Abstract
Vaccination is the most efficient prophylaxis against a variety of infectious diseases. New vaccination strategies rely on the incorporation of effective adjuvants, which stimulate the innate immune response and, in turn, activate the adaptive immune response. It is well established that flagellin induces inflammatory responses through the activation of antigen-presenting cells (APCs). In order to evaluate whether flagellin can serve as a carrier for the development of adjuvants or vaccines, we prepared a flagellin-enhanced green fluorescent protein (EGFP) fusion protein. Our results demonstrate that a flagellin-EGFP fusion protein is capable of stimulating APCs, resulting in the maturation of these cells and secretion of proinflammatory cytokines. Furthermore, APCs pulsed with the flagellin-EGFP fusion protein effectively process and present EGFP antigens. More importantly, animals immunized with the flagellin-EGFP fusion protein developed specific anti-EGFP T-cell responses. In contrast, recombinant EGFP was not able to stimulate APCs, nor did it induce a T-cell response. Thus, recombinant-flagellin fusion proteins may be suitable carriers as adjuvants or vaccines for the development of new vaccination strategies to induce and boost immune responses against infectious diseases and cancer.
Vaccination remains the most efficacious and valuable tool in the prevention of infectious diseases (6, 7, 18). However, new vaccination strategies that induce the cellular and humoral compartments of the immune response are needed for the development of effective prophylaxis against a number of infectious diseases. The immune system has two components: the innate immune response, which is a rather unspecific, first line of defense against infections (19), and the adaptive immune response, which represents specific resistance to a wide variety of antigens, weak at first, but which develops in the long term into strong memory responses (15). Adaptive responses are initiated when T and B cells recognize virtually any foreign molecule expressed on antigen-presenting cells (APCs) (37), through their unique T- and B-cell receptors, respectively. Innate responses are largely mediated by white blood cells such as neutrophils, monocytes, macrophages (Mφ), and dendritic cells (DCs) that recognize common specific antigenic structures found exclusively in microbial pathogens, termed pathogen-associated molecular patterns (PAMPs) (3).
Interestingly, the recognition of PAMPs by the innate immune system can stimulate the induction of adaptive immune responses (17). For example, DCs respond to some microbial product by taking up the antigen and concurrently synthesizing a wide variety of inflammatory mediators and cytokines that amplify the overall immune performance. In addition, DCs can process and present antigens, resulting in the activation of T- and B-cell responses and the establishment of protective immunity. As such, a number of microbial products are thought to function as effective adjuvants due to their effects on APCs, which in turn can boost the activation of an adaptive immune response. In this context, the identification of safe and effective adjuvants is of crucial importance for the development of effective vaccines. A number of novel adjuvants have been reported during the past few years, including bacterial toxins such as cholera toxin (25), endogenous immunomodulators like cytokines (33), bacterial wall components (45), and others (32).
Recent progress in understanding the innate immune responses is beginning to yield insight into the elucidation of the receptors and pathways involved and is leading to the characterization of immunostimulatory molecules that enhance these processes (20, 31). More than a decade ago, Janeway postulated that recognition of microbial PAMPs must be accomplished by specific receptors in the APCs (21). Recent studies have demonstrated that recognition of PAMPs by APCs is mediated by the Toll-like receptor (TLR) family (23, 28). There are currently 10 known TLRs capable of sensing bacterial wall components such as lipopolysaccharide (LPS) (TLR-2/4), lipoteichoic acids (TLR-2/4), CpG DNA (TLR-9), flagellin (TLR-5), and others (42). TLRs are expressed in immature and mature DCs, Mφ, and monocytes. All TLRs share a common intracellular domain (similar to the one in interleukin-1 receptors) that, upon binding of PAMPs, initiates a signaling cascade in the APCs through the adaptor protein MyD88 (43). The signal leads to the activation of NF-κB and mitogen-activated protein kinases (29) and ultimately triggers the maturation and activation of APCs (1), which include upregulation of major histocompatibility complex (MHC) and costimulatory molecules and secretion of proinflammatory cytokines and chemokines (13). Maturation of APCs significantly increases their ability to prime naive T cells. In this way, TLRs link pathogen recognition with the induction of adaptive immune responses.
Recent evidence suggests that the flagellum, the structure that confers motility (5, 8, 16) to gram-positive and -negative bacteria, enhances their pathogenicity by promoting adherence to host tissues and by directly activating host inflammatory signaling pathways (12). The component of the flagellum responsible for eliciting host immune responses is the filament protein flagellin (24). Purified or recombinant flagellin causes inflammatory responses on treated cells in vitro (22) or when systemically administered in vivo (46). This effect is mediated by the activation of TLR-5 (14) present on target APCs. It has been reported that flagellin is an effective adjuvant capable of enhancing T-cell responses in vivo (9, 27). Moreover, it has been hypothesized that flagellin can be used as an adjuvant in vaccines to induce and boost immune responses. For example, there is evidence that epitopes inserted into the flagellin of Salmonella and used as live vaccines induce specific T-cell responses against these epitopes (34, 35, 44). However, if the antigen incorporated into the flagellin interferes with the folding, transport, or assembly of the flagella, bacteria cannot present it on their surface (40), which is a major limitation to this approach. Furthermore, even the use of attenuated bacteria in vaccination represents an unknown risk. For these reasons, we hypothesized that a recombinant fusion protein incorporating both the antigen of interest and the adjuvant (flagellin) would eliminate these problems. In addition, the fusion of both elements in one single molecule would have the advantage of targeting the antigen to the APCs for uptake, processing and presentation. As a proof of concept we prepared a flagellin-EGFP fusion protein. We demonstrate that the flagellin-EGFP fusion protein activates APCs and is capable of stimulating specific T-cell responses. These data suggest that flagellin fusion proteins are suitable carriers for the development of vaccines to induce strong and specific immune responses against antigens of interest.
MATERIALS AND METHODS
Mice and cell lines.
BALB/c mice were purchased from Harlan (Indianapolis, Ind.) and housed under pathogen-free conditions. The cell lines BM-185-w.t. (wild type), BM-185 expressing EGFP (BM-185-EGFP), and BM-185 expressing EGFP and CD80 (BM-185-EGFP-CD80) were kindly provided by D. Kohn at the University of Southern California, Los Angeles. All cell lines were maintained in complete RPMI medium (RPMI 1640) supplemented with 10% fetal calf serum, 2 mM glutamine, 5 × 10−5 M 2-mercaptoethanol, and gentamicin (50 μg/ml).
Generation of a flagellin-EGFP fusion protein.
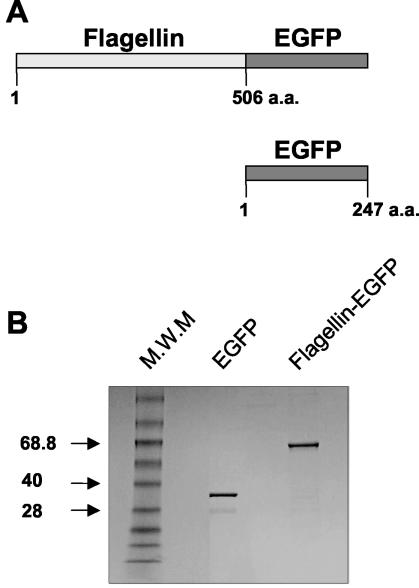
A flagellin-EGFP chimeric protein was prepared by PCR amplification of the entire fljB gene from Salmonella enterica serovar Typhimurium LT2 and the EGFP gene. The products were inserted in frame into the pRSET-A expression vector (Invitrogen) to form the chimeric gene (Fig. 1). The pRSET-A-flagellin-EGFP plasmid was used to transform E. coli BL21. One selected colony was grown at 37°C in Luria broth containing ampicillin (100 μg/ml). Stationary-phase bacteria were transferred to fresh broth, grown to an optical density of ∼0.5 at 600 nm, and supplemented with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) to induce the fusion protein synthesis. After 4 h of induction, bacteria were harvested and resuspended in chilled STE buffer (10 mM Tris [pH 8], 50 mM NaCl, 1 mM EDTA) containing lysozyme (100 μg/ml) and 0.2 mM phenylmethylsulfonyl fluoride and incubated 30 min on ice. Cells were lysed by two freeze-thaw cycles followed by sonication, and the lysates were cleared by centrifugation (15,000 × g for 30 min). This recombinant His-tagged flagellin fusion protein was purified by affinity chromatography on Ni-nitrilotriacetic acid agarose columns (Qiagen). Potential LPS contamination of the purified protein was neutralized with a polymyxin B column, and the levels of LPS were tested by the chromogenic Limulus amebocyte test. The proteins used in the experiments described below do not contain any detectable levels of endotoxin. Purified proteins were dialyzed against PBS, and each preparation was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 1) to check the size and purity of the protein, which was quantified by the Bradford assay. For control purposes, we prepared a recombinant EGFP protein using the same protocols (Fig. 1).
FIG. 1.
Schematic representation of EGFP and flagellin-EGFP fusion protein. EGFP and flagellin-EGFP were synthesized as described in Materials and Methods. (A) Diagram of EGFP and flagellin-EGFP proteins. a.a., amino acids. (B) An aliquot of each protein was loaded onto a 10% polyacrylamide gel and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Molecular weight markers (M.W.M) are indicated in thousands.
Isolation of APCs.
Bone marrow cells from BALB/c mice were depleted of lymphocytes with magnetic beads conjugated with anti-CD4, anti-CD8, and anti-B220 antibodies (Dynal, Oslo, Norway). The remaining cells were cultured in complete RPMI medium containing 3% granulocyte-macrophage colony-stimulating factor (GM-CSF) (supernatant from J558L cells transfected with the murine GM-CSF gene, obtained from R Steinman, Rockefeller University, New York, N.Y.). The medium was changed every second day, each time applying fresh RPMI medium containing 3% GM-CSF. On day 8, the cells were ready to use. These cells are a pool of activated APCs, including DCs, Mφ, and monocytes (data not shown).
Binding of flagellin-EGFP and EGFP proteins to APCs.
We took advantage of the fluorescent properties of the EGFP (which are conserved in the chimeric protein upon fusion with flagellin) for detection and localization purposes. Bone marrow-derived APCs were recovered on day 8 and plated (106) on Lab-Tek II chambered coverglasses (Nalge Nunc Int., Naperville, Ill.). They were incubated in complete medium at 37°C for 1 h with a 10-μg/ml concentration of purified flagellin-EGFP or EGFP proteins. Then, APCs were washed twice with fluorescence-activated cell sorter (FACS) buffer (PBS supplemented with 0.5% serum and 0.01% NaN3), cell fluorescence intensity was analyzed by flow cytometry (FACScan; Becton Dickinson, San Diego, Calif.), and data analysis was performed using the CellQuest software.
Maturation of APCs induced by flagellin-EGFP fusion proteins.
The maturation of pulsed APCs was confirmed by FACS analysis evaluating the levels of B7-1 and class II MHC expression. For these experiments 103 freshly isolated APCs (not cultured with GM-CSF) were incubated with 10-μg/ml concentrations of flagellin-EGFP, EGFP, and LPS (used as control, known to induce the maturation of APCs). After 24 h cells were washed and the expression of class II MHC and B7.1 was evaluated by FACS analysis. To detect the expression of surface molecules, APCs were stained with a primary murine biotinylated antibody directed against the B7.1 (PharMingen, San Diego, Calif.) or class II MHC (Pharmingen) molecules, followed by incubation with fluorescein-conjugated streptavidin. Samples were analyzed by flow cytometry and data analysis was performed using the CellQuest software.
Measurement of nitric oxide (NO) and tumor necrosis factor alpha (TNF-α) production by APCs.
NO− was measured using the Griess reaction. Culture supernatants (100 μl) from stimulated APCs were added to 100 μl of 1% sulfanilamide-0.1% naphthylethylene diamine dihydrochloride-2% H3PO4 and incubated for 10 min at room temperature. The absorbance at 562 nm was measured on an ELISA plate reader. A serial dilution of NaNO2 was used as standard. The limit of detection of this assay was 10 μM NO2−. The production of TNF-α was determined with a commercial ELISA kit (R&D Systems) on the same supernatants used for NO− measurement.
Determination of processing and presentation of antigens derived from flagellin-EGFP fusion proteins.
Cultured APCs (106) were incubated with a 10-μg/ml concentration of flagellin-EGFP, EGFP, or adenovirus-EGFP. After 24 h of incubation APCs were washed and the cytotoxic activity of anti-EGFP-specific T cells (previously established in our laboratory) against these pulsed cells was measured by a 51Cr release assay. Pulsed APCs were incubated with 150 μCi of [51Cr]sodium chromate for 1 h at 37°C. Cells were washed three times and resuspended in complete RPMI medium. For the cytotoxic assay, 51Cr-labeled target cells (104) were incubated with various concentrations of effector cells in a final volume of 200 μl in U-bottom 96-well microtiter plates. Supernatants were recovered after 6 h of incubation at 37°C and the percent lysis was determined by the formula: percent specific lysis = 100 × (experimental release − spontaneous release)/(maximum release − spontaneous release).
Stimulation of T-cell responses by a flagellin-EGFP protein.
BALB/c mice were immunized with a subcutaneous injection of flagellin-EGFP or EGFP (50 μg/injection). As a control, animals were immunized with an adenovirus-EGFP vector. Two weeks later, spleens from primed animals were removed and splenocytes were restimulated in vitro with BM-185-EGFP-CD80 cells. After 5 days, CTLs were assayed for lytic activity. The BM-185-w.t., BM-185-w.t. pulsed with the H2-Kd-HYLSTQSAL peptide (a Kd-EGFP-derived immunodominant epitope), and BM-185-EGFP cells were incubated with 150 μCi of [51Cr]sodium chromate for 1 h at 37°C. Cells were washed three times and resuspended in complete RPMI medium. For the cytotoxic assay we followed the same protocol as described above. For the CD4 proliferation assays, CD4+ T cells were enriched from spleens of immunized animals and incubated for 4 days with APCs pulsed with adenovirus-EGFP. After 3 days of incubation, tritiated thymidine (1 μCi/well for the last 18 h) was added, and thymidine incorporation was measured.
RESULTS
Binding and activation of APCs by flagellin-EGFP proteins.
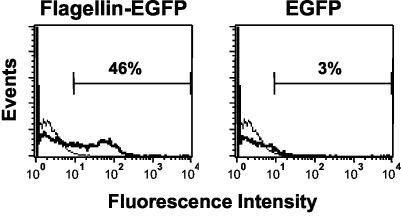
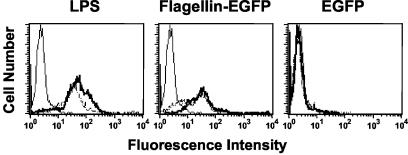
It is well established that flagellin interacts with APCs (14). To test the antigenic and immunostimulatory properties of the flagellin-EGFP fusion protein, we first analyzed whether the flagellin-EGFP protein has the capacity to bind APCs. Bone marrow-derived APCs were incubated with a 10-μg/ml concentration of the purified flagellin-EGFP or EGFP for 1 h. As demonstrated by FACS analysis (Fig. 2), almost 50% of the APCs incubated with flagellin-EGFP were fluorescent, whereas APCs incubated with EGFP showed that only 3% of the cells were fluorescent. Moreover, after only 1 h, some flagellin-EGFP is internalized into the APCs, as indicated by the appearance of green fluorescence in the cytoplasm, visualized under fluorescence microscopy (data not shown). No internalization of the recombinant-EGFP was observed after this time. Critical for any adjuvant is the effect it would have on APCs. Maturation of APCs is a requirement for the optimal stimulation of a T-cell response. Therefore, we analyzed whether flagellin-EGFP would induce the maturation of APCs. When maturation occurs, APCs increase the expression of surface molecules such as class II MHC and B7.1 (13). Freshly isolated APCs were incubated overnight in the presence of purified flagellin-EGFP or EGFP proteins or commercial LPS (used as a control known to induce maturation of APCs) and the expression of class II MHC and B7.1 molecules were analyzed. As shown in Fig. 3, APCs treated with flagellin-EGFP, as well as those treated with LPS, induced the expression of these molecules, while treatment of APCs with EGFP did not. We also evaluated the expression of B7.2. Interestingly, we observed that after stimulation with the flagellin-EGFP fusion protein, APCs also expressed B7.2, but to a lower extent than B7.1 (data not shown).
FIG. 2.
APCs are bound by flagellin-EGFP but not by EGFP. Bone marrow-derived APCs were incubated for 1 h with flagellin-EGFP or EGFP (thick lines). Thin lines represent APCs left untreated, as controls. Cells were analyzed by flow cytometry for fluorescence in the FLH-1 channel. Numbers within the plots are the percentage of APCs that showed increased fluorescence. Experiments were repeated three times with similar results. One representative experiment is shown.
FIG. 3.
Maturation of APCs is induced by LPS and by flagellin-EGFP, but not by EGFP. Freshly isolated APCs were incubated for 24 h with LPS (10 μg/ml), flagellin-EGFP, or EGFP (each at 10 μg/ml). APCs were stained to detect B7.1 (thick line) and class II MHC (broken line) molecules, or were left unstained as control (solid line), and analyzed by flow cytometry. Experiments were repeated three times with similar results. One representative experiment is shown.
A flagellin-EGFP fusion protein induces a proinflammatory response.
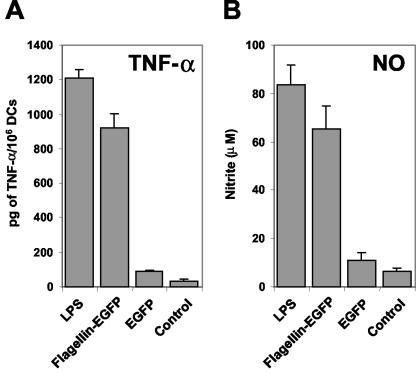
After confirming that flagellin-EGFP binds and induces the maturation of APCs, we examined whether this adjuvant-antigen chimeric protein is also capable of stimulating APCs to produce a proinflammatory response. To this end, the production of TNF-α and NO was measured. TNF-α and NO are known to be produced upon APC stimulation and to participate in the proinflammatory response (2). Cultured APCs were incubated overnight with flagellin-EGFP, EGFP, and LPS. As shown in Fig. 4, APCs treated with flagellin-EGFP produce TNF-α and NO to almost the same extent as APCs treated with LPS. In contrast, no production of TNF-α or NO is detected after treatment with recombinant EGFP. Taken together these results demonstrate not only that the flagellin-EGFP fusion protein can bind specifically to APCs, but also that this protein is capable of inducing the maturation and stimulation of APCs.
FIG. 4.
A flagellin-EGFP fusion protein induces a proinflammatory response. Cultured APCs were incubated with a 10-μg/ml concentration of LPS, flagellin-EGFP, or EGFP or were left unstimulated (control). After 24 h the cell supernatants were collected and TNF-α and NO secretion were measured. (A) TNF-α production in each culture was measured by ELISA. (B) The same supernatants were used to analyze NO production, as measured by the Griess reaction. Data are the means of three independent experiments (performed with duplicates) (error bars, standard deviations). A significant (P ≤ 0.001 [Student's t test]) difference was found between the control group and the flagellin-EGFP group.
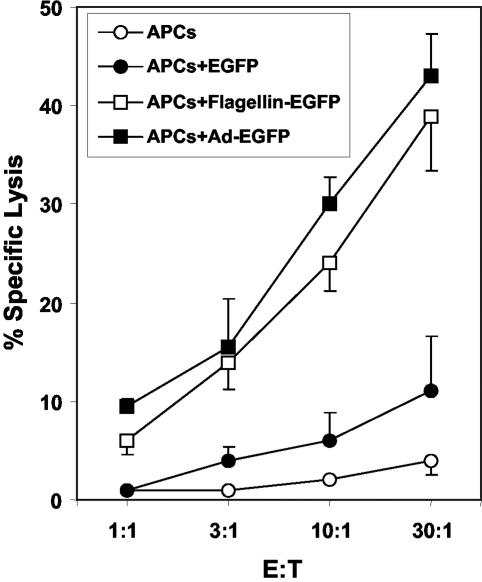
APCs pulsed in vitro with flagellin-EGFP effectively present EGFP-derived peptides.
For the induction of a specific immune response, antigens must be internalized, processed, and presented by APCs. To confirm that the antigen in the flagellin-EGFP fusion protein was efficiently processed and presented, the cytotoxic activity of specific-EGFP CD8+ T cells was tested against APCs that had been pulsed overnight with (i) flagellin-EGFP, (ii) EGFP, or (iii) adenovirus-EGFP (as a positive control for comparison). As demonstrated in Fig. 5, anti-EGFP-specific CD8+ T cells were able to effectively kill APCs pulsed with flagellin-EGFP or adenovirus-EGFP vector, while no cytotoxic activity was observed on APCs pulsed with the EGFP protein. No killing activity was observed on APCs that were not pulsed, indicating that the killing was specific. These experiments demonstrate that antigens from flagellin-EGFP are more effectively processed and presented by APCs than antigens from recombinant EGFP.
FIG. 5.
APCs pulsed with flagellin-EGFP process and present EGFP. APCs cells were pulsed for 18 h with Ad-EGFP, flagellin-EGFP, or EGFP or were unpulsed. The cytotoxic activity of an anti-EGFP-specific CD8+-T-cell line was analyzed against the pulsed APCs in a 6-h 51Cr release assay at the indicated ratios of effector to target cells (E:T). Data are the means of two independent experiments (performed with triplicates) (error bars, standard deviations). A significant (P ≤ 0.001 [Student's t test]) difference was found between the control group and the flagellin-EGFP group.
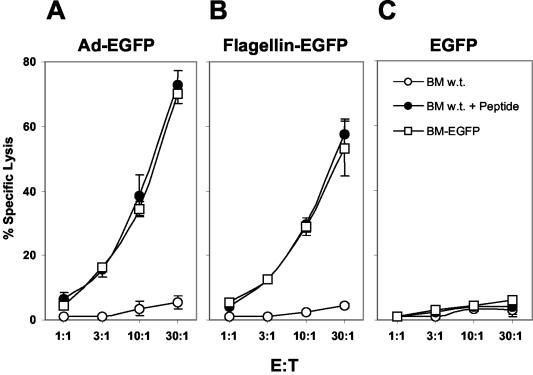
A flagellin-EGFP fusion protein induces specific T-cell responses in vivo.
The preceding results demonstrate that a flagellin-EGFP recombinant protein is bioactive and specifically activates APCs. Critical for the development of a vaccine is the induction of specific immune responses in vivo. We next tested whether mice immunized with the flagellin-EGFP fusion protein developed specific T-cell responses against EGFP. BALB/c mice were immunized with flagellin-EGFP, EGFP, and adenovirus-EGFP. For the evaluation of CD8+-T-cell responses against EGFP, we utilized the identified EGFP restricted H2-Kd-HYLSTQSAL peptide (11) and BM-185-EGFP cells (41). As shown in Fig. 6, CD8+ T cells derived from mice immunized with either adenovirus-EGFP (Fig. 6A) or flagellin-EGFP (Fig. 6B) induced comparable cytotoxic activities against both BM-185-EGFP cells and H2-Kd-HYLSTQSAL-pulsed BM-185-w.t. cells. No cytolytic activity was observed against the parental BM-185-w.t. cells, demonstrating that the immune response is specific. In contrast, immunization with recombinant EGFP did not induce a CD8+-T-cell response (Fig. 6C).
FIG. 6.
Induction of specific CD8+-T-cell responses by flagellin-EGFP fusion protein. BALB/c mice were immunized with adenovirus-EGFP (A), flagellin-EGFP (B) and EGFP (C). After 2 weeks spleens were removed, and T cells were stimulated in vitro for 5 days. Cytotoxic activity was tested against BM-185-w.t, BM-185-w.t. pulsed with the H2-Kd-EGFP-peptide, and BM-185-EGFP cells in a 6-h 51Cr release assay at the indicated ratio of effector cells to target cells (E:T). Data represent the means of results for three individually analyzed mice per group in two independent experiments (error bars, standard deviations). A significant (P ≤ 0.001 [Student's t test]) difference was found between the control group and mice immunized with flagellin-EGFP.
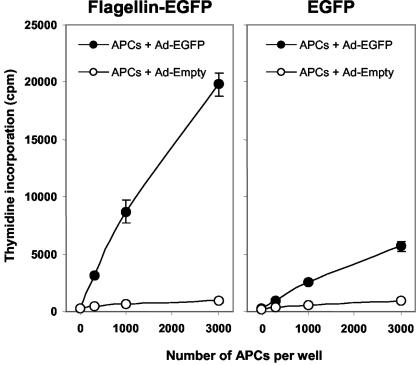
It is well established that CD4+-T-cell responses are also critical for an optimal immune response. Next, we assayed whether animals immunized with the flagellin-EGFP fusion protein stimulate a CD4+-T-cell response. BALB/c mice were immunized subcutaneously with flagellin-EGFP or EGFP proteins. Two weeks later spleens were removed and CD4+ T cells were enriched. CD4+-T-cell responses were measured in a proliferation assay against APCs pulsed with adenovirus EGFP. Our data demonstrate that CD4+ T cells from flagellin-EGFP-immunized mice proliferate after in vitro stimulation while minimal proliferation is observed on CD4+ T cells from EGFP-immunized mice (Fig. 7). Taken together, these results demonstrate that (i) the flagellin-EGFP fusion protein is phagocytosed with high efficiency by APCs when compared to soluble antigen (EGFP) and (ii) the antigen fused to the flagellin was properly processed and presented, which results in the induction of specific CD4+- and CD8+-T-cell responses.
FIG. 7.
Activation of specific CD4+ T cells by flagellin-EGFP fusion protein. BALB/c mice were immunized with flagellin-EGFP or EGFP. After 2 weeks spleens were removed, and CD4+ T cells were enriched. CD4+ T cells were incubated for 4 days with APCs pulsed with adenovirus-EGFP or empty adenovirus. After 3 days of incubation, tritiated thymidine (1 μCi/well for the last 18 h) was added, and thymidine incorporation was measured. Data show the means of results for three individually analyzed mice per group in two independent experiments (error bars, standard deviations). A significant (P ≤ 0.001 [Student's t test]) difference was found between the control group and mice immunized with flagellin-EGFP.
DISCUSSION
A major consideration in the design of vaccines is to overcome the low immunogenicity of the antigen itself, which is commonly achieved by coadministering antigens with an immunogenic carrier or adjuvant. In the development of efficient vaccines, a great need exists for the identification of safe and effective adjuvants. A number of microbial products are thought to function as effective adjuvants due to effects on APCs, which in turn promote the activation of an immune response. In this regard, it has been demonstrated that flagellin, which signals via the TLR-5 pathway (14), induces the activation of proinflammatory signals such as the secretion of TNF-α and NO (9). We hypothesize that flagellin fusion proteins can serve as adjuvants or vaccines for the induction of specific immune responses. As a proof of concept we generated a flagellin-EGFP fusion protein. Herein, we demonstrate that flagellin-EGFP is capable of inducing the maturation of APCs and the secretion of TNF-α and NO. These proinflammatory signals elicited by flagellin-EGFP are specific and derived from the flagellin component, since treatment of APCs with recombinant EGFP does not stimulate these cells. These data demonstrate that the flagellin component of the fusion protein retains its stimulatory effects. Moreover, we show that APCs loaded with the flagellin fusion protein take up, process, and present the antigen, because they are specifically recognized by anti-EGFP T cells. More importantly, animals immunized with flagellin-EGFP develop specific CD8+- and CD4+-T-cell responses, while no immune response is detected in animals immunized with recombinant EGFP. These results suggest that flagellin fusion proteins are suitable carriers for the development of adjuvants or vaccines to induce and boost immune responses against the antigen of interest.
One of the advantages of using flagellin fusion proteins is that it might be possible to effectively target APCs for the stimulation of specific immune responses. We do not yet understand the mechanism through which flagellin fusion proteins are internalized by the APCs. Sparwasser et al. (39) linked CpG-DNA to the soluble protein OVA, and under these conditions the CpG-DNA facilitates the uptake of OVA by DCs through a receptor-mediated mechanism and, at the same time, these CpG-DNA complexes retain their stimulatory potential. It is possible that not only does the binding of flagellin-EGFP to TLR-5 on APCs trigger the activation of these cells, but also the internalization of the complex and subsequent processing and presentation of the antigen result in the activation of an immune response. Further studies are needed to understand the regulation and activation of APCs following interaction of TLR-5 with flagellin. Then it would be possible to design more efficient vaccination strategies to achieve complete protective immunity.
Recently, Means et al. showed that they could not detect expression of TLR-5, by reverse transcription-PCR, on DCs isolated from C57BL/6 mice (30). In their experiments, murine DCs were not responsive to flagellin. However, Renshaw et al. (36) demonstrated that murine peritoneal Mφ express TLR-5 and that treatment with flagellin induces the production of interleukin-6 and TNF-α. Moreover, McSorley et al. demonstrated that flagellin is able to boost CD4+-T-cell responses in vivo (27). Furthermore, Madrazo et al. found that murine osteoblasts are activated after stimulation with flagellin (26). Sebastiani et al. also showed that TLR-5 is expressed in lungs and liver (38). Taken together, these results indicate that TLR-5 is expressed in different tissues and cell types, which are thus capable of interacting with flagellin. It is possible that the murine strain or the ex vivo culture conditions of the DCs affect the expression of TLR-5 and the responsiveness of these cells to flagellin and are responsible for the results obtained by Means et al. We do not discard the possibility that murine DCs do not express TLR-5. In fact, in our experiments we used a pool of APCs derived from bone marrow that in addition to DCs includes Mφ and monocytes, and these cells have the capacity to respond to the stimulation with the flagellin-EGFP fusion protein. In addition, we cannot discard the possibility that flagellin also binds to a receptor different from TLR-5 and that the effect observed by the flagellin-EGFP fusion protein may not be exclusively mediated by the TLR-5. Nevertheless, our results demonstrate that the flagellin fusion protein is active and capable of activating specific immune responses. We still need to understand how this flagellin-EGFP fusion protein activates the immune responses in vivo. Further studies are necessary to elucidate the effect that flagellin and flagellin fusion proteins exert on the different APCs.
There are several reports demonstrating that certain epitopes inserted into the flagellin of Salmonella and used as live vaccines induce specific T-cell responses against these epitopes, which further demonstrates the adjuvant abilities of flagellin (34, 35, 44). The major drawback of this approach is that if the antigen incorporated into the fusion protein interferes with the folding, transport, or assembly of flagellin, bacteria cannot express this molecule in its surface (40). Other advantages for the use of flagellin fusion proteins over epitopes inserted into flagellin and used as live vaccines are as follows. First, fusion proteins eliminate the limitation of using known epitopes of restricted size. Instead, it will be possible to incorporate in one molecule single or multiple antigens of larger size, which promises very interesting possibilities. Second, recombinant fusion proteins will be rid of the safety risk associated with live attenuated vaccines. Third, a very weak or no significant antiflagellin immune response appears to be developed upon administration of flagellin (4). A major concern for the efficacy of an adjuvant is that prior or subsequent exposures to the adjuvant may induce an immuno-neutralizing response against it, resulting in a diminished or suppressed immune response against the antigen of interest. In this regard, Ben-Yedidia and Arnon (4) demonstrated that prior exposure to flagellin did not result in an antiflagellin response and did not suppress the immune responses against influenza antigens. We have preliminary data showing that immunization with the flagellin-EGFP induces a minimal immune response against flagellin (data not shown), further supporting the findings of Ben-Yedidia and Arnon. A simple explanation as to why a strong antibody response is not induced against flagellin could be because hosts are tolerant or partially tolerant to flagellin. It is well established that a natural flora of bacteria exists in the body very early in life. As such, we might have developed some level of tolerance over time whereby T- or B-cell responses are not induced or are minimally induced against the flagellin. However, the interaction of flagellin with TLR-5 expressed on DCs, monocytes and other cells induces the activation of these cells. Finally, another advantage of using flagellin fusion proteins is that less antigen and less adjuvant might be needed relative to other vaccination methods, because the adjuvant (flagellin) would also target the fusion protein to the APCs.
Even though only weak responses are generated against the flagellin, it is still possible that any antiflagellin response could diminish the full potential of flagellin-derived vaccines. Flagellin contains conserved structures at the N- and C-terminal ends and an intervening hypervariable region (10). It has been hypothesized that the binding of flagellin to the TLR-5 is mediated by the recognition of the conserved structures in the flagellin protein. In this manner, TLR-5 expressed on APCs can guarantee the recognition of any flagellin. Recently, Eaves-Pyles et al. (10) demonstrated that the constant regions of flagellin, but not the hypervariable region, were capable of inducing a proinflammatory response in cultured human monocytes. The results indicate that the conserved region of flagellin contains the binding domain to TLR-5. The identification of flagellin minimal binding domains will facilitate the construction of smaller effective molecules, allowing easier tissue penetration and perhaps evoking milder immune responses against flagellin. The construction of other flagellin fusion proteins containing other antigens (e.g., against infectious pathogens) will determine the capacity of these proteins to provide protective immunity.
In conclusion, have we shown that flagellin fusion proteins are capable of stimulating APCs and, more importantly, that they have the capacity to induce specific immune responses. Therefore, flagellin fusion proteins are suitable carriers for the development of adjuvants or vaccines to induce and boost specific immune responses.
Acknowledgments
We thank D. Kohn (University of Southern California, Los Angeles) for providing the cell lines BM-185, BM-185-EGFP, and BM-185-EGFP-CD80 and Lynn Zacker for administrative assistance.
This work was supported by NIH grant CA 78759 (to J.L.). M.M. was supported by NIH grants AI34829 and AI52237.
Editor: D. L. Burns
REFERENCES
- 1.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 3.Barton, G. M., and R. Medzhitov. 2002. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 14:380-383. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Yedidia, T., and R. Arnon. 1998. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunol. Lett. 64:9-15. [DOI] [PubMed] [Google Scholar]
- 5.Chilcott, G. S., and K. T. Hughes. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, J., and E. Marshall. 2001. Bioterrorism. Vaccines for biodefense: a system in distress. Science 294:498-501. [DOI] [PubMed] [Google Scholar]
- 7.Curtiss, R., III. 2002. Bacterial infectious disease control by vaccine development. J. Clin. Investig. 110:1061-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeRosier, D. J. 1998. The turn of the screw: the bacterial flagellar motor. Cell 93:17-20. [DOI] [PubMed] [Google Scholar]
- 9.Eaves-Pyles, T., K. Murthy, L. Liaudet, L. Virag, G. Ross, F. G. Soriano, C. Szabo, and A. L. Salzman. 2001. Flagellin, a novel mediator of Salmonella-induced epithelial activation and systemic inflammation: I kappa B alpha degradation, induction of nitric oxide synthase, induction of proinflammatory mediators, and cardiovascular dysfunction. J. Immunol. 166:1248-1260. [DOI] [PubMed] [Google Scholar]
- 10.Eaves-Pyles, T. D., H. R. Wong, K. Odoms, and R. B. Pyles. 2001. Salmonella flagellin-dependent proinflammatory responses are localized to the conserved amino and carboxyl regions of the protein. J. Immunol. 167:7009-7016. [DOI] [PubMed] [Google Scholar]
- 11.Gambotto, A., G. Dworacki, V. Cicinnati, T. Kenniston, J. Steitz, T. Tuting, P. D. Robbins, and A. B. DeLeo. 2000. Immunogenicity of enhanced green fluorescent protein (EGFP) in BALB/c mice: identification of an H2-Kd-restricted CTL epitope. Gene Ther. 7:2036-2040. [DOI] [PubMed] [Google Scholar]
- 12.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 107:99-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 167:1882-1885. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 15.Heine, H., and E. Lien. 2003. Toll-like receptors and their function in innate and adaptive immunity. Int. Arch. Allergy Immunol. 130:180-192. [DOI] [PubMed] [Google Scholar]
- 16.Homma, M., H. Fujita, S. Yamaguchi, and T. Iino. 1987. Regions of Salmonella typhimurium flagellin essential for its polymerization and excretion. J. Bacteriol. 169:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, Q., D. Liu, P. Majewski, L. C. Schulte, J. M. Korn, R. A. Young, E. S. Lander, and N. Hacohen. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870-875. [DOI] [PubMed] [Google Scholar]
- 18.Hunter, R. L. 2002. Overview of vaccine adjuvants: present and future. Vaccine 20(Suppl. 3):S7-S12. [DOI] [PubMed] [Google Scholar]
- 19.Imler, J. L., and J. A. Hoffmann. 2001. Toll receptors in innate immunity. Trends Cell Biol. 11:304-311. [DOI] [PubMed] [Google Scholar]
- 20.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 21.Janeway, C. A., Jr. 1989. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harbor Symp. Quant. Biol. 54:1-13. [DOI] [PubMed] [Google Scholar]
- 22.Jeon, S. H., and R. Arnon. 2002. Immunization with influenza virus hemagglutinin globular region containing the receptor-binding pocket. Viral Immunol. 15:165-176. [DOI] [PubMed] [Google Scholar]
- 23.Kaisho, T., and S. Akira. 2002. Toll-like receptors as adjuvant receptors. Biochim. Biophys. Acta 1589:1-13. [DOI] [PubMed] [Google Scholar]
- 24.Lee, L. H., E. Burg III, S. Baqar, A. L. Bourgeois, D. H. Burr, C. P. Ewing, T. J. Trust, and P. Guerry. 1999. Evaluation of a truncated recombinant flagellin subunit vaccine against Campylobacter jejuni. Infect. Immun. 67:5799-5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee, S. F., S. A. Halperin, D. F. Salloum, A. MacMillan, and A. Morris. 2003. Mucosal immunization with a genetically engineered pertussis toxin S1 fragment-cholera toxin subunit B chimeric protein. Infect. Immun. 71:2272-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madrazo, D. R., S. L. Tranguch, and I. Marriott. 2003. Signaling via Toll-like receptor 5 can initiate inflammatory mediator production by murine osteoblasts. Infect. Immun. 71:5418-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McSorley, S. J., B. D. Ehst, Y. Yu, and A. T. Gewirtz. 2002. Bacterial flagellin is an effective adjuvant for CD4+ T cells in vivo. J. Immunol. 169:3914-3919. [DOI] [PubMed] [Google Scholar]
- 28.Means, T. K., D. T. Golenbock, and M. J. Fenton. 2000. Structure and function of Toll-like receptor proteins. Life Sci. 68:241-258. [DOI] [PubMed] [Google Scholar]
- 29.Means, T. K., D. T. Golenbock, and M. J. Fenton. 2000. The biology of Toll-like receptors. Cytokine Growth Factor Rev. 11:219-232. [DOI] [PubMed] [Google Scholar]
- 30.Means, T. K., F. Hayashi, K. D. Smith, A. Aderem, and A. D. Luster. 2003. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol. 170:5165-5175. [DOI] [PubMed] [Google Scholar]
- 31.Medzhitov, R., and C. Janeway, Jr. 2000. Innate immunity. N. Engl. J. Med. 343:338-344. [DOI] [PubMed] [Google Scholar]
- 32.Morse, M. A., and H. K. Lyerly. 2000. Clinical applications of dendritic cell vaccines. Curr. Opin. Mol. Ther. 2:20-28. [PubMed] [Google Scholar]
- 33.Murtaugh, M. P., and D. L. Foss. 2002. Inflammatory cytokines and antigen presenting cell activation. Vet. Immunol. Immunopathol. 87:109-121. [DOI] [PubMed] [Google Scholar]
- 34.Newton, S. M., C. O. Jacob, and B. A. Stocker. 1989. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science 244:70-72. [DOI] [PubMed] [Google Scholar]
- 35.Newton, S. M., T. M. Joys, S. A. Anderson, R. C. Kennedy, M. E. Hovi, and B. A. Stocker. 1995. Expression and immunogenicity of an 18-residue epitope of HIV1 gp41 inserted in the flagellar protein of a Salmonella live vaccine. Res. Microbiol. 146:203-216. [DOI] [PubMed] [Google Scholar]
- 36.Renshaw, M., J. Rockwell, C. Engleman, A. Gewirtz, J. Katz, and S. Sambhara. 2002. Cutting edge: impaired Toll-like receptor expression and function in aging. J. Immunol. 169:4697-4701. [DOI] [PubMed] [Google Scholar]
- 37.Schijns, V. E. 2001. Induction and direction of immune responses by vaccine adjuvants. Crit. Rev. Immunol. 21:75-85. [PubMed] [Google Scholar]
- 38.Sebastiani, G., G. Leveque, L. Lariviere, L. Laroche, E. Skamene, P. Gros, and D. Malo. 2000. Cloning and characterization of the murine toll-like receptor 5 (Tlr5) gene: sequence and mRNA expression studies in Salmonella-susceptible MOLF/Ei mice. Genomics 64:230-240. [DOI] [PubMed] [Google Scholar]
- 39.Sparwasser, T., R. M. Vabulas, B. Villmow, G. B. Lipford, and H. Wagner. 2000. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T cell responses to soluble proteins. Eur. J. Immunol. 30:3591-3597. [DOI] [PubMed] [Google Scholar]
- 40.Stocker, B. A., and S. M. Newton. 1994. Immune responses to epitopes inserted in Salmonella flagellin. Int. Rev. Immunol. 11:167-178. [DOI] [PubMed] [Google Scholar]
- 41.Stripecke, R., M. Carmen Villacres, D. Skelton, N. Satake, S. Halene, and D. Kohn. 1999. Immune response to green fluorescent protein: implications for gene therapy. Gene Ther. 6:1305-1312. [DOI] [PubMed] [Google Scholar]
- 42.Takeda, K., and S. Akira. 2003. Toll receptors and pathogen resistance. Cell Microbiol. 5:143-153. [DOI] [PubMed] [Google Scholar]
- 43.Takeuchi, O., and S. Akira. 2002. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr. Top. Microbiol. Immunol. 270:155-167. [DOI] [PubMed] [Google Scholar]
- 44.Verma, N. K., H. K. Ziegler, B. A. Stocker, and G. K. Schoolnik. 1995. Induction of a cellular immune response to a defined T-cell epitope as an insert in the flagellin of a live vaccine strain of Salmonella. Vaccine 13:235-244. [DOI] [PubMed] [Google Scholar]
- 45.Verthelyi, D., R. T. Kenney, R. A. Seder, A. A. Gam, B. Friedag, and D. M. Klinman. 2002. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J. Immunol. 168:1659-1663. [DOI] [PubMed] [Google Scholar]
- 46.Wyant, T. L., M. K. Tanner, and M. B. Sztein. 1999. Potent immunoregulatory effects of Salmonella typhi flagella on antigenic stimulation of human peripheral blood mononuclear cells. Infect. Immun. 67:1338-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]