Abstract
The mucosal and cellular responses of mice were studied, following mucosal-route administration of recombinant Lactococcus lactis expressing tetanus toxin fragment C (TTFC), which is a known immunogen protective against tetanus. A TTFC-specific T-cell response with a mixed profile of T-helper (Th) subset-associated cytokines was elicited in the intestine, with a Th2 bias characteristic of a mucosal response. These results correlated with the humoral response, where equivalent titers of anti-TTFC immunoglobulin G1 (IgG1) and IgG2a in serum were accompanied by an elevated IgA-specific response at more than one mucosal site. The route of vaccination had an important role in determining the immune response phenotype, as evidenced by the fact that an IgG1-biased subclass profile was obtained when lactococci were administered parenterally. Stimulation of splenic or mesenteric lymph node cells with lactococci resulted in their proliferation and the secretion of gamma interferon via antigen-specific and innate immune mechanisms. The data therefore provide further evidence of the potential of recombinant lactococcal vaccines for inducing systemic and mucosal immune responses.
The development of effective strategies for the mucosal delivery of vaccine antigens has received considerable attention over the past decade, because this route of administration has the potential to elicit local immune responses at mucosal surfaces, the major portals of entry to the body for many pathogens (10). The key effector molecule of the mucosal immune response is secretory immunoglobulin A (sIgA), which can play a key role in protecting against infection by inhibiting viral infectivity and bacterial colonization and by neutralizing the activity of microbial toxins (4, 21, 36, 43).
As mucosal delivery vehicles, recombinant bacterial vaccine vectors offer several practical advantages, including avoidance of culturing large quantities of pathogens, no need to purify antigenic components or subunits, and the ability to express immunogens in their native conformation. Many lactic acid bacteria (LAB) are acid and bile resistant and thus are well adapted to oral delivery. In addition, extensive fermentation know-how has been developed for these bacteria, and the genetics of LAB has progressed considerably during the past 2 decades, facilitating the construction of recombinant strains producing a variety of heterologous antigens (5, 12, 30, 45). The potential for the use of harmless LAB as mucosal delivery vehicles has been reviewed recently (20, 39, 40). Lactococcus lactis and Lactobacillus plantarum are the best-studied LAB for use as vaccine vectors. L. plantarum is a common commensal of the human urogenital and gastrointestinal tracts and is used in the food industry and as a probiotic organism (23). When fed to healthy subjects, it has been shown to survive in the human gastrointestinal tract longer than L. lactis (38), which is noncolonizing and noninvasive. Since L. lactis does not naturally colonize the intestines of humans or animals, it is perhaps more analogous to inert microparticle vaccine delivery systems (41).
To date, the majority of immunization studies with L. lactis have been carried out with recombinant strains producing tetanus toxin fragment C (TTFC) as the model antigen. TTFC is a 47-kDa nontoxic polypeptide carrying the ganglioside binding domain of the holotoxin, which has been shown to be immunogenic in mice and guinea pigs (11). Previous studies demonstrated that intragastric (i.g.) or intranasal (i.n.) administration of TTFC-expressing recombinant lactococci to mice induced systemic antibody responses at levels sufficient to be protective against a lethal challenge with tetanus toxin (26, 30), but no comparisons of efficacy with conventional vaccine delivery systems were carried out. The serological responses consisted predominantly of the IgG subclasses IgG1 and IgG2a, pointing to their regulation by an unbiased T-helper subset response (30); however, the cellular response was not investigated. The lactococcal vaccine strains also elicited increased concentrations of TTFC-specific IgA in the intestinal tract, which could be detected by assays of fecal extracts (30). Mucosal antibody responses at other sites, however, were not investigated.
Recent studies have indicated that certain probiotic strains of LAB have a profound effect on the secretion of cytokines from immune cells of both human and animal origin (3, 6, 8). In order to develop recombinant L. lactis further as a vaccine delivery system, it is important to determine how the innate properties of the bacterial carrier itself might influence the T-helper cell-associated cytokine response to a vaccine antigen. In addition, the effect of the route of administration on the magnitude and kinetics of the mucosal antibody response and cellular responses to the vaccine antigen need to be determined.
The aim of this study was to characterize the mucosal antibody and cellular responses of mice following i.g., i.n., or intraperitoneal (i.p.) administration of recombinant L. lactis expressing TTFC. Systemic and mucosal cytokine profiles in response to the lactococcal vaccine, and to a “gold standard” injected vaccine using tetanus toxoid (TT) in Freund's complete adjuvant (FCA), were examined to determine whether there was indeed a correlation of T-helper subset responses with serum antibody isotypes. These investigations revealed dramatic differences in the response profiles elicited by lactococcal vaccines given via mucosal and parenteral routes. The responses induced by recombinant lactococci given parenterally were lower in level than those obtained with TT in FCA, but i.g. and i.n. administration also induced elevated TTFC-specific IgA levels at several mucosal sites. A mixed profile of T-helper-associated cytokines was elicited in the intestine, together with an unbiased ratio of serum IgG1 and IgG2a subclasses. The results provide further evidence of the potential of recombinant lactococcal vaccines to induce immune protection at mucosal surfaces.
MATERIALS AND METHODS
Preparation of lactococcal strains for immunization.
Strains UCP1060, harboring the constitutive expression vector pTREX1-TTFC, and UCP1401, the control nonexpressor strain harboring pTREX1, were administered i.p., i.n., or i.g. as previously described (30). Briefly, L. lactis was cultured at 30°C in M17 broth (Difco Laboratories, East Molesey, United Kingdom) supplemented with 0.5% glucose (Sigma-Aldrich Company Ltd., Poole, United Kingdom) and 5 μg of erythromycin (Sigma)/ml for 18 h. Cells were washed with sterile phosphate-buffered saline (PBS) and resuspended at 109/ml for i.p. injection. For i.n. and i.g. immunizations, cells were resuspended in 0.2 M sodium bicarbonate-5% casein hydrolysate-0.5% glucose at 5 × 1010 CFU/ml.
Immunization of mice.
Specific-pathogen-free female C57BL/6 mice aged 6 to 8 weeks were purchased from Harlan UK (Oxon, United Kingdom). All experiments were carried out in accordance with Home Office and institutional regulations. Groups of six mice were immunized i.g., i.n., or i.p. with vaccine strain pTREX1 or pTREX1-TTFC. A naïve, nonvaccinated control group was included in each experiment. For i.g. immunization, doses of 5 × 109 CFU were administered via a gavage tube on days 0, 1, 2, 28, 29, 30, and 35. For i.n. immunization, doses of 1 × 109 CFU (20 μl) were administered on days 0, 14, and 28 to the nostrils of lightly anesthetized mice by using a pipette. i.p. injections of 108 CFU of recombinant L. lactis were administered on days 0, 14, and 28.
Collection of lung and gut lavage fluids.
By use of a method based on that of Wu and Russell (44), gut lavage fluids were obtained by flushing the excised small intestine with 3 ml of PBS containing 50 mM EDTA and 0.1 mg of soybean trypsin-chymotrypsin inhibitor (Sigma)/ml. The contents were collected and retained on ice for processing, whereupon the fluids were vortexed and centrifuged at 650 × g for 10 min at 4°C. A 30-μl volume of 100 mM phenylmethylsulfonyl fluoride (PMSF) (Sigma) was added to the supernatants before they were vortexed and spun at 27,000 × g for 20 min at 4°C. A further 20 μl of PMSF, 100 μl of fetal bovine serum (FBS), and 20 μl of 1% sodium azide (Sigma) were added to the supernatants before they were dispensed into aliquots and frozen.
Lung lavage fluids were obtained post mortem by inserting a nylon cannula into the exposed trachea, which was tied in place. A hypodermic needle and syringe were attached and used to inject and withdraw 0.7 ml of 2 mM PMSF in PBS three times. The fluid samples were retained on ice before centrifugation at 27,000 × g for 20 min at 4°C, and the supernatants were then stored in aliquots at −80°C.
ELISA detection of antigen-specific antibodies in serum.
As described previously (30), enzyme-linked immunosorbent assay (ELISA) plates were coated with 50 ng of recombinant TTFC (Roche Diagnostics Ltd., Lewes, United Kingdom)/well and blocked with 3% bovine serum albumin (BSA) (Sigma). Sera were tested in twofold dilution series, including replicate wells of a 1/50 dilution of preimmune serum on every plate. Anti-mouse IgG, IgA, IgM, IgG1, and IgG2a antibodies conjugated to alkaline phosphatase (Southern Biotechnology Associates, Birmingham, Ala.) were applied before development using Sigma 104 phosphatase substrate tablets. End point titers were calculated as the dilution resulting in the same optical density (at 405 nm) as the 1/50 dilution of pooled preimmune serum.
ELISA detection of antigen-specific mucosal IgA.
Intestinal and lung lavage fluids were tested for both TTFC-specific and total IgA by a method based on that of Medaglini et al. (19). Portions of each plate were coated, respectively, with an anti-mouse IgA monoclonal antibody (Sigma) and TTFC. In addition to the diluted samples, a dilution series of purified IgA was applied to each plate to provide a standard curve. After incubation with alkaline phosphatase-conjugated anti-mouse IgA and development with the substrate as described above, the concentrations of TTFC-specific and total IgA were determined from the standard curve. In order to address the possibility that increased IgA concentrations were the result of a polyclonal nonspecific response to mucosal stimulation, responses were expressed as the ratio of specific to total IgA.
Tissue preparation and enzyme-linked immunospot (ELISPOT) assay for TTFC-specific IgA-producing cells.
Mesenteric lymph nodes (MLN) were excised and disrupted by rubbing through sterile gauze, and the red blood cells were lysed by treatment with 0.85% ammonium chloride solution. The cells were resuspended in complete medium, consisting of RPMI 1640 containing 2 g of sodium bicarbonate/liter, 0.1 mM sodium pyruvate, 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10% FBS (Sigma).
Peyer's patches (PP) were removed from small intestines that had been flushed with RPMI 1640 medium containing 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10 μg of gentamicin/ml. The tissues were rubbed through sterile gauze and digested in Joklik's modified minimum essential medium (Sigma) containing 1 U of dispase (Roche)/ml for 5 min at 37°C before resuspension in complete medium containing 10 μg of gentamicin/ml.
In order to flush blood from the lungs, the abdominal aorta and vena cava were cut before PBS injection into the right ventricle of the heart. The lungs were then removed, cut into small pieces, and digested in 2 ml of PBS containing 0.5 U of collagenase A/ml and 0.25 mg of DNase I/ml (Roche) at 37°C for 1 h with shaking. The suspension was passed through gauze to remove debris, and red blood cells were lysed with ammonium chloride. Lymphocytes were purified by differential centrifugation through discontinuous 75 and 40% Percoll (Amersham Biosciences, Amersham, United Kingdom) gradients and then resuspended in complete medium.
As described previously (28), the wells of 96-well sterile microfiltration plates [Millipore (UK) Ltd., Watford, United Kingdom] were coated overnight with 1 μg of TTFC/ml in carbonate-bicarbonate buffer and blocked for 1 h with 5% FBS in RPMI 1640 medium. After a wash, cells were added to triplicate wells at 2 × 105, 1 × 105, and 5 × 104 per ml in 100 μl of complete medium. Plates were incubated at 37°C for 20 h under 5% CO2. The plates were then washed three times with PBS and three times with PBS-0.05% Tween 20 before addition of peroxidase-conjugated anti-mouse IgA (Sigma) diluted in PBS-0.05% Tween 20-1% FBS to the wells and incubation for 1 h at room temperature. Plates were washed before addition of 4-chloro-1-naphthol substrate solution (Sigma). After color development, plates were washed with distilled water, and spots were counted by using a dissection microscope.
T-cell proliferation assay.
Thirty-five days after primary immunization, spleens and MLN were removed and disrupted by rubbing though sterile gauze, as described previously (29). Red blood cells were lysed by treatment with ammonium chloride solution, and the remaining cells were washed, counted, and resuspended at 106/ml in complete medium. Aliquots (0.2 ml) of the spleen and MLN cell suspensions (SPLC and MLNC, respectively) were placed in quadruplicate wells of 96-well plates. By use of a protocol based on that of VanCott et al. (37), the cells were incubated for 5 days at 37°C under 5% CO2 in medium alone or in medium containing either 5 μg of concanavalin A (Sigma)/ml, 5 × 106 latex particles coated with TTFC (LP-TT)/ml, 5 × 106 uncoated latex particles (LP-U)/ml, or 5 × 106 formalin-fixed L. lactis(pTREX1) or L. lactis(pTREX1-TTFC) organisms/ml. One microcurie of [3H]thymidine (Amersham) was added to each well for the final 18 h of culture. The cells were harvested, and proliferation was detected by measurement of cellular [3H]thymidine incorporation. Stimulation indices (SI) were calculated as the ratio of mean counts per minute from stimulated cells to mean counts per minute from unstimulated cells.
Cytokine assays.
SPLC and MLNC were cultured for 48 h in the presence of 5 × 106 LP-TT/ml or 5 × 106 formalin-fixed L. lactis(pTREX1) or L. lactis(pTREX1-TTFC) cells/ml in quadruplicate wells of 96-well plates. Supernatants were collected and assayed for the presence of gamma interferon (IFN-γ) and interleukin 4 (IL-4) by ELISA using matched monoclonal antibody pairs and protein standards (Pharmingen, San Diego, Calif.) as described previously (1). The concentrations of IFN-γ and IL-4 were interpolated from the appropriate recombinant cytokine standard curve.
Isolation of LPL.
Small intestines were collected, and PP, blood vessels, adherent fat, and mesentery were removed as described above. The intestine was opened longitudinally, washed thoroughly, and cut into 0.5-cm-thick pieces. These were placed on ice in Hanks balanced salts solution (HBSS) supplemented with 1 mM dithiothreitol, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10 μg of gentamicin/ml (Sigma). The tissues were shaken at 210 rpm in a 37°C incubator for 20 min, transferred to fresh medium, and shaken twice more for 30 min each time until the supernatant was clear. Tissue samples were examined microscopically to confirm that the epithelium had been removed before enzymatic digestion was used to release cells from the lamina propria. The tissues were shaken twice for 30 min each time in HBSS containing 0.1 mg of collagenase-dispase/ml and 0.1 mg of DNase I/ml (Roche). Supernatants were passed through a 70-μm mesh to remove large clumps of debris before cells were pelleted and washed with HBSS. The cells were layered over Lympholyte-M (Cedarlane Laboratories Ltd., Hornby, Ontario, Canada) and spun at 500 × g for 20 min. Lamina propria lymphocytes (LPL) were collected at the interface of HBSS and Lympholyte-M, washed, and resuspended at 106/ml in complete medium.
Intracellular cytokine staining and flow cytometry.
As previously described (27, 31), 106 LPL were incubated overnight with 10 μg of recombinant TTFC (Roche)/ml and 10 U of recombinant murine IL-2 (Sigma)/ml. The cells were then incubated for a further 6 h in the presence of 50 ng of phorbol myristate acetate/ml, 500 ng of ionomycin/ml, and 10 μg of brefeldin A/ml (Sigma). Cells were fixed with 2% paraformaldehyde in PBS-0.5% BSA for 20 min on ice. The cells were then washed and permeabilized with a solution of PBS-1% BSA-0.5% saponin (Sigma) before being stained with phycoerythrin-Cy5-conjugated anti-CD4, phycoerythrin-conjugated anti-αβ T-cell receptor (TCR) or anti-γδ TCR, or fluorescein isothiocyanate-conjugated anti-IL-4, anti-IL-5, anti-IL-10, or anti-IFN-γ (Pharmingen). Finally, the cells were washed with PBS-BSA-saponin and then resuspended in 2% paraformaldehyde solution. Flow cytometry was performed on a FACScan with Lysis II software (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). In all experiments, unstained cells and cells stained separately with each fluorochrome-labeled antibody were included to optimize compensation settings. Fluorochrome-labeled isotype control antibodies were used to confirm the specificity of labeling and to determine the placing of quadrants during analysis. Cells were incubated and labeled in duplicate, and typically data were acquired on 10,000 events from each tube. Data analysis was performed by using WinMDI, version 2.8 (http://facs.scripps.edu/).
Statistics.
Statistical analysis was carried out by using the Mann-Whitney U test. A significant difference was taken to exist when P was ≤0.05.
RESULTS
Serum antibody responses elicited by lactococcal vaccination.
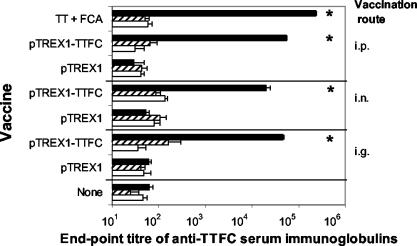
Immunization of mice with recombinant L. lactis expressing TTFC via i.p., i.g., and i.n. routes induced significantly elevated levels of TTFC-specific IgG in serum (Fig. 1). At 35 days post-primary immunization, the levels of IgA and IgM were not significantly elevated above the titers of nonimmunized mice or those inoculated with the pTREX1 control strain. i.g., i.n., and i.p. immunization with the lactococcal pTREX1-TTFC strain elicited mean serum anti-TTFC IgG titers of 4.6 × 104, 2.0 × 104, and 5.5 × 104, respectively, while the responses observed with the vector control strain (pTREX1) and in unvaccinated groups of mice were lower (titers of 60 to 62) and did not differ significantly from those of the naïve group. The i.p. route of lactococcal vaccination elicited 2.8-fold higher serum IgG titers than the i.n. route (P < 0.05). The antibody titers obtained by i.p. injection with lactococci were also significantly greater than those obtained via the i.g. route (P < 0.05); although the levels of the responses were similar, i.p. injections of TT in FCA elicited fourfold-higher serum IgG titers (2.4 × 105) than those obtained after injection of recombinant lactococci expressing TTFC.
FIG. 1.
Serum anti-TTFC IgG, IgA, and IgM titers elicited by recombinant lactococci or purified TT. Groups of six mice were immunized, either i.g., i.n., or by i.p. injection, with lactococci expressing TTFC (pTREX1-TTFC) or a control strain (pTREX1). For comparison, a group of mice were injected with TT in FCA. On day 35 post-initial treatment, blood samples were collected and TTFC-specific serum IgG (solid bars), IgA (striped bars), and IgM (open bars) titers were measured by ELISA. Bars, mean titers; error bars, standard deviations. Asterisks indicate values significantly different from those for unvaccinated controls (P < 0.01).
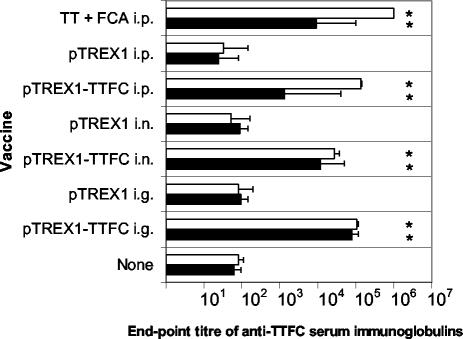
The TTFC-specific serum IgG1 and IgG2a subclasses were measured in order to determine which T-helper subset responses were elicited by the lactococci (Fig. 2). Mucosal-route vaccination with L. lactis elicited approximately equal levels of antigen-specific IgG1 and IgG2a subclass immunoglobulins in serum, with IgG1/IgG2a ratios of 1.3 and 2.3 for the i.g. and i.n. routes of immunization, respectively. In contrast, the parenteral route of administration elicited much higher levels of antigen-specific IgG1 than IgG2a. The serum anti-TTFC IgG1/IgG2a ratios elicited by i.p. administration of the lactococcal vaccine strain and i.p. immunization with TT in FCA were 105.4 and 110.9, respectively.
FIG. 2.
Serum anti-TTFC IgG1 and IgG2a titers elicited by recombinant lactococci or purified TT. Groups of six mice were immunized, either i.g., i.n., or by i.p. injection, with lactococci expressing TTFC (pTREX1-TTFC) or a control strain (pTREX1). For comparison, a group of mice was injected with TT in FCA, while another group received no treatment. On day 35 post-initial treatment, blood samples were collected, and serum TTFC-specific IgG1 (open bars) and IgG2a (solid bars) titers were measured by ELISA. Bars, mean titers; error bars, standard deviations. Asterisks indicate values significantly different from those for nonvaccinated controls (P < 0.05).
Mucosal antibody responses elicited by lactococcal vaccination.
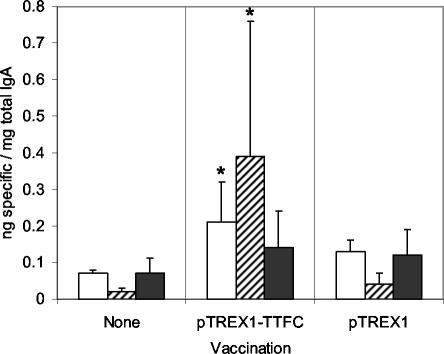
Significantly elevated TTFC-specific IgA responses could be detected in the intestinal lavage fluids of mice immunized by i.g. administration of pTREX1-TTFC lactococci (Fig. 3) but not in those of mice given the control strain. On days 6 and 20, mean ratios of specific to total IgA detected for immunized mice were 3- and 20-fold higher than those for unvaccinated controls (P < 0.05). On day 46, a twofold-higher ratio was detected, but this difference was not statistically significant. No significant differences could be detected between mice vaccinated with the pTREX1 control strain and unvaccinated groups at any of the time points.
FIG. 3.
Ratios of specific to total IgA in intestinal lavage fluids of mice immunized i.g. with lactococci expressing TTFC (pTREX1-TTFC) or a control strain (pTREX1). Intestinal lavage fluids were collected from groups of six mice on days 6 (open bars), 20(striped bars), and 46 (shaded bars) post-initial treatment. The samples were tested by ELISA for TTFC-specific and total IgA. Bars, mean ratios; error bars, standard deviations. Asterisks indicate values significantly different from those for nonvaccinated controls (P < 0.05).
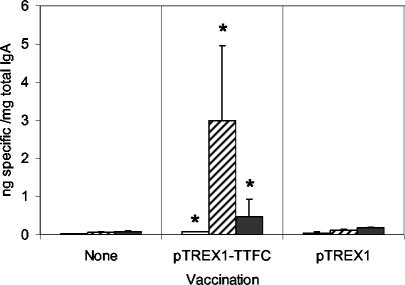
Significantly elevated TTFC-specific IgA responses were also detected in lung lavage fluids from mice immunized by i.n. administration of pTREX1-TTFC lactococci (Fig. 4) but not in those of mice receiving the control strain. On days 7, 20, and 45, 4-, 52-, and 6-fold-higher mean ratios of specific to total IgA were detected (P < 0.05, P = 0.001, and P < 0.05, respectively) relative to those for unvaccinated controls.
FIG. 4.
Ratios of specific to total IgA in lung lavage fluids of mice immunized i.n. with lactococci expressing TTFC (pTREX1-TTFC) or a control strain (pTREX1). Lung lavage fluids were collected from groups of six mice on days 7 (open bars), 20(striped bars), and 45 (shaded bars) post-initial treatment. The samples were tested by ELISA for TTFC-specific and total IgA. Bars, mean ratios; error bars, standard deviations. Asterisks indicate values significantly different from those for nonvaccinated controls (P < 0.05).
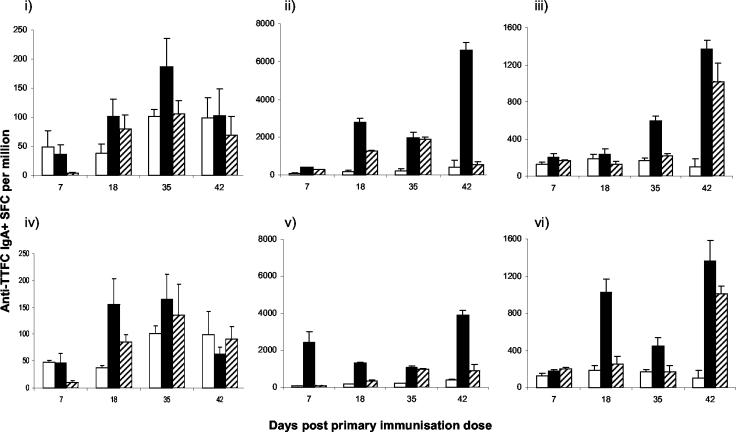
ELISPOT assays were conducted to assess the numbers of TTFC-specific IgA-secreting cells in lymphoid and mucosal tissues over 42 days following lactococcal immunization (Fig. 5). After i.g. immunization with the pTREX1-TTFC strain, the mean number of spot-forming cells per million (SFCPM) in the MLN reached a significantly higher level than that seen in naïve animals at day 35 (P < 0.05) only. In the PP, however, significantly elevated SFCPM relative to those of naïve controls were detected at each time point, with a 4-fold increase on day 7 (P < 0.05), a 17-fold increase on day 18 (P = 0.001), a 10-fold increase on day 35 (P = 0.001), and a second peak with a 16-fold increase on day 42 (P = 0.001) following the final immunizations. Lung tissues contained significantly elevated SFCPM on days 35 (3-fold; P = 0.001) and 42 (13-fold; P = 0.001) after primary immunization by the i.g. route.
FIG. 5.
Quantification of TTFC-specific IgA-secreting cells in lymphoid and mucosal tissues over 42 days after lactococcal immunization. Groups of six mice were immunized i.g. (i, ii, and iii) or i.n. (iv, v, and vi) with lactococci expressing TTFC (pTREX1-TTFC) (solid bars) or a control strain (pTREX1) (striped bars). A control group remained untreated (open bars). On days 7, 18, 35, and 42 post-initial treatment, MLN (i and iv), PP (ii and v), and PBS-perfused lung (iii and vi) cell suspensions were cultured and tested by ELISPOT for the presence of TTFC-specific IgA-secreting cells. Bars, mean frequencies of SFCPM; error bars, standard deviations.
Following i.n. immunization with the pTREX1-TTFC strain, the peak in SFCPM occurred in the MLN at days 18 and 35, with a fourfold increase over the value for naïve controls on day 18 (P < 0.05). SFCPM were at levels similar to those found with the i.g. route of immunization. In the lung, significantly elevated SFCPM were observed at days 18 (5-fold; P = 0.001) and 42 (13-fold; P = 0.001), whereas in the PP, peak responses were observed on days 7 (25-fold; P = 0.001) and 42 (10-fold; P = 0.001).
Fluctuations in the SFCPM of cells from unvaccinated animals occurred throughout the time course. Administration of the non-TTFC-expressing control lactococcal strain induced significantly elevated numbers of TTFC-specific IgA-secreting cells at several points and in several tissues, probably through polyclonal stimulation and up-regulation of IgA.
Cellular responses elicited by i.p. and i.g. immunization.
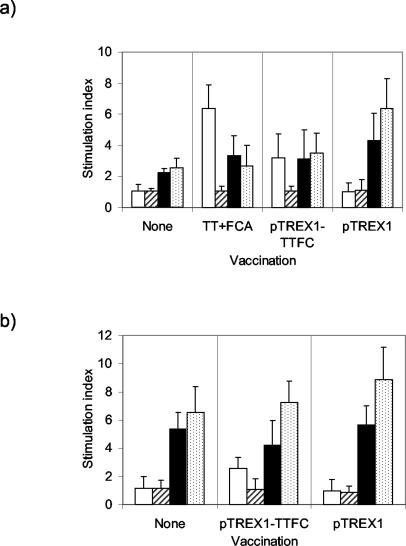
SPLC harvested on day 35 from mice injected with TTFC in FCA proliferated in response to stimulation with LP-TT, giving a mean SI of 6.35 (Fig. 6a). The response was significantly greater than that induced by LP-U (P = 0.001), which did not stimulate these cells or any of the other cultures to proliferate. SPLC from mice injected with the pTREX1-TTFC strain proliferated in response to LP-TT, with a mean SI of 3.18 (P < 0.05 compared with LP-U stimulation), whereas no significant response was detected with cells from the group injected with the L. lactis vector control strain (mean SI, 1.02). LP-TT stimulation induced proliferative responses of MLNC from mice immunized i.g. with pTREX1-TTFC (Fig. 6b). These SI (mean, 2.58) were significantly greater than the response to LP-U stimulation (P < 0.05) and were similar to those obtained from the SPLC of mice to which lactococci were administered via the i.p. route. No response was detected in MLNC isolated from mice administered the lactococcal control strain. Cells from all of the groups proliferated when incubated with the lactococcal strains in vitro. All cultures of SPLC and MLNC responded to conconavalin A stimulation with SI of >16.5.
FIG. 6.
Proliferative responses of SPLC from groups of six mice immunized i.p. with lactococci expressing TTFC (pTREX1-TTFC), a control strain (pTREX1), or TT in FCA (a) and of MLNC from mice immunized i.g. with recombinant lactococci (b). Control nonvaccinated groups were also included. SPLC and MLNC were cultured with LP-TT (open bars), LP-U (striped bars), or formalin-fixed pTREX1-TTFC (solid bars) or pTREX1 (speckled bars) lactococci. Unstimulated cells were also cultured. Proliferation was determined by [3H]thymidine incorporation, and results were expressed as SI (ratios of counts from stimulated cells to counts from unstimulated cells). Bars, mean SI. Error bars, standard deviations.
T-helper subset-associated cytokine responses elicited by i.g. and i.p. immunization.
MLNC harvested on day 35 from untreated control mice or those immunized i.g. with recombinant lactococci were cultured for 48 h in the presence of LP-TT. Culture supernatants were then tested by ELISA for the presence of IFN-γ and IL-4 (Table 1). LP-TT-stimulated MLNC from pTREX1-TTFC-vaccinated mice secreted significantly lower IFN-γ concentrations (P < 0.05) than mice in the unvaccinated group, resulting in an IFN-γ/IL-4 ratio of 1.77 (Table 1). The responses of cells from pTREX1-immunized and untreated control groups to LP-TT were not significantly different, with IFN-γ/IL-4 ratios of 3.6 and 3.3, respectively.
TABLE 1.
IL-4 and IFN-γ concentrations in culture supernatants of MLNC from mice immunized by i.g. administration of the pTREX1 or pTREX1-TTFC lactococcal strain
| Stimulation | Cytokine concn (pg/ml) for mice immunized with:
|
|||||
|---|---|---|---|---|---|---|
| Nothing
|
L. lactis (pTREX1-TTFC)
|
L. lactis (pTREX1)
|
||||
| IL-4 | IFN-γ | IL-4 | IFN-γ | IL-4 | IFN-γ | |
| None | 172.7 ± 8.8 | 616.5 ± 12.3 | 172.7 ± 7.8 | 652.0 ± 13.4 | 157.3 ± 14.3 | 608.5 ± 16.7 |
| LP-TT | 158.7 ± 8.3 | 528.5 ± 13.5 | 210.0 ± 7.5 | 372.0 ± 10.7 | 188.2 ± 9.6 | 687.5 ± 7.4 |
SPLC harvested on day 35 from mice immunized by i.p. injection of either TT in FCA, the TTFC-expressing strain (pTREX1-TTFC), or the vector control strain (pTREX1) were also cultured for 48 h in the presence of LP-TT or formalin-fixed lactococci. SPLC from mice immunized with TT-FCA produced predominantly IFN-γ in response to LP-TT stimulation, with an IFN-γ/IL-4 ratio of 5.6 (Table 2). In comparison, LP-TT-stimulated SPLC from nonvaccinated animals showed a ratio of 3.8. LP-TT stimulation of SPLC from pTREX1-TTFC-vaccinated mice produced significantly higher levels of IL-4 than those observed in unvaccinated controls (P < 0.05) but no elevation in IFN-γ, resulting in a ratio of 2.3.
TABLE 2.
IL-4 and IFN-γ concentrations in culture supernatants of SPLC from mice immunized by i.p. injection of TT in FCA or the pTREX1 or pTREX1-TTFC lactococcal strain
| Stimulation | Cytokine concn (pg/ml) for mice immunized with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Nothing
|
TT-FCA
|
L. lactis (pTREX1-TTFC)
|
L. lactis (pTREX1)
|
|||||
| IL-4 | IFN-γ | IL-4 | IFN-γ | IL-4 | IFN-γ | IL-4 | IFN-γ | |
| None | 103.2 ± 6.6 | 292.0 ± 5.4 | 102.1 ± 4.0 | 607.5 ± 12.5 | 123.2 ± 5.5 | 275.0 ± 5.0 | 124.3 ± 5.0 | 275.0 ± 5.5 |
| LP-TT | 95.0 ± 5.8 | 365.0 ± 12.0 | 113.2 ± 5.1 | 637.0 ± 12.4 | 153.4 ± 6.1 | 354.0 ± 68.8 | 94.5 ± 4.6 | 279.5 ± 5.9 |
| pTREX1-TTFC | 148.7 ± 9.7 | 4,080 ± 118.6 | 193.4 ± 8.1 | 6,409 ± 182.2 | 161.6 ± 7.8 | 23,266 ± 456.3 | 98.1 ± 4.2 | 29,250 ± 558.0 |
| pTREX1 | 141.9 ± 6.3 | 4,080 ± 124.9 | 149.9 ± 6.5 | 6,352 ± 172.1 | 227.1 ± 10.3 | 20,306 ± 460.1 | 161.6 ± 7.8 | 40,502 ± 801.0 |
The lactococcus stimulation of SPLC from all of the groups induced significantly higher levels (at least ninefold) of IFN-γ (P < 0.01) than those observed in corresponding unstimulated cells (Table 2). Significantly higher IFN-γ responses were obtained from groups immunized with recombinant lactococci of either strain than from mice receiving TT-FCA injection. pTREX1-TTFC-stimulated SPLC from mice administered the pTREX1-TTFC or pTREX1 strain via the i.p. route secreted a mean IFN-γ concentration of 23 or 29 ng/ml, respectively (standard deviations, 0.45 and 0.56; P < 0.01). In comparison, lactococcus-stimulated cells from untreated control mice or from mice injected with TT-FCA secreted only 4.0 or 6.4 ng/ml, respectively (standard deviations, 0.12 and 0.18).
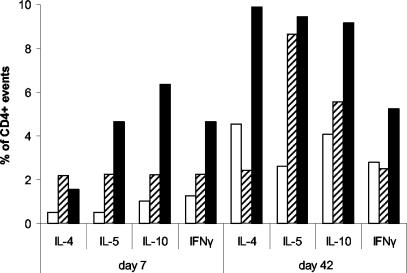
In order to investigate the mucosal cellular response and also to determine the source of the cytokines measured, lamina propria mononuclear cells were isolated from the small intestines of groups of untreated mice and from those immunized i.g. with lactococcal strains harboring pTREX1-TTFC or pTREX1. After stimulation in vitro, the cells were fixed, permeabilized, and stained for surface markers and intracellular cytokines. Cells from untreated mice contained 37% αβ+, 9.72% γδ+, and 24.74% CD4+ T cells. Cells from mice immunized with the control strain carrying pTREX1 contained 35.14% αβ+, 6.55% γδ+, and 21.72% CD4+ T cells, whereas cells from mice immunized with the pTREX1-TTFC vaccine strain contained 41.16% αβ+, 4.04% γδ+, and 34.3% CD4+ T cells. At day 7 post-primary immunization, there were higher proportions of IL-4 (threefold)-, IL-5 (ninefold)-, IL-10 (sixfold)-, and IFN-γ (threefold)-positive events in CD4 cells from mice immunized with TTFC-expressing lactococci than in those from control unimmunized mice (Fig. 7). Similarly, at day 42 after primary vaccination, CD4+ cells from mice immunized with lactococci expressing TTFC revealed higher proportions of IL-4 (2-fold)-, IL-5 (3.5-fold)-, IL-10 (2.2-fold)-, and IFN-γ (1.8-fold)-positive events than those from the unvaccinated group. Additionally, the former group of mice showed a >2-fold-higher proportion of IL-4+ cells at day 42 post-primary vaccination than either the unvaccinated or the control group (Fig. 7). In all cases, more than 60% of the cytokine-producing cells were αβ TCR positive. The ratio of IFN-γ+ to IL-4+ total events in cells from untreated mice on day 42 was 1.23, compared with 0.36 from mice immunized with TTFC-expressing lactococci. When only CD4+ events were considered, the ratio of IFN-γ+ to IL-4+ events was 0.53, also exhibiting a bias toward Th2.
FIG. 7.
Percentages of cytokine-positive events among CD4-positive LPL. Mice were immunized i.g. with lactococci expressing TTFC (pTREX1-TTFC) (solid bars) or a control strain (pTREX1) (striped bars). Control unvaccinated mice were also included (open bars). LPL were isolated from the small intestine on days 7 and 42, cultured, fixed, and stained for CD4 and the intracellular cytokines IL-4, IL-5, IL-10, and IFN-γ.
DISCUSSION
Recombinant lactococci expressing TTFC have previously been shown to induce both systemic and mucosal antibody responses after i.g. or i.n. immunization (30). The main aim of the present study was to characterize the cellular immune response at systemic and mucosal sites, particularly T-helper subsets, which play an important role in regulating the humoral response.
The comparative efficacy of vaccination with recombinant L. lactis was determined by also investigating the serum TTFC-specific antibody level elicited by i.p. injection of lactococci expressing TTFC or of TT in FCA, which has long been known as an extremely potent adjuvant (15). In accordance with previous data, the i.g. and i.n. mucosal routes of immunization with recombinant lactococci elicited high-level serological responses. The resulting antibody titers were only 1.2- and 2.7-fold lower, respectively, than those obtained by i.p. injection of TTFC-expressing lactococci without adjuvant. The responses to TT-FCA, in comparison, resulted in IgG titers approximately fourfold higher than those obtained after mucosal immunization with the lactococcal vaccine strain. These results indicate that, although the antibody titers reached protective levels (30), steps should be taken in the future to improve the immunogenicity of recombinant lactococcal vaccines for mucosal delivery, especially when weak immunogens are being expressed. To this end, it has previously been shown that coexpression of a heterologous antigen with cytokines (2, 35) or prolonging the in vivo persistence of the antigen-delivering strain (13) may enhance immune responses to recombinant LAB vaccine strains. Preliminary observations with TTFC suggest that increasing the amount of antigen expressed in the vaccine strain might also enhance immunogenicity (unpublished data).
The serum TTFC-specific IgG subclass response elicited by i.g. or i.n. lactococcal vaccination has previously been shown to be dominated by IgG1 and IgG2a, and the levels of IgG2b and IgG3 did not rise significantly above those of negative controls (30). The present study has confirmed that IgG1 and IgG2a were produced at approximately equal levels following mucosal immunization, but interestingly, a significant predominance of IgG1 was observed following i.p. administration of the lactococcal vaccine. This result correlates with the data of Medaglini et al. (19), who found that mucosal administration of recombinant Streptococcus gordonii expressing TTFC elicited equivalent serum IgG1 and IgG2a levels, while parenteral immunization resulted in a predominantly IgG1 response. The route of administration of recombinant lactococci is therefore important in determining the type of immune response elicited.
The mucosal immune response is initiated by exposure of antigen-presenting cells and lymphocytes to antigen in lymphoid follicles, e.g., PP in the intestine. Cells pass from this inductive site into the efferent lymphatics to the MLN, where lymphocytes undergo clonal expansion before entering distant effector sites via the thoracic duct and systemic circulation. These cell migratory pathways are the basis for the observation that mucosal immunization can result in the induction of antibody responses at distant mucosal sites, i.e., via a common mucosal immune system (33). Previous work demonstrated that i.n. immunization with lactococci expressing TTFC induced elevated numbers of IgA-secreting cells in the nasal mucosa and lungs (26), while i.g. immunization induced increased levels of TTFC-specific IgA in feces (30). Similar results have been obtained by immunization with recombinant L. plantarum (13) or S. gordonii (19) expressing TTFC. In the present study, mucosal antibody responses elicited by lactococcal immunization were investigated over a time course of 42 days in order to determine whether mucosal antibody responses could be detected at more than one mucosal site and also to discover if such responses were sustained or merely transient.
Following i.g. immunization, a rapid increase in TTFC-specific IgA levels could be detected in intestinal lavage fluids, but this declined by day 46 to nonsignificant levels. Increasing numbers of IgA-producing cells were detected in the MLN of immunized animals, reaching a peak after administration of the second set of doses but also declining by day 42. The numbers of IgA-producing cells in the PP, an inductive site for immune responses in the gastrointestinal tract, were significantly elevated at all time points and peaked on days 18 and 42, approximately 2 weeks after the i.g. primary and booster doses, respectively. The discovery of anti-TTFC IgA-secreting cells in the lungs was particularly interesting, especially since the numbers of these cells gradually increased throughout the time course of the experiment. Clearly, i.g. vaccination with a lactococcal vaccine can elicit responses at distal mucosal sites in the body.
Immunization via the i.n. route induced significantly elevated levels of IgA in the lungs that could be detected in lung lavage fluids over the whole time course. Significantly increased frequencies of IgA-secreting cells were found in the lungs of i.n.-immunized mice from day 18 onward. The presence of such cells in tissues associated with the gut could also indicate the translocation of lymphocytes primed in the nasal mucosa to other mucosal effector sites. An alternative explanation is that a proportion of the inoculum applied to the nostrils was eventually swallowed and reached inductive sites for mucosal immune responses in the small intestine. Although significantly elevated numbers of antigen-specific IgA-secreting cells could be detected throughout the study, the data indicated that the mucosal antibody response fluctuated greatly. It appears, therefore, that a protective mucosal antibody response would not be sustained for very long after vaccination, although immunological memory may ensure a rapid mucosal response to the vaccine antigen upon subsequent reexposure.
A major objective of this study was to investigate the cellular immune response induced by lactococcal vaccination. SPLC and MLNC from mice immunized i.p. or i.g. with recombinant lactococci expressing TTFC, but not with the control strain, proliferated in response to LP-TT, indicating an antigen-specific cellular response. The SI obtained were not very high but were similar to those obtained by VanCott et al. (37) following oral vaccination of mice with recombinant Salmonella enterica serovar Typhimurium expressing TTFC.
SPLC from mice immunized by injection with TT-FCA secreted predominantly IFN-γ in response to stimulation with LP-TT. There was no increase in IL-4 levels, indicating a Th1 response, despite the fact that the predominant IgG antibody subclass was IgG1. Conversely, SPLC from mice vaccinated i.p. with pTREX1-TTFC produced significantly elevated levels of IL-4 but not IFN-γ in comparison with the unimmunized control group. This result correlated with the IgG subclass data and indicated a predominant Th2 response. MLNC from mice immunized i.g. with pTREX1-TTFC also produced significantly elevated concentrations of IL-4. It is difficult to speculate on the T-helper subset response when the cellular source of the cytokines detected in culture supernatants is unknown. The factors measured in the present study could have been secreted by many cell types (14, 24, 25). In order to more fully investigate the T-helper subset response induced by mucosal immunization with recombinant lactococci, cells were taken from a mucosal effector site, intracellular cytokines and cell surface markers were stained with fluorochrome-labeled antibodies, and flow cytometry was performed. Following i.g. immunization with pTREX1-TTFC lactococci, LPL from the small intestine contained higher frequencies of IL-4-, IL-5-, IL-10-, and IFN-γ-positive CD4 cells. When total IL-4- and IFN-γ-positive events were examined, a bias was noted in favor of IL-4. This was less pronounced but followed the same trend when the CD4-positive cytokine response was investigated, even though CD4 events contributed to less than 50% of total cytokine-producing cells.
Stimulation of cells from vaccinated and unvaccinated groups with recombinant lactococci (pTREX1 or pTREX1-TTFC strains) resulted in cellular proliferation and elevated levels of IFN-γ in culture supernatants. This could be due to prior exposure to antigenically similar bacteria or an innate immune mechanism. Some LAB produce immunomodulatory components which enhance the in vitro proliferation of peripheral blood mononuclear cells (17). Corinti et al. (7) showed that S. gordonii bacteria are capable of enhancing antigen presentation and T-cell proliferation. Significantly higher IFN-γ concentrations were also found in supernatants of cells from lactococcal vaccine recipients, indicating that the response could have both antigen-specific and innate components. Lactococci are known to be immunogenic, since mucosal administration of pTREX1-TTFC or pTREX1 strains elicited lactococcus-specific serum IgG responses (30). It has previously been shown that L. lactis can stimulate cytokines from human peripheral blood mononuclear cells in a nonspecific manner (22). Additionally, Lactobacillus casei is known to innately activate NF-κB-mediated cytokine signaling pathways via Toll-like receptor 2 (TLR2) (18). The interactions of L. lactis with pattern recognition receptors such as TLRs (16) have yet to be studied, but it appears likely that stimulation of such cytokine responses occurs via mechanisms similar to those observed with L. casei.
In summary, a mixed profile of Th1/Th2 cytokines was elicited at a mucosal effector site in the intestine by i.g. administration of recombinant lactococci expressing TTFC, with a bias toward Th2, which is characteristic of a mucosal response. The T-cell response correlated with the humoral response, where similar titers of the serum IgG subclasses IgG1 and IgG2a were detected together with elevated levels of TTFC-specific IgA at more than one mucosal site. A different IgG subclass profile was obtained when lactococci were administered i.p., confirming the importance of the vaccination route in determining the immune response phenotype (32).
An ideal multipurpose recombinant vaccine vehicle should be capable of inducing systemic responses relevant for protection against a variety of pathogens and should also elicit IgA at mucosal surfaces to prevent the entry of pathogens into the body. We and others have shown that L. lactis has the potential to act as an effective mucosal delivery system for bacterial (30) and viral (45) antigens. The present study shows that lactococcal immunization induces mixed IgG subclass and T-helper responses, allowing the induction of protection against a variety of infectious agents and potentially at several mucosal surfaces. The plasmids used for expression of TTFC in this system are structurally and segregationally stable during culture in vitro (42) and provide a useful model for exploring the characteristics of this vaccine delivery system. However, before recombinant LAB can be used in humans, it will be necessary to increase the potency of this system (i.e., to obtain high-level antibody responses to a variety of antigens with fewer doses) and to demonstrate protective-level immune responses in different animal models. It will also be necessary to construct strains for human or animal use that will meet the safety requirements of the regulatory bodies. The existing food grade expression systems should be further developed for this purpose (9). Recently a biologically contained L. lactis strain secreting human IL-10 was approved by the Dutch authorities for use in phase I clinical trials as an experimental therapy for humans with inflammatory bowel disease (34). As our knowledge of mucosal immunology and factors affecting the efficacy of LAB as delivery vehicles increases, there will be considerable potential to improve the existing technology.
Acknowledgments
J.M.W., M.C.L., and R.W.F.L.P. gratefully acknowledge grant support from the BBSRC. K.R. was supported by a research grant from The Wellcome Trust. J.M.W. also acknowledges financial support from the European Commission for research on “oral delivery of vaccine and therapeutic products using non-pathogenic lactic acid bacteria” (LABDEL project QLRT-2000-00340).
Editor: J. N. Weiser
REFERENCES
- 1.Bellaby, T., K. Robinson, and D. Wakelin. 1996. Induction of differential T-helper-cell responses in mice infected with variants of the parasitic nematode Trichuris muris. Infect. Immun. 64:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez-Humaran, L. G., P. Langella, N. G. Cortes-Perez, A. Gruss, R. S. Tamez-Guerra, S. C. Oliveira, O. Saucedo-Cardenas, R. Montes de Oca-Luna, and Y. Le Loir. 2003. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect. Immun. 71:1887-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borruel, N., M. Carol, F. Casellas, M. Antolin, F. de Lara, E. Espin, J. Naval, F. Guarner, and J. R. Malagelada. 2002. Increased mucosal tumour necrosis factor alpha production in Crohn's disease can be downregulated ex vivo by probiotic bacteria. Gut 51:659-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandtzaeg, P. 2003. Role of secretory antibodies in the defence against infections. Int. J. Med. Microbiol. 293:3-15. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain, L. M., J. M. Wells, K. Robinson, K. M. Schofield, and R. W. F. Le Page. 1997. Mucosal immunization with recombinant Lactococcus lactis, p. 83-106. In G. Pozzi and J. M. Wells (ed.), Gram-positive bacteria as vaccine vehicles for mucosal immunization. Landes Bioscience, Austin, Tex.
- 6.Christensen, H. R., H. Frokiaer, and J. J. Pestka. 2002. Lactobacilli differentially modulate expression of cytokines and maturation surface markers in murine dendritic cells. J. Immunol. 168:171-178. [DOI] [PubMed] [Google Scholar]
- 7.Corinti, S., D. Medaglini, A. Cavani, M. Rescigno, G. Pozzi, P. Ricciardi-Castagnoli, and G. Girolomoni. 1999. Human dendritic cells very efficiently present a heterologous antigen expressed on the surface of recombinant gram-positive bacteria to CD4+ T lymphocytes. J. Immunol. 163:3029-3036. [PubMed] [Google Scholar]
- 8.Cross, M. L., R. R. Mortensen, J. Kudsk, and H. S. Gill. 2002. Dietary intake of Lactobacillus rhamnosus HNOO1 enhances production of both Th1 and Th2 cytokines in antigen-primed mice. Med. Microbiol. Immunol. (Berlin) 191:49-53. [DOI] [PubMed] [Google Scholar]
- 9.de Vos, W. M. 1999. Gene expression systems for lactic acid bacteria. Curr. Opin. Microbiol. 2:289-295. [DOI] [PubMed] [Google Scholar]
- 10.Eriksson, K., and J. Holmgren. 2002. Recent advances in mucosal vaccines and adjuvants. Curr. Opin. Immunol. 14:666-672. [DOI] [PubMed] [Google Scholar]
- 11.Fairweather, N. F., V. A. Lyness, and D. J. Maskell. 1987. Immunization of mice against tetanus with fragments of tetanus toxin synthesized in Escherichia coli. Infect. Immun. 55:2541-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, C., K. Robinson, R. W. Le Page, and J. M. Wells. 2000. Heterologous expression of an immunogenic pneumococcal type 3 capsular polysaccharide in Lactococcus lactis. Infect. Immun. 68:3251-3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grangette, C., H. Muller-Alouf, M. Geoffroy, D. Goudercourt, M. Turneer, and A. Mercenier. 2002. Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria: impact of strain viability and in vivo persistence. Vaccine 20:3304-3309. [DOI] [PubMed] [Google Scholar]
- 14.Harris, D. P., L. Haynes, P. C. Sayles, D. K. Duso, S. M. Eaton, N. M. Lepak, L. L. Johnson, S. L. Swain, and F. E. Lund. 2000. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 1:475-482. [DOI] [PubMed] [Google Scholar]
- 15.Jollès, P., and A. Paraf. 1973. Chemical and biological basis of adjuvants, p. 53-80. Chapman and Hall, London, United Kingdom. [DOI] [PubMed]
- 16.Kopp, E., and R. Medzhitov. 2003. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 15:396-401. [DOI] [PubMed] [Google Scholar]
- 17.Laffineur, E., N. Genetet, and J. Leonil. 1996. Immunomodulatory activity of beta-casein permeate medium fermented by lactic acid bacteria. J. Dairy Sci. 79:2112-2120. [DOI] [PubMed] [Google Scholar]
- 18.Matsuguchi, T., A. Takagi, T. Matsuzaki, M. Nagaoka, K. Ishikawa, T. Yokokura, and Y. Yoshikai. 2003. Lipoteichoic acids from Lactobacillus strains elicit strong tumor necrosis factor alpha-inducing activities in macrophages through Toll-like receptor 2. Clin. Diagn. Lab. Immunol. 10:259-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medaglini, D., A. Ciabattini, M. R. Spinosa, T. Maggi, H. Marcotte, M. R. Oggioni, and G. Pozzi. 2001. Immunization with recombinant Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine 19:1931-1939. [DOI] [PubMed] [Google Scholar]
- 20.Mercenier, A., H. Muller-Alouf, and C. Grangette. 2000. Lactic acid bacteria as live vaccines. Curr. Issues Mol. Biol. 2:17-25. [PubMed] [Google Scholar]
- 21.Michetti, P., M. J. Mahan, J. M. Slauch, J. J. Mekalanos, and M. R. Neutra. 1992. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 60:1786-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miettinen, M., J. Vuopio-Varkila, and K. Varkila. 1996. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect. Immun. 64:5403-5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molin, G. 2001. Probiotics in foods not containing milk or milk constituents, with special reference to Lactobacillus plantarum 299v. Am. J. Clin. Nutr. 73:380S-385S. [DOI] [PubMed] [Google Scholar]
- 24.Moore, K. W., R. de Waal Malefyt, R. L. Coffman, and A. O'Garra. 2001. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19:683-765. [DOI] [PubMed] [Google Scholar]
- 25.Moser, M., and K. M. Murphy. 2000. Dendritic cell regulation of TH1-TH2 development. Nat. Immunol. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 26.Norton, P. M., J. M. Wells, H. W. Brown, A. M. Macpherson, and R. W. Le Page. 1997. Protection against tetanus toxin in mice nasally immunized with recombinant Lactococcus lactis expressing tetanus toxin fragment C. Vaccine 15:616-619. [DOI] [PubMed] [Google Scholar]
- 27.Openshaw, P., E. E. Murphy, N. A. Hosken, V. Maino, K. Davis, K. Murphy, and A. O'Garra. 1995. Heterogeneity of intracellular cytokine synthesis at the single-cell level in polarized T helper 1 and T helper 2 populations. J. Exp. Med. 182:1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson, K., T. Bellaby, and D. Wakelin. 1995. Immune response profiles in vaccinated and non-vaccinated high- and low-responder mice during infection with the intestinal nematode Trichinella spiralis. Parasitology 110:71-78. [DOI] [PubMed] [Google Scholar]
- 29.Robinson, K., T. Bellaby, and D. Wakelin. 1994. Vaccination against the nematode Trichinella spiralis in high- and low-responder mice. Effects of different adjuvants upon protective immunity and immune responsiveness. Immunology 82:261-267. [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson, K., L. M. Chamberlain, K. M. Schofield, J. M. Wells, and R. W. Le Page. 1997. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat. Biotechnol. 15:653-657. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, K., K. R. Neal, C. Howard, J. Stockton, K. Atkinson, E. Scarth, J. Moran, A. Robins, I. Todd, E. Kaczmarski, S. Gray, I. Muscat, R. Slack, and D. A. Ala'Aldeen. 2002. Characterization of humoral and cellular immune responses elicited by meningococcal carriage. Infect. Immun. 70:1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romagnani, S. 2000. Cytokines and the Th1/Th2 paradigm, p. 71-102. In F. Balkwill (ed.), The cytokine network. Oxford University Press, Oxford, United Kingdom.
- 33.Russell, M. W. 2002. Immunization for protection of the reproductive tract: a review. Am. J. Reprod. Immunol. 47:265-268. [DOI] [PubMed] [Google Scholar]
- 34.Steidler, L., S. Neirynck, N. Huyghebaert, V. Snoeck, A. Vermeire, B. Goddeeris, E. Cox, J. P. Remon, and E. Remaut. 2003. Biological containment of genetically modified Lactococcus lactis for intestinal delivery of human interleukin 10. Nat. Biotechnol. 21:785-789. [DOI] [PubMed] [Google Scholar]
- 35.Steidler, L., K. Robinson, L. Chamberlain, K. M. Schofield, E. Remaut, R. W. Le Page, and J. M. Wells. 1998. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 66:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor, H. P., and N. J. Dimmock. 1985. Mechanism of neutralization of influenza virus by secretory IgA is different from that of monomeric IgA or IgG. J. Exp. Med. 161:198-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.VanCott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, M. Yamamoto, M. Coste, P. B. Carter, H. Kiyono, and J. R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 156:1504-1514. [PubMed] [Google Scholar]
- 38.Vesa, T., P. Pochart, and P. Marteau. 2000. Pharmacokinetics of Lactobacillus plantarum NCIMB 8826, Lactobacillus fermentum KLD, and Lactococcus lactis MG 1363 in the human gastrointestinal tract. Aliment. Pharmacol. Ther. 14:823-828. [DOI] [PubMed] [Google Scholar]
- 39.Wells, J. M., and A. Mercenier. 2003. Lactic acid bacteria as mucosal delivery systems, p. 260-291. In B. J. B. Wood and P. J. Warner (ed.), Genetics of lactic acid bacteria. Kluwer Academic/Plenum, Dordrecht, The Netherlands.
- 40.Wells, J. M., and G. Pozzi. 1997. An overview of Gram-positive bacteria as vaccine vehicles for mucosal immunization, p. 1-8. In G. W. Pozzi and J. M. Wells (ed.), Gram-positive bacteria as vaccine vehicles for mucosal immunization. Springer-Verlag, Berlin, Germany.
- 41.Wells, J. M., K. Robinson, L. M. Chamberlain, K. M. Schofield, and R. W. Le Page. 1996. Lactic acid bacteria as vaccine delivery vehicles. Antonie Leeuwenhoek 70:317-330. [DOI] [PubMed] [Google Scholar]
- 42.Wells, J. M., and K. M. Schofield. 1996. Cloning and expression vectors for lactococci, p. 37-62. In T. F. Bozoglu and B. Ray (ed.), Lactic acid bacteria: current advances in metabolism, genetics and applications. NATO ASI series, vol. H98. Springer-Verlag, Berlin, Germany. [Google Scholar]
- 43.Winner, L., III, J. Mack, R. Weltzin, J. J. Mekalanos, J. P. Kraehenbuhl, and M. R. Neutra. 1991. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect. Immun. 59:977-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu, H. Y., and M. W. Russell. 1993. Induction of mucosal immunity by intranasal application of a streptococcal surface protein antigen with the cholera toxin B subunit. Infect. Immun. 61:314-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin, K. Q., Y. Hoshino, Y. Toda, S. Igimi, Y. Kojima, N. Jounai, K. Ohba, A. Kushiro, M. Kiwaki, K. Hamajima, D. Klinman, and K. Okuda. 2003. Immunogenicity and protective efficacy of orally administered recombinant Lactococcus lactis expressing surface-bound HIV Env. Blood 102:223-228. [DOI] [PubMed] [Google Scholar]