Abstract
Upper gastrointestinal (UGI) bleeding secondary to ulcer disease occurs commonly and results in significant patient morbidity and medical expense. After initial resuscitation, carefully performed endoscopy provides an accurate diagnosis of the source of the UGI hemorrhage and can reliably identify those high-risk subgroups that may benefit most from endoscopic hemostasis.
Large channel therapeutic endoscopes are recommended. Endoscopists should be very experienced in management of patients with UGI hemorrhage including the use of various hemostatic devices.
For patients with major stigmata of ulcer hemorrhage – active arterial bleeding, non-bleeding visible vessel and adherent clot – combination therapy with epinephrine injection and either thermal coaptive coagulation (with multipolar or heater probe), or endoclips is recommended. High dose intravenous proton pump inhibitors are recommended as concomitant therapy with endoscopic hemostasis of major stigmata. Patients with minor stigmata or clean-based ulcers will not benefit from endoscopic therapy and should be triaged to less intensive care and be considered for early discharge. Effective endoscopic hemostasis of ulcer bleeding can significantly improve outcomes by reducing rebleeding, transfusion requirement, and need for surgery, as well as reduce cost of medical care.
Keywords: Endoscopic hemostasis, UGI Bleeding, Peptic ulcer, Thermal therapy, Endoclips, Combination therapy
INTRODUCTION
Upper gastrointestinal (UGI) bleeding occurs frequently and is a common cause of hospitalization or inpatient bleeding. Such bleeding results in substantial patient morbidity, mortality, and medical care expense. Ulcer disease is the most common cause of severe UGI hemorrhage, causing about 40–50% of the cases, and UGI bleeding is the most common complication of peptic ulcer disease [1]. Although other nonvariceal conditions such as Mallory-Weiss tear, angiodysplasia, or Dieulafoy’s lesion may also cause UGI hemorrhage, these occur much less frequently [2]. Our purposes are to focus on the important aspects of the diagnosis and treatment of bleeding from ulcers.
METHODS
Initial Approach to the Patient
The initial management of the patient with UGI bleeding should include evaluation of severity of the hemorrhage, patient resuscitation, a medical history and physical examination and consideration of possible interventions [1]. Clinical assessment should focus on the patient’s comorbidities and hemodynamic state, with a view to early resuscitation. Initial medical therapy should be aimed at restoring blood volume by fluid replacement to ensure that tissue perfusion and oxygen delivery are not compromised. Airway protection with endotracheal intubation should be strongly considered in patients with ongoing hematemesis, altered mental or respiratory status, or severe neuromuscular disorders to prevent aspiration [1,2].
Intravenous erythromycin (a motilin receptor agonist that stimulates gastrointestinal motility) may improve the quality of endoscopic exams in patients with UGI hemorrhage by promoting the emptying of intragastric blood. A recent cost-effectiveness study confirmed that giving intravenous (IV) erythromycin prior to endoscopy for acute UGI bleeding resulted in cost savings and an increase in quality-adjusted life-years [3]. Because of these benefits, IV erythromycin is recommended prior to endoscopy in patients with severe UGI hemorrhage, when clots or blood are anticipated and may obscure the bleeding site.
After initial resuscitation and initiation of medical therapy, urgent endoscopy is the preferred procedure for diagnosis and treatment because of its high accuracy and low complication rate. Endoscopy using large single or double-channel therapeutic endoscopes is diagnostic in about 95% of patients with severe UGI bleed. Endoscopy may also reveal stigmata of recent hemorrhage (SRH) on ulcers that have important prognostic value, helping to risk stratify patients for rebleeding and to triage patients into low and high risk. Whereas some SRH are associated with increased rebleeding, patients without stigmata of hemorrhage or low risk SRH rarely rebleed. By consensus, SRH are divided into either active bleeding, ( i.e. arterial spurting or oozing,) (Figure 4) or recent hemorrhage, (i.e. non-bleeding visible vessel (NBVV) (Figure 5), adherent clot without other SRH, or flat, dark slough or spots [1]. From analysis of the Center for Ulcer Research and Education (CURE) randomized controlled trials (RCTs), medically treated patients on histamine-2-receptor antagonists (H2RAs) had significantly different rebleeding rates according to their stigmata of ulcer hemorrhage. Without endoscopic therapy, the rebleeding rate of ulcers with active arterial bleeding was 90%, with NBVV was 50%, and with non-bleeding adherent clots was 33% [1]. Ulcer with oozing bleeding(without other SRH), flat spots, or clean bases have much lower rebleeding rates of 10%, 7%, and 3%, respectively. Based on the high rebleeding rates with medical treatment alone, endoscopic therapy of all patients with active arterial bleeding, NBVV and adherent clots is currently recommended. Although rebleeding on medical therapy occurs less frequently, persistent oozing may also be treated endoscopically. A large US multicenter trial illustrates the prevalence of these stigmata. Of 4090 hospitalized patients (duodenal ulcer – 2033, gastric ulcer – 2057), 10.3% had active bleeding (arterial or oozing), 12.2% had NBVV, 8.3% had adherent clot, 9.9% had flat spot, and 58.4% had clean ulcer base [4].
Figure 4.
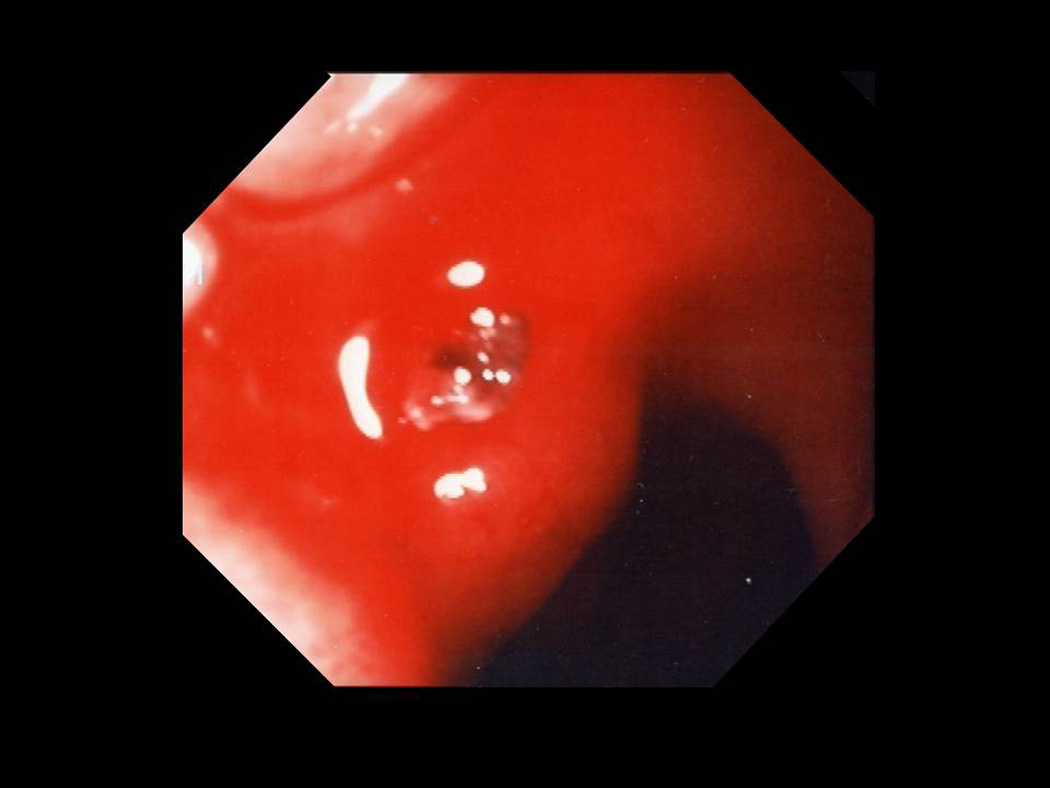
Ulcer with arterial bleeding and visible vessel.
Figure 5.
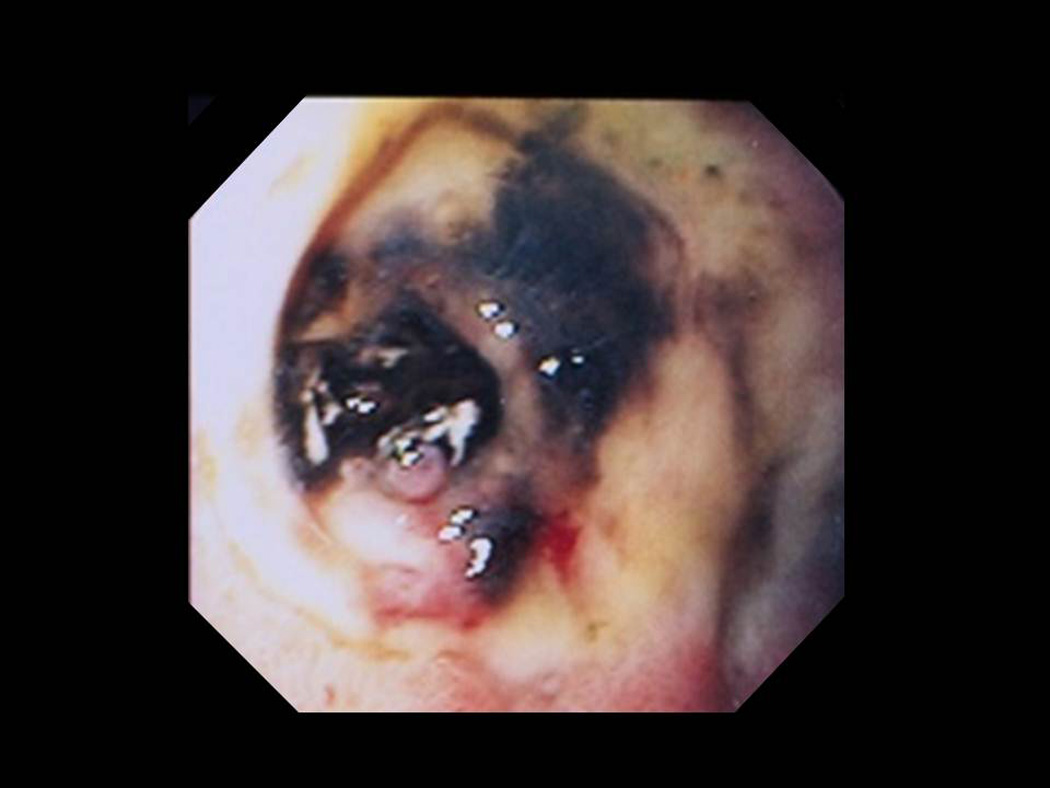
Clot over a non-bleeding visible vessel.
DOPPLER ULTRASOUND
Newer techniques such as endoscopic Doppler ultrasound probe (DUP) may provide more objective findings about risk stratification for patients with ulcer hemorrhage and other non-variceal gI hemorrhage (NVGJH). Prior reports suggest that there is substantial interobserver disagreement in the interpretation of visual endoscopic SRH. Even among an expert international panel, close correlation only occurred with active bleeding. For determination of rebleeding potential, it may be even more critical to determine if there is continued blood flow under the SRH and to determine if blood flow has stopped. Doppler ultrasound probe (DUP) technology has been used to interrogate nonvariceal and variceal bleeding lesions. DUP uses a small (2 mm diameter), flexible pulsed-wave, 16 or 20-MHz probe (Figure 6) that is passed through the endoscope’s biopsy channel directly onto the bleeding lesion (5). The output signal is expressed as an audible signal. Based on DUP signal, scanning depths, and DUP placement on the lesion, this technology permits evaluation of arterial or venous blood flow, depth of the blood vessel and position of the blood vessel (5). Use of DUP has shown that most NBVVs demonstrate an arterial signal while some ulcers with a clean base or pigmented spot also show an underlying arterial signal. Persistence of a positive Doppler signal after endoscopic treatment correlates with the potential for rebleeding. Therefore, endoscopic DUP may be a useful guide to the completion of hemostasis. If endoscopic treatment is continued until the underlying blood flow signal is extinguished the rebleeding rate of NVGIB is very low [6]. A prospective study in a group of severely bleeding ulcer patients with active arterial bleeding, NBVV and adherent clot, showed that DUP–based endoscopic treatment provided a significantly reduced rate of recurrent hemorrhage at 30 days than standard therapy based on endoscopic stigmata alone (7). A recent decision-analysis comparing DUP of acute ulcer hemorrhage with standard treatment, demonstrated an average cost savings ranging from $560 to $1160 per patient in the DUP-directed group [8]. In summary, the current studies of DUP suggest that 1) there is a close correlation between a positive signal and endoscopic stigmata; 2) DUP-positive ulcers are more likely to rebleed than DUP-negative ulcers; 3) persistence of a DUP-positive signal in ulcers after endoscopic coagulation results in an increased risk of ulcer rebleeding.
Figure 6.

Doppler ultrasound unit and probe (Vascular Technology Inc.)
NBVVs in ulcers have also been evaluated using a combination of magnification endoscopy and chromendoscopy with methylene blue (9). In a pilot study, the authors reported a diagnostic gain of 33% after reclassifying routine endoscopic findings with the results of magnification endoscopy. The clinical impact of these findings is uncertain since all patients in this study underwent successful endoscopic hemostasis (9).
ENDOSCOPIC THERAPY FOR ULCER HEMOSTASIS
Several different techniques have been developed for endoscopic treatment of ulcer bleeding. An ideal endoscopic hemostasis technique should posses the following features: a) reproducible effectiveness, b) easy and rapid application, c) low complications rate, d) low cost, e) portability to the bedside, and f) widespread availability. Endoscopic techniques have been grouped into three general types and are categorized according to whether or not tissue contact is necessary to achieve hemostasis. A combined therapy group (dilute epinephrine injection plus thermal or mechanical treatment) is considered separately.
The major thermal endoscopic therapies include multipolar probes (MPEC), heater probe, and argon plasma coagulator (APC). The contact probes (heater and MPEC probes) can be applied en face or tangentially in peptic ulcers with major (SRH). Target irrigation, suctioning using therapeutic endoscopes, and tamponade of the bleeding point allow the localization of the ulcer stigma and permit endoscopic treatment. Large diameter probes (3.2 mm) and slow coagulation provide the most effective thermal hemostasis and prevention of rebleeding by coaptive coagulation of the underlying artery in the ulcer base [1, 4]. APC coagulates poorly through blood and provides only superficial coagulation (≤ 1 mm unless it touches the mucosa and becomes a monopolar coagulator) which is ineffective for the treatment of larger underlying vessels [1].
Injection techniques use epinephrine (usually 1:10,000 or 1:20,000 in saline), sclerosants, or clotting factors (not available in the USA) and are the most frequently used technique in non-USA countries. either alone or in combination with thermal or mechanical for emergency hemostasis. Mechanical techniques such as hemoclips may provide hemostasis by grasping underlying vessels and can be used to close acute lesions that are bleeding and accelerate their healing.
ENDOSCOPIC THERAPY
INJECTION TREATMENT
Injection therapy for ulcer bleeding has been advocated because it is easy to use, inexpensive, widely available and many endoscopists have had prior experience sclerosing esophageal varices [1, 4].
Epinephrine injection 1:10,000 to 1:20,000 in saline, provides local tamponade, vasoconstriction, and improved platelet aggregation to promote hemostasis. Saline injection alone, causes local vessel compression or tamponade. Sclerosants such as alcohol, ethanolamine, and polidocanol cause tissue necrosis. Alcohol may predispose to ulceration, hemorrhage and possible perforation. Tissue adhesives such as thrombin, fibrin glue and N-butyl-2-cyanoacrylate, have also been used for the therapy of bleeding ulcers, although less frequently in the United States than in Europe and Asia. Human-derived thrombin is available in the U.S., fibrin glue is also available but not labeled for endoscopic use, and cyanoacrylate is not commercially available for endoscopic use in the U.S. The tissue adhesives have been not been evaluated as extensively as diluteepinephrine and the sclerosants for bleeding ulcers, and are more difficult to inject and expensive (10). These agents are not commonly used in clinical practice with the exception of cyanoacrylate for the eradication of bleeding gastric varices.
The technique involves injection through a sclerotherapy catheter (Figure 1) with a 25-gauge retractable needle in 4 quadrants around actively bleeding point or non-bleeding vessel. Dilute epinephrine/saline solution (1:10,000 – 1:20,000) is injected in 0.5–1.5 ml increments up to a total of 25 – 30 ml. If alcohol is used, 0.1 to 0.2 ml increments are injected up to a maximum of 1 ml. Caution is recommended to avoid tissue damage, necrosis, and perforation with alcohol, and not to exceed 1 ml injection volume. Alcohol injection should not be repeated if rebleeding occurs. Further, alcohol injection should not be combined with other thermal modalities.
Figure 1.
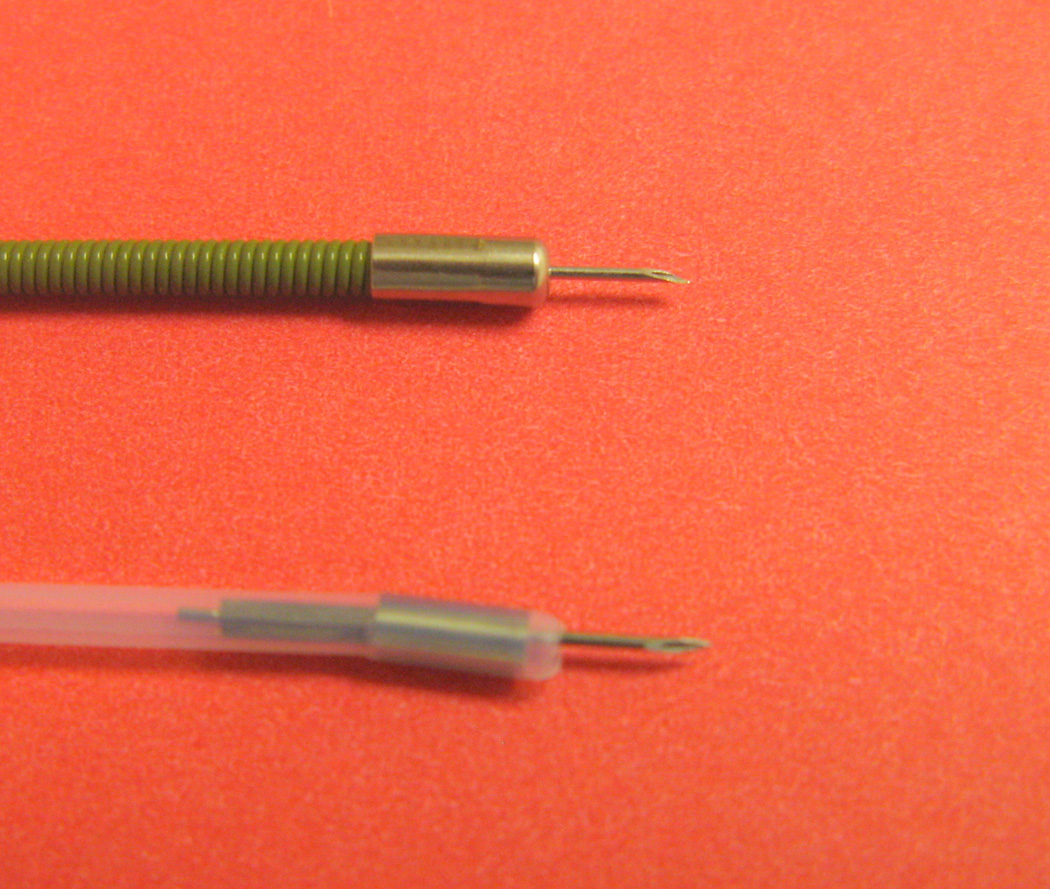
Two different injectors. Top-US Endoscopy. Bottom-American Endoscopy.
This technique is effective for active ulcer bleeding (arterial or oozing) and prevention of NBVV rebleeding. Adding a second endoscopic treatment to epinephrine injection significantly reduces the rate of recurrent bleeding, surgery, and mortality [11]. A Cochrane Database Review confirmed that in patients with bleeding ulcers and major stigmata of hemorrhage, the risk of further bleeding was significantly reduced independent of which second procedure (electrocoagulation, heater probe, or endoclip) was added to injection of epinephrine [12].
ELECTROCOAGULATION
Electrical current from a probe in contact with tissue generates heat which can coagulate tissue, including underlying arteries. In bipolar or multipolar electrocoagulation (MPEC), the current flows between two or more electrodes separated by 1 to 2 mm at the probe tip. Current flow is concentrated closer to the tip than with a monopolar probe, providing less depth of tissue injury and lesser potential for perforation [13].
Coaptive coagulation involves applying a large diameter probe (3.2 mm diameter) directly on the ulcer stigmata or bleeding site to compress the underlying vessel with moderate appositional (tamponade) pressure before coagulation. The pressure on the stigmata temporarily interrupts blood flow through the underlying vessel, reduces the heat sink effect, and with application of heat can coaptively seal arteries up to 2 mm in diameter. Use of low energy (12–16 W on a bipolar coagulation generator), long duration (5–10 seconds) can weld the walls of arteries up to 2 mm in diameter (Table 1). Coaptive coagulation with low power settings and long duration provides deeper coagulation which is especially useful for therapy of large chronic ulcers or large arteries [13]. MPEC coagulation is effective for treatment of actively bleeding ulcers, NBVV or adherent clot and prevention of rebleeding by coaptively coagulating the artery underlying these SRH.
Table 1.
Comparison of Thermal Coagulation vs. Hemoclipping for Nonvariceal UGI Hemorrhage
| Thermal Coagulation | Hemoclipping | |
|---|---|---|
| Ease of emergency use | Easy | Relatively easy |
| Tangential treatment | Easy | More difficult |
| Irrigation with device | Yes | No |
| Different sizes of probes or clips | Yes | Yes |
| Different brands of devices | Yes | Yes |
| Increase tissue injury (Lesion size/depth) |
Yes | No |
| Time to lesion healing | Longer | Shorter |
HEATER PROBE
This probe effectively transfers heat from its end or sides to tissues, allowing heat transfer whether applied perpendicularly or tangentially. The teflon coating of heater probes lessens sticking. The technique involves use of a large (3.2 mm) heater probes and firm tamponade directly on the ulcer SRH to coagulate with a power setting of 25–30 joules per pulse on the SRH in the ulcer base, using 4–5 pulses (total of 125–150 J) per tamponade station (before changing the probe position) [13]. (Table 1). The heater probe is effective for major SRH-active arterial bleeding ulcer, NBVV and adherent clot.
ENDOCLIPS
Several devices including metallic clips, endoloops, and rubber band ligation have been described for the mechanical endoscopic treatment of bleeding ulcers. Endoclips have been the most extensively studied [14]. Clipping devices (Figure 2) are designed to grasp into the submucosa, seal the underlying patent blood vessels, and/or to approximate the sides of lesions during endoscopy, to potentially accelerate lesion healing. The clips can produce hemostasis similar to surgical ligation, if properly applied. They do not cause significant tissue damage and do not interfere with ulcer healing [14].
Figure 2.
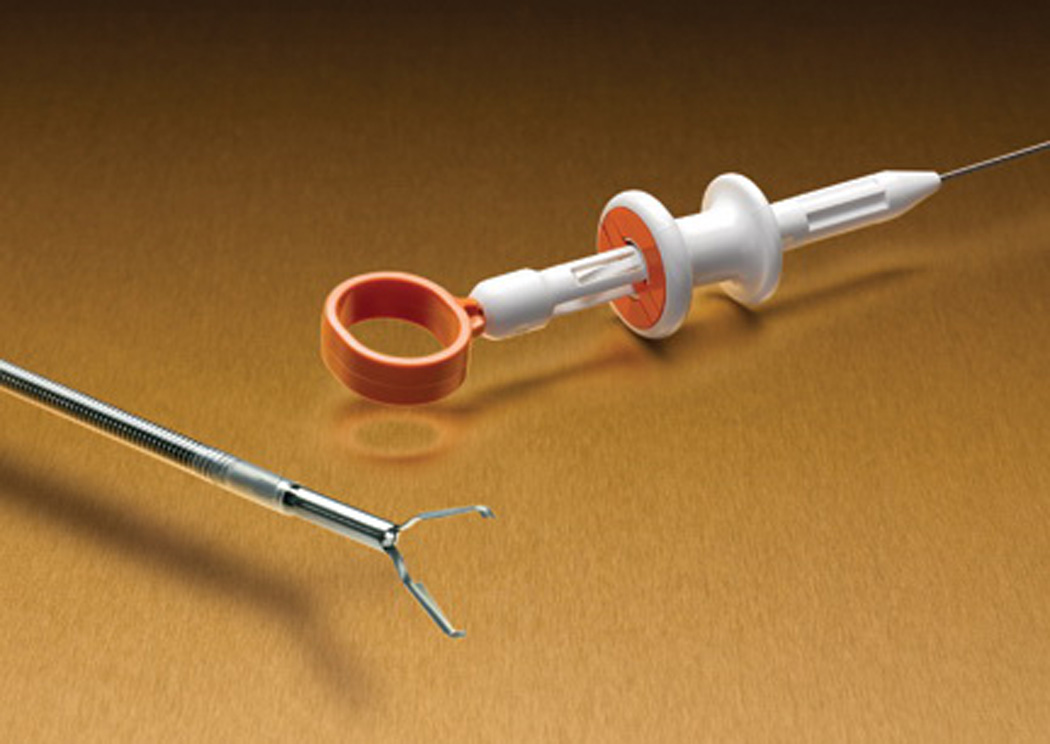
Hemoclip open (Boston Scientific Corp).
Precise deployment to stop the acute bleeding and to occlude the underlying patent artery is critical. En face approach allows optimal capture of the target site and surrounding tissue. A single clip may be sufficient to stop some active bleeding. However, placing 2 additional clips to ligate proximally and distally from the bleeding point to occlude the underlying artery is recommended (Table 1). Endoclips are effective for active arterial bleeding, NBVV and adherent clot [15]. A recent meta-analysis compared the effects of hemoclips (Olympus clips primarily) to epinephrine injection or thermocoagulation (heater probe or electrocoagulation) for bleeding ulcer treatment. Hemoclips significantly improved definitive hemostasis in comparison to injection alone, and were comparable to thermocoagulation [16].
Endoclipping may be limited by the vessel size (> 2 mm in diameter), difficulty in accessing ulcers (such as proximal lesser curve of the stomach, posterior wall gastric body and posterior duodenal bulb), fibrotic lesions and single clip deployment (although multiple clips often needed) [14]. Studies have shown that not all clips are alike (Figure 7). They differ in size, shape, deployment characteristics, ability to grasp and release a bleeding point and to rotate, and in long-term clip retention 17] as well as clinical efficacy [18]. In a chronic canine ulcer model comparing 3 different clips, both hemoclipping time and ulcer healing was similar with all 3 clips, but retention time was significantly prolonged with the Resolution clip (19). In a pilot study evaluating a specific clip brand, the overall hemostasis failure rate was 33%, and the clips were dislodged in 41% at the follow-up endoscopy 24 hours after placement [20]. In another comparative trial, hemoclips were superior to triclips in achieving primary hemostasis in patients with major stigmata of ulcer hemorrhage [18]. All hemoclips appear to be safe and do not cause significant tissue inflammation or injury. Although all commercially available hemoclips are labeled as magnetic resonance imaging (MRI) incompatible, a study in a porcine model suggested that some clips were compatible. Under the experimental conditions, the Resolution Clip, the Quick Clip and the TriClip, all showed physical deflection, but only the TriClip actually detached from pig gastric tissue. An Ethicon Endo-surgery Clip was unaffected and was felt to be compatible with MRI but it is no longer commercially available (21).
Figure 7.
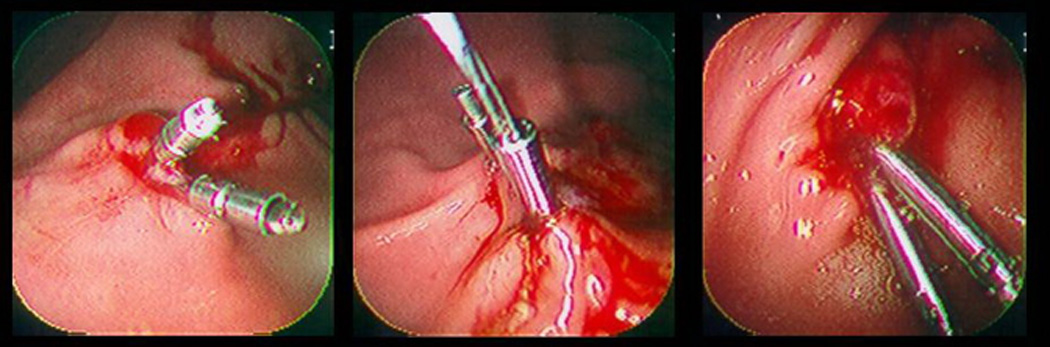
Commercially available hemoclips. Left: Olympus America QuickClip 2; Middle: Cook Endoscopy TriClip; Right: Boston Scientific Resolution Clip.
COMBINATION THERAPY
Combination treatment with epinephrine injection and thermal therapy (multipolar or heater probe) (Figure 3) or endoclips has theoretical advantages since each technique has different mechanisms of action for hemostasis. Combination therapy combines the mechanism of action of each hemostasis technique, providing a potential, beneficial additive effect. Both epinephrine injection and thermal devices activate platelet coagulation and produce tamponade of the underlying vessel. Epinephrine also produces vessel constriction and thermal probes cause coaptive coagulation. Endoclips cause vessel ligation, and can be used to close lesions [1]. The technique involves dilute epinephrine injection into four quadrants around stigmata in the ulcer base followed by thermal coagulation with heater probe or multipolar probe, or deployment of endoclips. Combination therapy has become the standard treatment for actively bleeding ulcers and non-bleeding adherent clot. A recent meta-analysis compared combination therapy (epinephrine injection plus other injection or thermal or mechanical method) to monotherapy (injection, thermal or mechanical alone) in high risk bleeding ulcer patients. The authors reported that dual therapy achieved significantly better outcomes than epinephrine injection alone, but was not significantly superior to thermal or hemoclips [22].
Figure 3.
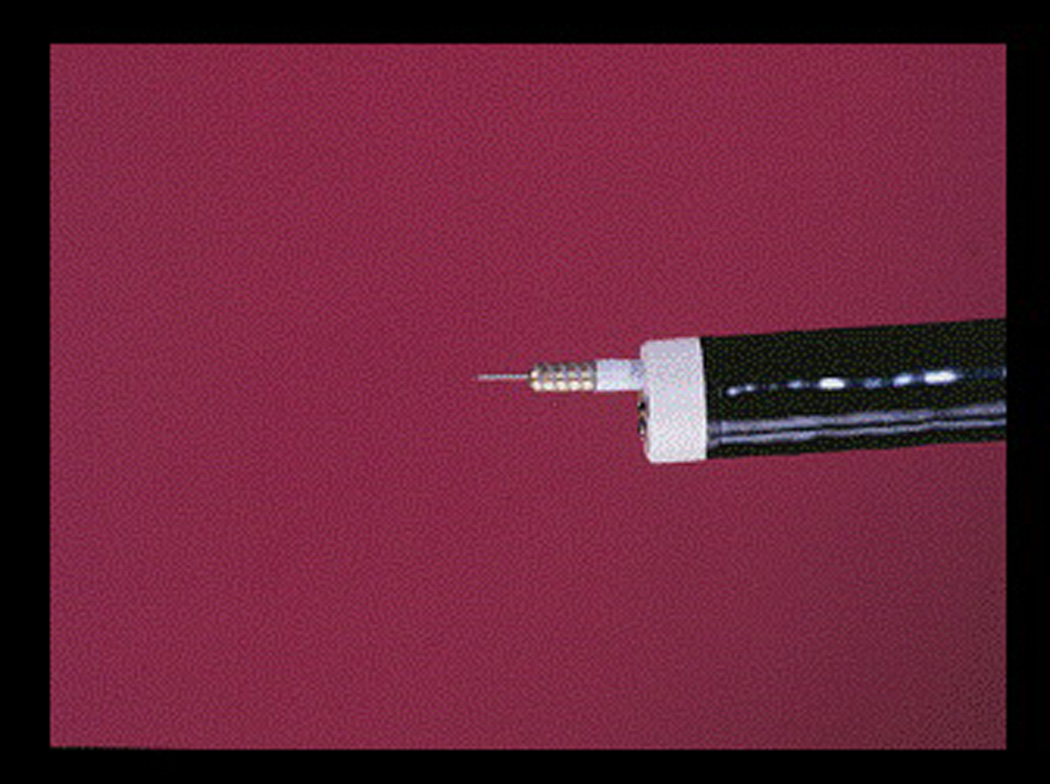
Injector – MPEC probe (Boston Scientific Corp)
RESULTS OF ENDOSCOPIC THERAPY BASED ON META-ANALYSES OF CONTROLLED TRIALS
Five recent meta-analyses have evaluated the results of controlled trials of endoscopic therapy for patients with high-risk endoscopic stigmata on bleeding ulcers. All the studies with hemoclips were reported for older Olympus hemoclips and did not include other, newer hemoclips for which better results have been reported in prospective studies. Marmo and colleagues showed that dual treatment (epinephrine injection plus other injection or thermal or hemoclips) significantly decreased the rebleeding rates and need for surgery in comparison to monotherapy ( injection, thermal, or hemoclips). Subgroup analysis revealed that combination therapy significantly reduced the rates of rebleeding, surgery and mortality compared to injection treatment alone. However, dual therapy did not improve outcomes compared to single treatment with either thermal coagulation or hemoclips (22).
Sung et al reported that hemoclip use with or without injection significantly increased the rate of definitive hemostasis and decreased rebleeding and need for surgery compared to injection alone. Clipping did not improve outcomes such as rebleeding, surgery or death rates compared to thermal therapy (16). Yuan et al described similar findings when comparing clipping alone to other hemostasis techniques such as injection or thermal therapy alone or combination treatment with injection and thermal therapy. In this review, clipping did not improve the rates of initial hemostasis, rebleeding, emergency surgery and mortality compared to other endoscopic modalities (23).
Another meta-analysis confirmed that epinephrine injection should not be used alone, and that several techniques such as thermal modalities, clips and injection of sclerosants, fibrin glue and thrombin were effective endoscopic therapies. Although limited, the data also suggested that epinephrine injection prior to other endoscopic treatments may benefit patients with an actively spurting ulcer (24).
Other authors, based on different studies, concluded that combination treatment may benefit patients more than thermal therapy alone, and that clipping was superior to injection or thermal therapy alone (25).
RECOMMENDATIONS FOR ENDOSCOPIC THERAPY BASED ON STIGMATA OF ULCER HEMORRHAGE
Active Arterial Ulcer Bleeding
Combination therapy with epinephrine injection (1:10,000 or 1:20,000 in saline) and thermal coagulation (multipolar or heater probe) (Figure 8) or hemoclipping is recommended. Coaptive coagulation is the goal with thermal therapies. Combination therapy with epinephrine and hemoclipping is another alternative (13,26,27,28). Successful endoscopic hemostasis occurs in nearly 100% of lesions. Rebleeding occurs in 10–30% (higher with severe comorbidities) compared to continued bleeding or rebleeding rated of 85–95% on medical therapy [1,13, 26].
Figure 8.
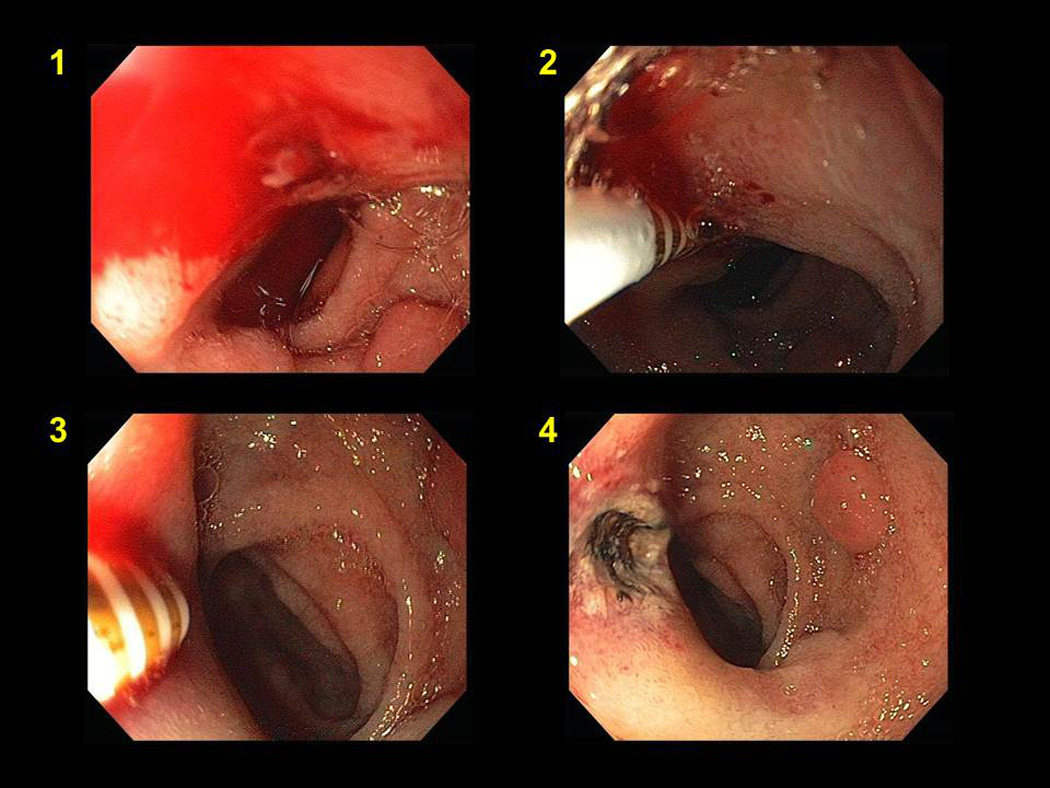
Combined epinephrine injection (panel #2) and multipolar coagulation (panel #3) therapy of a bleeding duodenal ulcer (panel #1). Panel #4 shows appearance after hemostasis.
Ulcer Oozing Without Other Stigmata of Hemorrhage
If oozing from an ulcer base persists despite irrigation and observation, any monotherapy (thermal probes or hemoclipping) is effective. Rebleeding rates are less than 5% compared to rebleeding rates varying from 10% to 27% on medical therapy alone [1,4,26].
Non-Bleeding Visible Vessel (NBVV)
Monotherapy with thermal coagulation (heater or multipolar probe) is effective if coaptive coagulation is done. With large diameter probes (3.2 mm in diameter), firm tamponade, slow coagulation with low power setting to flatten the visible vessel and coagulat the underlying artery, rebleeding rates are less than 5–20% compared to 50% rebleeding rate with medical therapy alone [13,26]. Hemostasis of NBVV with hemoclips provides similar beneficial outcomes as thermal therapy for such patients with ulcers (16, 25, 26).
Adherent Non-Bleeding Clot
Combination therapy including:
four-quadrant dilute epinephrine injection close to the attachment of the clot, in the ulcer base
a rotatable polypectomy snare to shave down the clot using a cold-guillotine technique, without monopolar coagulation
thermal coaptive coagulation or hemoclipping to treat the residual clot or NBVV (Figure 9 and 10).
Figure 9.
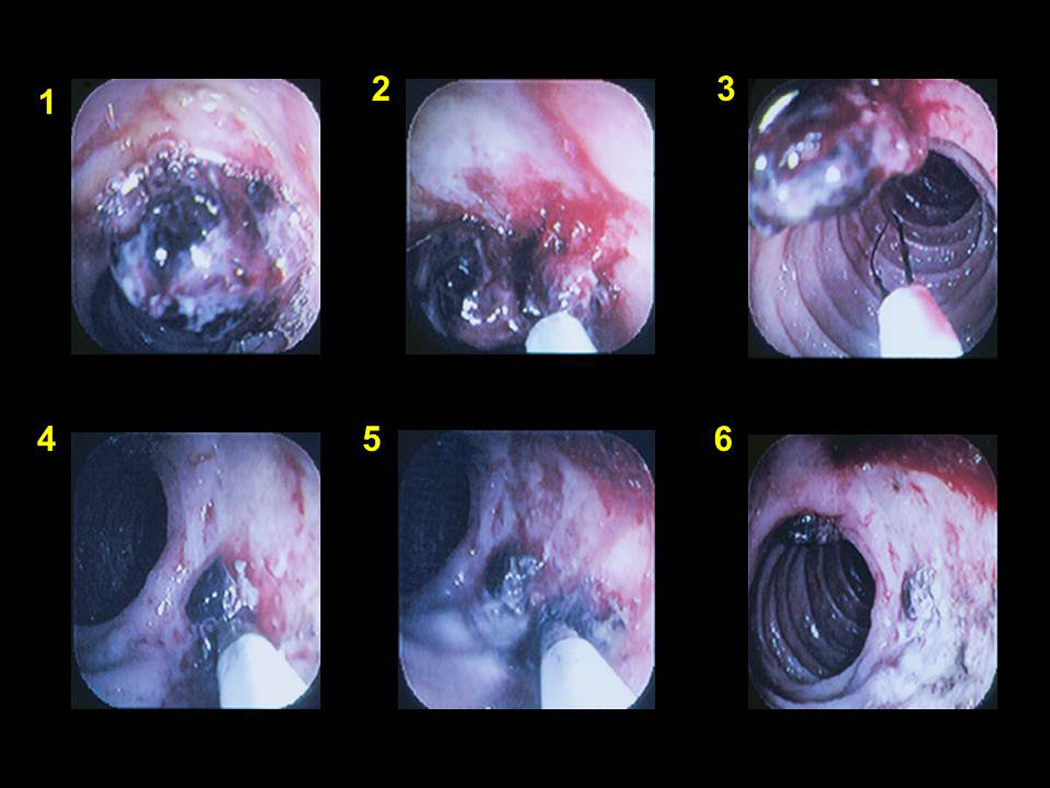
Combination therapy of a clot over a duodenal ulcer (panel #1) with epinephrine injection (panel #2), cold guillotining (panel # 3) and multipolar coagulation (panel #4/5). Panel #6 shows appearance after treatment.
Figure 10.
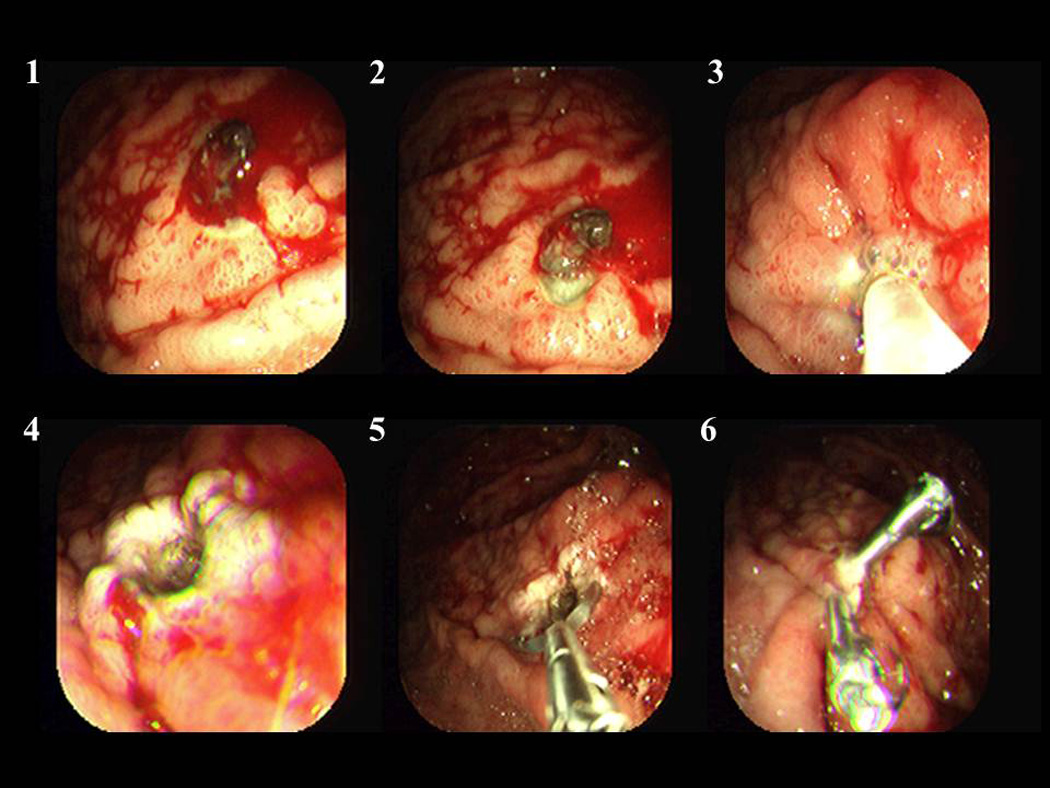
Combination therapy of a clot over a gastric ulcer (panel #1–2) with epinephrine injection, cold guillotining, multipolar coagulation (panel #3) and hemoclips (panel #5/6).
The rebleeding rate of patients after combination therapy in a CURE trial for adherent non-bleeding clots was less than 5% compared to 35% rebleeding rate with medical therapy alone [29]. A recent meta-analysis confirmed the benefit of endoscopic combination therapy for adherent clot overlying an ulcer [30].
Flat Spots or Clean Based Ulcers
No benefit from endoscopic hemostasis, since patients with these endoscopic findings have a very low rebleeding rate – 7% and 3 % respectively on medical therapy alone (26). The exception is large ulcers with both a flat spot and another SRH such as a clot or NBVV. In DUP studies of such patients with 2 SRH in large ulcers, arterial blood flow beneath the 2 SRH and between them was detected in 63% of such patients and to prevent rebleeding, both SRH and between the SRH in the artery, required endoscopic treatment to significantly reduce the rebleeding risk.
RETREATMENT
Rebleeding after endoscopic therapy of UGI ulcers occurs in 10–25% of patients and represents a challenging problem [31]. One large randomized trial showed a significant reduction in complication rates in patients re-treated endoscopically with epinephrine injection and heater probe compared with emergency surgery. These results together with our own experience, suggest that either use of DUP to ascertain obliteration of blood flow under SRH or repeat endoscopic therapy after clinical rebleeding is warranted for rebleeding after initial hemostasis for ulcer hemorrhage. One potential limitation of repeat endoscopic coagulation is the increased complication rate attributed to repeat treatments. Meta-analyses suggested that about half of the perforations associated with heater probe use occurred in patients undergoing retreatment (24). It may be that any thermal coagulation technique that induces tissue injury is more likely to cause a complication with repetitive use. Endoscopic combination therapy for retreatment of ulcer bleeding is recommended. The use of clips, which do not produce significant tissue damage, may provide an additional safety feature as part of this combination retreatment
Other UGI Nonvariceal Focal Lesions
The same principles of endoscopic techniques as described for ulcers can be applied to other focal lesions with SRH such as Mallory-Weiss tears or Dieulafoy’s lesion. We favor hemoclipping now because no significant tissue injury occurs (unlike thermal coagulation) and the efficacy of newer hemoclips is very good. Retreatment is also safe and effective if rebleeding occurs.
Second Look Endoscopy
Since the risk of peptic ulcer rebleeding after primary hemostasis ranges from 10–25%, some endoscopists routinely schedule a follow-up endoscopy the day after endoscopic coagulation.
However, in the future this may prove unnecessary if DUP is used to confirm that endoscopic hemostasis successfully obliterates the underlying blood flow.
The first meta-analysis of four randomized trials showed that second-look endoscopy produced a 6.2% absolute risk reduction in ulcer rebleeding (32). There was no significant benefit on rates of surgery or mortality. These trials used epinephrine injection alone as the endoscopic therapy (not considered adequate anymore for major ulcer SRH), and predated high-dose proton pump inhibitor infusion treatment. A second meta-analysis showed that endoscopic retreatment with injection alone did not reduce ulcer rebleeding after second-look endoscopy compared to single endoscopy. However, retreatment with heater probe during second-look endoscopy significantly decreased rebleeding compared to single endoscopy, (4.2% vs. 15.7%) (33). There was no significant benefit in need for transfusion or surgery, or length of hospital stay or mortality rates. The authors did not recommend routine second-look endoscopy due to the limited benefit and substantial cost from second-look endoscopy. Recent guidelines from an international panel do not recommend routine second-look endoscopy, unless there is a high risk ulcer (such as active bleeding, large ulcer, ulcer on high gastric lesser curve or posterior duodenal bulb) or if endoscopy was incomplete. (26). High dose intravenous PPI infusion for 72 hours after successful primary endoscopic hemostasis appears to be the treatment of choice.
ENDOSCOPIC HEMOSTASIS COMPLICATIONS
Potential complications include perforation or precipitation of bleeding from a NBVV. In a meta-analysis comparing controlled trials of endoscopic hemostasis with no endoscopic therapy, pooled rates for complications associated with endoscopic treatment were 0.8% (24). Clips and epinephrine had the lowest complication rates. In a meta-analysis evaluating various endoscopic modalities, the induced bleeding rate was similar for either monotherapy or combination therapy. However, perforations occurred significantly more frequently in patients receiving combination treatment, such as injection plus thermal coagulation or dual injection (epinephrine followed by a sclerosant) than single therapy (22). Perforations were more frequent after endoscopic retreatment with thermal coagulation[1, 24].
INITIAL MEDICAL MANAGEMENT AFTER SUCCESSFUL ENDOSCOPIC HEMOSTSIS OF MAJOR SRH
Several randomized controlled trials have demonstrated the efficacy of high-dose PPI infusion for 72 hours after successful endoscopic therapy of patients with bleeding ulcers and high-risk stigmata of hemorrhage (34, 35). Lau and coworkers showed that after primary hemostasis had been achieved by endoscopic coagulation, high dose omeprazole infusion reduced the rate of rebleeding, transfusion requirements and duration of hospitalization (35). Sung and colleagues reported similar prevention of recurrent bleeding in ulcer patients with NBVV and adherent clots with combination endoscopic therapy and omeprazole infusion compared to omeprazole infusion alone (36). These studies illustrated the benefits of IV PPI infusion after endoscopic hemostasis, but not as a stand-alone therapy. More recently, several reviews and meta-analyses of PPI use in peptic ulcer bleeding confirm that PPIs reduce rebleeding, surgery, transfusion requirements and duration of hospitalization without decreasing mortality (37, 38) .
After the initial bleed is treated endoscopically and hemostasis achieved, medical management is recommended with oral PPIs for 6–8 weeks, unless the patient is also Helicobacter pylori positive, requires low dose aspirin maintenance or uses a non-selective NSAID. H. pylori positive patients should receive eradication therapy and should be retested to document H. pylori eradication 6–10 weeks after completion of antibiotics. Patients needing long-term aspirin or NSAIDs should receive PPI maintenance treatment to indefinitely reduce ulcer recurrence [1, 4]. Patients with bleeding ulcers who require chronic aspirin or other antiplatelet medications for cardiovascular and cerebrovascular prophylaxis are a difficult problem. A recent study in patients with bleeding ulcers who were on low-dose aspirin compared the outcomes after either stopping the aspirin (80 mg daily) for 8 weeks or resuming aspirin while on proton pump inhibitors (PPIs). The authors reported that early (before 30 days) aspirin resumption increased rebleeding but decreased cardiovascular and cerebrovasular-related mortality. (39). After an ulcer bleed, we suggest resuming aspirin or other antiplatelet agents within 5–7 days while on PPIs.
CONCLUSION
UGI bleeding secondary to ulcer hemorrhage is a frequent cause of hospitalization and inpatient bleeding, resulting in substantial patient morbidity and mortality. Randomized controlled trials and meta-analyses show that PPIs improve clinical outcomes in patients with ulcer hemorrhage, after successful endoscopic hemostasis of high risk SRH. Patients with high-risk endoscopic stigmata should receive high-dose IV PPI after successful endoscopic treatment. Patients with low-risk endoscopic stigmata should receive oral PPI at twice the usual clinical dose. For patients with ulcers that have major stigmata of ulcer hemorrhage – active arterial bleeding, NBVV, and adherent clot – combination therapy with epinephrine injection and either thermal coagulation (multipolar or heater probe) or endoclips is recommended. Patients with minor stigmata or clean-based ulcers usually do not benefit from endoscopic hemostasis and should be triaged to less intensive care and be considered for early discharge. Doppler ultrasound probe for detection of blood flow under SRH seen on endoscopy has been reported to change risk stratification for rebleeding and to determine whether endoscopic hemostasis is complete.
Contributor Information
Thomas O.G. Kovacs, CURE Digestive Diseases Research Center, David Geffen School of Medicine at UCLA, Ronald Reagan Medical Center, and VA Greater Los Angeles Healthcare System, Los Angeles, CA
Dennis M. Jensen, CURE Digestive Diseases Research Center, David Geffen School of Medicine at UCLA, Ronald Reagan Medical Center, and VA Greater Los Angeles Healthcare System, Los Angeles, CA
References
- 1.Kovacs TOG, Jensen DM. Recent advances in the endoscopic diagnosis and therapy of upper gastrointestinal, small intestinal, and colonic bleeding. Med Clin N Am. 2002;86:1319–1356. doi: 10.1016/s0025-7125(02)00079-2. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs TOG. Mallory-Weiss Tears, Angiodysplasia, Watermelon Stomach and Dieulafoy’s: A Potpourri. Tech Gastrointest Endosc. 2005;7:139–147. [Google Scholar]
- 3.Winstead NS, Wilcox CM. Erythromycin prior to endoscopy for acute upper gastrointestinal hemorrhage: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2007;26:1371–1377. doi: 10.1111/j.1365-2036.2007.03516.x. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs TOG, Jensen DM. Endoscopic Treatment of Peptic Ulcer Bleeding. Curr Treat Opt Gastroenterol. 2007;10:143–148. doi: 10.1007/s11938-007-0066-3. [DOI] [PubMed] [Google Scholar]
- 5.Wong RCK. Non-variceal upper gastroitestinal hemorrhage: Probing beneath the surface. Gastroenterology. 2009;137:1897–1902. doi: 10.1053/j.gastro.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Wong RCK. Endoscopic Doppler US probe for acute peptic ulcer hemorrhage. Gastrointest Endosc. 2004;57:557–560. doi: 10.1016/s0016-5107(04)02046-2. [DOI] [PubMed] [Google Scholar]
- 7.Jensen DM, Ohning GV, Singh B, et al. For severe UGI hemorrhage Doppler ultrasound probe is more accurate and helpful for complete endoscopic hemostasis than lesion stigmata alone (abstract) Gastrointest Endosc. 2008;67:AB81. (abs #264) [Google Scholar]
- 8.Chen V, Wong RCK. Endoscopic Doppler Ultrasound versus Endoscopic Stigmata – directed management of acute peptic ulcer hemorrhage: a multimodel cost analysis. Dig Dis Sciences. 2007;52:149–160. doi: 10.1007/s10620-006-9506-5. [DOI] [PubMed] [Google Scholar]
- 9.Cipolletta L, Bianco MA, Salerno R, et al. Improved characterization of visible vessels in bleeding ulcers by using magnification endoscopy: results of a pilot study. Gastrointest Endosc. 2010;72:413–418. doi: 10.1016/j.gie.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Park WG, Yeh RW, Triadafilopoulos G. Injection therapies for nonvariceal bleeding disorders. Gastrointest Endosc. 2007;66:343–354. doi: 10.1016/j.gie.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Calvet X, Vergara M, Brullet E, et al. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology. 2004;126:441–450. doi: 10.1053/j.gastro.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Vergara M, Calvet X, Gisbert JP. Cochrane Database Syst Rev. 2007 Apr 18;:CD005584. doi: 10.1002/14651858.CD005584.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Jensen DM, Machicado GA. Endoscopic hemostasis of ulcer hemorrhage with injection, thermal, and combination methods. Tech Gastrointest Endosc. 2005;7:124–131. [Google Scholar]
- 14.Technology Assessment Committee. Technology status evaluation report: Endoscopic clip application devices. Gastrointest Endosc. 2006:746–750. doi: 10.1016/j.gie.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 15.Saltzman JR, Strata LL, Di Sena V, et al. Prospective trial of endoscopic clips versus combination therapy in upper GI bleeding. Am J Gastroenterol. 2005;97:1503–1508. doi: 10.1111/j.1572-0241.2005.41561.x. [DOI] [PubMed] [Google Scholar]
- 16.Sung JJ, Tsai KK, Lai LH, Wu JC, Lan JY. Endoscopic clipping versus injection and thermo-coagulation in the treatment of non-variceal upper gastrointestinal bleeding: a meta-analysis. Gut. 2007;56:1364–1373. doi: 10.1136/gut.2007.123976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen DM, Machicado GA, Hirabayashi K. Randomized controlled study of three different types of hemoclips for hemostasis of bleeding canine acute gastric ulcers. Gastrointest Endosc. 2006;64:768–773. doi: 10.1016/j.gie.2006.06.031. [DOI] [PubMed] [Google Scholar]
- 18.Lin H-J, Lo WC, Cheng Y-C, Peing C-L. Endoscopic hemoclip versus triclip placement in patients with high-risk peptic ulcer bleeding. Am J Gastroenterol. 2007;102:539–543. doi: 10.1111/j.1572-0241.2006.00962.x. [DOI] [PubMed] [Google Scholar]
- 19.Jensen DM, Machicado GA. Hemoclipping of chronic canine ulcers: a randomized prospective study of initial deployment success, clip retention rates and ulcer healing. Gastrointest Endosc. 2009;70:969–975. doi: 10.1016/j.gie.2009.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan CY, Yan KK, Siu WT, et al. Endoscopic hemostasis by using the Triclip for peptic ulcer hemorrhage: a pilot study. Gastrointest Endosc. 2008;67:35–39. doi: 10.1016/j.gie.2007.05.042. [DOI] [PubMed] [Google Scholar]
- 21.Gill KRS, Pooley RA, Wallace MB. Magnetic resonance imaging compatibility of endoclips. Gastrointest Endosc. 2009;70:532–536. doi: 10.1016/j.gie.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Marmo R, Rotondano G, Piscopo R, et al. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol. 2007;102:279–289. doi: 10.1111/j.1572-0241.2006.01023.x. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y, Wang C, Hunt RH. Endoscopic clipping for acute nonvariceal upper-gi bleeding: a meta-analysis and critical appraisal of randomized controlled trials. Gastrointest Endosc. 2008;68:339–351. doi: 10.1016/j.gie.2008.03.1122. [DOI] [PubMed] [Google Scholar]
- 24.Laine L, McQuaid R. Endoscopic therapy for bleeding ulcers: an evidence based approach based on meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2009;7:33–47. doi: 10.1016/j.cgh.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Barkun AN, Martel M, Toubouti Y, et al. Endoscopic hemostasis in peptic ulcer bleeding for patients with high-risk lesions: a series of meta-analyses. Gastrointest Endosc. 2009;69:786–799. doi: 10.1016/j.gie.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with non-variceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101–113. doi: 10.7326/0003-4819-152-2-201001190-00009. [DOI] [PubMed] [Google Scholar]
- 27.Park C-H, Joo Y-E, Kim H-S, et al. A prospective, randomized trial comparing mechanical methods of hemostasis plus epinephrine injection to epinephrine injection alone for bleeding peptic ulcer. Gastrointest Endosc. 2004;60:173–179. doi: 10.1016/s0016-5107(04)01570-6. [DOI] [PubMed] [Google Scholar]
- 28.Lo CC, Hsu PI, Lo GH, et al. Comparison of hemostatic efficacy for epinephrine injection alone and injection combined with hemoclip therapy in treating high-risk bleeding ulcers. Gastrointest Endosc. 2006;63:767–773. doi: 10.1016/j.gie.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 29.Jensen DM, Kovacs TOG, Jutabha R, et al. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology. 2002;123:407–413. doi: 10.1053/gast.2002.34782. [DOI] [PubMed] [Google Scholar]
- 30.Kahi CJ, Jensen DM, Sung JJY, et al. Endoscopic therapy versus medical therapy for bleeding peptic ulcer with adherent clot: a meta-analysis. Gastroenterology. 2005;129:855–862. doi: 10.1053/j.gastro.2005.06.070. [DOI] [PubMed] [Google Scholar]
- 31.Lau JJ, Sung JJY, Lam Y, et al. Endoscopic retreatment compared with surgery in patients with recurrent bleeding after initial endoscopic control of bleeding ulcers. N Engl J Med. 1999;340:751–756. doi: 10.1056/NEJM199903113401002. [DOI] [PubMed] [Google Scholar]
- 32.Marmo R, Rotondano G, Blanco MA, et al. Outcome of endoscopic treatment for peptic ulcer bleeding: is a second look necessary? A meta-analysis. Gastrointest Endosc. 2003;57:62–67. doi: 10.1067/mge.2003.48. [DOI] [PubMed] [Google Scholar]
- 33.Tsoi KKF, Chan HCH, Chiu PWY, et al. Second-look endoscopy with thermal coagulation injection for peptic ulcer bleeding: A meta-analysis. J Gastroenterol Hepatol. 2010;25:8–13. doi: 10.1111/j.1440-1746.2009.06129.x. [DOI] [PubMed] [Google Scholar]
- 34.Lin HJ, Lo WC, Cheng YC, et al. Role of intravenous omeprazole in patients with high- risk peptic ulcer bleeding after successful endoscopic epinephrine injection: a prospective, randomized, comparative trial. Am J Gastroenterol. 2006;101:500–505. doi: 10.1111/j.1572-0241.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 35.Lau JY, Sung JJ, Lee KK, et al. Effects of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000;343:310–316. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 36.Sung JJY, Chan FKL, Lau JYW. The effect of endoscopic therapy in patients receiving omeprazole for bleeding ulcers with non-bleeding visible vessels or adherent clots. Ann Intern Med. 2003;139:237–243. doi: 10.7326/0003-4819-139-4-200308190-00005. [DOI] [PubMed] [Google Scholar]
- 37.Andriulli A, Annese V, Caruso N, et al. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcers: a series of meta-analyses. Am J Gastroenterol. 2005;100:207–219. doi: 10.1111/j.1572-0241.2005.40636.x. [DOI] [PubMed] [Google Scholar]
- 38.Leontiadis GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2006;1:CD002094. doi: 10.1002/14651858.CD002094.pub3. [DOI] [PubMed] [Google Scholar]
- 39.Sung JJ, Lau JY, Chung JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1–9. doi: 10.7326/0003-4819-152-1-201001050-00179. [DOI] [PubMed] [Google Scholar]


