Abstract
Propionibacterium acnes (P. acnes), a Gram-positive anaerobic bacterium, is a commensal organism in human skin. Like human cells, the bacteria produce porphyrins, which exhibit fluorescence properties and make bacteria visible with a Wood’s lamp. In this review, we compare the porphyrin biosynthesis in humans and P. acnes. Also, since P. acnes living on the surface of skin receive the same radiation exposure as humans, we envision that the changes in porphyrin profiles (the absorption spectra and/or metabolism) of P. acnes by radiation may mirror the response of human cells to radiation. The porphyrin profiles of P. acnes may be a more accurate reflection of radiation risk to the patient than other biodosimeters/biomarkers such as gene up-/down-regulation, which may be non-specific due to patient related factors such as autoimmune diseases. Lastly, we discuss the challenges and possible solutions for using the P. acnes response to predict the radiation risk.
Keywords: Biomarker, biosynthesis, commensal bacteria, cancer, gamma radiation, microbiome, P. acnes, porphyrins, radiation risk, skin
1. INTRODUCTION
Under healthy conditions, most people are unwilling to provide their tissues or blood to clinicians for determination of whether they are at risk of developing diseases, such as cancers. Thus, the feasibility of using biomarkers identified from human cells or tissues as predictors for disease initiation in clinical practice may be limited. Although various human biomarkers have been revealed via characterization of the responses of tissues/organs to disease initiators, detection of these biomarkers probably requires taking live human tissues and varies significantly depending on human immune status. The reaction of human skin commensal bacteria to environmental hazards may serve as a novel and early biomarker for prediction of the risk of environmental hazard-initiated diseases because the bacteria are exposed to the same field of hazards as human body. P. acnes, an auto-fluorescent anaerobic bacterium, is part of the commensal flora present on human skin. The fluorescence is due to the presence of endogenous porphyrins. The production of porphyrins in P. acnes and its medical application for prediction of radiation risk are discussed below.
2. P. acnes
The propionibacterium is a member of the anaerobic organisms, an established and proliferating member of the cutaneous micro-flora. Three main species have been identified on human skin: P. acnes, P. granulosum and P. avidum. P. acnes bacteria are the most prevalent species and usually the most numerous among the three cutaneous propionibacteria on human skin. P. acnes is an opportunistic pathogen, which belongs to the ‘high GC’ group of Gram-positive bacteria. With an estimated density of 102 to 105–6 per cm2 on the skin, it accounts for approximately half of the total skin microbiota [1]. As a resident of human bacterial flora member, P. acnes is found predominantly in the sebaceous gland-rich areas of the skin. The highest level of P. acnes is found on the face and on the scalp. On the other hand, P. acnes can also be detected at a very low level in the limbs, which only ranges 102 per cm2. The reasons that P. acnes can range differently are that the sebaceous gland produces high amounts of lipid and fatty acids and those compounds can be utilized by P. acnes as its major nutritional source to grow. P. acnes can also be found normally in areas that are rich in eccrine sweat and mucosal areas [2]. P. acnes also secrete protease, hyaluronidase, and lipases that are contributed to not only support with carbon/energy source, but also favor the interaction of P. acnes with the surrounding free fatty acids. Thus, P. acnes flourishes in these areas [3]. Nowadays, the influence of the P. acnes involvement in acne pathogenesis is still a hot controversy because P. acnes belong to the resident microbiota [3]. The propionibacteria species might be habitual and innocuous inhabitants of gastric environments [4]. Recently, the question about the pathogenic potential of P. acnes has been raised again due to the genome decoding of this kind of bacteria. This possibility is further supported by the observation of P. acnes and its induction of the expression of antimicrobial peptides (e.g. β-defensin-2) and pro-inflammatory cytokines/chemokines [e.g. tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-8 (IL-8)] from various cell types. Although some scientists indicated P. acnes to be a harmless organism [5], some demonstrated that P. acnes are not only in the development of inflammatory acne lesions but also in the formation of the microcomedo [6, 7]. However, how this kind of bacteria comes into human body and why it transforms from commensal bacteria into the cause of some diseases still need to be fathomed.
P. acnes has been divided into two distinct phenotypes, type I and type II, by serological agglutination tests and cell-wall sugar analysis for nearly 40 years. Additional studies have shown that these biovars display differences in the fermentation of sugar and sugar alcohols, as well as in the susceptibility to bacteriaphage infection [8]. However, recently, based on the recA gene sequence analysis of P. acnes, scientists have found a new P. acnes type, type III, and also revealed that these three types of P. acnes correspond to phylogenetically different cluster or lineages [9]. Although sequencing of the 16S rRNA gene is still considered the ‘gold standard’ for investigating the phylogenetic relationship between bacterial organisms, potential problems associated with this ability to resolve the relationships between closely related species are due to an extremely low rate of neutral mutations that have been recognized. In contrast, protein-encoding genes with housekeeping function, such as recA, often provide a better foundation for bacterial systematic and differentiation for closely related organisms. This is due to a higher neutral mutation rate with such genes, which are a consequence of the redundancy of the genetic code. Therefore, the gene results in synonymous substitutions at the third codon position that have no effect on the function of the resulting protein. Analyses of recA gene and Christie Atkins Munch-Petersen (CAMP) factor have also identified a subcluster strains within P. acnes type I that is designated to as type I B. These organisms do not react with monoclonal antibodies QUBPa 1, but variable labeling with the QUBPa 2, ranging from no reaction to a weak reaction can be observed. QUBPa 1 and QUBPa 2 react with a proteinaceous and carbohydrate/glycolipid-containing antigen on type IA and II individually [10, 11]. These strains of P. acnes have their own characteristics. Compared with the classical coryneform morphology normally seen with type I and type II, such as clubs, tadpole forms, and short bifid forms, type III consists individual cells of variable length and long slender filaments that form very large tangled aggregates. While type I isolates are found to be variable for α- and β-haemolytic activity [9], type III is negative for lecithinase activity as well as α- and β-hemolysis. These strains also have regional variations. Type I B and type II are more frequently isolated from radical prostatectomy specimens of subjects with prostate cancer that suggest P. acnes distribution exhibits gender relationship [12]. Furthermore, P. acnes isolates have been recovered from orthopedic implants. Type I is more frequently presented than type II [12]. The main different production of virulence factors by P. acnes type I and type II is β-hemolysis [9] indicate that β-hemolysis is a possible factor that affects the distribution of these two types. It is unclear whether the distinct recA lineages of P. acnes differ significantly with respect to virulence and their distribution.
3. PORPHYRINS
3.1. Porphyrins in Humans
Porphyrins are groups of organic compounds. Several porphyrins play major roles in diverse process as oxygen transportation and photosynthesis. Heme, protoporphyrin, coproporphyrin, and uroporphyrin are the most common porphyrins found in the human body. Porphyrins are pigmented compounds. While exposed to long wavelength ultraviolet light near 400 nm, porphyrins will expose red fluorescence [13]. Heme is essential to the human circulatory system. Although serving as parts of other proteins structures, heme is best known for comprising units of hemoglobin, a metalloprotein which transports oxygen. Heme contains iron inside of its porphyrin ring, thus allowing oxygen binding.
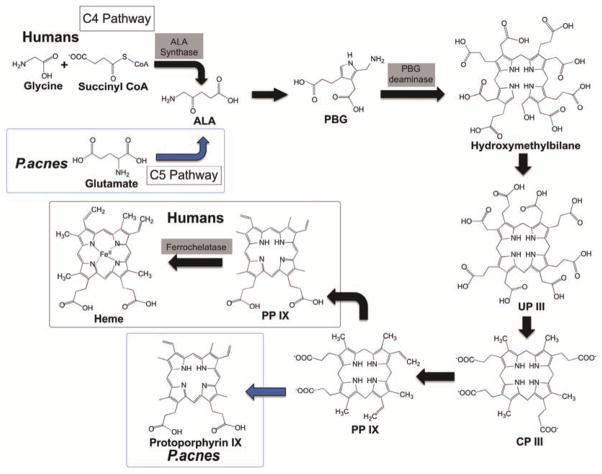
Heme biosynthesis happens between the mitochondrial and cytosol of the cell. The starting step of the porphyrin biosynthesis is the production of 5-aminolevulinic acid (ALA), which takes place in the mitochondrion matrix Fig. (1). It involves the synthesis of one glycine and one succinyl CoA that is catalyzed by the enzyme ALA synthase and is required the cofactor pytidoxal phosphate [13, 14]. In the following step, ALA is transported from the mitochondrial to the cytosol, where ALA dehydratase (also called porphobilinogen synthase) takes place to catalyze the reaction. During this step, in order to form the monopyrrole porphobilinogen (PBG), two moles of ALA are condensed and two moles of water are lost.
Fig. (1).
Porphyrin biosynthesis pathways in humans and P. acnes. In humans, ALA is synthesized through C4 pathway (a black arrow), which is the condensation of glycine and succinyl CoA catalyzed by ALA synthase. P. acnes can go through the C5 pathway (a blue arrow) to convert glutamate to ALA. In humans, the majority end product in the biosynthesis pathway is heme. However, in P. acnes, the end product can be protoprophyrin IX (a blue arrow) or coproporphyrin III. (The color version of the figure is available in the electronic copy of the article).
In the next step of the pathway, four molecules of PBG are condensed in head-to-tail manner, which are catalyzed by the enzyme, PBG deaminase, to form hydroxymethylbilane [13]. With the uroporphyrinogen (UP) III synthase, hydroxymethylbilane is in a region-specific manner to form UP II. Hydroxymethylbilane can also function in the absence of enzyme to form UP I. However, UP I and its metabolites are not biologically active. Then, in the cytosol, the enzyme UP decarboxylase will decarboxylate the acetate substitute of UP. The resultant products have methyl groups in place of acetate, which are coproporphyrinogens (CP), in which the CP III is the major normal intermediate in heme synthesis [13, 14].
After the production of CP III, the CP III enzyme activity will take place at the outer surface of the inner mitochondrial membrane and is specific for the CP III. In this step, two propionate residues are decarboxylated, yielding vinyl substituents on the 2-pyrrole rings. The product of this reaction is protoporphyrinogen (PP) IX, which is colorless [13]. In the inner mitochondrial membrane, PP IX is converted to protoporphyrin IX catalyzed by the enzyme PP IX oxidase. The oxidase reaction requires molecular oxygen and causes the loss of 6 protons and 6 electrons, yielding a completely conjugated ring system. The ring system is responsible for the red color of the hemes [13, 14]. The final reaction in heme synthesis also takes place in the mitochondrion. The iron atom is added into the ring system, catalyzed by the enzyme ferrochelatase to produce the final product, heme. Human excreteuroporphyrin and coproporphyrin are eliminated as waste products in urine or feces. Both the presence and amount of these prophyrins can be analyzed as signals from the body, especially as indicators of porphyria.
Porphyria, a disruption in the mechanism for producing heme, is a common human illness involving porphyrins. Patients with the life-long disease suffer from a lack of or deficiency in certain enzymes that are needed in the heme-synthesizing process. Symptoms of porphyria include abdominal pain, light sensitivity, and nervous system problems. Treatments include phelobotomies, beta-carotene supplements, intravenous applications of hematin (enzyme inhibitor), and avoidance of possible triggers. Additionally, the disruption of porphyrin metabolism can signal the onset of some diseases as well. The aforementioned coproporphyrin and uroporphyrins (found in excretions) along with ALA were found to be present in higher concentrations in children suffering from malignant diseases including leukemia, lymphoma, and Hodgkin’s disease. Presented as a group, due to the disruption of enzyme synthesis pathways and thus another form of porphyria, “free erythrocytes” are increased. Specifically for leukemia, porphyrin metabolism changes result in a block at the end of an enzyme synthesis pathway. This late blockage produces some porphyrins that accumulate, but do not finish as completed molecules. The observation made for leukemia could be extended as a conclusion for many diseases where the disruption of porphyrin-metabolism signals onset. In general, porphyrin build-up and eventual excretion at higher levels (of uroporphyrin and coproporphyrin) could result from plentiful substrate in early stages of multi-step enzyme synthesis pathways. However, later blockages can prevent the process from completing a finished end product. An enzyme product is produced, but it is not the correct end result of the pathway.
3.2. Porphyrins in P. acnes
Porphyrins are involved in many major metabolic processes of prokaryotic and eukaryotic cells including respiration, biological oxidation, photosynthesis, sulfate reduction, and rearrangement of the carbon backbone. P. acnes produce porphyrins, which fluoresce on Wood’s light examination, most notably on the nose Fig. (2) and the forehead, and produce endogenous porphyrins like many other cell types. Porphyrins produced by P. acnes might contribute to the perifollicular inflammatory reaction through their cytotoxic effect and by stimulating expression of keratinocyte-derived IL-8 [15]. After rupture of the follicle epithelia, porphyrins secreted perifollicularly could also contribute to the inflammatory reaction of the follicle or its environment by favoring the development of cytotoxic substances such as squaleneperoxide possibly via singlet oxygen [16].
Fig. (2).
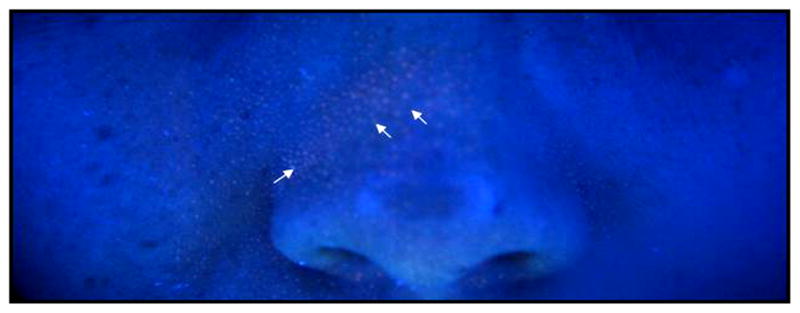
Imaging auto-fluorescent P. acnes in human facial skin. A Wood’s lamp (SkinMate, Tulsa, OK) with a UV light source was used to illustrate the auto-fluorescent P. acnes (red fluorescence, arrows) in human facial skin [60].
A review of the literature on porphyrin production by P. acnes reveals contrasting results. In P. acnes, the terminal product in the sequence of metabolic reactions is protoporphyrin IX. While in plant cell, Mg2+ ions are further inserted in the center of protoporphyrin IX to produce a chlorophyll molecule. In animal cells, the insertion of Fe2+ ions leads to the production of a heme. In P. acnes, under anaerobic conditions protoporphyrin IX is accumulated, while under semianaerobic conditions, uroporphyrin III and coproporphyrin III are formed from the corresponding porphyrinogens, which are earlier steps in the reaction pathway leading to protoporphyrin IX. In contrast, most studies using fluorescence analyses have identified the predominance of coproporphyrin III in these bacteria, followed by lower amounts of protoporphyrin [17–20]. Kjeldstad [21] studied one isolate of P. acnes and found that the proportions of porphyrins vary with the pH of the growth medium and the length of the incubation. However, the amount of protoporphyrin IX produced is always more than the amount of coproporphyrin III. One study has revealed the presence of uroporphyrin, as well ascopro- and protoporphyrin in the analyzed material [22]. The addition of ALA to the growth medium of P. acnes results in two different porphyrin patterns in the bacteria. While in some colonies a predominance of protoporphyrin can be observed, in others, the porphyrin pattern includes mostly uro- and coproporphyrin [23]. These differences may be related to the environmental conditions in which the P. acnes were studied with the serotypes of propionibacteria, the growth medium, the cell fraction, and the activity of the enzymes, e.g. UP decarboxylase or CP oxidase. Ricardo [24] studied one to two week incubation periods under standardized conditions of the in vitro synthesis of P. acnes porphyrins from patients with acne vulgaris. Their high performance liquid chromatography (HPLC) analysis results showed two different porphyrin patterns in P. acnes. One group produced mostly protophorphyrin and traces of coproporphyrin III. On the other hand, the results revealed an overwhelming production of polar porphyrins, including uroporphyrin, heptacarboxyporphyrin and pentacarboxyporphyrins I/III, followed by small amounts of coproporphyrin I/III.
4. COMPARE AND CONTRAST BETWEEN HUMAN AND BACTERIAL PORPHYRIN BIOSYNTHESIS
There are a few differences and similarities in human and P. acnes porphyrin biosynthesis. First, both biosynthesis requires the production of ALA. ALA can be synthesized through two pathways, the C4 pathway (Shemin pathway) and the C5 pathway Fig. (1) [25]. In humans, yeasts, fungi and certain bacteria, ALA is formed through the C4 pathway, which is the condensation of glycine and succinyl CoA catalyzed by ALA synthase. Plants and some bacteria undergo the C5 pathway, which uses glutamate. ALA in P. acnes could be synthesized via both pathways. However, P. acnes KPA171202 does not require ALA in the porphyrin biosynthesis because it does not have the ALA synthase gene present. Second, the terminal product is also different in both biosynthesis. The majority terminal product in humans is the production of heme. However, in P. acnes, the terminal product in the biosynthesis pathway can be affected by the environmental conditions. Under anaerobic conditions, protoprophyrin IX is dominant. On the other hand, under aerobic conditions, coproporphyrin III is predominantly the final product of the P. acnes porphyrin metabolic pathway. The structural difference between heme and P. acnes porphyrins (protoprophyrin IX or coproporphyrin III) is that heme has the addition of metallation into the middle ring structure of protoporphyrin IX catalyzed by enzyme ferrochelatase. Due to mutations in the ferrochelatase gene, reduction in activity of heme synthesis in humans leads to erythropoietic porphyria. A symptom of this disease is light-sensitive dermatitis caused by overproduction of protoporphyrin and its deposition in skin and liver. In some cases, erythropoietic porphyria may even lead to fatal liver damage [17–19, 21].
5. MEDICAL APPLICATION OF PORPHYRINS
Porphyrins currently have many uses in various fields. One application is in tumor detection and fluorescence delineation of porphyrin-containing tumor cells. Due to the sensitivity of the method, diagnostics use of fluorescence can have a shorter testing period. Another method of using porphyrins in medical treatment is photodynamic therapy. Due to increased binding ability between side chains and cell membranes, tumor-bearing tissues retain porphyrins to a greater extent than surrounding healthy tissues. Photodynamic therapy can inactivate cell membranes and cause problems in areas such as cell division, membrane synthesis, and eventual cell lysis. Cross-linking due to porphyrin photosensitization may also cause these symptoms. Most photodynamic therapy has been utilized on tumor cells but the investigation on bacterial cells is able to complete and extend the knowledge of light induced porphyrin photosensitization. Photodynamic therapy on P. acnes in vitro has been demonstrated. In clinical tests, using both red and blue light in photodynamic therapy is becoming prevalent. It is unknown if distinct phenotypes of P. acnes produce the different levels of P. acnes. P. acnes KPA171202 does not express ALA synthase for its porphyrin biosynthesis. There are 8 completely and more than 70 partially sequenced P. acnes strains in the GenBank. Currently, it is not fully clear whether there is any correlation between P. acnes strains and their distribution in the human body. Results from our laboratory have revealed that exposure of P. acnes to ultraviolet (UV) decreased the production of porphyrins in the human facial bacteria containing different strains of P. acnes and other skin bacteria [26]. Besides P. acnes, other skin resident bacteria (e.g. Corynebacterium minutissimum [27]) can also produce porphyrins, although they are not predominant bacteria in human skin. Several commensal bacteria (e.g. Porphyromonas gingivalis [28]) that are present in the human oral cavity can produce porphyrins. It has been reported that detection of porphyrin fluorescence can be a promising method to diagnose carious lesions, calculus and plaque [29].
Illumination of P. acnes will excite protoporphyrin IX or other prophyrins in the cells into higher singlet states, which will give rise to both fluorescence emission [19] and a population of triplet state (3PP IX) due to intersystem crossing. 3PP IX is a metal stable state, which can either donate the excited state energy to a ground state oxygen molecule, whereby a singlet oxygen molecule is created, or abstract an electron from a neighboring molecule, whereby radical species are formed. The first type of process is called a Type II reaction, while an electron transfer process is called a Type I reaction. In both cases, the result may be lethal to the bacteria. P. acnes can be inactivated by the combined action of light and an endogenous photosensitizer [30]. Porphyrins can absorb energy when excited by light of a certain wavelength. There are different absorption spectra for every chromophore. The different spectra of porphyrins have been associated with the presence of a free base, a monocationic and a dicationic form. As shown in Fig. (3), a free-based porphyrin, e.g. PP IX, displays a four-band visible absorption spectrum. The strong intense absorption band in the blue wavelength region characteristic of the porphyrins is called the Soret band, named after its discoverer Jacques-Louis Soret. It provides the basis for the spectrophotometric quantitation of porphyrins. The Soret band is known to subject to changes in wavelength and intensity according to the state of ionization of the pyrrolicnitrogens of the porphin nucleus. Visible spectra of porphyrins also show several weaker absorption peaks (Q-bands) at longer wavelengths (450 to 700 nm).
Fig. (3).
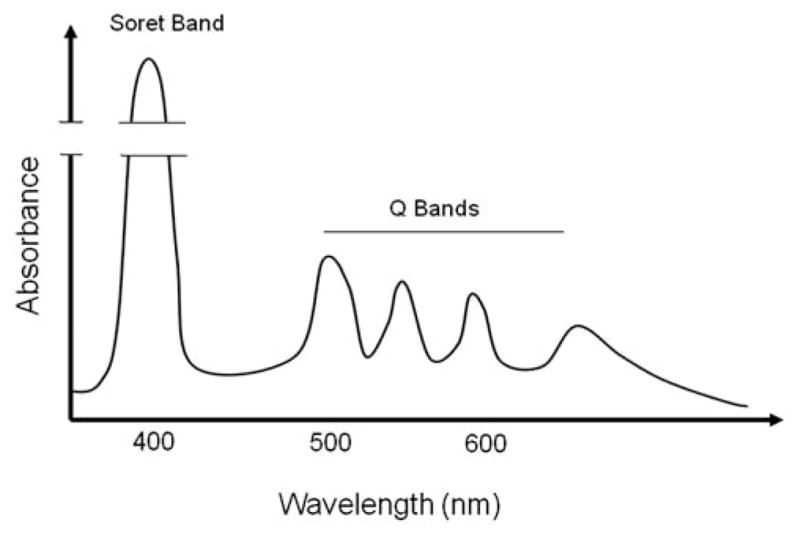
An absorption spectrum of porphyrin. Porphyrins possess an intense Soret band at approximately 400 nm and moderate Q bands between 450 and 700 nm.
Radiation, such as gamma radiation and x-irradiation, can alter the absorption spectra of porphyrins. Recent research has shown that increasing dose and temperature of gamma radiation can produce increasing shifts in characteristic peaks and significantly decreases in the Soret bands [31] Fig. (2). Under gamma radiation, 50% of porphyrin rings were destroyed. Therefore, the intensity peak of porphyrin decreased. Furthermore, gamma radiation also induced the decomposition of the metal-ion in the middle of the ring structure of porphyrin [32]. The absorption spectra peak shifted as well. Porphyrin can also be attacked by X-irradiation. X-irradiation can produce oxidation of porphyrin, which is manifested by a decrease of all the absorption Soret bands. Consequently, there is a linear relationship between the decrease in the height of the Soret band and the increase of X-irradiation dose [33]. Results in our recent publication have demonstrated that the production of porphyrins in P. acnes was significantly reduced with increasing doses of gamma radiation and UV-B [26]. The porphyrin reduction was detectable in both P. acnes and human skin bacterial isolates. Exposure of UV-B to P. acnes-inoculated mice resulted in a significant decrease in porphyrin production in a single colony of P. acnes and simultaneously induced the formation of cyclobutane pyrimidine dimers (CPD) in the epidermal layers of mouse skin. In addition, porphyrin reduction in P. acnes has a longer detection time than CPD formation in mouse skin, indicating that the response of P. acnes to radiations may be more stable than DNA damage in host cells. These studies indicate that detection of the change in the amounts and/or absorption spectra of porphyrins by radiation may serve as a radiation bio-dosimeter. The radiation-induced changes in the amounts and/or absorption spectra of porphyrins can be developed as radiation biomarkers for prediction of the risk of radiation injury.
6. CURRENT DETECTION OF RADIATION INJURY
Radiation injury can take days or weeks to present clinical manifestations and some of the delayed radiation injuries may not develop for months or years after exposure. This latency in symptoms may lead to delays in treatment decisions, thus resulting in increased morbidity and loss of lives. Lack of good methods for radiation detection is a major factor of delays in treatment decisions. For example, one of the detection methods uses an apoptosis assay, such as in situ terminal deoxynucleotidyl transferase (TdT), with cultured peripheral lymphocytes. The sensitivity of this method was reported as 0.05–0.1 gray (Gy). The reaction peaks at 72 h after irradiation. However, the assay is difficult to scale up since blood drawing and culture are required. The cytogenetic aberration is a hallmark of irradiation injuries. The cytogenetic aberrations have been used in clinics as a diagnosis of radiation injury [34, 35]. Despite its specificity and sensitivity, this assay is very labor intensive. Although many genes/proteins, eg. γ-H2AX [36], are derived from tissues/organs have been identified as radiation biomarkers, due to patient related factors such as underlying illnesses and immune status, they may be non-specific. The fluorescent in situ hybridization (FISH) is used to measure more stable and long lasting chromosome translocations. However, this method requires very skilled personnel to perform. Other methods, including cytokinesis block micronucleus (CBMN) assays [37], premature chromosome condensation (PCC) assays [38], and an electron spin resonance (ESR) based assay [39] have been subsequently developed. However, a limitation of CBMN assays is background variation in the human population related to age and lifestyle factors (e.g. smoking). PCC assays require cell fusion and obtaining blood samples within several hours following exposure. The procedure of ESR based assays is quick (5 min) but requires expensive instruments. Although the sensitivity of ESR is at 0.05–0.1 Gy with material from tooth enamel extraction, there are only limited bodily areas that can be used for this technique.
7. PROSPECTS OF USING BACTERIAL RESPONSES AS RADIATION BIOMARKERS
No methods above can predict the radiation risk before human cells are damaged by radiation exposure. Although many qualitative/quantitative radiation bio-dosimeters have been developed, it is very inconvenient and impracticable for everyone to carry these bio-dosimeters at all times, as radiation accidents are unpredictable. Many researchers have identified radiation biomarkers by characterizing the response of tissues/organs to radiation [40, 41]. These biomarkers may be non-specific because they can be greatly affected by other physiological factors such as age, illness and immune statue. Collection of biological fluids (such as urine) presents a non-invasive way for identification of radiation biomarkers, but fluid secretion may be a late response of humans to radiation. As mentioned above, each individual carries approximately 10 times more bacterial cells than human cells [38]. The skin is the human body’s largest organ, colonized by a diverse milieu of microorganisms (skin microbiome). Since they are harmless or even beneficial to their hosts, most of those microorganisms are commensals. The skin microbiome has been initially characterized by shotgun metagenomic sequencing and 16S rDNA amplicon sequencing [42]. In skin microbiome, P. acnes predominated (>60% of total bacteria) in the facial skins [42, 43]. Nearly everyone hosts P. acnes [41, 42], which accounts for approximately half of the total skin microbiome [44–46]. In addition, the commensal bacterium was found at a very high percentage on the foreheads [103 to 107 colony-forming unit (CFU)] of normal individuals in Seattle (92%) and Alaska (81%) [47, 48], suggesting no differences of bacterial colonization between different populations during various seasons. Besides porphyrins that are highlighted in this review, there are many fluorophores in P. acnes that potentially can be used to reflect the radiation damage. These intrinsic fluorophores include the amino acids tryptophan and tyrosine, and the coenzymes NADH, NADPH, and flavins [49]. Our recent results have illustrated that an internal peptide of a Lsr2 family protein (Q6AB31) was oxidized in gamma irradiated P. acnes [26]. The oxidized peptide of a Lsr2 family protein can potentially be developed as a biomarker for acute radiation sickness during nuclear accidents. Chronic radiation exposure to astronauts could be a significant obstacle for long duration manned space exploration. It is worthwhile to identify the radiation-induced mutant P. acnes in astronauts and establish a pattern of genome mutation of P. acnes as a radiation index for long-term space travel. Detection of the responses of P. acnes to radiations (including the metabolisms of intrinsic fluorophores, protein/peptide oxidation, and bacterial mutation) as radiation biomarkers may be a more accurate reflection of radiation injury since 1) P. acnes resides on the human skin surface with a high density; 2) the bacteria receive the same radiation exposure as human body; 3) sample collection from the skin surface is readily accessible and requires no trained personnel; 4) the response of live P. acnes on human faces can be monitored in a real time manner; and 5) bacterial responses to radiation are less affected by internal physiological conditions of the individuals.
8. CHALLENGES IN DEVELOPPING BACTERIA-BASED RADIATION BIO-DOSIMETRY
The changes in the absorption spectra and/or metabolism of porphyrins may occur commonly when P. acnes is exposed to environmental stress (e. g. heat [50]). Our recent results showed that both gamma radiation and UV-B caused a reduction in the production of porphyrins although they generated distinguishable signatures of protein oxidation/de-oxidation of P. acnes [26]. Thus, establishment of a radiation-specific porphyrin profile reflecting differential porphyrin metabolites (absorption spectra) may be needed when P. acnes response is used as a radiation biomarker. In addition, various strains of P. acnes, as mentioned in Section 2, existing in human skin may respond differentially to radiations. Clinical evidence indicated that radiation <2 Gy induces mild cytopenias without significant bone marrow damage [51, 52]. In addition, consensus guidance [52] based on a threshold whole-body or significant partial body radiation exposure suggests starting antibiotics and cytokine therapy at exposure dose of 2 Gy. However, low doses (<2 Gy) of gamma radiation will extend the latency period for onset of cancers [53]. Thus, it is important to determine if the P. acnes porphyrin profile is detectable following exposure to low-dose (<2 Gy) radiation. If the concept of using P. acnes response as radiation biomarkers is applied to humans in the future, tape stripping [54] will be an option for collection of P. acnes living on the human skin. Sampling follicles using cyanoacrylate skin surface stripping revealed that about 25% of all bacteria found on the skin are hair follicle derived [55]. The method allows collecting the P. acnes deep inside the hair follicle. Due to the sensitivity limitations, tape-stripped skin samples with a relatively low concentration of P. acnes porphyrins may be not quantifiable. Thus, development of sensitive porphyrin detectors [56] may be required. In an ongoing study in our laboratory, we are able to collect the human facial P. acnes using a plate coated with a P. acnes-specific monoclonal antibody (data not shown). The technique enables us to collect the P. acnes from tape-tripped skin samples and analyze the porphyrin metabolism in individual P. acnes.
Exposure of bacteria with intense radiation can kill bacteria through production of free radicals [57]. Theoretically, reduction in the number of bacterial counts may be able to reflect the amount of radiation exposure. However, the number of P. acnes residing on the surface of our skins varies from individual to individual, making it difficult to use the bacterial reduction as a parameter for radiation exposure. Furthermore, bacterial colonization is driven by the ecology of the skin surface, which is highly variable depending on topographical location [38]. It has been documented that genome-equivalents of P. acnes are proportional to the number of P. acnes on human skin [58]. P. acnes genome-equivalents can be determined by reverse transcription polymerase chain reaction (RT-PCR) targeting 16S rRNA gene [58]. Thus, to circumvent variations in P. acnes density, the changes in the porphyrin profiles can be normalized to genome-equivalents of P. acnes. Another challenge is that porphyrins produced by skin cells may interfere with the detection of bacterial porphyrins. However, monitoring the porphyrin profile by quantification of the transcript levels of P. acnes-specific-enzymes (ALA synthase, and PBG deaminase) involved in porphyrin biosynthesis may circumvent the interference of skin cell prophyrins. In addition, a method developed in our laboratory by quantification of the prophyrin amounts in single colonies, not bacterial population of P. acnes [26], may eliminate the skin cell prophyrins and minimize the effect of variations in bacterial numbers on porphyrin production.
9. CONCLUSION
Porphyrins are naturally synthesized in human cells. The endogenous porphyrins in tumor cells have been used for tumor detection. Porphyrins are also produced by human commensal bacteria such as P. acnes in human skin. Photodynamic therapy by activating the endogenous porphyrins of P. acnes has been applied for treatment of acne vulgaris, a common human skin disease. Although the biosynthesis of porphyrins in humans and P. acnes shares similarity, the end products of porphyrin biosynthesis in humans and P. acnes are distinguishable. As a commensal bacterium, P. acnes is a component part of every human being. P. acnes’ constant and consistent presence on human skin may make it an excellent endogenous radiation biodosimetry. The large-scale nuclear accidents - as the nuclear disaster in the Japan [59] and atomic bombings during World War II, Marshall Island and Chernobyl - emitted intense gamma radiation that is harmful or even lethal to humans. During these nuclear accidents, people feared they may have been exposed to radiation. Although many radiation detectors are available, due to the unpredictability of radiation accidents, no one is willing to carry these detectors at all times. Since they can change in response to other physiological conditions, such as illness and aging, biomarkers identified from tissues/organs may be not radiation-specific. The concept of detecting the changes in the porphyrin profile of P. acnes as a reflection of human responses to environments can be applied to the development of various disease monitors. These monitors include detecting the risk of radiations in a battlefield, space, nuclear accidents, terrorism, or cancer detection (e.g. X-irradiation) and treatment (e.g. radiotherapy).
Acknowledgments
The authors thank Justin Song for joining this discussion. This work was supported by a NIH grant (1R41AR056169).
ABBREVIATIONS
- ALA
5-Aminolevulinic Acid
- CAMP
Christie Atkins Munch-Petersen
- CBMN
Cytokinesis Block Micronucleus
- CFU
Colony-Forming Unit
- CP
Coproporphyrinogen
- CPD
Cyclobutane Pyrimidine Dimers
- ESR
Electron Spin Resonance
- FISH
Fluorescent In Situ Hybridization
- Gy
Gray
- HPLC
High Performance Liquid Chromatography
- IL-1β
Interleukin-1 beta
- IL-8
Interleukin-8
- P. acnes
Proprionibacterium acnes
- P. avidum
Proprionibacterium avidum
- P. granulosum
Proprionibacterium granulosum
- RT-PCR
Reverse Transcription Polymerase Chain Reaction
- TNF-α
Tumor Necrosis Factor-Alpha
- PBG
Porphobilinogen
- PP
Protoporphyrinogen
- TdT
Deoxynucleotidyl Transferase
- PCC
Premature Chromosome Condensation
- UP
Uroporphyrinogen
- UV
Ultraviolet
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
References
- 1.McGinley KJ, Webser GF, Leyden JJ. Regional variations of cutaneous propionic bacteria. Appl Environ Microbiol. 1987;35:62–66. doi: 10.1128/aem.35.1.62-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGinley KJ, Webster GF, Ruggieri MR, Leyden JJ. Regional variations in Density of Cutaneous Propionibacteria: Correlation of Propionibacterium acnes population with Sebaceous Secretion. J Clin Microbiol. 1980;12:672–675. doi: 10.1128/jcm.12.5.672-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dessinioti C, Katsambas AD. The role of Propionibacteriium acnes in acne pathogenesis facts and controversies. Clin Dermatol. 2010;28:2–7. doi: 10.1016/j.clindermatol.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Baltasar A, Perez N, Serra C, Bou R, Bengochea M, Borrás F. Weight loss reporting: predicted body mass index after bariatric surgery. Obes Surg. 2011;21:367–372. doi: 10.1007/s11695-010-0243-7. [DOI] [PubMed] [Google Scholar]
- 5.Eady EA, Ingham E. Propionibacterium acnes-friend or foe. Rev Med Microbiol. 1994;5:163–173. [Google Scholar]
- 6.Jarrousse V, Castex-Rizzi N, Khammari A, Charveron M, Dreno B. Modulation of integrins and filaggrin expression by Propionibacterium acnes extracts on Keratinocytes. Arch Dermatal Res. 2007;299:441–447. doi: 10.1007/s00403-007-0774-5. [DOI] [PubMed] [Google Scholar]
- 7.Nagy I, Pivarcsi A, Kemeny L. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokinesin human sebocytes. Microbes Infect. 2006;8:2195–2205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Funke G, von Graevvenitz A, Charridge JE, Bernard KA. Clinical microbiology of coryneformbacteria. Clin Microbiol Rev. 1997;10:125–159. doi: 10.1128/cmr.10.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDowell A, Perry AL, Lambert PA, Patrick SA. new phylogenetic group of Propionibacterium acnes. J Med Microbiol. 2008;57:218–224. doi: 10.1099/jmm.0.47489-0. [DOI] [PubMed] [Google Scholar]
- 10.McDowell A, Valanne S, Ramage G, Tunney MM, Glenn JV, McLorinan GC, Bhatia A, Maisonneuve JF, Lodes M, Persing DH, Patrick S. Propionibacterium acnes type I and II represent phylogenetically distinct groups. J Clin Micro. 2005;43:326–334. doi: 10.1128/JCM.43.1.326-334.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valanne S, McDowell A, Ramage G, Tunney MM, Einarsson GG, O’Hagan S, Wisdom GB, Fairley D, Bhatia A, Maisonneuve JP, Lodes M, Persing DH, Patrick S. CAMP factor homologues in Propionbacterium acnes: a new protein family differentially expressed by type I and II. Microbol. 2005;151:1369–1371. doi: 10.1099/mic.0.27788-0. [DOI] [PubMed] [Google Scholar]
- 12.Samperdro MF, Piper KE, McDowell A, Patrick S, Mandreker JN, Rouse MS, Steckelberg JM, Patel P. Species of Propionibacterium and Propionibacterium acnes phylotypes associated with orthopedic implants. Diagn Micr Infec Dis. 2009;64:138–145. doi: 10.1016/j.diagmicrobio.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 13.King MW. The Medical Biochemistry Page, 2011. May 16, 2011. Iron, Heme and Porphyrin Metabolism. [Google Scholar]
- 14.Ashkenazi H. Eradication of Propionibacterium acnes by its endogenic porphyrins after illumination with high intensity blue light. FEMS Microbiol Immunol. 2002;16:17–24. doi: 10.1111/j.1574-695X.2003.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 15.Schaller M, Loewenstein M, Borelli C, Jacob K, Vogeser M, Burgdorf W. Induction of a chemoattractive proinflammatory cytokine response after stimulation of keratinocytes with Propionibacterium acnes and coproporphyrin III. Br J Dermatol. 2005;153:66–71. doi: 10.1111/j.1365-2133.2005.06530.x. [DOI] [PubMed] [Google Scholar]
- 16.Saint-Leger D, Bague A, Cohen E, Chivot MA. Possible role for squalene in the pathogenesis of acne. I In vitro study of squalene oxidation. Br J Dermatol. 1986;114:535–542. doi: 10.1111/j.1365-2133.1986.tb04060.x. [DOI] [PubMed] [Google Scholar]
- 17.Cornelius CE, Ludwig GD. Red fluorescence of comedones: production of porphyrins by Corynebacterium acnes. J Invest Dermatol. 1976;49:368–370. [PubMed] [Google Scholar]
- 18.Fanta D, Formanek I, Poitschek CH, Thurner J. Die Porphyrinproduktion der Propioni-Bakterien in Abhängigkeit von externenEinflüssen. Arch Dermatol Res. 1981;271:127–133. [Google Scholar]
- 19.Johnsson A, Kjeldstad B, Melo TB. Fluorescence from pilosebaceous follicles. Arch Dermatol Res. 1987;279:190–193. doi: 10.1007/BF00413256. [DOI] [PubMed] [Google Scholar]
- 20.Lee WLS, Shalita AR, Poh-Fitzpatrick MB. Comparative studies of porphyrin production in Propionibacterium acnes and Propionibacterium granulosum. J Bacteriol. 1978;133:811–815. doi: 10.1128/jb.133.2.811-815.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjeldstad B, Johnsson A, Sandberg S. Influence of pH on porphyrin production in Propionibacterium acnes. Arch Dermatol Res. 1984;276:396–400. doi: 10.1007/BF00413361. [DOI] [PubMed] [Google Scholar]
- 22.Melo TB, Johnsson M. In vivo porphyrin fluorescence from Propionibacterium acnes: A characterization of the fluorescing pigments. Dermatologica. 1982;164:167–174. [PubMed] [Google Scholar]
- 23.Ramstad S, Futsaether CM, Johnsson A. Porphyrin sensitization and intracellular calcium changes in the prokaryote Propionibacterium acnes. J Photochem Photobiol B. 1997;14:275–292. doi: 10.1016/s1011-1344(97)00039-0. [DOI] [PubMed] [Google Scholar]
- 24.Romiti R, Schaller M, Plewig KJG. High-performance liquid chromatography analysis of porphyrins in Propionibacterium acnes. Arch Dermatol Res. 2000;292:320–322. doi: 10.1007/s004030000122. [DOI] [PubMed] [Google Scholar]
- 25.Kiatpapan P, Murooka Y. Genetic manipulation system in propionibacteria. J Biosci Bioeng. 2003;93:1–8. [PubMed] [Google Scholar]
- 26.Wang Y, Zhu W, Shu M, Jiang Y, Gallo RL, Liu YT, Huang CM. The Response of Human Skin Commensal Bacteria as a Reflection of UV Radiation: UV-B Decreases Porphyrin Production. PLoS One. 2012;7:e47798. doi: 10.1371/journal.pone.0047798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darras-Vercambre S, Carpentier O, Vincent P, Bonnevalle A, Thomas P. Photodynamic action of red light for treatment of erythrasma: preliminary results. Photodermatol Photoimmunol Photomed. 2006;22:153–156. doi: 10.1111/j.1600-0781.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- 28.Jacobs NJ, Jacobs JM, Brent P. Formation of protoporphyrin from coproporphyrinogen in extracts of various bacteria. J Bacteriol. 1970;102:398–403. doi: 10.1128/jb.102.2.398-403.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koenig K, Schneckenburger H. Laser-induced autofluorescence for medical diagnosis. J Fluoresc. 1994;4:17–40. doi: 10.1007/BF01876650. [DOI] [PubMed] [Google Scholar]
- 30.Kjeldstad B, Johnsson A. A 31P-NMR study of Propionibacterium acnes, including effects caused by near-ultraviolet irradiation. Biochim Biophys Acta. 1987;927:184–189. doi: 10.1016/0167-4889(87)90133-9. [DOI] [PubMed] [Google Scholar]
- 31.Kamarei AR, Karel M, Wierbicki E. Spectral studies on the role of ionizing radiation in color changes of radappertized beef. J Food Sci. 1979;44:25–32. [Google Scholar]
- 32.Dunning HN, Moore JW. Decomposition of Metal-porphyrin Complexes by Gamma Irradiation. Ind Eng Chem Res. 1959;51:161–164. [Google Scholar]
- 33.Barron ESG, Flood V. Studies on the mechanism of action of ionizing radiations. IX. The effect of x-irradiation on cytochrome c. Arch Biochem Biophys. 1952;41:203–211. doi: 10.1016/0003-9861(52)90520-1. [DOI] [PubMed] [Google Scholar]
- 34.Leonard A, Rueff J, Gerber GB, Leonard ED. Usefulness and limits of biological dosimetry based on cytogenetic methods. Radiat Prot Dosimetry. 2005;115:448–454. doi: 10.1093/rpd/nci061. [DOI] [PubMed] [Google Scholar]
- 35.Miller LP, Miller DR. Acute lymphoblastic leukemia in children: current status, controversies, and future perspective. Crit Rev Oncol Hematol. 1983;1:29–97. doi: 10.1016/s1040-8428(83)80007-9. [DOI] [PubMed] [Google Scholar]
- 36.Rothkamm K, Horn S. gamma-H2AX as protein biomarker for radiation exposure. Ann Ist Super Sanita. 2009;45:265–271. [PubMed] [Google Scholar]
- 37.Vral A, Fenech M, Thierens H. The micronucleus assay as a biological dosimeter of in vivo ionising radiation exposure. Mutagenesis. 2011;26:11–17. doi: 10.1093/mutage/geq078. [DOI] [PubMed] [Google Scholar]
- 38.Ottolenghi A, Ballarini F, Merzagora M. Modelling radiation-induced biological lesions: from initial energy depositions to chromosome aberrations. Radiat Environ Biophys. 1999;38:1–13. doi: 10.1007/s004110050132. [DOI] [PubMed] [Google Scholar]
- 39.Fattibene P, Callens F. EPR dosimetry with tooth enamel: A review. Appl Radiat Isot. 2010;68:2033–2116. doi: 10.1016/j.apradiso.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 40.Blakely WF, Ossetrova NI, Whitnall MH, Sandgren DJ, Krivokrysenko VI, Shakhov A, Feinstein E. Multiple parameter radiation injury assessment using a nonhuman primate radiation model-biodosimetry applications. Health Phys. 2010;98:153–159. doi: 10.1097/HP.0b013e3181b0306d. [DOI] [PubMed] [Google Scholar]
- 41.Taki K, Wang B, Nakajima T, Wu J, Ono T, Uehara Y, Matsumoto T, Oghiso Y, Tanaka K, Ichinohe K, Nakamura S, Tanaka S, Magae J, Kakimoto A, Nenoi M. Microarray analysis of differentially expressed genes in the kidneys and testes of mice after long-term irradiation with low-dose-rate gamma-rays. J Radiat Res. 2009;50:241–252. doi: 10.1269/jrr.09011. [DOI] [PubMed] [Google Scholar]
- 42.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahn CY, Ko CY, Wagar EA, Wong RS, Shaw WW. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstr Surg. 1996;98:1225–1229. doi: 10.1097/00006534-199612000-00016. [DOI] [PubMed] [Google Scholar]
- 44.Brook I, Frazier EH. Infections caused by Propionibacterium species. Rev Infect Dis. 1991;13:819–822. doi: 10.1093/clinids/13.5.819. [DOI] [PubMed] [Google Scholar]
- 45.Leyden JJ, McGinley KJ, Vowels B. Propionibacterium acnes colonization in acne and nonacne. Dermatology. 1998;196:55–58. doi: 10.1159/000017868. [DOI] [PubMed] [Google Scholar]
- 46.McGinley KJ, Webster GF, Leyden JJ. Regional variations of cutaneous propionibacteria. Appl Environ Microbiol. 1978;35:62–66. doi: 10.1128/aem.35.1.62-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin WS, Wong F, Anderson R. Role of superoxide in radiation-killing of Escherichia coli and in thymine release from thymidine. Biochem Biophys Res Commun. 1987;147:778–786. doi: 10.1016/0006-291x(87)90998-3. [DOI] [PubMed] [Google Scholar]
- 48.Shi Y, Elmets CA, Smith JW, Liu YT, Chen YR, Huang CP, Zhu W, Ananthaswamy HN, Gallo RL, Huang CM. Quantitative proteomes and in vivo secretomes of progressive and regressive UV-induced fibrosarcoma tumor cells: mimicking tumor microenvironment using a dermis-based cell-trapped system linked to tissue chamber. Proteomics. 2007;7:4589–4600. doi: 10.1002/pmic.200700425. [DOI] [PubMed] [Google Scholar]
- 49.Koenig K, Schneckenburger H. Laser-induced autofluorescence for medical diagnosis. J Fluorescence. 1994;4:17–40. doi: 10.1007/BF01876650. [DOI] [PubMed] [Google Scholar]
- 50.Ramstad S, Le Anh-Vu N, Johnsson A. The temperature dependence of porphyrin production in Propionibacterium acnes after incubation with 5-aminolevulinic acid (ALA) and its methyl ester (m-ALA) Photochem Photobiol Sci. 2006;5:66–72. doi: 10.1039/b512837d. [DOI] [PubMed] [Google Scholar]
- 51.Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program. 2003:473–496. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- 52.Mettler FA, Jr, Voelz GL. Major radiation exposure--what to expect and how to respond. N Engl J Med. 2002;346:1664–1561. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 53.Mitchel RE, Jackson JS, Morrison DP, Carlisle SM. Low doses of radiation increase the latency of spontaneous lymphomas and spinal osteosarcomas in cancer-prone, radiation-sensitive Trp53 heterozygous mice. Radiat Res. 2003;159:320–327. doi: 10.1667/0033-7587(2003)159[0320:ldorit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 54.Wang CY, Maibach HI. Why minimally invasive skin sampling techniques? A bright scientific future. Cutan Ocul Toxicol. 2011;30:1–6. doi: 10.3109/15569527.2010.517230. [DOI] [PubMed] [Google Scholar]
- 55.Teichmann A, Jacobi U, Ossadnik M, Richter H, Koch S, Sterry W, Lademann J. Differential stripping: determination of the amount of topically applied substances penetrated into the hair follicles. J Invest Dermatol. 2005;125:264–269. doi: 10.1111/j.0022-202X.2005.23779.x. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Liu Z, Ma L, Hossu M, Chen W. Interaction of porphyrins with CdTe quantum dots. Nanotechnology. 2011;22:195501. doi: 10.1088/0957-4484/22/19/195501. [DOI] [PubMed] [Google Scholar]
- 57.He YY, Häder D. Reactive oxygen species and UV-B: effect on cyanobacteria. Photochem Photobiol Sci. 2002;1:729–736. doi: 10.1039/b110365m. [DOI] [PubMed] [Google Scholar]
- 58.Miura Y, Ishige I, Soejima N, Suzuku Y, Uchida K, Kawana S, Eishi Y. Quantitative PCR of Propionibacterium acnes DNA in samples aspirated from sebaceous follicles on the normal skin of subjects with or without acne. J Med Dent Sci. 2010;57:65–74. [PubMed] [Google Scholar]
- 59.Kintisch E. Japan disaster. Pool at stricken reactor holds answers to key safety questions. Science. 2011;332:24–25. doi: 10.1126/science.332.6025.24. [DOI] [PubMed] [Google Scholar]
- 60.Youn SW, Kim JH, Lee JE, Kim SO, Park KC. The facial red fluorescence of ultraviolet photography: is this color due to Propionibacterium acnes or the unknown content of secreted sebum. Skin Res Technol. 2009;15:230–236. doi: 10.1111/j.1600-0846.2009.00360.x. [DOI] [PubMed] [Google Scholar]