Abstract
The importance of the neurotransmitter dopamine (DA) in the nervous system is underscored by its role in a wide variety of physiological and neural functions in both vertebrates and invertebrates. Binding of dopamine to its membrane receptors initiates precise signaling cascades that result in specific cellular responses. Dopamine receptors belong to a super-family of G-protein coupled receptors (GPCRs) that are characterized by seven trans-membrane domains. In mammals, five dopamine receptors have been identified which are grouped into two different categories D1- and D2-like receptors. The interactions of DA receptors with other proteins including specific Gα subunits are critical in deciding the fate of downstream molecular events carried out by effector proteins. In this mini-review we provide a synopsis of known protein-protein interactions of DA receptors and a perspective on the potential synergistic utility of Caenorhabditis elegans as a model eukaryote with a comparatively simpler nervous system to gain insight on the neuronal and behavioral consequences of the receptor interactions.
Keywords: Dopamine receptor, G-protein, GPCR, Gα, Caenorhabditis elegans
Background
Dopamine (DA) is a catecholamine neurotransmitter that plays a central role in nervous system development and its apt function later in life. DA is known to regulate many neuronal and physiological activities ranging from movement control to cognition, emotion, positive reinforcement, food intake, and endocrinal regulation. In mammals, cell bodies of dopaminergic neurons originate from the ventral tegmental area (VTA) of the midbrain extending processes to the cortex and limbic areas of forebrain to establish the mesocortical and mesolimbic pathways reviewed in [1]. Dysregulation of these pathways can lead to pathological states including Parkinson’s disease, Huntington’s disease, schizophrenia, psychoses, and attention deficit hyperactivity disorder reviewed in [2]. In addition, a number of socio-behavioral disorders such as alcoholism, drug addiction, and depression are also suggested to be based on DA dysfunction reviewed in [3]. As with most other complex neuronal pathways, dopamine cross talks with other neurotransmitters and a subset of traditional dopaminergic neuronal subpopulations within VTA may also co-transmit the excitatory neurotransmitter glutamate along with dopamine reviewed in [4]. Availability of a number of comprehensive reviews including those referenced above, on various aspects of dopaminergic transmission underscore the enormous effort by researchers on uncovering the functional dynamics of this key neurotransmitter. Readers are cautioned that this mini-review restricts its perspective on the utility of Caenorhabditis elegans, a model eukaryote with a simple nervous system and well-defined neural connectivity to synergize investigations into the biological relevance of molecular interactions of DA receptors, and is not intended as a comprehensive structural-functional review of dopamine triggered signaling. The worm model, offers phenomenal level of genetic accessibility, knowledge of neuronal connectivity circuits and behavioral assays. Studies in a whole organism can be useful in understanding the functional consequences of the DA receptor interactions as well as in developing potential therapeutic targets.
Precise molecular interactions determine the specificity and efficacy of dopaminergic signaling pathways, which are largely conserved in both invertebrates and vertebrates The effect of DA is transduced through cell surface G-protein coupled receptors (GPCRs) that are characterized by seven transmembrane domains and based on pharmacological profiles and binding specificity to hetero-trimeric G-proteins, they are classified as D1-like or D2-like receptors [5,6]. G-proteins consist of α, β and γ subunits and binding of DA to its receptor causes a GDP to GTP exchange in the α subunit resulting in its activation and release from the βγ heterodimer so as to recruit downstream effectors [7]. D1-like receptors signal by coupling to Gαs (stimulatory G-protein) and closely related Gαolf proteins. D2-like receptor signaling is mediated through inhibitory, Gαi/o class of G-proteins that are sensitive to pertussis toxin. Studies in the last two decades have shown that besides adenylate cyclase, there are various other effectors including ion channels, archidonic acid, mitogen activated protein kinases (MAPKs), sodium proton exchangers that can be activated by dopamine receptors [8,9]. In addition, investigators have unearthed several proteins, such as calcium-binding proteins, cytoskeletal proteins, chaperones and endoplasmic reticulum associated proteins and the more common Gα subunits, which can directly interact with D1- and D2-like receptors [9]. Here, an overview of known protein-protein interactions of dopamine receptors is provided with a focus on the utility of a model eukaryote with a comparatively simpler nervous system Caenorhabditis elegans to integrate understanding of cellular dopaminergic signaling with behavioral correlates at the whole organism level.
C. elegans is a eukaryotic experimental model that is widely used in contemporary research in molecular neurobiology due to its simpler nervous system, small genome, and availability of convenient forward and reverse genetic tools. C. elegans has been established as a successful model system to investigate various aspects of metabolic, genetic, neurobiological and neurodegenerative diseases prevalent in humans [10]. At a fundamental level, the organism shares cellular and molecular mechanisms with humans. This microscopic nematode worm has ~19,800 predicted protein-coding genes, and the fate of each of the 959 somatic cells present in the adult hermaphrodite has been tracked through development, and the connectivity for all its 302 neurons is known [11,12]. In a C. elegans hermaphrodite which is the predominant sex (>99% of wild type population) there are just eight dopaminergic neurons [11]. The completely sequenced C. elegans genome reveals that it has homologues for a majority of human genes, and this model organism has helped pioneer the identification of key genes involved in a number of important processes including development, signal transduction, cell death, neural function, and drug discovery [13]. Neuronal studies in C. elegans are favorable due to high-resolution live imaging of neurons in its transparent body and the development of various behavioral assays including development of automated worm tracking programs that allow the simultaneous monitoring of different behaviors including their endo-phenotypes [14,15].
Dynamics of dopamine signaling via G-protein coupling
Mammalian model systems have provided a broader understanding of dopamine signaling which can be divided into three major stages: (i) activation of dopamine receptors at the cell surface by ligands, (ii) interaction of receptor with heterotrimeric G-proteins, and (iii) signal transduction through effector molecules to generate cellular responses. Dopamine receptors transduce signals by coupling to G-proteins, composed of α, β and γ subunits. There are 20 known Gα subunits, grouped into 4 subfamilies (Gαs, Gαi, Gαq, and Gα12), 5 Gβ subunits and 12 Gγ subunits which participate in a wide range of cellular activities from development to signaling [16]. D1-like receptors transduce signals by coupling to Gαs (stimulatory G-protein) and a closely related Gαolf (G-protein involved in olfaction), which lead to activation of adenylyl cyclase (AC), resulting in an increase in cAMP production. D2-like receptor signaling is mediated through inhibitory Gαi/o class of G-proteins which are sensitive to pertussis toxin and cause inhibition of adenylate cyclase activity. Adenylate cyclase further activates Protein Kinase A (PKA) that results in the phosphorylation of specific downstream effector molecules some of which influence gene expression [6,17,18]. Both D1-like and D2-like receptors can also alternatively couple to other Gα proteins and Gβγ subunits. This coupling of D1-like or D2-like dopamine receptors to a diverse group of G-proteins can occur simultaneously or alternately [18].
Dopamine receptors like other GPCR proteins are characterized by 3 intracellular loops (il1-3), 3 extracellular loops (ol1-3), an extracellular amino terminus (Nt) and cytoplasmic C terminal (Ct) tail [19]. While the transmembrane regions are generally conserved amongst the GPCRs, there are variations in other regions of these proteins. Comparison between D1-like and D2-like receptors reveals that C terminal (Ct) tail in D1-like receptors is significantly longer as compared to D2-like receptors (Figure 1). On the other hand, D1-like receptors have short third intracellular loop (il3) whereas D2-like receptors are characterized by a long third intracellular loop [20,21]. Multiple isoforms of mammalian receptors exist in vivo. Isoforms of D2-like receptors are generated due to alternative splicing of the pre-mRNA in the region coding for their third intracellular loop. Two isoforms of human D1 receptor are known and result from alternate use of transcriptional initiation sites. The human D2 receptor is known to have 3 isoforms, Short, D2-Long, and D2-Longer [5,22]. The presence of splice variants for these receptors provides isoforms with a wider range of choices to couple to G-proteins. The diversity of dopamine receptor subtypes, along with the heterotrimeric nature of the G-proteins themselves, provides key components for complex signaling networks within the neuronal cell. A number of studies indicate that the third intracellular loop of DA receptors is essential for G-protein coupling and specific regions of interaction lie near the N- and C-terminal regions of the loop [23-27]. It remains to be determined if other regions of the receptor facilitate or actively participate in G-protein coupling. Additionally, the mechanism by which a receptor can selectively discriminate between closely related subtypes of G-proteins remains unclear or whether such coupling is dictated by the receptor itself. Furthermore, several aspects of receptor-G protein coupling remain unresolved. These include deducing reliable consensus sequences on the receptor for each G-protein subtype. Investigations in these directions can help unveil mechanisms governing how signals are transmitted and fine-tuned, within cells through regulation of DA receptors.
Figure 1.
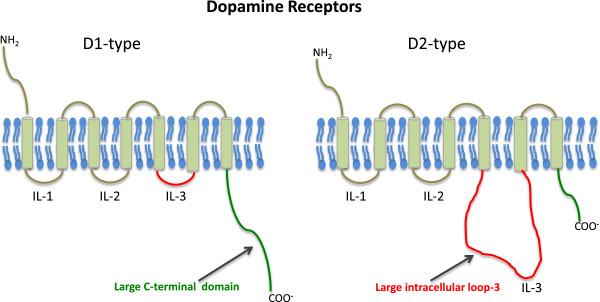
Schematic of structures highlighting comparative general differences between D1- and D2-like dopamine receptors.
Dopamine receptor protein-protein interactions
Receptors are amongst the primary targets for drugs, and the need to identify dopamine receptors’ interacting proteins that are likely to directly influence dopaminergic pathways remains fundamentally important to assess their suitability as therapeutic targets. DA receptors can interact with various proteins either through their extracellular loops, intracellular loops or C-terminal regions. Their intracellular interactions are known to occur with a variety of proteins including ion channels, cytoskeleton proteins, signaling proteins, scaffolding and adapter proteins. Even though, in general, D2-receptors act antagonistically to D1 receptors and inhibit cAMP activity, they may also show synergistic effects when co-expressed in the same neurons where they may form hetero-oligomers [28,29]. Together, D1 and D2 receptors in mammals are found to induce calcium release that is distinct from D1 or D2 receptor pathways activated independently [30-32]. It has been suggested that unusual or excessive formation of D1-D2 hetero-dimer complexes may play a part in major depression in humans, and uncoupling of such complexes in the rat model can have antidepressant effects [33]. Dopamine receptors can also crosstalk to molecular pathways initiated by other receptors or with scaffold proteins which then help integrate the two pathways. Proteins known to interact with D1- and D2-like receptors include: a chaperone protein calnexin [34], DRiP78 (Dopamine receptor-interacting protein 78) [35], calcium binding proteins S100B, cytoskeletal proteins 4.1 N [36], arrestin 2 and arrestin 3 [23]. Arrestins are important trafficking proteins that help in receptor desensitization and re-sensitization during GPCR signaling. After being exposed to continued or repeated stimulation with an agonist, receptor desensitization may occur. During desensitization, agonist induced activation of receptors is followed by receptor phosphorylation through GPCR kinases (GRKs) or Protein Kinase A [37]. Arrestins are also known to act as a scaffolding protein, promoting the stable association of signaling proteins with the receptor. Phosphorylated receptors are bound to arrestins or other interacting proteins that terminate GPCR signaling, resulting in rapid desensitization of the receptor by inhibiting receptor binding to G-proteins. Arrestins can also target receptors to clathrin-coated pits for internalization and degradation, thereby preventing re-sensitization [38,39]. Protein-protein associations of DA receptors have been suggested to determine the expression of receptors at the cell surface and maintain their density. Several cytoskeletal proteins (filamin A, protein 4.1 N and actin binding protein-ABP280) and an endoplasmic reticulum protein (calnexin) interact with D1/D2 receptors and control surface expression and receptor signaling [34,36,40]. Calcium binding proteins S100B and calmodulin bind to dopamine receptor D2 to regulate the activation of effector ERK signaling pathway [41]. D2 receptor also interacts with spinophilin (also called neurabin) that links the receptors and various downstream molecules to the actin cytoskeleton, thus regulating signaling within neurons that receive dopaminergic input [42]. A comprehensive list of known specific proteins that interact with mammalian dopamine receptors is provided along with their cellular function, and their C. elegans counterparts’ are included with the mutant phenotype of the latter (Table 1).
Table 1.
Cellular function of mammalian proteins known to interact with DA receptors and the mutant phenotypes of their worm homologues
| Mammalian DA receptor interacting proteins | C. elegans orthologues | ||
|---|---|---|---|
|
Protein |
Function [Reference] |
Orthologues |
Mutant phenotype [Reference] |
| Calcium binding protein S100B |
Increased D2 receptor stimulation of extracellular signal-regulated kinases and inhibition of adenylate cyclase [41]. |
CNB-1 |
cnb-1 mutants show thinner cuticle, decreased brood size and delayed egg laying [43]tax-6 mutants are defective in thermosensation [44]. The specific role of CAL-1 is not known. |
| CAL-1 | |||
| CNA-1 (TAX-6) | |||
| CaM Kinase II |
Phosphorylation by CaMKII sensitizes D3 receptor [45]. |
UNC-43 |
unc-43 mutation lengthens the period of the motor output as well as reduces locomotor activity [46-48]. |
| NCS-1 |
Desensitization of D2 receptor through calcium sensing [49]. |
NCS-1 |
ncs-1 knockout animals show deficits in isothermal tracking behavior [50]. |
| Arrestin 2 |
Desensitization through inhibition of D2 receptor-G-protein interaction [23]. |
ARR-1 |
arr-1 mutants are defective in olfactory adaptation [51,52]. |
| ER protein Calnexin |
Trafficking of D1 and D2 receptors to the cell surface [34]. |
CNX-1 |
At 25°C cnx-1 mutants show increased lethality, slow growth, and lower brood size [53]. |
| Protein 4.1 N |
Cell surface expression and co-localization and stability of D2 receptor subtypes [54]. |
FRM-4 |
Not known |
| FRM-10 | |||
| Spinophilin (Neurabin) |
Provides scaffold for D2 receptors and relay molecules [42]. |
NAB-1 |
nab-1 mutants display reduced synapse density and resistance to paralysis on aldicarb [55]. |
| Neurofilament M |
Regulates D1 cell surface expression, and receptor desensitization [56]. |
Not known |
Not known |
| Filamin- A |
Adaptor for linking D2-like receptors with cytoskeletal actin [40,57]. |
FLN-1 |
fln-1 mutants have defective spermatheca and reduced brood size [58]. |
| Paralemmin |
Attenuation of D2 mediated as well as receptor-independent generation of cAMP [59]. |
LMN-1 |
lmn-1 mutants show major deficits due to abnormal condensation of chromatin, abnormal distribution of nuclear pore complexes and chromosome loss in some cells [60]. |
| CLIC6 |
Interaction with D2 receptors potentially regulates chloride channel [61]. |
EXC-4 |
The tubular excretory cell lumen in exc-4 mutants is disrupted by swellings similar to cysts found in tubulocystic kidney disease [62,63]. |
| β-catenin |
D2 receptors’ interaction with β-catenin inhibits wnt/calcium signaling pathway [64]. |
SYS-1 |
sys-4 mutants display gonadogenesis defects [65]. |
| NCAM-180 |
Internalization of D2 receptors [66]. |
NCAM-1 |
Not known |
| Transient receptor potential channel TRPC1 |
D2 interaction enhances TRP1 delivery to cell surface [67]. |
TRP-4 |
trp-4 mutants are defective in proprioception and mechanosensation [68,69]. |
| GIPC |
Attenuates D2 signaling through regulator of G-protein RGS19 [70]. |
C35D10.2 |
Not known |
| Gamma COP |
Transport of D2 receptors to neuronal membrane [71]. |
T14G10.5 |
RNAi knock-down of T14G10.5 causes defects in locomotion and reduced fertility [72]. |
| DRiP78 | Transport of D1 receptors from ER to neuronal membrane [35]. | DNJ-5 | Not known |
It is emerging that dopamine receptors like other GPCRs are dynamic complexes, that involve interactions between receptor–receptor, receptor–G-protein and receptor-interacting proteins, all of which help control the intricate and finely tuned process of signal propagation. A consequence of the complexity of dopaminergic signaling is that there remain significant gaps in understanding the basic organization of GPCRs and their effectors, and therefore, there is limited understanding of precise role of dopaminergic signaling pathways in neurological diseases. Dopamine signaling studies done via ectopic expression in heterogenous cell lines have been very informative, however it is important to recognize that they do not recapitulate the multifaceted milieu provided by a functional nervous system inside an organism. Thus utilizing synergistic information obtained through a whole organism model such as Caenorhabditis elegans in analogous studies can be advantageous. This model eukaryote overcomes the isolated environment of in vitro or cellular models and allows the study of complex neural mechanisms in an intact native environment. This “whole animal system” provides the additional advantage of tracking actual behavioral responses in the animal.
Dopamine receptor function in C. elegans
In C. elegans dopamine regulates neuronal functions and participates in a wide array of nematode behaviors such as locomotion, food sensation, egg laying, defecation, learning and memory (Table 1) [73-80]. Primarily, studies with this organism have utilized either reduction in dopamine synthesis by laser ablation of dopaminergic neurons, genetic mutants, and pharmacological antagonists or enhancement of dopamine levels by providing exogenous dopamine or agonists. In the hermaphrodite worm, dopamine is synthesized in eight neurons: two anterior deirid neurons (ADEs), two posterior deirid neurons (PDEs) and four cephalic neurons (CEPs) while an additional six dopaminergic neurons are also located in the male tail [11,81,82]. Four dopamine receptors have been characterized in C. elegans and two additional receptor genes have been recently curated in the worm genome. Based on its sequence profile and pharmacological properties DOP-1 is classified as a D1-like receptor [83], DOP-2 and DOP-3 are D2-like receptors, while DOP-4 is an invertebrate specific receptor [77,84,85]. Unlike mammalian D1-like receptor isoforms that result from alternate transcriptional initiation, the four known DOP-1 variants in worms result from alternate splicing [77,86,87]. D2-like receptors in both mammals and C. elegans have two or more splice variants with differences limited primarily to the lengths of their third intracellular loops [84,88]. However DOP-4 does not have any splice variants. DOP-1 mutant studies have elucidated its role in locomotion, basal slowing and habituation response to tap stimulus [77,78,89]. The DOP-2 auto-receptor has been reported to modulate dopamine release in associative learning [79]. DOP-3 works antagonistic to the DOP-1 mediated physiological processes and signaling [90,91]. DOP-3 and DOP-4 plays a role in avoidance response to aversive soluble repellents [92,93]. Two newly curated open reading frames namely T02E9.3 and C24A8.1 represent dop-5 and dop-6 and the gene product of the latter may act redundantly with DOP-2 [94,95]. Table 2 summarizes known interactions of the C. elegans dopamine receptors along with their known roles in behavior. Additional details on specific mutant allele phenotypes, expression patterns and pharmacological profiles of DA receptors are available [85].
Table 2.
A summary of known effectors of C. elegans dopamine receptors and their physiological functions
| Receptor | G-proteins | Effectors | Physiological role |
|---|---|---|---|
| DOP-1 |
EGL-30/ Gαq |
ITR-1, PKC-1, EGL-8 |
Tap habituation, locomotion, acetylcholine release [77,78,90,91]. |
| DOP-2 |
GPA-14, GOA-1 |
Unknown |
Associative learning, anterior touch habituation, suppression of octopamine-mediated CREB activation [32,78,79,88]. |
| DOP-3 |
GOA-1/ Gαo/i |
DAG kinase (DGK-1) |
Enhancement of 2-nonanone avoidance, negative regulation of locomotion, and octanol hyper-sensitization [90,92,96]. |
| RGS protein (EAT-16) | |||
| RGS-3 | |||
| DOP-4 | Not known | Not known | General enhancement of avoidance responses [93]. |
The C. elegans genome encodes for 21 Gα, 2Gβ and 2 Gγ proteins. Four of the Gα genes goa-1, gsa-1, egl-30, gpa-12 belong to mammalian classes of Gα family Gαi/o, Gs, Gq and G12, respectively. The remaining 17 Gα genes are closer in sequence similarity to Gαi/o proteins but display limited similarity to the mammalian classes [97,98]. The Gα proteins in C. elegans also interact with dopamine receptors to transmit signal upon activation. Gαq encoded by EGL-30 was shown to mediate dopamine signaling through DA receptor DOP-1 and DOP-3 signaling is mediated through Go protein GOA-1 [78,90,91]. These two pathways function antagonistically in C. elegans and has been suggested to explain the functional effects of analogous pathways in mammalian brain that regulate neurotransmitter release by working in a manner opposite to each other [91]. Dopamine regulates various aspects of behavior in C. elegans which has been investigated using mutants in the individual DA receptors: dop-1 mutants are deficient in habituation, a simple form of non-associative learning [77,78] and dop-2 mutants are defective in habituation as well as associative learning. These abnormalities are rescued by the application of exogenous dopamine [79,80]. Upon encountering food, worms display basal slowing, which is a decrease in their rate of locomotion. Loss of function mutants of the D2-like receptor dop-3 display abnormal basal slowing responses, and mutations in dop-1 have been shown to rescue this defective response [90-98]. In addition, dop-3 deletion mutants have abnormal octanol avoidance responses (62). DOP-4 enhances repellant responses towards copper and this deficit is not rescued by providing exogenous dopamine [93]. With the exception of DOP-1, the remaining worm dopamine receptors have been shown to be involved in amphetamine (AMPH) dependent behaviors such as swimming induced paralysis (SWIP), when worms were placed in water [99]. Interestingly, the transcription factor HLH-17 influences the expression of dop-1, dop-2 and dop-3, and hlh-17 mutants display deficits in behaviors requiring a functional dopaminergic signaling [100]. It has been suggested that another transcription factor CREB known for its role in long-term memory, is modulated antagonistically by DOP-1 and DOP-3 [91,101]. In summary, studies with C. elegans have helped bridge the gap between dopamine function at a molecular and cellular level on one hand, and the neuronal output affecting behavior on the other.
Conclusions
The importance of dopamine receptors in neuronal signaling has led to numerous elegant studies that have produced an enormous amount of valuable information relevant to the molecular signaling pathways used by the D1- and D2- receptor types. The vast and sometimes contradictory results from cell culture systems, combined with the complexity of the pathways involved has inspired many researchers to study the precise role of dopamine receptors with a holistic perspective. Since the dopamine receptor interacting proteins are conserved between vertebrates and invertebrates therefore, investigating the functional role of dopamine receptors and their interacting proteins in a whole organism such as C. elegans provides a contiguous “molecule to behavior” in vivo system. Towards this end, other basic biomedical research models including fruit flies, zebra fish and mice also offer similar platforms aimed at the functional dissection of dopamine receptors’ interactions in terms of their in vivo and overt biological consequences. The advantage of C. elegans is that its behavior is controlled by a limited set of neurons whose connections can be precisely traced to provide fundamental insights in understanding complex processes in higher organisms. Greater insight into the precision and subtleties involved in dopamine receptor regulation from a simple yet complete system, will eventually require extrapolation to mammalian systems in the future.
Abbreviations
GPCRs: G-protein coupled receptors; G-protein: Guanine nucleotide-binding protein; Gαs: Stimulatory G-alpha subunit; Gαi: Inhibitory G-protein.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors (PP, MDM and HSD) contributed synergistically to conceive, write, update and review the manuscript, and approve the final manuscript.
Authors’ information
PP is a Research Scientist at the Indian Institute of Scientific Research and Education, MDM is a Graduate student at Delaware State University and HSD is an Associate Professor of Neuroscience at Delaware State University.
Contributor Information
Pratima Pandey, Email: Pratima.Sharma@babulab.org.
Mahlet D Mersha, Email: Mahlet.Mersha@gmail.com.
Harbinder S Dhillon, Email: HSDhillon@desu.edu.
Acknowledgements
Thanks to Dr. Jeff Rosen (University of Delaware) and Rosaria Formisano (Delaware State University) for useful suggestions. MDM and HSD acknowledge NIH support through NIGMS-2P20RR-016472 and NIGMS-1P20GM103653-01A1, respectively.
References
- Sillitoe RV, Vogel MW. Desire, disease, and the origins of the dopaminergic system. Schizophr Bull. 2008;8:212–219. doi: 10.1093/schbul/sbm170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast T, Heinz A. Dopamine and the diseased brain. CNS Neurol Disord Drug Targets. 2006;8:109–131. doi: 10.2174/187152706784111560. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Del’guidice T, Sotnikova TD, Leasson M, Gainetdinov RR. Beyond cAMP: The regulation of Akt and GSK3 by dopamine receptors. Front Mol Neurosci. 2011;8:1–13. doi: 10.3389/fnmol.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JI. Co-transmission of dopamine and glutamate. J Gen Physiol. 2012;8:93–96. doi: 10.1085/jgp.201110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich JA, Caron MG. Recent advances in the molecular biology of dopamine receptors. Annu Rev Neurosci. 1993;8:299–321. doi: 10.1146/annurev.ne.16.030193.001503. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;8:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- New DC, Wong YH. Molecular mechanisms mediating the G protein-coupled receptor regulation of cell cycle progression. J Mol Signal. 2007;8:2. doi: 10.1186/1750-2187-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Feng J, Fienberg AA, Greengard P. D2 dopamine receptors induce mitogen-activated protein kinase and cAMP response element-binding protein physphorylation in neurons. Proc Natl Acad Sci U S A. 1999;8:11607–11612. doi: 10.1073/pnas.96.20.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. Recept Signal Transduct Res. 2004;8(3):165–205. doi: 10.1081/RRS-200029981. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Gabel C, Ferree A, Guillily M, Eata A. Watching worms whither: modeling neurodegeneration in C. elegans. Prog Mol Biol Transl Sci. 2011;8:499–514. doi: 10.1016/B978-0-12-384878-9.00015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system in the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;8:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Hillier LW, Marth GT, Quinlan AR, Dooling D, Fewell G, Barnett D, Fox P, Glasscock JI, Hickenbotham M, Huang W, Magrini VJ, Richt RJ, Sander SN, Stewart DA, Stromberg M, Tsung EF, Wylie T, Schedl T, Wilson RK, Mardis ER. Whole-genome sequencing and variant discovery in C. elegans. Nat Methods. 2008;8:183–188. doi: 10.1038/nmeth.1179. [DOI] [PubMed] [Google Scholar]
- Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;8:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- Dusenbery DB. Using a microcomputer and video camera to simultaneously track 25 animals. Comput Biol Med. 1985;8(4):169–175. doi: 10.1016/0010-4825(85)90058-7. [DOI] [PubMed] [Google Scholar]
- Swierczek NA, Giles AC, Rankin CH, Kerr RA. High-throughput behavioral analysis in C. elegans. Nat Methods. 2011;8(7):592–598. doi: 10.1038/nmeth.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA. Greengard P the neurobiology of dopamine signaling. Arch Neurol. 2004;8:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Huff RM, Chio CL, Lajiness ME, Goodman LV. Signal transduction pathways modulated by D2-like dopamine receptors. Adv Pharmacol. 1998;8:454–457. doi: 10.1016/s1054-3589(08)60786-3. [DOI] [PubMed] [Google Scholar]
- Sidhu A, Kimura K, Uh M, White BH, Patel S. Multiple coupling of human D5 dopamine receptors to guanine nucleotide binding proteins Gs and Gz. J Neurochem. 1998;8:2459–2467. doi: 10.1046/j.1471-4159.1998.70062459.x. [DOI] [PubMed] [Google Scholar]
- Strader CD, Fong TM, Tota MR, Underwood D, Dixon RA. Structure and function of G protein-coupled receptors. Annu Rev Biochem. 1994;8:101–132. doi: 10.1146/annurev.bi.63.070194.000533. [DOI] [PubMed] [Google Scholar]
- Probst WC, Snyder LA, Schuster DI, Brosius J, Sealfon SC. Sequence alignment of the G-protein coupled receptor superfamily. DNA Cell Biol. 1992;8:1–20. doi: 10.1089/dna.1992.11.1. [DOI] [PubMed] [Google Scholar]
- Civelli O, Bunzow JR, Grandy DK. Molecular diversity of the dopamine receptors. Annu Rev Pharmacol Toxicol. 1993;8:281–307. doi: 10.1146/annurev.pa.33.040193.001433. [DOI] [PubMed] [Google Scholar]
- Vernier P, Cardinaud B, Valdenaire O, Philippe H, Vincent JD. An evolutionary view of drug-receptor interaction: the bioamine receptor family. Trends Pharmacol Sci. 1995;8:375–381. doi: 10.1016/S0165-6147(00)89078-1. [DOI] [PubMed] [Google Scholar]
- Macey TA, Gurevich VV, Neve KA. Preferential interaction between the dopamine D2 receptor and arrestin2 in neostriatal neurons. Mol Pharmacol. 2004;8:1635–1642. doi: 10.1124/mol.104.001495. [DOI] [PubMed] [Google Scholar]
- Simonovic M, Soskic V, Joksimovic J. Quantification of human dopamine D2s receptor interactions with G αi,1,2 and Gα proteins. Neurochem Int. 1998;8:271–275. doi: 10.1016/S0197-0186(98)00031-X. [DOI] [PubMed] [Google Scholar]
- Filteau F, Veilleux F, Lévesque D. Effects of reciprocal chimeras between the C-terminal portion of third intracellular loops of the human dopamine D2 and D3 receptors. FEBS Lett. 1999;8:251–256. doi: 10.1016/S0014-5793(99)00290-2. [DOI] [PubMed] [Google Scholar]
- Senogles SE, Heimert TL, Odife ER, Quasney MW. A region of the third intracellular loop of the short form of the D2 dopamine receptor dictates Gi coupling specificity. J Biol Chem. 2004;8:1601–1606. doi: 10.1074/jbc.M309792200. [DOI] [PubMed] [Google Scholar]
- Johnston CA, Siderovski DP. Structural basis for nucleotide exchange on Gαi-subunits and receptor coupling specificity. Proc Natl Acad Sci U S A. 2007;8:2001–2006. doi: 10.1073/pnas.0608599104. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Waszczak BL, Martin LP, Finlay HE, Zahr N, Stellar JR. Effects of individual and concurrent stimulation of striatal D1 and D2 dopamine receptors on electrophysiological and behavioral output from rat basal ganglia. J Pharmacol Exp Ther. 2002;8:850–861. doi: 10.1124/jpet.300.3.850. [DOI] [PubMed] [Google Scholar]
- Nolan EB, Harrison LM, Lahoste GJ, Ruskin DN. Behavioral synergism between D(1) and D(2) dopamine receptors in mice does not depend on gap junctions. Synapse. 2007;8:279–287. doi: 10.1002/syn.20371. [DOI] [PubMed] [Google Scholar]
- Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O'Dowd BF, George SR. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;8:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci U S A. 2007;8:654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo S, Culotti JG, Van Tol HH. Dopamine counteracts octopamine signalling in a neural circuit mediating food response in C. elegans. EMBO J. 2009;8:2437–2448. doi: 10.1038/emboj.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Li S, Wang M, Diwan M, Anisman H, Fletcher PJ, Nobrega JN, Liu F. Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat Med. 2010;8:393–395. doi: 10.1038/nm.2263. [DOI] [PubMed] [Google Scholar]
- Free RB, Hazelwood LA, Cabrera DM, Spalding HN, Namkung Y, Rankin ML, Sibley DR. D1 and D2 dopamine receptor expression is regulated by direct interaction with the chaperone protein calnexin. J Biol Chem. 2007;8:21285–21300. doi: 10.1074/jbc.M701555200. [DOI] [PubMed] [Google Scholar]
- Bermak JC, Li M, Bullock C, Zhou QY. Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol. 2001;8:492–498. doi: 10.1038/35074561. [DOI] [PubMed] [Google Scholar]
- Binda F, Dipace C, Bowton E, Robertson SD, Lute BJ, Fog JU, Zhang M, Sen N, Colbran RJ, Gnegy ME, Gether U, Javitch JA, Erreger K, Galli A. Syntaxin 1A interaction with the dopamine transporter promotes amphetamine-induced dopamine efflux. Mol Pharmacol. 2008;8:1101–1108. doi: 10.1124/mol.108.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;8:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- Pippig S, Andexinger S, Lohse MJ. Sequestration and recycling of beta 2-adrenergic receptors permit receptor resensitization. Mol Pharmacol. 1995;8:666–676. [PubMed] [Google Scholar]
- Tsao LI, Hayashi T, Su TP. Blockade of dopamine transporter and tyrosine hydroxylase activity loss by D-Ala(2), D-Leu(5)]enkephalin in methamphetamine-treated CD-1 mice. Eur J Pharmacol. 2000;8:89–93. doi: 10.1016/S0014-2999(00)00616-6. [DOI] [PubMed] [Google Scholar]
- Li M, Bermak JC, Wang ZW, Zhou QY. Modulation of dopamine D(2) receptor signaling by actin-binding protein (ABP-280) Mol Pharmacol. 2000;8:446–452. doi: 10.1124/mol.57.3.446. [DOI] [PubMed] [Google Scholar]
- Liu Y, Buck DC, Neve KA. Novel interaction of the dopamine D2 receptor and the Ca2+ binding protein S100B: role in D2 receptor function. Mol Pharmacol. 2008;8:371–378. doi: 10.1124/mol.108.044925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FD, Oxford GS, Milgram SL. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J Biol Chem. 1999;8:19894–19900. doi: 10.1074/jbc.274.28.19894. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay J, Lee J, Lee J, Yu J, Jee C, Cho J, Jung S, Hee Lee M, Zannoni S, Singson A, Kim D, Koo H, Ahnn J. Calcineurin, a Calcium/Calmodulin-dependent Protein Phosphatase, Is Involved in Movement, Fertility, Egg Laying, and Growth in Caenorhabditis elegans. Mol Biol Cell. 2002;8:3281–3293. doi: 10.1091/mbc.E02-01-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhara A, Inada H, Katsura I, Mori I. Negative regulation and gain control of sensory neurons by the C. elegans calcineurin TAX-6. Neuron. 2002;8:751–763. doi: 10.1016/S0896-6273(02)00607-4. [DOI] [PubMed] [Google Scholar]
- Guo ML, Liu XY, Mao LM, Wang JQ. Regulation of dopamine D3 receptors D3 receptors by protein-protein interactions. Neurosci Bull. 2010;8:163–167. doi: 10.1007/s12264-010-1016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner DJ, Newton EM, Tian H, Thomas JH. Diverse behavioural defects caused by mutations in Caenorhabditis elegans unc-43 CaM kinase II. Nature. 1999;8:199–203. doi: 10.1038/46072. [DOI] [PubMed] [Google Scholar]
- Robatzek M, Thomas JH. Calcium/calmodulin-dependent protein kinase II regulates Caenorhabditis elegans locomotion in concert with a G(o)/G(q) signaling network. Genetics. 2000;8:1069–1082. doi: 10.1093/genetics/156.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemura T, Rapp P, Rongo C. The role of regulatory domain interaction in UNC-43 CaMKII localization and trafficking. J Cell Sci. 2005;8:3327–3338. doi: 10.1242/jcs.02457. [DOI] [PubMed] [Google Scholar]
- Kabbani N, Negyessy L, Lin R, Goldman-Rakic P, Levenson R. Interaction with neuronal calcium sensor NCS-1 mediates desensitization of the D2 dopamine receptor. J Neurosci. 2002;8:8476–8486. doi: 10.1523/JNEUROSCI.22-19-08476.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, Bartfai T, Bargmann CI, Nef P. Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron. 2001;8:241–248. doi: 10.1016/S0896-6273(01)00276-8. [DOI] [PubMed] [Google Scholar]
- Fukuto HS, Ferkey DM, Apicella AJ, Lans H, Sharmeen T, Chen W, Lefkowitz RJ, Jansen G, Schafer WR, Hart AC. G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron. 2004;8:581–593. doi: 10.1016/S0896-6273(04)00252-1. [DOI] [PubMed] [Google Scholar]
- Palmitessa A, Hess HA, Bany IA, Kim YM, Koelle MR, Benovic JL. Caenorhabditis elegans arrestin regulates neural G protein signaling and olfactory adaptation and recovery. J Biol Chem. 2005;8:24649–24662. doi: 10.1074/jbc.M502637200. [DOI] [PubMed] [Google Scholar]
- Lee W, Lee TH, Park BJ, Chang JW, Yu JR, Koo HS, Park H, Yoo YJ, Ahnn J. Caenorhabditis elegans calnexin is N-glycosylated and required for stress response. Biochem Biophys Res Commun. 2005;8:1018–1030. doi: 10.1016/j.bbrc.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Binda A, Kabbani N, Lin R, Levenson R. D2 and D3 Dopamine recetor cell surface localization mediated by interaction with protein 4.1 N. Mol Pharmacol. 2002;8:506–513. doi: 10.1124/mol.62.3.507. [DOI] [PubMed] [Google Scholar]
- Sieburth D, Ch’ng Q, Dybbs M, Tavazoie M, Kennedy S, Wang D, Dupuy D, Rual JF, Hill DE, Vidal M, Ruvkun G, Kaplan JM. Systematic analysis of genes required for synapse structure and function. Nature. 2005;8:510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- Kim O, Ariano MA, Lazzarini RA, Levine MS, Sibley DR. Neurofilament-M interacts with the D1 dopamine receptor to regulate cell surface expression and desensitization. J Neurosci. 2002;8:5920–5930. doi: 10.1523/JNEUROSCI.22-14-05920.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Leneson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci U S A. 2001;8:5258–5263. doi: 10.1073/pnas.011538198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic I, Cram EJ. FLN-1/filamin is required for maintenance of actin and exit of fertilized oocytes from the spermatheca in C. elegans. Dev Biol. 2010;8:247–257. doi: 10.1016/j.ydbio.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile M, Lin R, Kabbani N, Karpa K, Kilimann M, Simpson I, Kester M. Paralemmin interacts with D3 dopamine receptors: implications for membrane localization and cAMP signaling. Arch Biochem Biophys. 2006;8:60–68. doi: 10.1016/j.abb.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Liu J, Ben-Shahar TR, Riemer D, Treinin M, Spann P, Weber K, Fire A, Gruenbaum Y. Essential roles for Caenorhabditis elegans lamin gene in nuclear organization, cell cycle progression, and spatial organization of nuclear pore complexes. Mol Biol Cell. 2000;8:3937–3947. doi: 10.1091/mbc.11.11.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffon N, Jeanneteau F, Prieur F, Diaz J, Sokoloff P. CLIC6, a member of the intracellular chloride channel family, interacts with dopamine D2-like receptors. Brain Res Mol Res. 2003;8:47–57. doi: 10.1016/S0169-328X(03)00283-3. [DOI] [PubMed] [Google Scholar]
- Berry KL, Bulow HE, Hall DH, Hobert O. A C. elegans CLIC-like protein required for intracellular tube formation and maintenance. Science. 2003;8:2134–2137. doi: 10.1126/science.1087667. [DOI] [PubMed] [Google Scholar]
- Buechner M, Hall DH, Bhatt H, Hedgecock EM. Cystic canal mutant in Caenorhabditis elegans are defective in apical membrane domain of the renal (excretory) cell. Dev Biol. 1999;8:227–241. doi: 10.1006/dbio.1999.9398. [DOI] [PubMed] [Google Scholar]
- Min C, Cho DI, Kwon KJ, Kim KS, Shin CY, Kim KM. Novel regulatory mechanism of canonical Wnt signaling by dopamine D2 receptor through direct interaction with beta-catenin. Mol Pharmacol. 2011;8:68–78. doi: 10.1124/mol.111.071340. [DOI] [PubMed] [Google Scholar]
- Siegfried KR, Kidd AR, Chesney MA, Kimble J. The sys-1 and sys-3 genes cooperate with Wnt signaling to establish the proximal-distal axis of the Caenorhabditis elegans gonad. Genetics. 2004;8:171–186. doi: 10.1534/genetics.166.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao MF, Xu JC, Tereshchenko Y, Novak D, Schachner M, Kleene R. Neural cell adhesion molecule modulates dopaminergic signaling and behavior by regulating dopamine D2 receptor internalization. J Neurosci. 2009;8:14752–14763. doi: 10.1523/JNEUROSCI.4860-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan MA, Kabbani N, Paspalas CD, Leneson R. Interaction with dopamine D2 receptor enhances expression of transient receptor potential channel 1 at the cell surface. Biochem Biophys Acta. 2008;8:974–982. doi: 10.1016/j.bbamem.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Gao J, Schafer WR, Xie Z, Xu XZ. C. elegans TRP family protein TRP-4 is a pore-forming subunit of a native mechanotransduction channel. Neuron. 2010;8:381–391. doi: 10.1016/j.neuron.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Feng Z, Sternberg PW, Shawn XZX. A C. elegans stretch receptor neuron revealed by a mechanosensitive TRP channel homologue. Nature. 2006;8:684–687. doi: 10.1038/nature04538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F, Diaz J, Sokoloff Griffon NP. Interactions of GIPC with dopamine D2 and D3 but not D3 receptors define a novel mode of regulation of G protein-coupled receptor. Mol Biol Cell. 2004;8:696–705. doi: 10.1091/mbc.E03-05-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermak JC, Li M, Bullock C, Weingarten P, Zhou QY. Interaction of gamma-COP with a transport motif in the D1 receptor C-terminus. Eur J Cell Biol. 2002;8:77–85. doi: 10.1078/0171-9335-00222. [DOI] [PubMed] [Google Scholar]
- Simmer F, Moorman C, van der Linden AM, Kuijk E, Van Den Berghe PV, Kamath RS, Fraser AG, Ahringer J, Plasterk RH. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 2003;8:E12. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer WR, Kenyon CJ. A calcium-channel homologue required for adaptation to dopamine and serotonin in Caenorhabditis elegans. Nature. 1995;8:73–78. doi: 10.1038/375073a0. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Garriga G, Thomas JH. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J Neurosci. 1995;8:6975–6985. doi: 10.1523/JNEUROSCI.15-10-06975.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;8:619–631. doi: 10.1016/S0896-6273(00)81199-X. [DOI] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci. 2004;8:1217–1225. doi: 10.1523/JNEUROSCI.1569-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hébert TW, van der Kooy D, Schafer WR, Culotti JG, Van Tol HH. Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J. 2004;8:473–482. doi: 10.1038/sj.emboj.7600057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR. Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron. 2007;8:662–676. doi: 10.1016/j.neuron.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N. A synaptic DEGENaC ion channel mediates learning in C. elegans by facilitating dopamine signaling. EMBO J. 2008;8:3288–3299. doi: 10.1038/emboj.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersha M, Formisano R, McDonald R, Pandey P, Tavernarakis N, Harbinder S. GPA-14, a Gαi subunit mediates dopaminergic behavioral plasticity in C. elegans. Behav Brain Funct. 2013;8:9–16. doi: 10.1186/1744-9081-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston J, Brenner S. Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol. 1975;8:215–226. doi: 10.1002/cne.901630207. [DOI] [PubMed] [Google Scholar]
- McDonald PW, Jessen T, Field JR, Blakely RD. Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol Neurobiol. 2006;8:593–618. doi: 10.1007/s10571-006-9003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S. Identification of a dopamine receptor from Caenorhabditis elegans. Neurosci Lett. 2002;8:13–16. doi: 10.1016/S0304-3940(01)02477-6. [DOI] [PubMed] [Google Scholar]
- Suo S, Sasagawa N, Ishiura S. Cloning and characterization of a Caenorhabditis elegans D2-like dopamine receptor. J Neurochem. 2003;8:869–878. doi: 10.1046/j.1471-4159.2003.01896.x. [DOI] [PubMed] [Google Scholar]
- Chase DL, Koelle MR. Biogenic amine neurotransmitters in C. elegans. WormBook, the online review of C. elegans biology. WormBook. 2007. 10.1895/wormbook.1.132.1. [DOI] [PMC free article] [PubMed]
- Sidhu A, Olde B, Humblot N, Kimura K, Gardner N. Regulation of human D1 dopamine receptor function and gene expression in SK-N-MC neuroblastoma cells. Neuroscience. 1999;8:537–547. doi: 10.1016/S0306-4522(98)00555-7. [DOI] [PubMed] [Google Scholar]
- Tsalik EL, Niacaris T, Wenick AS, Pau K, Avery L, Hobert O. LIM homeobox gene-dependent expression of biogenic amine receptors in restricted regions of the C. elegans nervous system. Dev Biol. 2003;8:81–102. doi: 10.1016/S0012-1606(03)00447-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Harbinder S. The Caenorhabditis elegans D2-like dopamine receptor DOP-2 physically interacts with GPA-14, a Gαi subunit. J Mol Signaling. 2012;8:1–10. doi: 10.1186/1750-2187-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Rankin CH. Analyses of habituation in Caenorhabditis elegans. Learn Mem. 2001;8:63–69. doi: 10.1101/lm.37801. [DOI] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 2004;8:1096–1103. doi: 10.1038/nn1316. [DOI] [PubMed] [Google Scholar]
- Allen AT, Maher KN, Wani KA, Betts KE, Chase DL. Coexpressed D1- and D2-like dopamine receptors antagonistically modulate acetylcholine release in Caenorhabditis elegans. Genetics. 2011;8:579–590. doi: 10.1534/genetics.111.128512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezak MJ, Ferkey DM. The C. elegans D2-like dopamine receptor DOP-3 decreases behavioral sensitivity to the olfactory stimulus 1-octanol. PLoS One. 2010;8:e9487. doi: 10.1371/journal.pone.0009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezcurra M, Tanizawa Y, Swaboda P, Schafer WR. Food sensitizes C. elegans avoidance behaviors through acute dopamine signaling. EMBO J. 2011;8:1110–11122. doi: 10.1038/emboj.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaye DD, Greenwald I. OrthoList: a compendium of C. elegans genes with human orthologs. PLoS One. 2011;8:e20085. doi: 10.1371/journal.pone.0020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Hashimshony T, Wagner F, Yanai I. Developmental milestones punctuate gene expression in the Caenorhabditis embryo. Dev Cell. 2012;8:1101–1108. doi: 10.1016/j.devcel.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Kimura KD, Fujita K. Katsura I enhancement of odor avoidance regulated by dopamine signaling in Caenorhabditis elegans. J Neurosci. 2010;8:16365–16375. doi: 10.1523/JNEUROSCI.6023-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G, Thijssen KL, Werner P, Van der Horst M, Hazendonk E, Plasterk RH. The complete family of genes encoding G-proteins of Caenorhabditis elegans. Nat Genet. 1999;8:414–419. doi: 10.1038/7753. [DOI] [PubMed] [Google Scholar]
- Cuppen E, van der Linden AM, Jansen G, Plasterk RH. Proteins interacting with Caenorhabditis elegans Gα subunits. Comp Funct Genom. 2003;8:479–491. doi: 10.1002/cfg.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli L, Matthies DS, Galli A. Molecular Mechanisms of amphetamine actions in Caenorhabditis elegans. Mol Pharmacol. 2010;8:151–156. doi: 10.1124/mol.109.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felton CM, Johnson CM. Modulation of dopamine-dependent behaviors by the Caenorhabditis elegans Olig homolog HLH-17. J Neurosci Res. 2011;8:1627–1636. doi: 10.1002/jnr.22694. [DOI] [PubMed] [Google Scholar]
- Suo S, Ishiura S. Dopamine modulates acetylcholine release via octopamine and CREB signaling in Caenorhabditis elegans. PLoS One. 2013;8:1–7. doi: 10.1371/journal.pone.0072578. [DOI] [PMC free article] [PubMed] [Google Scholar]


