Abstract
The receptor binding domains of the most potent mucosal adjuvants, bacterial toxins and plant lectins, are organized in repeat units to recognize specific sugar residues. The lectin-like structure of the C-terminal region of Clostridium difficile toxin A prompted us to investigate the mucosal adjuvant properties of a nontoxigenic peptide corresponding to amino acids 2394 to 2706 (TxAC314). We compared TxAC314 adjuvant activity to those of cholera toxin (CT) and Escherichia coli heat-labile enterotoxin subunit B (EtxB) coadministered orally or nasotracheally with poor peptide antigens (keyhole limpet hemocyanin [KLH] and hen egg lysozyme [HEL]). Levels of anti-KLH-specific serum immunoglobulin G (IgG) and IgA as well as that of mucosal IgA were significantly higher in animals immunized orally with TxAC314 plus KLH than with KLH alone, CT plus KLH, or EtxB plus KLH. Following intranasal immunization with TxAC314 plus HEL, levels of serum- and mucosa-specific antibodies were comparable to those induced by coadministering HEL with CT or EtxB. The TxAC314 adjuvant effect following oral, but not intranasal, immunization was dose dependent. The analysis of the subclasses of anti-KLH-specific IgG isotypes and the cytokines released from splenocytes of immunized mice challenged in vitro with KLH indicates the induction of a mixed Th1/Th2-type immune response, with prevalence of the Th1 branch. We conclude that TxAC314 enhances immune responses against mucosa-coadministered foreign antigens and represents a promising mucosal adjuvant, especially because its ability to stimulate mixed Th1/Th2 responses with a strong a Th1 component is extremely worthwhile against intracellular pathogens.
The vast majority of human and animal pathogens colonize and invade the host through mucosal surfaces. Therefore the induction of strong and persistent immune responses in these districts represents an attractive and rational approach to developing protective vaccines. Antigen administration through systemic routes generally stimulates sustained systemic immunity, but it does not guarantee effective mucosal immune responses (31). Indeed, following mucosal administration of nonreplicating antigens, local immune responses are quite weak and short-lived. The physicochemical barriers at the mucosal surfaces that impair antigen transport across the epithelium, preventing a direct interaction with immune cells, and the low reactivity of the mucosal immune system are the leading causes of weak responses triggered by mucosal vaccines (36). Thus, a variety of strategies including live bacterial vectors, biodegradable microparticles, liposomes, and mucosal adjuvants are under investigation to enhance the responses to mucosally delivered antigens (48, 55).
A few molecules are highly immunogenic when delivered to the mucosal immune system; indeed, some of these proteins are also able to enhance the immune response against coadministered antigens. Most of these highly immunogenic molecules are proteins or glycoproteins that share an interesting structural feature: all present lectin-like properties. These molecules possess a modular organization with at least one noncatalytic domain that reversibly binds to a specific mono- or oligosaccharide (47, 48). For example, the most powerful mucosal antigens/adjuvants identified to date, cholera toxin (CT) secreted by Vibrio cholerae and Escherichia coli heat-labile enterotoxin (LT), feature a lectin-like structure with five modules (B subunit) that bind to oligosaccharide residues on membrane receptors (1, 46). In keeping with this view, it has recently been reported that plant lectins, also showing a modular structure, such as mistletoe and Lycospersicum esculentum lectins, are potent mucosal immunogens and enhance the immune response to coadministered antigens (25, 50).
Clostridium difficile, the causative agent of hospital-acquired diarrhea, produces two large exotoxins, toxin A and toxin B (4). Both toxins have intracellular targets, as they specifically glucosylate Rho, Rac, and Cdc42 at threonine 37 using UDP-glucose as the cosubstrate, causing changes in cell morphology and physiology (21). Injection of purified toxin A into closed intestinal loops elicits an acute inflammatory diarrhea with massive fluid secretion, mucosal damage, and a prominent neutrophil infiltration driven by the release of proinflammatory cytokines (4). On the basis of its amino acid sequence, C. difficile toxin A can be roughly divided into enzymatic, translocation, and binding domains (8). Indeed the N terminus of the toxin includes a putative nucleotide binding domain and bears the catalytic activity, whereas the middle part of the molecule has a small hydrophobic region, assumed to be involved in membrane translocation (13). The carboxy terminus of toxin A is characterized by 38 continuous repeat units, occupying 853 amino acid (aa) residues out of a total of 2,710 aa (40). This region is the putative receptor binding domain of the molecule for it specifically recognizes a trisaccharide moiety and causes rabbit erythrocyte agglutination. Moreover, antibodies directed against this portion of the molecule neutralize toxin A toxicity both in vitro and in vivo (28, 30).
The paucity of adjuvants for human mucosal vaccines, because the toxicity of CT and LT limits their clinical application (38), prompted us to investigate whether the nontoxic receptor binding domain of C. difficile toxin A displayed any adjuvant activity against antigens coadministered via the oral or nasotracheal route. The hypothesis behind this study was that the carboxyl-terminal region of toxin A, consisting of identical repeat units, presents a striking structural homology with the most powerful mucosal adjuvants known. To test our hypothesis, we measured the immune responses to poor mucosal antigens (keyhole limpet hemocyanin [KLH] and hen egg lysozyme [HEL]) coadministered to mice, by the oral and nasotracheal routes, with a recombinant nontoxigenic fragment of toxin A (TxAC314).
MATERIALS AND METHODS
Cloning, expression, and purification of recombinant toxin A fragment. Genomic DNA was extracted by standard procedures from C. difficile strain VPI 10463 (American Type Culture Collection, Manassas, Va.) cultured under anaerobic conditions for 48 h in brain heart infusion broth (Gibco). A 942-bp DNA fragment encoding the carboxyl-terminus region of toxin A (bp 7338 to 8280, inclusive) was amplified by PCR using a proofreading thermostable polymerase (Vent polymerase; New England Laboratories, Beverly, Mass.). Primers were based on the published sequence from strain VPI 10463 (GenBank accession no. M30307). A 5′ sense primer for the 27-bp coding sequence of the 9-aa epitope of the hemagglutinin of influenza virus type A (HA) was synthesized to fuse the epitope coding sequence in frame with that for toxin A. The PCR amplification (35 cycles) involved melting the DNA strand at 94°C for 20 s, annealing at 64°C for 30 s, and extension at 72°C for 30 s. The amplified toxin A sequence was ligated into the pGEM easy vector (Promega) to generate the plasmid pGEM/TxAC314. For expression of the TxAC314 recombinant protein, the cloned toxin A coding sequence was excised with BamHI-EcoRI restriction sites engineered in the PCR primers and was inserted into similarly digested pGEX (Pharmacia). The coding sequence for the TxAC314 fragment was fused in frame with the glutathione S-transferase (GST) binding protein-encoding sequence to generate the construct named pGEX/TxAC314. Integrity of all recombinant constructs was verified by sequence analysis.
To express the recombinant proteins in E. coli, recombinant constructs were transformed into E. coli BL21(DE3). Small-scale (20-ml) and large-scale (1,000-ml) cultures of the recombinant clone were grown in Luria-Bertani medium containing ampicillin to reach an optical density at 600 nm of 0.9 to 1.0. The expression of the plasmid-encoded recombinant protein was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. Cultures were harvested by centrifugation (5,000 × g for 15 min) after 1 to 16 h of incubation. Preliminary experiments using small-scale cultures showed that maximal expression of the recombinant protein was achieved after 4 to 6 h of incubation following IPTG addition. Cells were placed in phosphate-buffered saline (PBS) containing 0.1% Triton X-100 and then lysed with three 45-s bursts of ultrasonic power. Cellular debris was removed by centrifugation (12,000 × g for 15 min), and the soluble fractions were recovered and sterilely filtered through 0.2-μm-pore-size filters (Amicon).
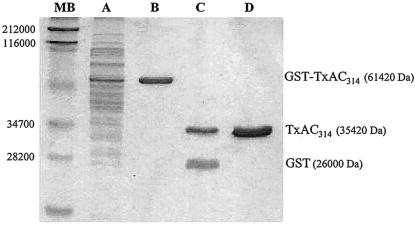
The recombinant toxin A fragments were purified by affinity chromatography using a glutathione-Sepharose 4B resin (Pharmacia Biotech.). Cellular extracts containing the GST fusion proteins were chromatographed in buffer (50 mM Tris containing 1% Triton X-100 and 0.3 M NaCl) to allow binding to the resin, and, following extensive washing (10 resin bed volumes), the recombinant toxin fragments were removed by overnight digestion with thrombin (1 U, 20-μl bed volume). The integrity, purity, and concentrations of the recombinant proteins were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis and a micro-Bradford test using the commercial Bio-Rad protein assay (Fig. 1). After a step of affinity chromatography using an endotoxin-removing gel (Pierce), the levels of endotoxin contamination in the recombinant protein preparations were determined to be less than 0.01 IU/ml (Limulus amoebocyte assay; BioWhittaker), which corresponds to less than 0.1 pg of lipopolysaccharide delivered per mouse (7). As expected (13, 40), the recombinant TxAC314 peptide was devoid of cytotoxic effects, as determined by cell morphology following incubation in vitro with CHO cells up to concentrations of 1 mg/ml (data not shown).
FIG. 1.
SDS-PAGE analysis of purified TxAC314HA. E. coli BL21(DE3) carrying the plasmid pGEX/TxAC314 was grown for 6 h at 37°C following induction with IPTG; cells were then collected by centrifugation and lysed. Soluble proteins were subjected to affinity chromatography purification on a glutathione-Sepharose 4B resin and then separated through SDS-12% PAGE gels stained with Coomassie blue. Lane A, total cell lysate following 6 h of induction with IPTG and before affinity column purification; lane B, recombinant protein (GST-TxAC314) obtained following affinity chromatography of cell lysate on glutathione-Sepharose 4B resin and collected by addition of reduced glutathione; lane C, purified GST and TxAC314 obtained following digestion of recombinant GST-TxAC314 with thrombin; lane D, purified recombinant TxAC314 obtained by overnight digestion with thrombin (1 U/20-ml resin bed volume) of cell lysate purified and immobilized on glutathione-Sepharose 4B resin; lane MW, molecular mass markers (apparent molecular masses are shown in daltons).
Mucosal adjuvants and antigens.
CT and HEL were obtained from Sigma, and endotoxin-free KLH was purchased from Clontech. E. coli heat-labile enterotoxin subunit B (EtxB) was expressed and purified to homogeneity in our laboratory, as previously described (27).
Mucosal immunization.
Male BALB/c mice were purchased from Charles River (Oderzo, Italy) and used at 6 to 8 weeks of age. Animals were housed in groups of four under controlled humidity and temperature conditions and were given free access to commercial rodent food and water. Experiments were carried out by following the guidelines recommended by the Institutional Animal Care and Use Committee of the University of Padua. Mice were bled 1 week prior to the first immunization and then on days 0 and 14 were immunized either orally or intranasally. Each experiment was performed two to four times for a total of 8 to 12 mice per experimental condition. For oral immunization, mice received 100 μl of sterile PBS containing KLH (5 mg) alone or supplemented with TxAC314 (0.1 to 10 μg), CT (10 μg), or EtxB (10 μg) via curved oral dosing needles. In a series of experiments TxAC314 (10 μg) was preincubated in vitro for 1 h at 22°C with an anti-toxin A antiserum and then coadministered with KLH by oral gavage. For intranasal immunization, mice were dosed through fine tips attached to a pipette. The antigen (HEL, 5 mg) was administered in a total volume of 30 μl of PBS (15 μl per nostril) supplemented with TxAC314 (0.1 to 10 μg), CT (10 μg), or EtxB (10 μg).
Collection of blood and feces.
Blood samples were collected on days 13 and 35 after the primary immunization by bleeding from the retro-orbital plexus. Blood was left at room temperature for 2 h and centrifuged, and serum was collected and stored at −20°C. Feces (two or three pellets) were collected on the same days and immediately placed in preweighed microcentrifuge tubes containing ice-cold PBS (1:10 ratio) and a protease inhibitor mixture (5 mM phenylmethylsulfonyl fluoride, 5 μg of aprotinin and leupeptin/ml). Samples were then vortexed vigorously, insoluble material was removed by centrifugation (7,000 × g for 15 min), and clear supernatants were collected and individually stored at −20°C until used.
Detection of specific antibodies by ELISA.
Enzyme-linked immunosorbent assays (ELISA) were set up to obtain measurements of specific murine immunoglobulin G (IgG), IgA, and IgG subtype responses. Sera and feces were diluted in the appropriate buffer and used to determine the antibody levels. High-bound-capacity microtiter plates (Corning-Costar, Cambridge, Mass.) were coated with 100 μl of the appropriate antigen solution/well (10 μg/ml) in 0.1 M carbonate-bicarbonate buffer (pH 9.6) and incubated for 16 h at 4°C. After being washed (PBS containing 0.1% Tween 20 [PBS-T]), plates were blocked with 4% bovine serum albumin in PBS-T (90 min at 22°C) prior to sample addition (2 h at 22°C). Following washing (three times) with PBS-T, plates were incubated with biotinylated antiserum (anti-IgG, anti-IgA, anti-IgG1, or anti-IgG2a) for 1 h at 22°C and then streptavidin-labeled peroxidase was added (1 h at 22°C) prior the addition of developing solution (3,3′,5,5′-tetramethylbenzidine; microwell peroxidase substrate; Sigma). The reaction was stopped by addition of 100 μl of 0.2 M H2SO4, and the optical density at 450 nm was determined with an ELISA plate reader (LX300; Epson Diagnostic). Optimal working dilutions of anti-IgG (1:4,000) and anti-IgA (1:2,000) biotinylated capture antibodies were determined after preliminary assays, whereas working dilutions of anti-IgG subtype antisera were as recommended by the manufacturer (Southern Biotechnology Associated, Inc.). To assess the level of specific anti-KLH and anti-HEL antibodies on each plate, a reference curve was constructed by coating microtiter plate wells with known amounts of purified mouse IgG, IgA, IgG1, and IgG2a (range, 250 to 3.0 ng/ml; ICN). The serum and fecal concentrations of specific anti-HEL and anti-KLH antibodies were determined by subtracting the mean values obtained in nonimmune animals (background) from those obtained in each experimental (immune) mouse. Then the concentrations of specific anti-HEL and anti-KLH antibodies were expressed as micrograms per milliliter.
Induction and measurement of cytokine production.
Spleens were removed aseptically 35 days after the primary immunization, and single-cell suspensions were prepared by forcing the tissue homogenates through a wire mesh (2 mm). Red blood cells were removed by density gradient centrifugation (3). Mononuclear cells were washed twice in the culture medium (RPMI medium supplemented with 20 mM 2-mercaptoethanol, 100 IU of penicillin/ml, 100 μg of streptomycin/ml, and 10% heat-inactivated fetal calf serum), seeded at 106/ml, and then restimulated in triplicate with the appropriate antigen (50 μg/ml) for 72 h in a humidified 5% CO2 incubator at 37°C. Positive (phytohemagglutinin at 5 μg/ml) and negative (cells with culture medium only) controls were run in each experiment. Following incubation, cultures were harvested by centrifugation and the supernatants were collected, fractionated, and stored at −80°C. The levels of interleukin-2 (IL-2), gamma interferon (IFN-γ), IL-10, and IL-4 were measured by commercially available ELISAs, running each sample in duplicate (Biosource, Camarillo, Calif.).
Confocal immunofluorescence studies.
To assess binding of TxAC314 to intestinal epithelial cells, closed 3- to 4-cm-long ileal loops (one for each animal) were prepared in overnight fasting mice as previously described (3). Ileal loops were then injected with 50 μl of 50 mM Tris buffer, either alone or containing 20 μg of TxAC314. The abdomen was then closed, and animals were left on a heating pad to keep the body temperature at 37°C. Animals were sacrificed at various time points (10 to 120 min), and the loops were removed, washed in PBS, and fixed in 4% buffered paraformaldehyde for 60 min. Tissues were placed in embedding medium for frozen tissue specimens, and longitudinal sections (10 μm thick) were cut with a cryostat. To determine TxAC314 binding to intestinal epithelial cells, sections were washed in PBS (twice for 5 min) and then incubated for 10 min in 50 mM NH4Cl in PBS to quench reactive aldehydes. Nonspecific protein binding was blocked with 10% donkey serum in PBS for 1 h at 22°C. Sections were then incubated (1 h at 22°C) with a rabbit polyclonal antibody (Santa Cruz Biotechnology) against the HA epitope genetically fused at the N terminus of TxAC314. Sections were then washed three times with PBS-T at room temperature and then incubated with fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG (1:200 dilution) for 1 h at 22°C. Finally, sections were washed extensively, and slides were mounted, with a drop of 1-mg/ml N-propylgallate (Sigma) to reduce photobleaching, in 90% glycerol-PBS. Sections were then analyzed and photographed with a Leica TCS-NT/SP2 confocal microscope using a 40× objective. Images were digitally stored with Leica software. As a control, slides were incubated with nonimmune rabbit IgG.
Statistics.
Data are expressed as the means ± standard errors. Statistical analysis was performed using analysis of variance, and Bonferroni's test was used to test for significance between groups. Statistical significance was considered for P values <0.05.
RESULTS
TxAC314 binds to enterocytes in mouse intestine.
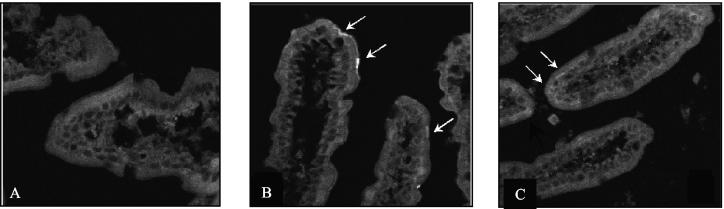
We first determined whether TxAC314 was able to bind to mucosal epithelial cells in vivo. As shown in Fig. 2, following injection in mouse ileal loops, TxAC314 readily bound to villus tips. The pattern of TxAC314 binding to intestinal villi in vivo was quite similar to the distribution described for toxin A binding to the rabbit ileum in vitro (15). TxAC314 binding was evident within 10 min following injection into the ileal loops, and after 2 h it was possible to observe the peptide inside the epithelial cells (Fig. 2).
FIG. 2.
Binding of TxAC314 to enterocytes in mouse intestine. Closed ileal loops were injected with buffer, either alone or containing 20 μg of TxAC314. After 10 to 120 min the animals were sacrificed; loops were removed, fixed in 4% buffered paraformaldehyde, and placed in embedding medium for frozen tissue specimens; and 10-μm-thick longitudinal sections were cut. To determine TxAC314 binding to intestinal epithelial cells, sections were incubated with a rabbit polyclonal antibody against the HA epitope fused at the N terminus of TxAC314. The immunocomplexes were detected with a FITC-conjugated donkey anti-rabbit IgG, and then sections were analyzed with a Leica TCS-NT/SP2 confocal microscope with a 40× objective. (A) Absence of signal in a loop injected with buffer. (B) Representative section obtained from an ileal loop incubated for 10 min with TxAC314. With the anti-HA antibody, it is possible to detect the presence of a strong signal at villus tips, indicating the presence of TxAC314 bound to the epithelium. (C) Representative section obtained from an ileal loop 120 min after injection with TxAC314. Following incubation with a rabbit antibody against the HA epitope fused to TxAC314 it is possible to observe the presence of a faint signal within the cytoplasm of the epithelial cells.
Adjuvant effect of TxAC314 on KLH-specific serum antibody responses following oral immunization.
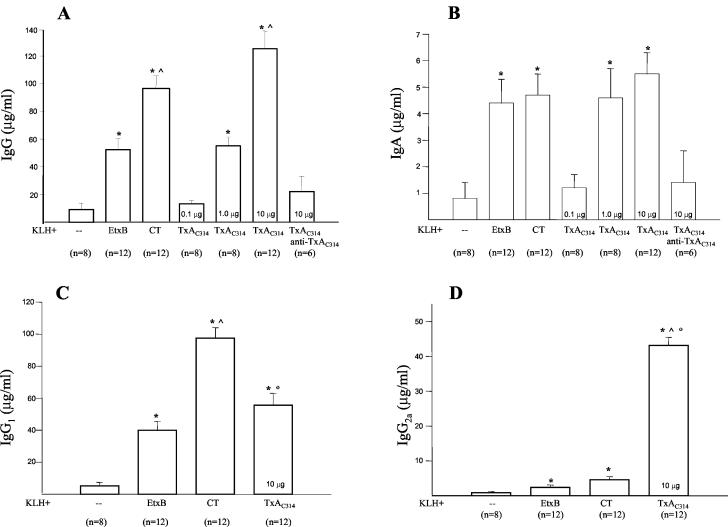
Low levels of KLH-specific IgG were detected after a single dose of orally administered antigen in 4 of 12 mice immunized with CT plus KLH or EtxB plus KLH and in 8 of 12 mice immunized with TxAC314 plus KLH. None of the animals that received only KLH showed a detectable antibody response. As expected, 3 weeks after the second immunization dose, low levels of KLH-specific serum IgG were detected in mice immunized orally with KLH alone (Fig. 3). However, anti-KLH-specific serum IgG significantly increased in mice immunized with CT plus KLH or EtxB plus KLH and also with TxAC314 plus KLH (Fig. 3). As shown in Fig. 3, at doses of 10 μg/mouse TxAC314 was 2.4-fold (P < 0.01) more effective at enhancing the anti-KLH-specific immune response than EtxB and slightly more effective (1.26-fold increase; P ≥ 0.05) than CT. TxAC314 at doses of 0.1 μg/mouse did not show any significant adjuvant activity but were as effective as 10 μg of EtxB at 1 μg/mouse (Fig. 3). However, we could not test doses of TxAC314 higher than 10 μg/mouse since it was not possible to obtain more-concentrated peptide solutions because of a certain degree of insolubility.
FIG. 3.
Levels of systemic KLH-specific antibodies in mice immunized by oral gavage. BALB/c mice (8 to 12 per group) were immunized orally on days 0 and 14 with KLH (5 mg) alone or supplemented with TxAC314 (0.1 to 10 μg), CT (10 μg), or EtxB (10 μg). At day 35, blood was collected by retro-orbital bleeding and serum concentrations of IgG (A), IgA (B), IgG1 (C), and IgG2a (D) reactive to KLH were measured by ELISA. In some experiments TxAC314 (10 μg/mouse) was preincubated in vitro with anti-C. difficile toxin A antiserum (anti-TxAC314) and then coadministered by oral gavage with KLH. The level of specific antibodies reacting to KLH was determined by preparing a reference curve by using mouse IgG, IgA, IgG1, or IgG2a and subtracting the background from each sample (nonimmune mice [n = 8]). Results are means ± standard errors. *, P < 0.01 versus KLH alone; ^, P < 0.01 versus KLH plus EtxB, °, P < 0.01 versus KLH plus CT.
Following two oral doses, KLH-specific serum IgA was detected in mice coadministered KLH with CT, EtxB, or TxAC314. As shown in Fig. 3, the highest levels of anti-KLH-specific IgA were detected in mice that received 10 μg of TxAC314 or CT as the adjuvant, whereas slightly lower levels were observed in mice dosed with EtxB as the adjuvant (P ≥ 0.05).
To determine whether the TxAC314-mediated adjuvant effect required binding to mucosal epithelial cells, we preincubated TxAC314 with a mouse polyclonal antibody raised against the carboxy terminus region of C. difficile toxin A, before coadministration with KLH. This antibody was effective at neutralizing toxin A-mediated toxic effects in vivo and in vitro (data not shown). As shown in Fig. 3, preincubation of TxAC314 with the neutralizing antibody completely abolished the TxAC314-mediated adjuvant effect.
KLH-specific IgG subclass patterns.
The levels of KLH-specific IgG1 and IgG2a were significantly higher in mice immunized with KLH plus CT, EtxB, or TxAC314 than in mice immunized with KLH alone (Fig. 3). KLH-specific IgG1 levels were 1.7-fold higher in mice immunized with CT plus KLH than in animals dosed with TxAC314 plus KLH (P < 0.01). In contrast, anti-KLH-specific IgG2a levels were significantly (P < 0.01) higher in mice immunized orally with TxAC314 plus KLH than in mice immunized with CT or EtxB and KLH (9.7- and 18.5-fold increases, respectively; Fig. 3).
Adjuvant effect of TxAC314 following oral immunization on KLH-specific mucosal IgA responses.
Specific anti-KLH IgA was detected at low levels in the feces of a few mice immunized by oral gavage with KLH alone (Fig. 4). However, following oral immunization with CT plus KLH, EtxB plus KLH, and TxAC314 plus KLH, specific anti-KLH IgA was present at significantly higher concentrations in the feces (Fig. 4). Furthermore, 1 μg of TxAC314 was as effective as 10 μg of CT and significantly more effective than 10 μg of EtxB to release mucosal KLH-specific IgA.
FIG. 4.
Fecal anti-KLH- and anti-HEL-specific antibodies. BALB/c mice (8 to 12 per group) were immunized on days 0 and 14 orally with KLH (5 mg) or intranasally with HEL (5 mg) either alone or supplemented with TxAC314 (0.1 to 10 μg), CT (10 μg), or EtxB (10 μg). Three weeks after the second inoculation, feces were collected and anti-KLH- (A) or anti-HEL (B)-specific IgA levels were measured by ELISA. Results are means ± standard errors. *, P < 0.01 versus KLH (A) or HEL (B) alone; °, P < 0.05 versus KLH plus EtxB; #, P < 0.05 versus HEL plus CT.
Adjuvant effect of TxAC314 following intranasal immunization on HEL-specific antibody responses.
To determine whether the protocol of immunization influenced the outcome of antibody production, we evaluated the immune responses triggered following nasotracheal immunization using HEL as the test antigen. Intranasal administration of HEL alone induced the appearance of low levels of specific serum IgG only after the second immunization dose; however the coadministration of either CT, EtxB, or TxAC314 with HEL triggered significant specific antibody responses (Fig. 5). As shown in Fig. 5, it was not possible to observe a dose-response effect in the serum antibody response obtained by coadministering HEL with TxAC314, since TxAC314 was effective only at 10 μg/mouse.
FIG. 5.
Levels of systemic anti-HEL-specific antibodies in mice immunized intranasally. BALB/c mice (8 to 12 per group) were immunized intranasally on days 0 and 14 with HEL (5 mg) alone or supplemented with TxAC314 (0.1 to 10 μg), CT (10 μg), or EtxB (10 μg). At day 35 blood was collected by retro-orbital bleeding and serum concentrations of IgG (A), IgA (B), IgG1 (C), and IgG2a (D) reactive to HEL were measured by ELISA. The level of specific antibodies reacting to HEL was determined by preparing a reference curve with mouse IgG, IgA, IgG1, or IgG2a and subtracting the background from each sample (nonimmune mice [n = 8]). Results are means ± standard errors. *, P < 0.01 versus HEL alone; °, P < 0.05 versus HEL plus EtxB.
Following intranasal immunization, significant levels of HEL-specific serum IgA were detected in mice that received CT, EtxB, or TxAC314 and HEL, but not HEL alone. As shown in Fig. 5, the highest levels of HEL-specific IgA were detected in mice that received 10 μg of either CT or TxAC314 as the adjuvant, whereas slightly lower levels were measured with EtxB as the adjuvant (not significant).
HEL-specific IgG subclass patterns.
As expected, the levels of anti-HEL-specific IgG1 and IgG2a were significantly higher in mice immunized with HEL plus CT, EtxB, or TxAC314 than in mice immunized with HEL alone (Fig. 5). Anti-HEL-specific IgG1 and IgG2a levels in mice immunized intranasally with CT plus HEL and TxAC314 plus HEL were comparable (Fig. 5). Indeed, immunization with CT plus HEL or TxAC314 plus HEL stimulated the production of anti-HEL-specific IgG1 levels that were significantly (P < 0.05) higher than IgG2a levels (Fig. 5).
Adjuvant effect of TxAC314 following intranasal immunization on HEL-specific mucosal IgA responses.
Following intranasal immunization with HEL alone (Fig. 4) anti-HEL IgA in feces was barely detectable. However, following intranasal immunization with CT plus HEL, EtxB plus HEL, or TxAC314 plus HEL, specific anti-HEL IgA was present at significant levels in the feces (Fig. 4). Coadministration of HEL with 10 μg of CT was significantly more effective (P < 0.05) than EtxB or TxAC314 in stimulating the release of anti-KLH-specific mucosal IgA. Indeed, EtxB and TxAC314 showed a similar adjuvant activities (Fig. 4).
Anti-CT- and -TxAC314-specific antibody responses.
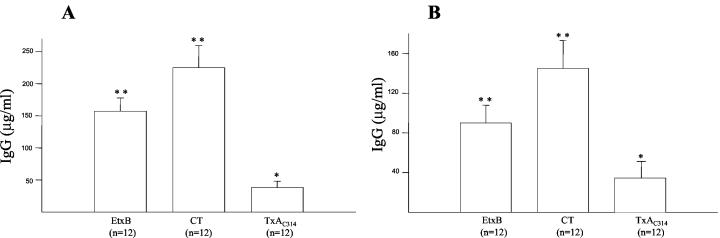
Anti-CT- and anti-EtxB-specific serum IgG was detected, as expected, in animals following oral administration of 10 μg of CT or EtxB (Fig. 6A) and at an even higher level following intranasal administration (Fig. 6). Indeed, following oral or intranasal coadministration of TxAC314 with either KLH or HEL the levels of anti-TxAC314 IgG in the serum were only barely detectable (Fig. 6). Similar results were obtained by measuring the levels of specific IgA in the feces of animals following either oral or intranasal immunization (data not shown).
FIG. 6.
Levels of systemic anti-CT, anti-EtxB, and anti-TxAC314 in mice immunized by oral gavage or nasotracheally. BALB/c mice (8 to 12 per group) were immunized twice orally with KLH (5 mg) or intranasally with HEL (5 mg) supplemented with 10 μg of either TxAC314, CT, or EtxB or with just buffer, and after 35 days blood was collected by retro-orbital bleeding. Following oral gavage (A) or intranasal (B) immunization, specific IgG anti-CT, anti-EtxB, and anti-TxAC314 in serum were measured by ELISA. The level of specific antibodies was determined by preparing a reference curve with mouse IgG and subtracting the background from each sample (nonimmune mice [n = 8]). Results are means ± standard errors. **, P < 0.01 versus control; *, P < 0.05 versus control.
Adjuvant effect of TxAC314 following oral immunization on KLH-specific cellular responses.
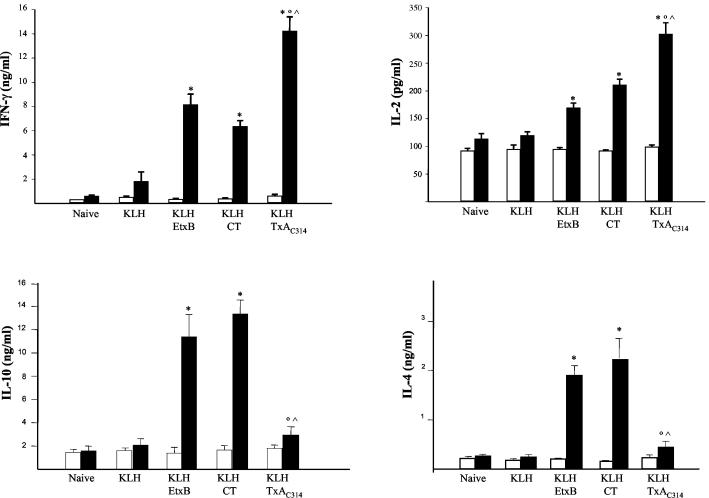
As shown in Fig. 7, spleen lymphocytes obtained from mice immunized orally with only KLH exhibited a modest release of IFN-γ, IL-2, IL-4, and IL-10 following in vitro challenge with KLH, as opposed to the more robust cytokine release observed in cells isolated from mice dosed with CT plus KLH, EtxB plus KLH, or TxAC314 plus KLH. Exposure of spleen cells purified from mice immunized with TxAC314 plus KLH produced significantly more IL-2 and IFN-γ following in vitro exposure to KLH than exposure of cells obtained from mice immunized with CT plus KLH or EtxB plus KLH (Fig. 7). Indeed, IL-4 and IL-10 secretion was significantly enhanced in cells purified from mice immunized with CT plus KLH or EtxB plus KLH and challenged in vitro with KLH but was only slightly evident in splenocytes obtained from mice immunized with TxAC314 plus KLH (Fig. 7). Indeed, phytohemagglutinin stimulation induced a robust production of the cytokines in all experimental groups (data not shown).
FIG. 7.
Cytokine production of spleen cells isolated from mice immunized by oral gavage. BALB/c mice were immunized orally on days 0 and 14 with KLH (5 mg) alone or supplemented with TxAC314 (10 μg), CT (10 μg), or EtxB (10 μg). On day 35 splenocytes were collected, seeded in culture (106/ml), and in vitro restimulated with KLH (50 μg/ml) (black bars) or left unstimulated (open bars). Culture supernatants collected after 72 h were assayed for cytokines IFN-γ, IL-2, IL-4, and IL-10 by ELISA. Results are means ± standard errors from three to six mice per group. *, P < 0.05 versus the respective nonstimulated cells; °, P < 0.05 versus mice immunized with KLH plus EtxB; ^, P < 0.05 versus mice immunized with KLH plus CT.
DISCUSSION
The growing body of knowledge on the immune system and human pathogens makes it possible to develop new recombinant vaccines. However, the development of effective mucosal vaccines is still jeopardized by the poor immunological properties of nonreplicating antigens and the lack of efficient delivery systems and mucosal adjuvants (14). To date the most effective mucosal adjuvants are CT and LT and their genetically detoxified mutant versions, which are used as vaccine carriers and immunomodulators (38). Here we report that a recombinant peptide, the nontoxic carboxy-terminus domain of C. difficile toxin A, demonstrates robust adjuvant properties when coadministered with poor antigens via mucosal routes. The profile of antigen-specific IgG subclasses and of cytokine release from immune splenocytes challenged in vitro with the test antigens used in this study is compatible with the induction of a mixed, Th1/Th2-type immune response.
C. difficile toxin A, a large clostridial toxin, acts at microgram concentrations in the rodent gut to stimulate fluid secretion, disrupt intestinal integrity, and trigger the release of proinflammatory mediators (4). Indeed, systemic administration of a few nanograms of toxin A holotoxin results in animal death (29). The amino acid sequences of C. difficile toxins suggest the existence of three functional domains within a single large peptide rather than the classical AB structure, with specialized binding and catalytic subunits, of the vast majority of cytotoxins acting on intracellular targets (42). Thus the catalytic domain, located at the N terminus of the molecule, is essential for cell rounding and death (18). Microinjections of C. difficile toxins into fibroblasts or the direct expression of the catalytic domain (aa 1 to 556) in cells mimics the morphological and physiological changes caused by exposure to the holotoxin (18). However, the role of the UDP-glucosyltransferase catalytic domain in intestinal inflammation and cytokine release from immune cells is not clear since mitochondrial oxygen radical generation, NF-κB activation, and IL-8 gene up-regulation appear to precede holotoxin-mediated Rho glucosylation (17). Whereas the carboxy terminus domain of C. difficile toxin A shows a lectin-like structure and mediates toxin binding to membrane receptors and erythrocyte agglutination (13, 28), recombinant peptides comprising the C terminus region of toxin A lack significant inflammatory or cytotoxic effect in vivo and in vitro (16, 30, 52). Although apparently devoid of intrinsic toxic and proinflammatory activity, the receptor binding portion of toxin A may still activate biological responses. In fact, membrane receptor clustering is an event known to trigger a variety of intracellular signaling pathways. Thus, antibody-mediated CD3 and T-cell receptor ligation or CT B-subunit-induced clustering of GM1 gangliosides on immune cells activates signal transduction cascades, cytokine release, and lymphocyte proliferation (9, 45). Indeed, we have observed in preliminary experiments that TxAC314, as well as anti-CD3 antibodies, stimulates IL-2 and IFN-γ release from normal mouse spleen cells in vitro (I. Castagliuolo and M. Sardina, unpublished observation), strongly suggesting that the carboxy terminus domain of C. difficile toxin A exhibits specific direct biological activities.
An intriguing common feature of powerful mucosal antigens and adjuvants is their hetero-oligomeric structure, composed of structurally and functionally distinct catalytic and binding subunits (44, 46). CT and LT, as well as mistletoe lectins, possess a catalytic domain and a carbohydrate recognition domain able to bind simultaneously several specific membrane receptors. Recent studies aimed at developing new effective mucosal adjuvants have generated nontoxic mutant CT and LT retaining mucosal immunogenicity and adjuvant activity (38). Thus, it is now evident that the enzymatic ADP ribosylation activity in CT and LT is not absolutely required to exert the adjuvant activity whereas the carbohydrate-binding domain can act as a mucosal adjuvant per se (38).
Our data suggest that the TxAC314 peptide is an effective mucosal adjuvant following either oral and nasotracheal administration. This is quite interesting since oral adjuvants have to survive the harsh gastric environment and avoid destruction from pancreatic proteases. Accordingly, bacterial toxins are generally more effective mucosal adjuvants following nasotracheal administration than following oral administration (38, 54) (Fig. 3 to 5). However, it has recently been reported that several plant lectins are stable in the rodent gut and retain the ability to interact with the mucosal epithelium following oral feeding (11). In addition, certain clostridial toxins, including C. difficile toxins, retain their biological activity following oral administration (22, 28). Indeed, C. difficile toxin A and the peptide TxAC314 are extremely resistant to protease digestion in vitro (2; I. Castagliuolo and V. De Filippis, unpublished observation). Interestingly, C. difficile toxins, like other bacterial toxins, undergo pH-dependent structural changes probably required to escape from the intracellular endocytic compartment in order to exert the full biological activity (41). Thus, exposure to the gastric pH may induce conformational changes that favor transmembrane translocation of TxAC314, enhancing its biological activity.
C. difficile toxin A, like other ADP-ribosylating toxins, must bind to intestinal epithelial cells in vivo to exert its adjuvant activity. It is possible that the differences in the immune responses elicited by CT, EtxB, and TxAC314 are due, at least in part, to different properties for binding to the gastrointestinal and nasopharyngeal mucosa (39, 46). Indeed, the sugar binding properties can confine lectins to specific cell populations, such as M cells in the Peyer's patches or the follicle-associated epithelium in nasopharyngeal tissue (5, 24). Thus, the repeating units in the toxin A carboxy terminus recognizing the trisaccharide Galα1-3Galβ1-4GlcNAc cause binding to rodent intestinal epithelial and immune cells (23). The fate of C. difficile toxins following receptor binding in vivo is not known; however, in vitro the toxin binds to and translocates across the plasma membrane within a few minutes to reach its intracellular target(s) (13, 16). Specific antigen binding and uptake by epithelial cells constitute an essential, although probably not sufficient, first step in eliciting an immune response following antigen delivery via a mucosal route. Binding to membrane receptors of LT, CT, or other ADP-ribosylating toxins, such as pertussis toxin, is a prerequisite for immunogenicity of these toxins since it may facilitate receptor-mediated transepithelial uptake, possibly leading to entry into a nonproteolytic intracellular environment (6). Indeed, following binding to the epithelial brush border, plant lectins as well as CT are translocated across the mucosal epithelium to reach and, eventually, activate lamina propria lymphocytes (26, 51). Thus, weak antigens fused to carrier peptides, such as bacterial toxins, can be efficiently delivered to the mucosal immune system (7). We observed that TxAC314 readily binds to and penetrates intestinal enterocytes; however our time course experiments cannot determine whether TxAC314 can directly reach the submucosal lymphocytes through the intact epithelium.
As previously claimed, we observed that orogastric or intranasal coadministration of soluble weak antigens, such as KLH and HEL, with CT or EtxB resulted in the induction of a mixed Th1/Th2-type responses (20, 32, 37). This was supported by the cytokine profile, by the release of significant amount of Th1- (IFN-γ and IL-2) and Th2 (IL-4 and IL-10)-type cytokines, and by the production of both IgG subclasses (IgG1 and IgG2a) although the predominant production of serum IgG1 was detected (Fig. 3 and 5). Our data indicate that mucosal immunization with antigens in combination with TxAC314 seems to induce also a mixed Th1/Th2-type immune response, as indicated by the production of both IgG subclasses (IgG1 and IgG2a). However, following oral immunization high levels of serum IgG2a (Fig. 3) are coupled to the release of Th1-type cytokines (Fig. 7). This complex and not well defined profile of the immune response is not completely unprecedented since, following mucosal immunization, Th1-type and Th2-type responses can be observed at different stages of strong immune responses and depend on specific antigen/adjuvant combinations (20, 34, 37, 43). The molecular mechanisms leading to a more prominent production of Th1- or Th2-type cytokines and different IgG isotypes are presently not completely known; however, we can speculate that they rely on the different effects of CT and TxAC314 on immune cells. Thus, CT induces intracellular cyclic AMP accumulation, leading to protein kinase A activation, whereas it fails to activate NF-κB, enhances major histocompatibility complex class II-restricted antigen presentation in vitro, activates the Th2-type CD4+ lymphocyte population, down-regulates inflammatory reactions associated with autoimmune disorders, and induces CD8+ lymphocyte apoptosis (33). On the other hand, C. difficile toxins cause intracellular Ca2+ influx, production of reactive oxygen radicals, and NF-κB activation, which eventually trigger the release of a variety of Th1-type cytokines from immune and nonimmune cells (12, 35, 53).
The most widely experimentally used mucosal adjuvants, CT and LT, are strongly immunogenic by themselves and potentiate the immune response to bystander antigens through several mechanisms (19). Indeed, we observed that TxAC314, although effective at enhancing the immune response against coadministered antigens, was poorly immunogenic by itself (Fig. 6). Although this is quite unusual for a mucosal adjuvant, recent studies have clearly demonstrated that cytokines (peptides or cDNA) can replace CT and LT in nasal and intragastric vaccination protocols (10, 49). Thus, mucosal administration of cytokines can enhance antigen presentation and promote humoral and cytotoxic T-lymphocyte-mediated immune responses. Therefore, we speculate that TxAC314-mediated adjuvant activity relies primarily on the ability of TxAC314 to stimulate cytokine release from mucosal immune cells, although further studies are necessary to elucidate its mechanism of action.
In conclusion, bacterial toxins and plant lectins are the most powerful mucosal adjuvants known to date, but significant problems result from the toxic effects of their catalytic domain. In this study we report for the first time that the carboxy terminus region of C. difficile toxin A, TxAC314, is an effective mucosal adjuvant following intragastric and intranasal administration. However further studies are required to prove that this peptide is devoid of significant toxic effects in vivo and that TxAC314 is able to stimulate protective immune responses against coadministered viral and bacterial antigens.
Editor: J. T. Barbieri
REFERENCES
- 1.Aizpurua, H. J., and G. J. Russell-Jones. 1988. Identification of classes of proteins that provoke an immune response upon oral feeding. J. Exp. Med. 167:440-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castagliuolo, I., J. T. LaMont, S. T. Nikulasson, and C. Pothoulakis. 1996. Saccharomyces boulardii protease inhibits Clostridium difficile toxin A effects in rat ileum. Infect. Immun. 64:5225-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castagliuolo, I., A. Keates, C. Wang, A. Pasha, L. Valenick, C. P. Kelly, S. Nikulasson, J. T. LaMont, and C. Pothoulakis. 1998. Clostridium difficile toxin A stimulates macrophage inflammatory protein-2 production in rat intestinal epithelial cells. J. Immunol. 160:6039-6045. [PubMed] [Google Scholar]
- 4.Castagliuolo, I., and J. T. LaMont. 1999. Pathophysiology, diagnosis and treatment of Clostridium difficile infection. Keio J. Med. 48:169-174. [DOI] [PubMed] [Google Scholar]
- 5.Clark, M. A., M. A. Jepson, N. L. Simmons, T. A. Booth, and B. H. Hirst. 1993. Differential expression of lectin-binding sites defines mouse intestinal M-cells. J. Histochem. Cyochem. 41:1679-1687. [DOI] [PubMed] [Google Scholar]
- 6.Cropley, I., G. Douce, M. Roberts, S. Chatfield, M. Pizza, I. Marsili, R. Rappuoli, and G. Dougan. 1995. Mucosal and systemic immunogenicity of a recombinant non-ADP-ribosylating pertussis toxin: effects of formaldehyde treatment. Vaccine 13:1643-1648. [DOI] [PubMed] [Google Scholar]
- 7.De Haan, L., A. R. Hearn, A. J. Rivett, and T. R. Hirst. 2002. Enhanced delivery of exogenous peptides into the class I antigen processing and presentation pathway. Infect Immun. 70:3249-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dove, C. H., S. Wang, S. B. Price, C. J. Phelps, D. M. Lyerly, T. D. Wilkins, and J. L. Johnson. 1990. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 58:480-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elson, C. O., S. P. Holland, M. T. Dertzbaugh, C. F. Cuff, and A. O. Andeson. 1995. Morphologic and functional alterations of mucosal T cells by cholera toxin and its B subunit. J. Immunol. 154:1032-1040. [PubMed] [Google Scholar]
- 10.Eo, S. K., S. Lee, U. Kumaragur, and B. T. Rouse. 2001. Immunopotentiation of DNA vaccine against herpes simplex virus via co-delivery of plasmid DNA expressing CCR7 ligands. Vaccine 19:4685-4693. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald, A. J., M. Jordinson, J. M. Rhodes, R. Singh, J. Calam, and R. A. Goodlad. 2001. Comparison of the effects of concanavalin-A and epidermal growth factor on epithelial cell proliferation in the rat intestine. Aliment. Pharmacol. Ther. 15:1077-1084. [DOI] [PubMed] [Google Scholar]
- 12.Flegel, W. A., F. Muller, W. Daubener, H. G. Fischer, U. Hadding, and H. Northoff. 1991. Cytokine response by human monocytes to Clostridium difficile toxin A and toxin B. Infect. Immun. 59:3659-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisch, C., R. Gerhard, K. Aktories, F. Hofmann, and J. Ingo. 2003. The complete receptor-binding domain of Clostridium difficile toxin A is required for endocytosis. Biochem. Biophys. Res. Commun. 300:706-711. [DOI] [PubMed] [Google Scholar]
- 14.Fujihashi K., T. Koga, F. W. van Ginkel, Y. Hagiwara, and J. R. McGhee. 2002. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine 20:2431-2438. [DOI] [PubMed] [Google Scholar]
- 15.Giannasca, P. J., Z. X. Zhang, W. D. Lei, J. A. Boden, M. A. Giel, T. P. Monath, and W. D. Thomas. 1999. Serum antitoxin antibodies mediate systemic and mucosal protection from Clostridium difficile in hamsters. Infect. Immun. 67:527-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, D., S. Hagen, C. Pothoulakis, M. Chen, N. D. Medina, M. Warny, and J. T. Lamont. 2000. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 119:139-150. [DOI] [PubMed] [Google Scholar]
- 17.He, D., S. Sougioultzis, S. Hagen, J. Liu, S. Keates, A. C. Keates, C. Pothoulakis, and J. T. Lamont. 2002. Clostridium difficile toxin A triggers human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology 122:1048-1057. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann, F., C. Busch, U. Prepens, I. Just, and K. Aktories. 1997. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J. Biol. Chem. 272:11074-11078. [DOI] [PubMed] [Google Scholar]
- 19.Holmgren J., C. Czerkinsky, K. Eriksson, and A. Mharandi. 2003. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine 21(Suppl. 2):89-95. [DOI] [PubMed] [Google Scholar]
- 20.Jones, H. P., L. M. Hodge, K. Fujihashi, H. Kiyono, J. R. McGhee, and J. W. Simecka. 2001. The pulmonary environment promotes Th2 responses after nasal-pulmonary immunization with antigen alone, but Th1responses are induced during instances of intense immune stimulation. J. Immunol. 167:4518-4525. [DOI] [PubMed] [Google Scholar]
- 21.Just, I., J. Selzer, M. Wllm, C. von Eichel-Streiber, M. Mann, and K. Aktories. 1995. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 375:500-503. [DOI] [PubMed] [Google Scholar]
- 22.Just, I., F. Hofmann, and K. Aktories. 2000. Molecular mode of action of the large clostridial cytotoxins. Curr. Top. Microbiol. Immunol. 250:55-83. [DOI] [PubMed] [Google Scholar]
- 23.Krivan, H. C., G. F. Clark, D. F. Smith, and T. D. Wilkins. 1986. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Galα1-3Galβ1-4GlcNAc. Infect. Immun. 53:573-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuper, C. F., P. J. Koornstra, D. M. H. Hameleers, J. Biewenga, B. J. Spit, A. M. Duijvestijn, P. J. van Breda Vriesman, and T. Sminia. 1992. The role of nasopharyngeal lymphoid tissue. Immunol. Today 12:219-224. [DOI] [PubMed] [Google Scholar]
- 25.Lavelle, E. C., G. Grant, A. Pusztai, U. Pfuller, and D. T. O'Hagan. 2001. The identification of plant lectins with mucosal adjuvant activity. Immunology 102:77-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindner, J., A. F. Geczy, and G. J. Russell-Jones. 1994. Identification of the site of uptake of the E. coli heat-labile enterotoxin, LTB. Scand. J. Immunol. 40:564-572. [DOI] [PubMed] [Google Scholar]
- 27.Loregian, A., T. R. Hirst, H. S. Marsden, and G. Palù. 1996. Use of Vibrio spp. for expression of E. coli enterotoxin B subunit proteins: purification and characterization of a chimera containing a C-terminal fragment of DNA polymerase from herpes simplex virus type 1. Prot. Expr. Purif. 8:381-389. [DOI] [PubMed] [Google Scholar]
- 28.Lyerly, D. M., D. E. Lockwood, H. Richardson, and T. D. Wilkins. 1985. Biological activities of toxins A and B. Infect. Immun. 35:1147-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lyerly, D. M., K. E. Saum, D. K. MacDonald, and T. D. Wilkins. 1985. Effects of Clostridium difficile toxins given intragastrically to animals. Infect. Immun. 47:349-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyerly, D. M., J. L. Johnson, S. M. Frey, and T. D. Wilkins. 1990. Vaccination against lethal Clostridium difficile enterocolitis with a nontoxic recombinant peptide of toxin A. Curr. Microbiol. 21:29-32. [Google Scholar]
- 31.MacKay, I. R., and F. S. Rosen. 2001. Vaccines and vaccination. N. Engl. J. Med. 345:1042-1053. [DOI] [PubMed] [Google Scholar]
- 32.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, and H. Bluethmann. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 33.Matousek, M. P., J. G. Nedrud, and C. V. Hardling. 1996. Distinct effects of recombinant cholera toxin B subunit and holotoxin on different stages of class II MHC antigen processing and presentation. J. Immunol. 156:4137-4145. [PubMed] [Google Scholar]
- 34.Nakagawa, I., I. Takahashi, H. Kiyono, J. R. McGhee, and S. Hamada. 1996. Oral immunization with the B subunit of the heat-labile enterotoxin of Escherichia coli induces early Th1 and late Th2 cytokine expression in Peyer's patches. J. Infect. Dis. 173:1428-1436. [DOI] [PubMed] [Google Scholar]
- 35.Ng, E. K., N. Panesar, W. E. Longo, M. J. Shapiro, D. L. Kaminski, K. C. Tolman, and J. E. Mazuski. 2003. Human intestinal epithelial and smooth muscle cells are potent producers of IL-6. Mediators Inflamm. 12:3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nossal, G. J. V. 1999. Vaccines, p. 1387-1425. In W. E. Paul (ed.), Fundamental immunology, 4th ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 37.Okahashi, N., M. Yamamoto, J. L. Vancott, S. N. Chatfield, M. Roberts, H. Bulethmann, T. Hiroi, H. Kiyono, J. R. McGhee. 1996. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect. Immun. 64:1516-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pizza, M., M. M. Giuliani, M. R. Fontana, E. Monaci, G. Douce, G. Dougan, K. H. Mills, R. Rappuoli, and G. Del Giudice. 2001. Mucosal vaccines: nontoxic derivatives of LT and CT as mucosal adjuvants. Vaccine 19:2534-2541. [DOI] [PubMed] [Google Scholar]
- 39.Pothoulakis, C., R. J. Gilbert, C. Claradas, I. Castagliuolo, G. Semenza, Y. Hitti, J. S. Montcrief, J. F. Linevsky, C. P. Kelly, S. T. Nikulasson, H. P. Desai, T. D. Wilkins, and J. T. LaMont. 1996. Rabbit sucrase-isomaltase is a functional intestinal receptor for Clostridium difficile toxin A. J. Clin. Investig. 98:641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Price, S. B., T. D. Phelps, T. D. Wilkins, and J. L. Johnson. 1987. Cloning of the carbohydrate-binding portion of the toxin A gene of Clostridium difficile. Curr. Microbiol. 16:82-86. [Google Scholar]
- 41.Qa'dan, M., L. M. Spyres, and J. D. Ballard. 2000. pH-induced conformational changes in Clostridium difficile toxin B. Infect. Immun. 68:2470-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rappuoli, R., and M. G. Pizza. 2000. Bacterial toxins, p. 193-220. In P. Cossart, P. Boquet, S. Normark, and R. Rappuoli (ed.), Cellular microbiology. American Society for Microbiology, Washington, D.C.
- 43.Roberts, M., A. Bacon, R. Rappuoli, M. Pizza, I. Cropley, G. Douce, G. Dougan, M. Marinaro, J. McGhee, and S. Chatfield. 1995. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect. Immun. 63:2100-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rudiger, H., and H. J. Gabius. 2001. Plant lectins: occurrence, biochemistry, functions and applications. Glycoconj. J. 18:589-613. [DOI] [PubMed] [Google Scholar]
- 45.Schwinzer, R., H. Sommermeyer, H. J. Schlitt, R. E. Schmidt, and K. Wonigeit. 1991. Activation of human thymocytes by antibodies to the CD3/T-cell receptor complex: triggering of different epitopes results in different signals. Cell. Immunol. 136:318-328. [DOI] [PubMed] [Google Scholar]
- 46.Sears, C. L., and J. B. Kaper. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 60:167-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simmons, C. P., M. Ghaem-Magami, L. Petrovska, L. Lopes, B. M. Chain, N. A. Williams, and G. Dougan. 2001. Immunomodulation using bacterial enterotoxins. Scand. J. Immunol. 53:218-226. [DOI] [PubMed] [Google Scholar]
- 48.Sizemore, D. R., A. A. Branstom, and J. C. Sadoff. 1995. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science 270:299-302. [DOI] [PubMed] [Google Scholar]
- 49.Staats, H. F., C. P. Bradney, W. M. Gwinn, S. S. Jackson, G. D. Sempowski, H. X. Liao, N. L. Letvin, and B. F. Haynes. 2001. Cytokine requirements for induction of systemic and mucosal CTL after nasal immunization. J. Immunol. 167:5386-5394. [DOI] [PubMed] [Google Scholar]
- 50.Van Damme, E. J. M., W. J. Peumans, A. Pusztai, and S. Bardocz. 1988. Plant lectins: a special class of plant proteins, p. 3-28. In E. J. M. Van Damme, W. J. Peumans, A. Pusztai, and S. Bardocz (ed.), Handbook of plant lectins: properties and biomedical applications. John Wiley and Sons, Chichester, United Kingdom.
- 51.Wang, Q., L. G. Yu, B. J. Campbell, J. D. Milton, and J. M. Rhodes. 1998. Identification of intact peanut lectin in peripheral venous blood. Lancet 352:1831-1832. [DOI] [PubMed] [Google Scholar]
- 52.Ward, S., G. Douce, G. Dougan, and B. W. Wren. 1999. Local and systemic neutralizing antibody responses induced by intranasal immunization with the nontoxic binding domain of toxin A from Clostridium difficile. Infect. Immun. 67:5124-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warny, M., and C. P. Kelly. 1999. Monocyte cell necrosis is mediated by potassium depletion and caspase-like proteases. Am. J. Physiol. 276:C717-C724. [DOI] [PubMed] [Google Scholar]
- 54.Williams, N. A., T. R. Hirst, and T. O. Nashar. 1999. Immune modulation by the cholera-like enterotoxins: from adjuvant to therapeutic. Immunol. Today 20:95-101. [DOI] [PubMed] [Google Scholar]
- 55.Zho, F., and M. R. Neutra. 2002. Antigen delivery to mucosa-associated lymphoid tissues using liposomes as a carrier. Biosci. Rep. 22:355-369. [DOI] [PubMed] [Google Scholar]