-12 p70 were determined by an enzyme-linked immunosorbent assay (ELISA) using pairs of mouse anti-human IL-12 p70 monoclonal antibody. Human recombinant IL-12 (R & D Systems Inc., Minneapolis, Minn.) was used as a standard. Quantities of IL-12 p70 were expressed as picograms per milliliter. The sensitivity of the ELISA was ≥10 pg/ml. All assays were performed in duplicate.
Phagocytosis assay.
Freshly prepared monocytes were labeled with phycoerythrin (PE)-conjugated monoclonal antibody CD14 (Dako, Glostrup, Denmark) for 30 min at 4°C. Ethanol-killed blastospores were labeled with fluorescein isothiocyanate (FITC) (0.01 mg/ml; Sigma Chemical Co., St. Louis, Mo.) in a 0.5 M carbonate/bicarbonate buffer (pH 9.5) for 30 min with agitation in a light-protected environment. The labeled blastospores were washed and stored in 5 × 106-cell aliquots at −80°C until use. Prior to the phagocytosis assay, the ethanol-fixed FITC-labeled blastospores were suspended in heat-activated human AB serum for 30 min at 37°C and washed three times with PBS. For the phagocytosis assay, 5 × 106 ethanol-fixed FITC-C. albicans blastospores were incubated with 1 × 106 PE-monocytes for 30 min at 37°C in a light-protected environment in a water bath with continuous agitation, to give a final volume of 400 μl. For discrimination between ingested, membrane-bound, and free C. albicans, ethidium bromide (EtBr) was added to the samples prior to flow cytometric analysis. Phagocytosis was determined by gating the phagocytes and calculating the percentage of phagocyte-associated green fluorescent cells. The cytometric analysis of phagocytosis was performed with the Elite ESP flow cytometry system (Coulter Electronics, Miami Lakes, Fla.). Multiparametric data were collected from 10,000 events and analyzed with Elite ESP, version 4.5, acquisition software (Coulter Electronics). Red fluorescence from PE was collected through a 575-nm long-pass filter, green fluorescence from FITC was collected by means of a 525-nm band-pass filter, and fluorescence from EtBr was measured through a 620-nm band-pass filter. Cytochalasin D was used as an inhibitor of phagocytosis and served as a positive control for the inhibition of phagocytosis.
Protein extraction and Western blot analysis.
Monocytes or monocytes cocultured with supernatants from C. albicans at 1, 5, and 15 min were lysed with lysis buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1 mM sodium EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg of leupeptin/ml, and 1 mM phenylmethylsulfonyl fluoride [Cell Signaling Technology, Inc., Beverly, Mass.]) by incubation on ice for 5 min. After sonication, the lysates were centrifuged at 13,000 × g at 4°C for 10 min, and the supernatant fluids were transferred to fresh tubes and stored at −70°C until used for assays. Protein concentrations of the lysates were determined by use of a Bio-Rad protein assay kit (Bio-Rad, Hercules, Calif.). Cell lysates (25 μg) from each sample were resolved by Nu-PAGE (4 to 12% bis-tris gel) before they were transferred to nitrocellulose. Nitrocellulose filters were incubated with washing buffer (20 mM Tris [pH 7.4], 0.9% NaCl, and 0.1% Tween 20 [Sigma]) containing 5% nonfat dry milk (Bio-Rad) for at least 1 h to block nonspecific protein binding. Primary antibodies to MAPK (ERK44/42, p38, and SAPK) (Cell Signaling Tech) were diluted in washing buffer containing 5% nonfat dry milk and were applied to the filters at 4°C overnight. After being washed, the blots were incubated with the appropriate horseradish peroxidase-conjugated secondary antibodies (diluted 1:1,000 or 1:2,000 in washing buffer with 5% milk) for 1 h at room temperature. Immunoreactive bands were visualized with the enhanced chemiluminescence system (Pierce Biotechnology, Inc., Rockford, Ill.). Densitometric quantitation was carried out with OPTIMAS image software.
Analysis of specificity of MAPK.
For determination of the specificity of MAPK, the specific inhibitor PD 98059 (20 μM) for ERK44/42 phosphorylation (Calbiochem, San Diego, Calif.) was employed. Dimethyl sulfoxide (DMSO) was used as the solvent (0.005%) and served as a control for the inhibitors. IFN-γ (1 ng/ml) and LPS (100 ng/ml) were used as stimulators of IL-12 production. The effect of IFN-γ plus LPS on MAPK expression by monocytes was correlated with IL-12 production in parallel experiments. For inhibition experiments, monocytes were first treated with PD 98059 for 45 min, followed by the addition of IFN-γ plus LPS and C. albicans supernatant as described in the figure legends.
Statistical analysis.
Results were expressed as means ± standard errors of the means for n number of replicated experiments. The statistical significance of differences was determined by the t test. P values of <0.05 were considered significant.
RESULTS
Soluble factors released from C. albicans inhibit IL-12 production by human monocytes.
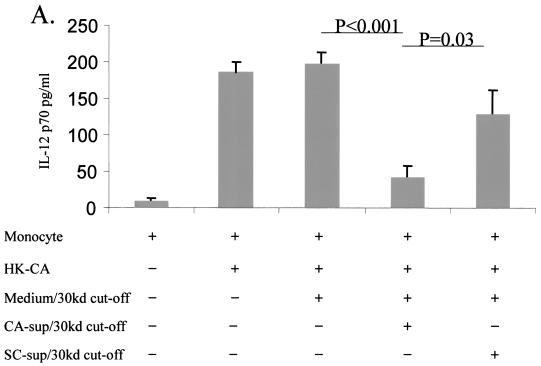
Our previous data showed that live C. albicans inhibits IL-12 production by monocytes. To explore whether C. albicans inhibits IL-12 production via a released soluble factor, independent of direct contact (6), we collected supernatant fluids from cultured yeast cells and concentrated them with Centricon columns as described above. A monocyte-Candida in vitro study model (26) was then employed to assess the ability of the supernatant fluid to inhibit IL-12 production by monocytes. To determine whether the SIIF was C. albicans specific, we compared supernatant fluids from C. albicans SC5314 and S. cerevisiae cultures. The results showed that the addition of concentrated supernatant fluid from C. albicans significantly inhibited heat-killed C. albicans (HKCA)-induced monocytic IL-12 production (43 ± 15 pg/ml) compared to the addition of medium with FBS after a 30-kDa cutoff (200 ± 14 pg/ml; P < 0.001) (Fig. 1). Monocytes incubated with HKCA only served as a second control. Both controls had similar results (Fig. 1A). This implies that FBS itself does not affect IL-12 produced by monocytes. In contrast, the supernatant fluid from S. cerevisiae failed to inhibit IL-12 production (130 ± 32 pg/ml) compared to the control (200 ± 14 pg/ml; P > 0.5), as shown in Fig. 1A. Importantly, IL-12 secretion by monocytes incubated with the supernatant fluid from C. albicans was significantly lower than that by monocytes incubated with the supernatant fluid from S. cerevisiae (P = 0.03; Fig. 1A). These data show that SIIF is C. albicans specific.
FIG. 1.
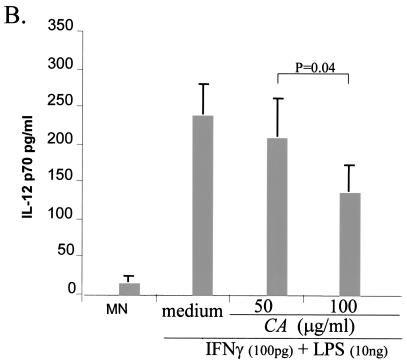
IL-12 production after treatment with supernatant fluids from C. albicans. (A) Supernatant fluids from C. albicans inhibited HKCA-induced IL-12 production by monocytes. Monocytes were incubated with HKCA (2:1) for 2 h, followed by the addition of concentrated supernatant fluids (>30 kDa) from C. albicans (equivalent to 1:0.3 monocytes to live C. albicans spores incubated overnight) or from S. cerevisiae. After overnight incubation, culture supernatant fluids were collected for IL-12 p70 quantitation. Monocytes only, monocytes plus HKCA, and monocytes, HKCA, and medium supplemented with FBS after filtration with a 30-kDa cutoff served as controls. CA-sup, supernatant fluids from C. albicans; SC-sup, supernatant fluids from S. cerevisiae. P values were determined by a paired t test (n = 3). (B) C. albicans culture supernatant fluid from FBS-free medium inhibits IL-12 p70 produced by monocytes in a dose-dependent manner. Supernatant fluids from C. albicans cultured in RPMI medium without FBS were collected. Protein concentrations were measured after concentration through Centricon 30-kDa cutoff filters. The amount of protein from the supernatant fluid was added as indicated to a monocyte (MN) culture (106/ml ) with the addition of IFN-γ (100 pg/ml) and LPS (10 ng/ml). Monocyte cultures with or without IFN-γ plus LPS were used as controls. CA, C. albicans protein. P values were determined by a paired t test (n = 3).
To determine if SIIF also exists in the FBS-free medium cultured with C. albicans, supernatant fluids were collected and IL-12 inhibition assays were performed as described above. Our results showed that SIIF also inhibited IL-12 and did so in a dose-dependent manner (Fig. 1B).
In order to corroborate the size of SIIF present in the concentrated supernatant fluids, we performed sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) combined with Centricon filtration. This analysis confirmed that SIIF present in the supernatant fluid has a molecular mass of >30 kDa. In this regard, the proteins retained by the filter had molecular masses ranging from 40 to 250 kDa.
We also tested the supernatant fluids after passing them through a Centricon filter with a cutoff ranging from 10 to 30 kDa. No inhibition of IL-12 production occurred when we used the supernatant fluid obtained from both C. albicans and S. cerevisiae (data not shown). These studies further confirmed that SIIF is present at a molecular mass of >30 kDa.
Preliminary biochemical analysis showed that C. albicans SIIF is glycoprotein in nature.
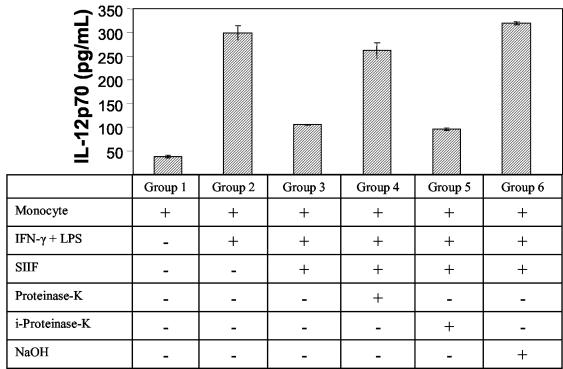
To gain some insight into the biochemical nature of SIIF, we subjected the supernatant to proteinase K digestion or mild alkali oxidation (Fig. 2). SIIF treated with proteinase K induced two times lower levels of monocytic IL-12 (105 pg/ml) than untreated SIIF (262 pg/ml). Moreover, SIIF treated with heat-inactivated proteinase K did not affect monocytic IL-12 production, indicating that the IL-12 inhibitory activity of SIIF is sensitive to proteinase K. A mild alkali treatment of SIIF also resulted in the loss of its ability to inhibit IL-12 production by monocytes, suggesting that carbohydrates play an important role in SIIF-associated IL-12 inhibition. Taken together, our preliminary studies indicate that the IL-12 inhibitory activity of C. albicans SIIF is due to the presence of a glycoprotein(s).
FIG. 2.
C. albicans SIIF is glycoprotein in nature. Equal amounts of C. albicans supernatant fluid (25 μl of SIIF) were added to different groups of monocytes, and their IL-12 levels were determined. Group 1 represents normal monocytes, while group 2 represents monocytes stimulated with supernatant fluids and IFN-γ plus LPS. The stimulated monocytes were incubated with untreated supernatant fluids (group 3), proteinase K-treated supernatant fluids (group 4), inactive proteinase K-treated supernatant fluids (group 5), or alkali-treated supernatant fluids (group 6). Data shown are means ± standard deviations of duplicate experiments.
Candidal inhibition of IL-12 production by monocytes is not related to inhibition of phagocytosis of blastospores by C. albicans supernatant.
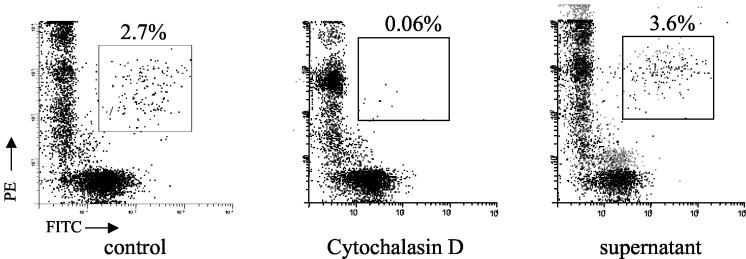
It has been shown that phagocytosis may cause IL-12 production by monocytes. The different efficiencies of Mycobacterium tuberculosis bacilli and purified protein derivative for the induction of IL-12 expression by monocytes are in part due to a requirement for phagocytosis. The phagocytosis of dead M. tuberculosis cells or inert 2-μm-diameter beads by monocytes induces IL-12 p40 mRNA (7). Thus, we sought to determine whether the inhibition of IL-12 production by monocytes incubated with the C. albicans supernatant fraction was due to the inhibition of phagocytosis. Flow cytometry analysis showed that 2.7% of monocytes phagocytosed ethanol-killed spores, whereas cytochalasin D clearly inhibited the phagocytosis to 0.06% of cells ingesting spores (Fig. 3). In contrast, the C. albicans supernatant failed to inhibit phagocytosis, with 3.6% of cells ingesting spores (Fig. 3). In addition, main channel fluorescence is an indirect measure of the number of yeast cells ingested. The mean channel fluorescence intensity (MFI) was not reduced by the C. albicans supernatant (MFI of 115 versus MFI of 115), indicating that the numbers of yeast cells ingested by monocytes were similar. Thus, the inhibition of IL-12 production by monocytes is not due to an upstream inhibition of phagocytosis by the supernatant fluid.
FIG. 3.
The >30-kDa supernatant of C. albicans does not inhibit phagocytosis of killed blastospores by monocytes. Monocytes were first labeled with PE-conjugated monoclonal antibody CD14. Ethanol-killed C. albicans blastospores were labeled with FITC and opsonized by suspension in human AB serum. Then opsonized FITC-blastospores were incubated with PE-monocytes at a ratio of 5:1. For discrimination between ingested, membrane-bound, and free C. albicans, EtBr was added to the samples prior to the flow cytometric analysis. Thus, internalized blastospores remained green, while adherent and nonphagocytosed blastospores stained red. C. albicans phagocytosis was determined by gating the phagocytes and calculating the percentage of phagocyte-associated green fluorescent cells (refer to Materials and Methods for further details). The numbers above each box indicate the percentages of killed C. albicans cells that were phagocytosed by the monocytes. Cytochalasin D is an inhibitor of phagocytosis and served as a control. The supernatant was obtained from C. albicans. Data are representative of three experiments.
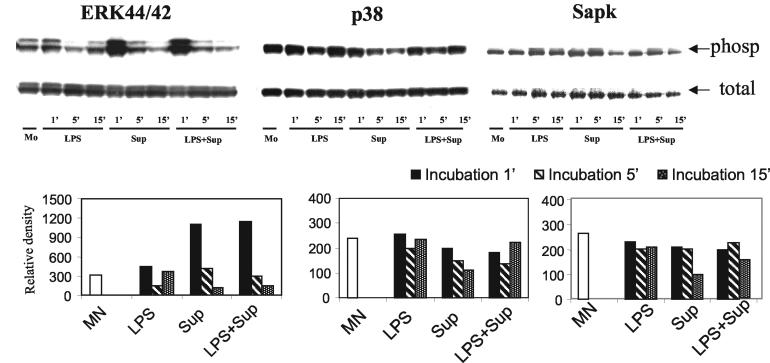
C. albicans supernatant fluid markedly induced phosphorylated ERK44/42 as a very early event.
Because MAPK family members may be involved in IL-12 regulation, we next determined whether MAPK family members play a role in C. albicans-mediated IL-12 inhibition. Indeed, the C. albicans fraction resulted in a rapid and robust induction of the phosphorylation of ERK p44/42 at the 1-min time point (Fig. 4). No increases in the phosphorylation of p38 and SAPK MAPK were found, although a late slightly inhibitory effect of phosphorylation of p38 at 15 min may occur (Fig. 4). Pooled results from the repeated experiments with ERK are shown in Table 1; the phosphorylation of ERK MAPK was significantly induced by the C. albicans supernatant in five individual experiments (P = 0.004). However, pooled data for the phosphorylation of p38 at 1 min from three individual experiments, expressed as relative intensities, were 410 ± 27 versus 370 ± 21, showing a slight inhibition by the C. albicans supernatant. No detectable change was seen for SAPK (Table 1).
FIG. 4.
Effect of C. albicans supernatant fluid on phosphorylation of ERK, p38, and SAPK MAPK in monocytes. Monocytes (MN) were stimulated with or without LPS (1 μg/ml) as indicated, followed by the addition of >30-kDa C. albicans supernatants (equivalent to 1:0.3 monocytes to live C. albicans cells) for 1, 5, and 15 min, and then the monocytes were lysed with lysis buffer. The lysates were resolved by Nu-PAGE followed by Western blotting. RDs of phosphorylated ERK/p38/SAPK relative to the total amount of ERK/p38/SAPK are expressed in bar charts. Data are representative of three experiments. Mo, monocytes.
TABLE 1.
Expression of phosphorylation of MAPK by monocytes after coculturing with C. albicans supernatantsa
| Protein | N | RD of MAPK
|
P value | |
|---|---|---|---|---|
| Monocytes | Monocytes plus supernatant | |||
| Phospho-ERK | 5 | 450 ± 93 | 790 ± 132 | 0.004 |
| Phospho-38 | 3 | 410 ± 27 | 370 ± 21 | 0.036 |
| Phospho-SAP | 3 | 540 ± 37 | 560 ± 36 | 0.641 |
Monocytes were cocultured with concentrated C. albicans supernatant fluid for 1 min at 37°C and then were lysed with lysis buffer. The lysates were resolved by Nu-PAGE followed by Western blotting. RDs. of phosphorylated ERK/p38/SAPK are expressed relative to the total ERK/p38/SAPK. Results are expressed as means ± standard errors of the means for N number of replicated experiments. P values were determined by a paired t test. P values of <0.05 are considered significant.
Specific inhibitor of ERK MAPK phosphorylation significantly inhibits C. albicans supernatant-induced phospho-ERK MAPK expression.
In order to determine whether ERK MAPK induction is critically involved in IL-12 inhibition, we used a specific inhibitor of ERK MAPK, PD 98059. DMSO was also used as a solvent control for the inhibitor. Additionally, IFN-γ plus LPS was added alone or along with the C. albicans supernatant to observe if the addition of IFN-γ plus LPS would affect the induction of ERK MAPK expression, because IFN-γ plus LPS would be used for the induction of monocytic IL-12 production in subsequent experiments. Monocytes alone had low ERK phosphoprotein levels (Fig. 5), which were only minimally increased by stimulation with IFN-γ plus LPS (relative density [RD], 368). In contrast, the addition of C. albicans supernatant to the IFN-γ-plus-LPS-stimulated monocytes highly induced phospho-ERK MAPK expression (RD, 670) (Fig. 5). Whereas the DMSO vehicle had a minimal effect on ERK phosphorylation (Fig. 5), monocytes incubated with C. albicans supernatant fluids, IFN-γ plus LPS, and inhibitor PD 98059 demonstrated a significant inhibition of the phosphorylation of ERK (RD, 810 ± 140 versus 570 ± 110) (Fig. 5). In contrast, the expression of phosphorylated p38 was decreased (9% decrease) after the addition of PD 98059 (data not shown).
FIG. 5.
C. albicans-induced phosphorylation of ERK MAPK in monocytes is blocked by the specific inhibitor of ERK phosphorylation, PD 98059. Monocytes (MN) were incubated with >30-kDa C. albicans supernatants (equivalent to 1:0.3 monocytes to live C. albicans cells) for 1 min, with or without IFN-γ (1 ng/ml) and LPS (100 ng/ml) (to stimulate IL-12, connecting with the following experiments), and then were lysed with lysis buffer. The lysates were resolved by Nu-PAGE, followed by Western blotting with antibodies for phospho-ERK MAPK and total ERK MAPK. White plus sign, positive standard for phospho-ERK MAPK; I+L, LPS plus IFN-γ; Sup, >30-kDa supernatant of SC5314; ERKi, PD 98059 inhibitor of phosphorylated ERK MAPK.
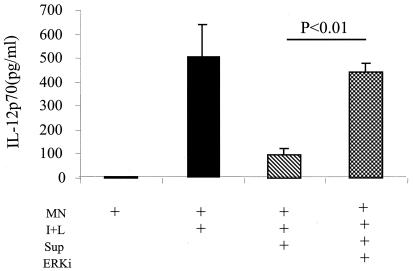
Inhibition of ERK MAPK phosphorylation by C. albicans supernatants restores monocytic IL-12 production.
In parallel experiments, we tested whether IL-12 p70 production could be restored if the ERK MAPK activity was inhibited. Unlike monocytes alone, which produced little IL-12 p70, IFN-γ-plus-LPS-treated monocytes induced IL-12 p70 production (Fig. 6). The C. albicans supernatant contained an activity capable of inhibiting IL-12 p70 production by monocytes (P = 0.05) (Fig. 6). The inhibitor PD 98059 was used to inhibit phospho-ERK MAPK with the addition of IFN-γ plus LPS to the culture, and IL-12 p70 production was significantly restored, to an RD of 440 ± 38 (from 96 ± 29 without the addition of inhibitor; P < 0.01). Thus, phospho-ERK MAPK expression in monocytes is induced by C. albicans in association with the inhibition of IL-12 p70, suggesting that C. albicans inhibits IL-12 through an ERK MAPK signaling mechanism.
FIG. 6.
Blocking of ERK phosphorylation in C. albicans-inhibited monocytes restores IL-12 production. Monocytes (MN) pretreated with or without PD 98059 (20 μM) for 45 min were stimulated with IFN-γ (1 ng/ml) and LPS (100 ng/ml), followed by incubation with the >30-kDa C. albicans supernatant (equivalent to 1:0.3 monocytes to live C. albicans cells) for 20 h. Supernatants were collected and IL-12 p70 was determined by ELISA. The P value is indicated (n = 4 tests). I+L, IFN-γ plus LPS; Sup, >30-kDa supernatant of SC5314; ERKi, PD 98059 inhibitor of phosphorylated ERK MAPK.
DISCUSSION
C. albicans infections occur mainly in immunocompromised individuals. However, C. albicans infections may further enhance existing immunosuppressive conditions, either via direct contact of C. albicans with immunocompetent cells of the host or indirectly via as yet unknown soluble factors secreted by C. albicans.
Local and systemic infections of C. albicans, as a rule, occur through primary invasion of the skin or mucous membranes. Thus, an epithelial barrier is the first line of defense of the host exposed to the organism. Both skin and mucous membranes contain dendritic cells, such as Langerhans cells, and macrophages as the immunocellular outposts in such peripheral tissues. Antigen-presenting cells such as Langerhans cells (12) can produce immunoregulatory cytokines such as IL-12 in response to stimulation, which is a critical component of developing effective type I immune responses to organisms such as C. albicans. However, tissue injury, such as UV damage to the skin, recruits monocytic/macrophagic cells into the epithelial and subepithelial layers, which eventually results in the production of IL-10 (27) with the consequent attenuation of the cellular immune response (11). Furthermore, the differentiation of monocytic precursors among the infiltrating monocytes into dendritic cells, such as Langerhans cells, can be modified by certain cytokines (8, 16) or products of complement activation in damaged tissue (22). Thus, C. albicans invasion and C. albicans protease-induced inflammation and tissue injury likely bring C. albicans into close proximity to constitutional dendritic cells as well as infiltrating monocytes/macrophages and monocytic precursors of dendritic cells. Alterations of the immunogenic potential of these cells and the ability to prevent such alterations may represent important mechanisms of C. albicans pathogenesis that can be therapeutically modified.
Previous studies (14, 26) have shown that direct interactions of monocytes and/or PBMC with C. albicans result in the inhibition of monocytic IL-12 and PBMC-produced IFN-γ, both of which are important cytokines relevant to type I immune responses. Recently, Angulo et al. (1) found that C. albicans infections enhanced immunosuppression in mice with a preimmunosuppressive condition induced by cyclophosphamide. In the present study, we showed that a soluble factor secreted from C. albicans plays an important role in the suppression of human monocytic IL-12 production via an ERK MAPK-dependent mechanism.
By acrylamide gel analysis, as mentioned in Materials and Methods, we showed that C. albicans-derived SIIF exists and has a molecular mass of >30 kDa. To concentrate and obtain the supernatant fluids at a feasible volume for the experiments, we used a 30-kDa molecular mass cutoff filter for all of the experiments. In order to get an idea of the nature of SIIF, we performed a preliminary biochemical analysis of SIIF, and the data showed that the inhibitory factor is glycoprotein in nature, consistent with the observation that almost all of the key molecules involved in the innate and adaptive immune responses are glycoproteins (21). Fractionation and characterization of SIIF are currently under way.
C. albicans is likely exposed to a serum environment once the host is infected. We used a medium supplemented with FBS to imitate in vivo conditions for C. albicans growth. However, to determine whether serum alone affected IL-12 production, we examined IL-12 levels in monocytes incubated with a medium supplemented with FBS in the absence of C. albicans as controls. As shown in Fig. 1A, the supernatant fluid from C. albicans significantly inhibited IL-12 production by monocytes, while medium plus FBS (in the absence of C. albicans cells) did not inhibit the production of this cytokine. This result indicates that SIIF is Candida specific. The FBS in the medium did not affect IL-12 production.
Microbes and their antigens can be potent inducers of IL-12 production by phagocytes. M. tuberculosis (7) and acapsular Cryptococcus neoformans (18) can induce IL-12 production by monocytes after phagocytosis. We investigated the possibility that SIIF inhibits IL-12 through the inhibition of phagocytosis of monocytes. The results showed that SIIF had no inhibitory effect on phagocytosis. Additional experiments showed that the induction of IL-12 by the exposure of monocytes to a soluble inducer (LPS plus IFN-γ) was also blocked by the addition of SIIF (Fig. 6). These data indicate that SIIF inhibits IL-12 production through a signaling pathway mechanism and is phagocytosis independent.
The present data show that the ERK and p38 MAPK signal transduction proteins undergo differential expression in human monocytes after SIIF treatment. The functional role of p38 MAPK in immune responses has been shown with mice deficient in p38 kinase MKK3. These mkk3−/− macrophages exhibit a selective defect in IL-12 production (15). Our data are in agreement with this finding, showing that SIIF inhibited the phosphorylation of p38 in three individual experiments (the relative intensity was 370 ± 21 [with SIIF] versus 410 ± 27 [without SIIF]; P = 0.035 by a paired t test). This is consistent with the above report that the mkk3−/− cells have a defect in IL-12 production.
The most interesting finding of this study was that the phosphorylation of ERK MAPK was dramatically enhanced by SIIF. We next asked if this differential SIIF regulation of ERK and p38 mirrors IL-12 production. Our data strongly indicate that the inhibition of ERK by a specific ERK inhibitor resulted in a restoration of IL-12 production, implying that enhanced phospho-ERK plays an inhibitory role in IL-12 production. Feng et al., using a murine macrophage cell line (J774), found that the inhibition of macrophage IL-12 production by Leishmania phosphoglycans is mediated by ERK MAP kinase (5). Phosphoglycans strongly enhance LPS-stimulated ERK MAPK activity even within <1 min. This is in agreement with our finding that the C. albicans supernatant fluid activation of ERK MAPK expression was an early event. Our data suggest that C. albicans affects the human immune response via the ERK MAPK pathway in at least two ways. (i) ERK MAPK plays a negative role in the regulation of IL-12. Enhanced ERK MAPK expression may result in a deficiency of IL-12-induced Th1-cell differentiation and maturation. (ii) ERK and p38 MAPK reciprocally regulate the differentiation of monocyte-derived dendritic cells (17). ERK MAPK inhibits monocytes from differentiating into dendritic cells, while p38 promotes this differentiation. Thus, high levels of ERK and low levels of p38 MAPK expression may impede in vivo dendritic cell development and result in a host diminution of Th1 immune responses.
Taken together, our studies indicate that pathogenic C. albicans secretes a soluble factor which inhibits IL-12 production by monocytes through an ERK-dependent mechanism. These findings may point to novel ways for preventing and treating candidiasis.
Acknowledgments
This work was supported by a pilot and feasibility grant (M.G. and K.K.) from a NIAMS Skin Diseases Research Center award (2P30AR39750) (K.D.C.) and in part by NIH grants AI-35097 (M.G.) and AI-41766 (K.D.C.), American Heart Association Scientist Development grant 0335313N (P.K.M.), and the University Hospitals of Cleveland Research and Education Fund.
Editor: T. R. Kozel
REFERENCES
- 1.Angulo, I., M. B. Jimenez-Diaz, J. F. Garcia-Bustos, D. Gargallo, F. G. de las Heras, M. A. Munoz-Fernandez, and M. Fresno. 2002. Candida albicans infection enhances immunosuppression induced by cyclophosphamide by selective priming of suppressive myeloid progenitors for NO production. Cell Immunol. 218:46-58. [DOI] [PubMed] [Google Scholar]
- 2.Castro, M., J. A. Bjoraker, M. S. Rohrbach, and A. H. Limper. 1996. Candida albicans induces the release of inflammatory mediators from human peripheral blood monocytes. Inflammation 20:107-122. [DOI] [PubMed] [Google Scholar]
- 3.de Waal Malefyt, R., J. Abrams, B. Bennett, C. G. Figdor, and J. E. De Vries. 1991. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J. Exp. Med. 174:1209-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong, C., R. J. Davis, and R. A. Flavell. 2002. MAP kinases in the immune response. Annu. Rev. Immunol. 20:55-72. [DOI] [PubMed] [Google Scholar]
- 5.Feng, G. J., H. S. Goodridge, M. M. Harnett, X. Q. Wei, A. V. Nikolaev, A. P. Higson, and F. Y. Liew. 1999. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 163:6403-6412. [PubMed] [Google Scholar]
- 6.Forsyth, C. B., E. F. Plow, and L. Zhang. 1998. Interaction of the fungal pathogen Candida albicans with integrin CD11b/CD18: recognition by the I domain is modulated by the lectin-like domain and the CED18 subunit. J. Immunol. 161:6198-6205. [PubMed] [Google Scholar]
- 7.Fulton, S. A., J. M. Johnsen, S. F. Wolf, D. S. Sieburth, and W. H. Boom. 1996. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect. Immun. 64:2523-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geissman, F., C. Prost, J.-P. Monnet, M. Dy, N. Brousse, and O. Hermine. 1998. Transforming growth factor b1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J. Exp. Med. 187:961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghannoum, M. A. 2000. Potential role of phospholipases in virulence and fungal pathogenesis. Clin. Microbiol. Rev. 13:122-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grunvald, E., M. Chiaramonte, S. Hieny, M. Wysocka, G. Trinchieri, S. N. Vogel, R. T. Gazzinelli, and A. Sher. 1996. Biochemical characterization and protein kinase C dependency of monokine-inducing activities of Toxoplasma gondii. Infect. Immun. 64:2010-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammerberg, C., S. K. Katiyar, M. C. Carroll, and K. D. Cooper. 1998. Activated complement component 3(C3) is required for UV induction of immunosuppression and antigenic tolerance. J. Exp. Med. 187:1133-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang, K., M. Kubin, K. D. Cooper, S. R. Lessin, G. Trinchieri, and A. H. Rook. 1996. IL-12 synthesis by human Langerhans cells. J. Immunol. 156:1402-1407. [PubMed] [Google Scholar]
- 13.Leidich, S. D., A. S. Ibrahim, Y. Fu, A. Koul, C. Jessup, J. Vitullo, W. Fonzi, F. Mirbod, N. Shigeru, Y. Nozawa, and M. A. Ghannoum. 1998. Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J. Biol. Chem. 273:26078-26086. [DOI] [PubMed] [Google Scholar]
- 14.Liu, L., K. Kang, M. Takahara, K. D. Cooper, and M. A. Ghannoum. 2001. Hyphae and yeasts of Candida albicans differentially regulate interleukin-12 production by human blood monocytes: inhibitory role of C. albicans germination. Infect. Immun. 69:4695-4697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu, H. T., D. D. Yang, M. Wysk, E. Gatti, I. Mellman, R. J. Davis, and R. A. Flavell. 1999. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 18:1845-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohamadzadeh, M., F. Berard, G. Essert, C. Chalouni, B. Pulendran, J. Davoust, G. Bridges, A. K. Palucka, and J. Banchereau. 2001. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J. Exp. Med. 194:1013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puig-Kroger, A., M. Relloso, O. Fernandez-Capetillo, A. Zubiaga, A. Silva, C. Bernabeu, and A. L. Corbi. 2001. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood 98:2175-2182. [DOI] [PubMed] [Google Scholar]
- 18.Retini, C., A. Casadevall, D. Pietrella, C. Monari, B. Palazzetti, and A. Vecchiarelli. 1999. Specific activated T cells regulate IL-12 production by human monocytes stimulated with Cryptococcus neoformans. J. Immunol. 162:1618-1623. [PubMed] [Google Scholar]
- 19.Romani, L., A. Mencacci, E. Cenci, R. Spaccapelok, P. Mosci, P. Puccetti, and F. Bistoni. 1993. CD4+ subset expression in murine candidiasis. Th responses correlate directly with genetically determined susceptibility or vaccine-induced resistance. J. Immunol. 150:925-931. [PubMed] [Google Scholar]
- 20.Romani, L., P. Puccetti, and F. Bistoni. 1996. Biological role of Th cell subsets in candidiasis. Chem. Immunol. 63:115-137. [PubMed] [Google Scholar]
- 21.Rudd, P. M., T. Elliott, P. Cresswell, I. A. Wilson, and R. A. Dwek. 2001. Glycosylation and the immune system. Science 291:2370-2376. [DOI] [PubMed] [Google Scholar]
- 22.Takahara, M., K. Kang, L. Liu, Y. Yoshida, T. S. McCormick, and K. D. Cooper. 2003. iC3b arrests monocytic cell differentiation into CD1c-expressing dendritic cell precursors: a mechanism for transiently decreased dendritic cells in vivo after human skin injury by ultraviolet B. J. Investig. Dermatol. 120:802-809. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri, G. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13:251-276. [DOI] [PubMed] [Google Scholar]
- 24.Vidalain, P. O., O. Azocar, C. Servet-Delprat, C. Rabourdin-Combe, D. Gerlier, and S. Manie. 2000. CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J. 19:3304-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittmann, M., P. Kienlin, S. Mommert, A. Kapp, and T. Werfel. 2002. Suppression of IL-12 production by soluble CD40 ligand: evidence for involvement of the p44/42 mitogen-activated protein kinase pathway. J. Immunol. 168:3793-3800. [DOI] [PubMed] [Google Scholar]
- 26.Xiong, J., K. Kang, L. Liu, Y. Yoshida, K. D. Cooper, and M. A. Ghannoum. 2000. Candida albicans and Candida krusei differentially induce human blood mononuclear cell interleukin-12 and gamma interferon production. Infect. Immun. 68:2464-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshida, Y., K. Kang, M. Berger, G. Chen, A. Gilliam, A. Moser, L. Wu, C. Hammerberg, and K. Cooper. 1998. Monocyte induction of IL-10 and down-regulation of IL-12 by iC3b deposited in ultraviolet-exposed human skin. J. Immunol. 161:5873-5879. [PubMed] [Google Scholar]