Abstract
Cryptosporidium parvum invasion of epithelial cells involves host cell membrane alterations which require a remodeling of the host cell actin cytoskeleton. In addition, an actin plaque, possibly associated with the dense-band region, forms within the host cytoplasm at the host-parasite interface. Here we show that Cdc42 and RhoA, but not Rac1, members of the Rho family of GTPases, are recruited to the host-parasite interface in an in vitro model of human biliary cryptosporidiosis. Interestingly, activation of Cdc42, but not RhoA, was detected in the infected cells. Neural Wiskott-Aldrich syndrome protein (N-WASP) and p34-Arc, actin-regulating downstream effectors of Cdc42, were also recruited to the host-parasite interface. Whereas cellular expression of a constitutively active mutant of Cdc42 promoted C. parvum invasion, overexpression of a dominant negative mutant of Cdc42, or depletion of Cdc42 mRNA by short interfering RNA-mediated gene silencing, inhibited C. parvum invasion. Expression of the WA fragment of N-WASP to block associated actin polymerization also inhibited C. parvum invasion. Moreover, inhibition of host cell Cdc42 activation by dominant negative mutation inhibited C. parvum-associated actin remodeling, membrane protrusion, and dense-band formation. In contrast, treatment of cells with a Rho inhibitor, exoenzyme C3, or cellular overexpression of dominant negative mutants of RhoA and Rac1 had no effect on C. parvum invasion. These data suggest that C. parvum invasion of target epithelia results from the organism's ability to activate a host cell Cdc42 GTPase signaling pathway to induce host cell actin remodeling at the attachment site.
Cryptosporidium parvum is a protozoan parasite that primarily infects intestinal epithelia, producing self-limited disease in immunocompetent persons. In contrast, C. parvum can also infect other types of epithelia, including biliary epithelial cells, and cause a potentially life-threatening illness in immunocompromised persons, especially those with the AIDS (10, 17, 41). To date, no consistently effective antimicrobial agent is available (12). When ingested, C. parvum oocysts excyst in the gastrointestinal tract and release infective sporozoites. Mediated by uncharacterized ligands on the sporozoite surface and unidentified receptors on the host cell plasma membrane, the sporozoite attaches to the apical membrane of the host epithelial cell inducing membrane protrusions that encapsulate the sporozoite and form a parasitophorous vacuole. Underlying the parasitophorous vacuole within the host cell cytoplasm, a dense-band structure of unknown composition is formed that presumably separates the organism from the host cell cytoplasm. Thus, the parasite exists in an intramembranous but extracytoplasmic compartment, a position that is different from that occupied by other microbes and that may protect the parasite from antimicrobial drugs (12). The molecular details of how C. parvum infection results in host cell membrane alterations and dense-band formation in this unusual process of invasion are unclear.
Actin is a critical component of receptor-mediated endocytosis and phagocytosis in a variety of cell types, including epithelial cells lining the intestinal tract and biliary tree (35). Recent studies have demonstrated that actin cytoskeleton remodeling induced by microbial pathogens facilitates infection. For example, Salmonella enterica serovar Typhimurium and Chlamydia trachomatis induce remodeling of host cell actin cytoskeleton for internalization (6, 25), while enteropathogenic Escherichia coli activates host cell actin aggregation to form a pedestal structure at the attachment site (26). Recent studies by us and others suggest that C. parvum infection results in host cell actin remodeling, with actin filaments accumulating at the host-parasite interface (9, 16, 18) and in the protrusive membranes that engulf the invading parasite (4). Moreover, actin-related protein 2/3 (Arp2/3), an important actin-binding protein complex and critical initiators of actin polymerization, is recruited to the host-parasite interface (17). An accumulation of cytoskeleton filaments is also observed by electron microscopy in the region of dense-band formation (1, 4). Indeed, C. parvum invasion of host epithelial cells appears to require host cell actin polymerization, while Toxoplasma gondii, another parasite in the same group, does not (14, 16-18). Cell invasion by C. parvum is blocked by cytochalasin B and cytochalasin D (9, 18) or by cellular expression of specific inhibitory fragments of actin-associated proteins, such as Scar-WA (17).
Various host cell signaling pathways have been implicated in host cell cytoskeleton-based invasion by pathogenic microbes, including parasites such as Trypanosoma cruzi, Leishmania amazonensis, and Plasmodium falciparum (13, 31, 43). We recently demonstrated that C. parvum attachment to cultured human biliary epithelial cells activates c-Src, a membrane-associated tyrosine kinase, resulting in tyrosine phosphorylation of cortactin, an actin-binding protein, and subsequently, actin remodeling at the host-parasite interface (11). However, inhibition of c-Src and cortactin function only partially blocked C. parvum-associated actin remodeling (11), suggesting involvement of other signaling pathways as well. Cdc42, RhoA and Rac1 are members of the Rho family of small guanosine triphosphatases (GTPases) and are key regulators of actin cytoskeletal remodeling induced by extracellular signals. Moreover, members of this family of GTPases have been implicated in the host cell invasion by many microbes including bacteria and protozoan pathogens (20, 22). Whether these GTPases are involved in C. parvum-associated host cytoskeleton remodeling and whether they thus may be required for C. parvum invasion of host cells remain unclear.
In the present study, we show that C. parvum recruits Cdc42 and RhoA, but not Rac1, to the host-parasite interface in an in vitro model of human biliary cryptosporidiosis. Activation of Cdc42, but not RhoA, was detected in infected cells. Both p34-Arc, an important member of the Arp2/3 complex and a downstream effector of Cdc42, and neural Wiskott-Aldrich syndrome protein (N-WASP), another downstream effector of Cdc42 previously reported to be recruited to the host-parasite interface during C. parvum infection of intestinal epithelial cells (17), were also recruited to the host-parasite interface in infected biliary epithelial cells. Inhibition of Cdc42 by overexpression in cells of a dominant mutant or suppression of Cdc42 mRNA through small interfering RNA (siRNA) silencing techniques were associated with a reduction of C. parvum-associated actin remodeling and membrane protrusion and, ultimately, C. parvum invasion. Treatment of cells with a Rho inhibitor, exoenzyme C3, or overexpression in host cells of dominant negative mutants to RhoA or Rac1, had no significant effect on C. parvum invasion. These findings demonstrate that C. parvum invasion of human biliary epithelial cells is facilitated by recruitment and activation of Cdc42 to the host-parasite interface, a process required for actin-associated host cell membrane protrusion and dense-band formation.
MATERIALS AND METHODS
C. parvum and H69 cells.
C. parvum oocysts of the Iowa strain were purchased from a commercial source (Pleasant Hill Farms, Troy, Idaho). Before infecting our human biliary epithelial cell line (see below), oocysts were treated with 1% sodium hypochlorite on ice for 20 min and then exposed to an excystation solution consisting of 0.75% taurodeoxycholate and 0.25% trypsin for 30 min at 37°C. After washing with Dulbecco's modified Eagle medium (DMEM)-F-12 medium (Bio Whittaker, Walkersville, Md.), freshly excysted sporozoites were collected by centrifugation at 1,000 × g for 5 min. The viability of excysted sporozoites was then determined as previously described by others (5, 32). Only those with a viability higher than 90% were used for experiments. H69 cells (a gift of D. Jefferson, Tufts University, Boston, Mass.) are simian virus 40-transformed human bile duct epithelial cells originally derived from a normal liver harvested for transplant and have been extensively characterized previously (21). For experiments, H69 cells were maintained for three passages without coculture cells to ensure that the culture was free of 3T3 fibroblasts, and cells between passage 23 and passage 30 were used.
In vitro models and infection assay.
H69 cells were seeded into four-well chamber slides or six-well Costar tissue culture plates (Becton Dickinson Labware, Franklin Lakes, N.J.), grown to 70 to 80% confluence, and then exposed to C. parvum. Infection with C. parvum was done in a culture medium consisting of DMEM-F-12, penicillin (100 U/ml), streptomycin (100 μg/ml) (Life Technologies, Carlsbad, Calif.), and freshly excysted C. parvum sporozoites (106 sporozoites/per slide well or culture plate). Inactivated organisms (treated at 65°C for 30 min) were used for sham-infection experiments (35).
Two in vitro models were employed to assay the attachment to and invasion of H69 cells by C. parvum: an attachment model and an attachment-invasion model as previously described (9). For the attachment model, H69 cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Fort Washington, Pa.) in phosphate-buffered saline (PBS) before exposure to C. parvum sporozoites. In this model, the organism can only attach to the fixed cell surface. For the attachment-invasion model, live cells (without prefixation) were directly exposed to C. parvum sporozoites, and thus the organism can both attach to and enter into host cells. An infection assay (attachment rate or attachment-invasion rate) was carried out after a 2-h incubation with the parasite employing an indirect immunofluorescent technique as previously described (9). Parasites infecting prefixed cells or nonfixed cells were counted and the results were expressed, respectively, as attachment rate or attachment-invasion rate (the number of parasites/total number of cells × 100). Up to 2,000 cells were counted for each assay. For the inhibitory experiments, a Rho inhibitor, exoenzyme C3 (10 μg/ml; Upstate Biotechnology, Lake Placid, N.Y.) was added to the medium 24 h before exposure to C. parvum. At a concentration of 10 μg/ml, exoenzyme C3 showed no cytotoxic effects on H69 cells.
Transfection of cells.
H69 cells for transient transfection were grown to 40 to 60% confluency on eight-well chamber slides and transfected with plasmid DNA (1 μg/well) using the Lipofectamine Plus reagent kit according to the manufacturer's recommendations. The plasmid constructs for transient transfection included the following: pKR5-Cdc42(61L)-Myc (a constitutively active form) and pKR5-Cdc42(17N)-Myc (a dominant negative form) (gifts from A. Hall, University College London, London, United Kingdom) (34, 38), pcDNA-RhoA(19N)-Myc and pcDNA-Rac1(17N)-Myc (dominant negative mutants for RhoA and Rac1) (33) (gifts from H. Cao, Mayo Medical School, Rochester, Minn.), and pCMV5/Myc3-bNWASP-WA (27, 30) (a gift from E. B. Leof, Mayo Medical School). The pFRT short hairpin-producing RNA (shRNA) suppression vector has previously been described (40). The 19-nucleotide targeting sequence used to deplete Cdc42 mRNA is 5′-TCTTCATTTGAAAACGTGA-3′. Empty vectors were used as controls. Twenty-four hours after cell transfection, cells were exposed to C. parvum sporozoites for the attachment and invasion assays. Transfected cells were identified by labeling of the Myc epitope tag using a monoclonal antibody to Myc (clone 9B11; Cell Signaling Technology). Cells transfected with shRNA to Cdc42 were identified by their nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI) (5 μM) but absence of Cdc42 expression using a monoclonal antibody (clone B8; Santa Cruz Biotechnology, Santa Cruz, Calif.). The parasites were visualized by indirect immunofluorescent staining (see below).
In some experiments, H69 cells were transfected with pcDNA4/V5-His-Cdc42(17N), a plasmid generated by the ligation of the Cdc42(17N) insert into the expression vector pcDNA4/V5-His (Invitrogen, Carlsbad, Calif.). Transfected cells were then selected by antibiotic selection (39) using Zeocin (600 μg/ml; Life Technologies) to the culture medium and were further confirmed by immunostaining with an antibody to the tagged peptide of V5 (Upstate Biotechnology). Selected cells were then exposed to C. parvum sporozoites and fixed for costaining of C. parvum with associated proteins or for scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Cells transfected with the empty vector were selected and used as the empty vector control.
Immunofluorescent microscopy.
H69 cells were exposed to C. parvum sporozoites as described above. After 2 h of incubation, cells were fixed (0.1 M 1,4-piperazinediethanesulfonic acid [pH 6.95], 1 mM EGTA, 3 mM magnesium sulfate [Sigma-Aldrich, Saint Louis, Mo.], 2% paraformaldehyde) at 37°C for 20 min and then permeabilized with 0.2% (vol/vol) Triton X-100 in PBS. For double-immunofluorescent labeling, fixed cells were incubated with primary monoclonal antibodies to proteins of interest mixed with a polyclonal antibody against C. parvum sporozoite membrane proteins (a generous gift from G. Zhu, Texas A&M University, College Station) followed by rhodamine-labeled anti-mouse and fluorescein isothiocyanate-labeled anti-rabbit antibodies (Molecular Probes, Eugene, Oreg.). Some cells were incubated with polyclonal antibodies to proteins of interest mixed with a monoclonal antibody against C. parvum (2H2; ImmunuCell, Portland, Maine) followed by rhodamine-labeled anti-rabbit and fluorescein-labeled anti-mouse antibodies. To confirm the specificity of the staining, multiple antibodies of different resources were used to the proteins including: two antibodies against Cdc42 (clone B8 [Santa Cruz Biotechnology] and a polyclonal antibody from Calbiochem-Novabiochem Corporation, San Diego, Calif.), one monoclonal antibody to RhoA (clone 26C4, Santa Cruz Biotechnology), and two antibodies to Rac1 (clone 23A8; Upstate Biotechnology and C-14, Santa Cruz Biotechnology). The polyclonal antibody to p34-Arc is a gift from L. Machesky (University of Birmingham, Birmingham, United Kingdom) and was used as previously described (29). A polyclonal anti N-WASP antibody was raised against a synthetic peptide (101-LLGRRQRKSEKRRDAPNGPNL-121) of N-WASP. The antibody was purified via an affinity column and assayed for N-WASP specificity using immunoblotting and immunofluorescent microscopy. For localization of actin with C. parvum, rhodamine-phalloidin (Sigma-Aldrich) was incubated with the secondary antibody step. Labeled cells were rinsed three times with PBS and once with distilled water and were then mounted with mounting medium (H-1000; Vector Laboratories, Burlingame, Calif.) and assessed by confocal laser scanning microscopy. The numbers of parasite attachment sites with and without accumulation of associated proteins were determined separately for quantitative analysis. Those with obvious accumulation of each associated protein were counted as positive, and the results were expressed as accumulation percentage (the number of parasite attachment sites with accumulation of the molecules/total number of attachment sites × 100); usually 500 to 1,000 attachment sites were randomly counted for each assay. Images obtained from the Zeiss 510 confocal microscope (Carl Zeiss, Inc. Oberkochen, Germany) were manipulated uniformly for contrast and intensity using the Adobe (Mountain View, Calif.) Photoshop software.
GST fusion proteins and GTPase activity assays.
Pull-down assays were employed to measure GTPase activation in H69 cells after exposure to C. parvum. To measure the activation of each GTPase, the following glutathione S-transferase (GST) fusion proteins were generated which specifically bind to the GTP-bound active form of each GTPase: GST-Cdc42/Rac interactive binding domain (GST-CRIB) specific to GTP-bound active Cdc42 was generated by amplification of a nucleotide fragment encompassing WASP amino acids 237 to 306 fused to GST; GST-Photekin Rho-binding domain (GST-RBD) for GTP-bound active RhoA was generated as previously described (36); and GST, the p21-binding domain of PAK1 (GST-PBD) for GTP-bound active Rac1, was a gift from Gary Bokoch (Scripps Research Institute, La Jolla, Calif.) (2). Those GST fusion proteins were produced in E. coli and purified by chromatography on glutathione-conjugated-agarose beads (Sigma-Alchale).
H69 cells were grown on 75 flasks to 95% confluence and exposed to 5 × 107 freshly excysted C. parvum sporozoites at 37°C for 1 h. Cells were then chilled on ice, washed with ice-cold PBS and lysed in buffer containing 50 mM Tris-hydrochloride (pH 7.4), 150 mM NaCl, 0.5% NP-40, 10% glycerol, 1 mM phenylmethanesulfonyl fluoride, leupeptin (1 μg/ml), and pepstatin (1 μg/ml). The cell lysates were then incubated with GST-CRIB, GST-RBD, or GST-PBD bound to glutathione-coupled agarose beads (Sigma-Aldrich) for 60 min at 4°C, washed with washing buffer (50 mM Tris [pH 7.5], 0.5% Triton X-100, 150 mM NaCl, 5 mM MgCl2, 1 mM dithiothreitol, aprotinin [1 μg/ml], leupeptin [1 μg/ml], 0.1 mM phenylmethylsulfonyl fluoride) and eluted with sodium dodecyl sulfate sample buffer with 200 mM dithiothreitol. GTP-bound active Cdc42, RhoA, and Rac1 were analyzed by Western blotting using monoclonal antibodies to Cdc42, RhoA, and Rac1. Whole-cell lysates were also analyzed for normalization.
Immunoelectron microscopy.
For immunogold labeling, cells were grown on 35 mm culture dishes to 70 to 80% confluence and exposed to C. parvum sporozoites for 2 h. After washing three times with DMEM-F-12 medium at 37°C, the cells for Cdc42 and RhoA labeling were fixed and processed according the postembedding labeling protocol as previously described (3). Sections were blocked in PBS containing 10% fetal calf serum. Grids were then incubated with primary antibodies diluted 1:100 in PBS with 2% fetal calf serum at 4°C overnight. Antibodies against Cdc42 (clone B8; Santa Cruz Biotechnology), RhoA (clone 26C4; Santa Cruz Biotechnology) and Rac1 (clone 23A8; Upstate Biotechnology) were used. After washing, the grids were incubated for 2 h in 10-nm-diameter-particle gold-conjugated goat anti-rabbit IgG (Sigma-Aldrich) at room temperature, diluted 1:30 in PBS containing 2% fetal calf serum. Samples were then stained with 2% methylcellulose and 0.5% uranyl acetate, and then examined with a JEOL 1200 electron microscope.
The relative distribution of Cdc42, RhoA and Rac1 in the sham- and C. parvum-infected biliary epithelial cells was determined by counting gold particles over cell profiles. Electron micrographs were printed at a final magnification of 20,000×, and the pictures were randomized for counting. Dense-band associated labeling for Cdc42, RhoA, and Rac1 was quantitated by counting the gold particles in the area within a 0.2-μm distance along the dense-band; totals were described as gold particles per square micrometer. Gold particles at randomly selected apical membrane areas in sham-infected cells were counted and used as the controls.
Statistical analysis.
All values are given as means ± standard errors. Means of groups were compared with the Student's t test (unpaired) or analysis of variance test when appropriate. P values less than 0.05 were considered statistically significant.
RESULTS
Accumulation and activation of Rho GTPases in C. parvum-infected biliary epithelial cells.
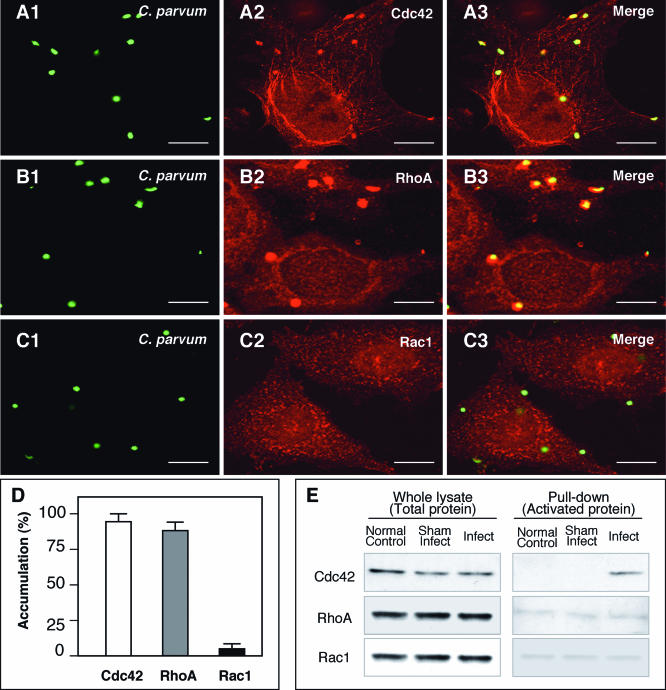
Cdc42, RhoA, and Rac1 are key regulators of cell adhesion and actin cytoskeletal remodeling. To test the role of these family members in the mechanism of host-cell actin-dependent invasion of epithelia by C. parvum, accumulation of Cdc42, RhoA, and Rac1 at the host-parasite interface was assessed by double immunofluorescent staining. A strong accumulation of Cdc42 and RhoA was found at the host-parasite interface (Fig. 1A -B3). Double staining using multiple antibodies showed similar results, confirming the specificity of staining (data not shown). No positive staining of Cdc42 and RhoA was found in the parasite itself with the procedure used in the experiments (data not shown). Rac1 did not show accumulation at the host-parasite interface by immunofluorescent staining employing multiple specific antibodies (Fig. 1C1 to C3). Quantitative analysis showed 94.6% ± 0.8% of the attachment sites accumulated Cdc42, 94.3% ± 0.9% accumulated RhoA, and only 5.24% ± 3.1% accumulated Rac1 (Fig. 1D) (means ± standard errors of the means). To test which of the Rho GTP-binding proteins in host cells is activated by C. parvum, pull-down experiments were performed. As shown in Fig. 1E, a strong band for Cdc42, but not RhoA and Rac1, was detected in infected cells compared with that in sham-infected controls, indicating that Cdc42 (but not RhoA and Rac1) is activated in infected host epithelial cells.
FIG. 1.
C. parvum recruits host cell Cdc42 and RhoA to the host-parasite interface but activates only Cdc42 in infected biliary epithelial cells. Biliary epithelial cells were exposed to C. parvum sporozoites and recruitment and activation of Rho GTP-binding proteins were then determined by confocal microscopy and pull-down approach, respectively. Accumulation of host cell Cdc42 (A1 to A3) and RhoA (B1 to B3) was found at the parasite-epithelial cell interface in infected biliary epithelial cells after exposure to C. parvum for 2 h. (C1 to C3) Rac1, another small GTP-binding protein, did not show accumulation at the host cell-parasite interface. Labels indicate staining of C. parvum or proteins or the mergers of the corresponding red and green panels. (D) Quantitative analysis of accumulation of Cdc42, RhoA and Rac1 at the host-parasite interface. (E) GST pull-down assay of Rho GTP-binding protein activation in biliary epithelial cells after exposure to C. parvum sporozoites for 1 h. The whole lysates showed similar bands to Cdc42, RhoA, or Rac1, suggesting no change at the total protein level in infected cells after C. parvum infection. GST pull-down using GST-CRIB (which specifically binds to the GTP-bound form of Cdc42) showed a much stronger band in C. parvum infected cells than that in the normal control or sham-infected cells, suggesting activation of Cdc42 in biliary epithelial cells after C. parvum infection. No significant increase of the band for RhoA or Rac1 was found in the pull-down assay using GST-RBD (which binds to GTP-RhoA) or GST-PBD (which binds to GTP-Rac1), respectively. Error bars, standard errors of the means; scale bars = 5 μm.
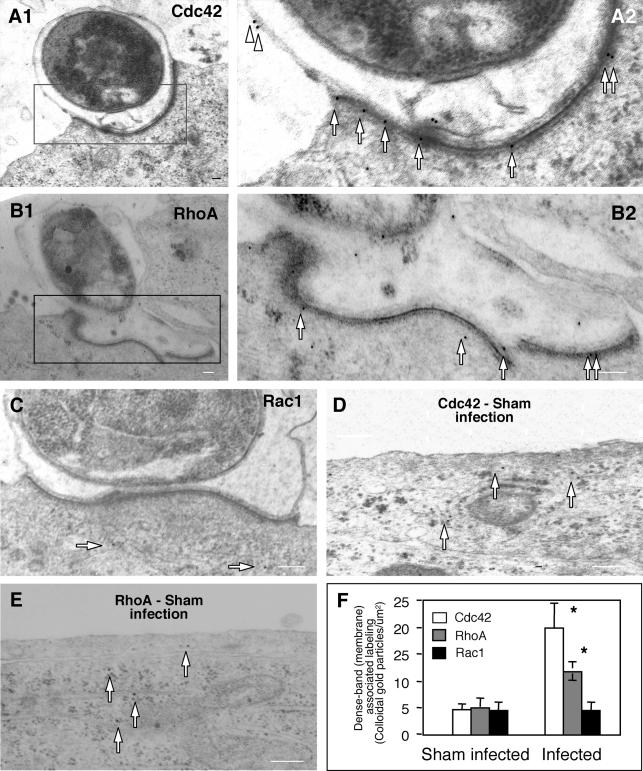
To further localize the accumulation of Cdc42 and RhoA at the host-parasite interface, cells exposed to C. parvum sporozoites were fixed and labeled with immunogold particles followed by electron microscopy. Accumulation of colloidal gold particles specific for both Cdc42 and RhoA was found at the host-parasite interface (Fig. 2A1 and B1). Images of a higher magnification show gold particle labeling of both Cdc42 and RhoA around the dense-band area (Fig. 2A2 and B2). Some labeling for Cdc42 was also observed at the parasitophorous vacuole membrane (Fig. 2A2), while fewer gold particles were found in the areas not directly adjacent to the parasite in infected cells (Fig. 2A1 and A2). While very few gold particles for Rac1 were found in the cytoplasm of infected cells (arrows in Fig. 2C), similar to the labeling in sham-infected cells (data not shown), no obvious accumulation of Racl gold particles was found along the dense-band area (Fig. 2C). Sham-infected cells showed diffuse cytoplasmic distribution of very few gold particles for both Cdc42 (arrows in Fig. 2D) and RhoA (Fig. 2E). Quantitative analysis showed a marked increase of gold particles for Cdc42 and RhoA, but not Rac1, along the dense-band after infection compared with sham-infection controls (Fig. 2F).
FIG. 2.
Accumulation of Cdc42 and RhoA at the host-parasite interface as revealed by immunoelectron microscopy. Biliary epithelial cells were exposed to C. parvum sporozoites for 2 h followed by immunoelectron microscopy. (A1 and B1) Immunogold labeling of Cdc42 (A1) and RhoA (B1) at the host-parasite interface in infected cells. (A2 and B2) Boxed regions of A1 and A2, respectively, shows at a higher magnification. (C) Immunogold labeling of Rac1 at the host-parasite interface. (E and F) Representative images of gold particle labeling of Cdc42 and RhoA in sham-infected control cells. (G) Quantitative analysis of immunogold particles for Cdc42, RhoA and Rac1 around the dense-band area. Immunogold particles at randomly selected apical membrane areas in sham-infected cells were used as the controls. *, P < 0.05 (compared with sham-infected controls); error bars, standard errors of the means; scale bars = 0.1 μm.
C. parvum invasion of biliary epithelial cells requires the activity of host cell Cdc42.
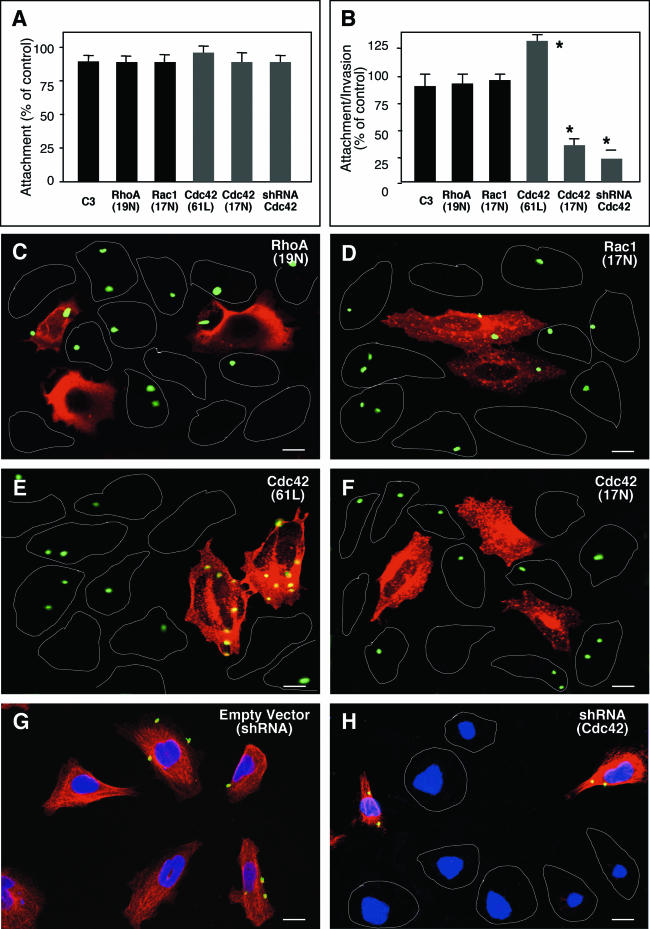
To further define if Rho GTP-binding proteins are involved in the attachment or invasion process of C. parvum into host epithelial cells, C. parvum attachment and invasion of cells transfected with constitutively active or dominant negative mutants of Cdc42, RhoA or Rac1 was observed by immunofluorescent microscopy. Transfected cells were identified by their labeling of the tagged proteins by immunofluorescent staining. An shRNA vector to Cdc42 was also used to suppress mRNA Cdc42 in some experiments. When biliary epithelial cells were prefixed and then exposed to C. parvum sporozoites, a model in which the organism attaches to but does not invade the fixed cell surface, no significant change of the attachment rate was found between all the treated cells, including cells transfected with Cdc42 (61L), Cdc42 (17N), RhoA (19N), and Cdc42 shRNA, or cells treated with exoenzyme C3 (Fig. 3A); these results suggest that C. parvum sporozoite attachment to biliary epithelial cells is not dependent upon host cell Rho GTP-binding proteins. When unfixed living biliary epithelial cells were exposed to C. parvum sporozoites, a model in which the organism can both attach to and enter into host cells, no significant change of attachment-invasion rate was found between untransfected cells and cells transfected with RhoA (19N) or Rac1 (17N), or cells treated with exoenzyme C3 (Fig. 3B). However, a significant decrease of attachment-invasion rate was found in cells transfected with Cdc42 (17N) or Cdc42 shRNA (Fig. 3B), whereas a significant increase of infection was detected in cells transfected with the constitutively active mutant of Cdc42 (61L) (Fig. 3B). As shown by immunofluorescent microscopy, a similar pattern of C. parvum infection was seen in nontransfected cells (as outlined in Fig. 3C and D) and cells transfected with RhoA (19N) (Fig. 3C) or Rac1 (17N) (Fig. 3D). Transfected cells were stained in red using an antibody to the tagged c-Myc (Fig. 3C and D). A significant increase of C. parvum infection was found in cells transfected with Cdc42 (61L) (in red in Fig. 3E) compared with nontransfected cells (as outlined in Fig. 3E). A significant decrease of C. parvum infection was found in cells transfected with Cdc42 (17N) (in red in Fig. 3F) or shRNA to Cdc42 (absence expression of Cdc42 in Fig. 3H) compared with nontransfected cells (as outlined in Fig. 3F and expression of Cdc42 in Fig. 3H, respectively) or the empty shRNA vector (Fig. 3G). Coupled with the observations of using the prefixed cells, the above data suggest that C. parvum sporozoite invasion (but not attachment) of biliary epithelial cells requires host cell activation of Cdc42.
FIG. 3.
C. parvum invasion of biliary epithelial cells requires the activation of host cell Cdc42, but not RhoA and Rac1. Cells were transfected with either a consitutively active mutant of Ccd42 or dominant negative mutants of Cdc42, RhoA, or Rac1 and then exposed to C. parvum followed by immunofluorescent microscopy. Some cells were transfected with a vector encoding an shRNA toward Cdc42 before exposure to C. parvum. (A) Attachment assay in prefixed cells after a 2-h exposure to C. parvum sporozoites shows no significant difference of C. parvum attachment in all the treated cells. (B) Attachment-invasion assay in nonfixed cells after a 2-h exposure to C. parvum sporozoites. A significant increase of infection rate was found in cells transfected with Cdc42 (Q61L) and a significant decrease of infection rate was detected in cells transfected withCdc42 (17N) or Cdc42 siRNA, but not RhoA (19N) and Rac1(17N) or cells treated with exoenzyme C3. (C to H) Representative confocal images of cells of various treatment exposed to C. parvum for 2 h. Transfected cells were identified by immunostaining using an antibody to the C-Myc epitope tag. No significant different of C. parvum infection was found between nontransfected cells as outlined (C and D) and cells transfected with RhoA (19N) (stained in red in C) or Rac1 (19N) (in red in D). More C. parvum parasites were detected in cells transfected with Cdc42 (61L) (in red in E) and much fewer C. parvum parasites were found in cells transfected with Cdc42 (17N) (in red in F) compared with nontransfected cells (as outlined in E and F). Whereas cells transfected with the empty shRNA vector displayed a normal Cdc42 cellular expression and a similar infection pattern as nontransfected cells (G), cells transfected with shRNA to Cdc42 showed no obvious expression of Cdc42 (with nucleus staining with DAPI but absence of Cdc42 expression in H) and a marked decrease of C. parvum infection compared with nontransfected cells (shown expression of Cdc42 in H). *, P < 0.05 (compared with no-inhibitor treated or nontransfected controls); error bars, standard errors of the means; scale bars = 5 μm.
Actin-regulating downstream effectors of Cdc42 are involved in C. parvum invasion of biliary epithelial cells.
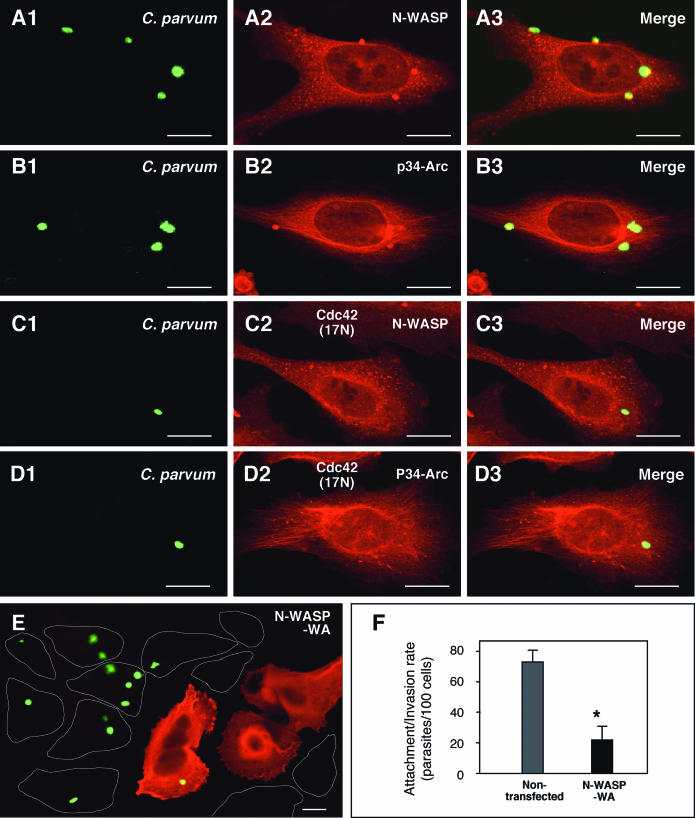
To further test the role of Cdc42 signaling pathways in the infection of host cells by C. parvum, accumulation of downstream effectors of Cdc42 at the host-parasite interface during C. parvum invasion of biliary epithelial cells was observed by immunoconfocal microscopy. Consistent with a previous report in a model of intestinal cryptosporidiosis (17), a strong accumulation of N-WASP was found at the host-parasite interface in infected biliary epithelial cells (Fig. 4A1 to A3). In addition, p34-Arc, another member in the Arp2/3 complex important in Cdc42-associated actin reorganization was found at the host-parasite interface (Fig. 4B1 to B3). When selected Cdc42 (17N)-transfected cells were exposed to C. parvum sporozoites, no obvious accumulation of either N-WASP or p34-Arc was detected (Fig. 4C1 to D3). A significant decrease of infection rate was found in cells transfected with the WA fragment of N-WASP (N-WASP-WA) (stained in red using an antibody to the C-Myc epitope tag in Fig. 4E) compared with nontransfected cells (as outlined in Fig. 4E). Quantitative analysis shows an up to 80% inhibition of infection in transfected cells compared with nontransfected cells (Fig. 4F).
FIG. 4.
Downstream effectors of Ccd42 and C. parvum invasion of biliary epithelial cells. (A and B) C. parvum stimulates the accumulation of host cell N-WASP and p34-Arc at the host-parasite interface in infected biliary epithelial cells. Biliary epithelial cells were exposed to C. parvum sporozoites for 2 h and then costained of C. parvum with associated proteins followed by confocal microscopy. (C and D) Inhibition of Cdc42 blocks C. parvum-induced N-WASP and p34-Arc accumulation at the host-parasite interface. Biliary epithelial cells were transfected with Cdc42(17N), selected by antibiotic section and then exposed to C. parvum sporozoites for 2 h followed by confocal microscopy. No obvious accumulation of N-WASP (C1 to C3) and p34-Arc (D1 to D3) was detected at the host-parasite interface in the transfected cells. (E) Representative confocal image of cells which were transfected with N-WASP-WA and then exposed to C. parvum for 2 h. Much fewer C. parvum parasites were found in cells transfected with N-WASP-WA (stained in red using an antibody to the C-Myc epitope tag in F) compared with nontransfected cells (as outlined in E). (F) Quantitative analysis of attachment-invasion of C. parvum in nonfixed cells transfected with N-WASP-WA after a 2-h exposure to C. parvum sporozoites. A significant decrease of infection rate was detected in cells transfected with N-WASP-WA. *, P < 0.05 (compared with nontransfected controls); error bars, standard errors of the means; scale bars = 5 μm.
Inhibition of host cell Cdc42 activation prevents actin reorganization, host cell membrane protrusion, and dense-band formation at the host-parasite interface.
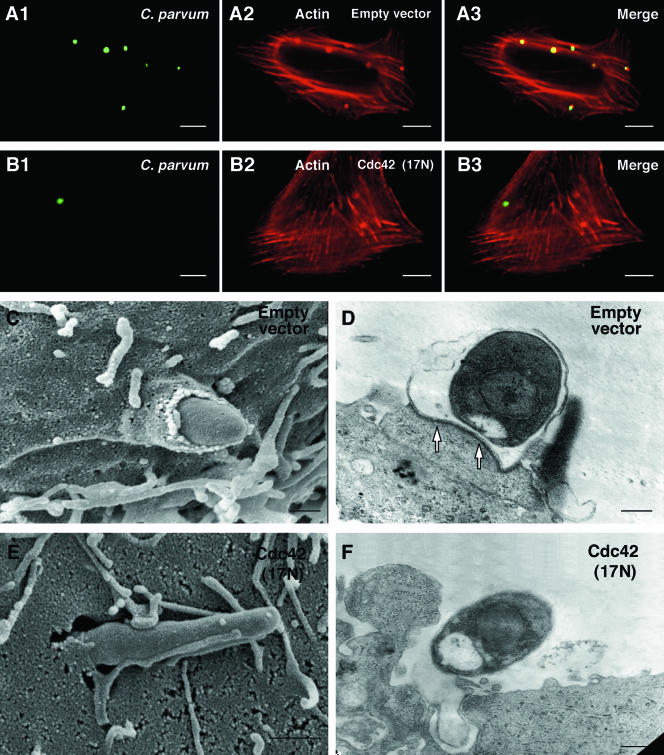
C. parvum-associated actin reorganization, host cell membrane protrusion, and dense-band formation in cells transfected with dominant negative mutant of Cdc42 and selected by antibiotic selection was observed by immunofluorescent or electron microscopy. Whereas a strong actin accumulation was found at the host-parasite interface in cells transfected with the empty vector (Fig. 5A1 to A3), no obvious actin accumulation was detected in the selected Cdc42 (17N) transfected cells (Fig. 5B1 to B3). Quantitative analysis shows an up-to-80% inhibition of actin accumulation in transfected cells compared with empty vector transfected cells (data not shown). When Cdc42 (17N)-transfected cells were selected and then exposed to C. parvum sporozoites, most of the C. parvum sporozoites observed by SEM were only attached to the membrane surface of the biliary epithelial cells (Fig. 5E and F): no host cell membrane protrusion at the host-parasite interface in the selected Cdc42 (17N) transfected cells was found compared with the empty vector controls. Moreover, the dense-band underlying the parasitophorous vacuole was rarely observed by TEM in selected Cdc42 transfected cells after a 2-h exposure to C. parvum sporozoites (Fig. 5F). In contrast, the cells transfected with the empty vector showed obvious membrane protrusion enveloping the organism, and dense-band formation (Fig. 5C and D).
FIG. 5.
Inhibition of host cell Cdc42 activation hampers C. parvum-induced actin reorganization, host cell membrane protrusion and dense-band formation at the host-parasite interface. Cdc42 (17N)-transfected cells were selected by antibiotic selection and then exposed to C. parvum for 2 h followed by confocal microscopy or SEM or TEM. (A and B) Confocal microscopy shows that C. parvum induces host cell actin accumulation at the host-parasite interface in empty vector-transfected cells (A1 to A3), but not in Cdc42 (17N)-transfected cells (B1 to B3). (C and D) Electron micrographs show C. parvum attachment and invasion of empty vector control cells, with host cell membrane protrusion and microvilli around the organism (C) and the dense-band underlying the parasitophorous vacuole (arrows in D). (E and F) C. parvum can only attach to the membrane surface of selected Cdc42 (17N) transfected cells. No obvious host cell membrane protrusion and dense-band formation at the host-parasite interface (F). Scale bar = 0.5 μm.
DISCUSSION
Invasion of epithelial cells by C. parvum is characterized by host cell membrane protrusions which rise up and enclose the invading parasite. As with other members of the phylum Apicomplexa, C. parvum develops within a parasitophorous vacuole. However, the niche occupied by C. parvum is unique in that it remains at the apex of the host cell because the parasite is separated from the host cytoplasm at the infection site by a dense-band of unknown composition. Whereas the function of this dense-band is unclear, it may prevent the internalized parasite from invading further into the host cell cytoplasm. Since C. parvum-induced host cell membrane alterations and dense-band formation are limited to the host-parasite interface, it seems plausible that the signals involved in the process may also be activated or localized at the area of attachment. Consistent with this hypothesis, we found, in the work described here, that C. parvum recruits Cdc42 and RhoA, but not Rac1, to the host-parasite interface during its infection of biliary epithelial cells in vitro. It appears, however, that only Cdc42, but neither RhoA nor Rac1, is activated in infected cells indicating that a Cdc42 activated signaling pathway is associated with C. parvum-associated host cell actin remodeling at the host-parasite interface. Such Cdc42-associated actin remodeling appears to be involved in C. parvum-associated epithelial cell membrane protrusion and dense-band formation at the host-parasite interface and is necessary for C. parvum invasion of host epithelial cells. These results provide the first direct experimental evidence suggesting that C. parvum activates a host cell Cdc42 signaling pathways and stimulates host cell actin remodeling to facilitate C. parvum invasion of epithelial cells.
The Rho family of small GTPases has been shown to coordinate signaling cascades that produce both morphological and nuclear responses to a variety of extracellular signals. In these cascades, Rac controls the subsequent formation of lamellopodia and membrane ruffling; Rho controls the formation of stress fibers and focal adhesions; and Cdc42 regulates the formation of microspikes and filopodia (23, 37). Recent studies suggest that many pathogenic microbes employ various mechanisms to manipulate host cell Rho GTPases to facilitate cellular invasion (22). In this study, we found that no significant change of C. parvum attachment in cells transfected with Cdc42 (17N) and N-WASP-WA. In contrast, a significant decrease of C. parvum invasion was found in cells transfected with function-deficient mutants of the Cdc42 pathways including Cdc42 (17N) or N-WASP-WA. Expression in host cells of a constitutively active mutant of Cdc42 also significantly increased C. parvum invasion. It was no surprise that C. parvum attachment does not require host cell Cdc42 pathways, since the attachment process is mediated by ligands on the sporozoite surface and receptors on the external surface of host cell plasma membrane (10), both of which remain to be identified (41).
C. parvum invasion of host epithelial cells appears to require host cell actin remodeling (9, 17, 18). Activation of Cdc42 can trigger various downstream effectors to induce actin remodeling. For example, Cdc42 activates WASP and Arp2/3 complexes to induce actin remodeling in many cell types, including epithelial cells (37). Indeed, we found that overexpression of Cdc42 in host cells with a dominant negative mutant or depletion of Cdc42 by siRNA significantly blocked C. parvum-associated actin accumulation. Significantly, neither host cell membrane protrusion, dense-band formation, nor morphological alterations associated with actin remodeling at the host-parasite interface, were observed by either SEM or TEM in Cdc42 (17N)-transfected cells exposed to C. parvum. Overexpression of the WA fragment of N-WASP, which inhibits specific actin polymerization in the host cell by constitutive activation of Arp2/3 throughout the host cell making it less available (27, 30), also diminished C. parvum invasion. The association of N-WASP and Arp2/3 complexes with C. parvum-induced host cell actin reorganization is further supported by a recent study in an in vitro model of intestinal cryptosporidiosis (17). Together, these findings suggest that the activation of host cell Cdc42 signaling pathways induces host cell actin remodeling at the parasite-host cell interface and thus facilitates C. parvum invasion (not attachment) of host epithelial cells. Similar processes have been reported in the bacterial invasion of bladder epithelial cells by E. coli (31). However, the molecular mechanisms by which actin remodeling at the host-parasite interface results in host cell membrane protrusion and is involved in dense-band formation remain to be elucidated. Since function-deficient mutation of host cells of Cdc42 does not completely block C. parvum invasion, other signaling pathways and additional actin associated proteins may also be required. Indeed, c-Src, a membrane-associated protein tyrosine kinase important in signal transduction and induced cytoskeleton reorganization, has recently been demonstrated to be involved in C. parvum cellular invasion (11).
Since Rac appears to be involved in the mechanisms of microbial induced membrane ruffling (28, 42), and assuming membrane ruffling and membrane protrusion are different processes, it was not surprising that Rac1 is not recruited to the host-parasite interface. In fact, host cell membrane protrusion, not membrane ruffling, at the host-parasite interface during C. parvum infection has previously been demonstrated (8, 11). However, while RhoA was recruited to the host-parasite interface, it was unexpected that no significant activation of RhoA was detected in infected cells. Consistent with RhoA not being involved in C. parvum attachment or invasion, inhibition of host cell Rho activity by exoenzyme C3 or transfection of biliary epithelial cells with dominant negative mutants of both RhoA and Rac1 did not significantly affect C. parvum sporozoite invasion of host cells, further suggesting that Rho and Rac1 may not be required for the process. Although it has been demonstrated that the Rho family of small GTPases are functionally linked with each other in some cell types, each individual GTPase can be activated separately and may function independently during microbial infection. For example, Salmonella selectively activates host cell Cdc42 and Rac but not Rho during its cellular entry (7, 19, 24). Our data suggest that C. parvum selectively activates host cell Cdc42 during cellular invasion. Nevertheless, it is also possible that activation of RhoA may simply be inhibited by other signals activated during C. parvum invasion, and thus RhoA will accumulate at the host-parasite interface but cannot be further activated. Indeed, it has been shown that Shigella entry into host epithelial cells activates c-Src, which in turn phosphorylates p190RhoGAP (a RhoA activating protein which converts the GTP-bound active RhoA to the GDP-bound inactive RhoA), thus inhibiting RhoA activation (15). Activation of c-Src in host epithelial cells has recently been described by us in the same in vitro model of biliary cryptosporidiosis (11). However, the functional interactions of c-Src with the GTPases during C. parvum infection remain to be defined.
In conclusion, using an in vitro model of biliary cryptosporidiosis, we demonstrated for the first time that C. parvum recruits Cdc42 to the host-parasite interface, an event involving host cell actin reorganization and recruitment of downstream effectors of Cdc42 to induce membrane protrusion and dense-band formation which facilitates parasitophorous vacuole formation and C. parvum invasion. Future studies aim to define the molecular mechanisms by which C. parvum activates Cdc42 and the role of other actin associated proteins.
Acknowledgments
We thank G. Zhu, A. H. Limper, and S. P. O'Hara for helpful discussions; A. Hall, D. Schlaepfer, L. Karnitz, E. B. Leof, G. Bokoch, and L. M. Machesky for antibodies or plasmids; and D. Hintz for secretarial assistance.
This work was supported by the National Institute of Health grants DK-57993 and DK-24031 (to N.F.L.) and DK-44650 (to M.A.M.), a cancer Research Institute Investigator Award (to D.D.B.), and the Mayo Foundation.
Editor: T. R. Kozel
REFERENCES
- 1.Aji, T., T. Flanigan, R. Marshall, C. Kaetzel, and M. Aikawa. 1991. Ultrastructural study of asexual development of Cryptosporidium parvum in a human intestinal cell line. J. Protozool. 38:82S-84S. [PubMed] [Google Scholar]
- 2.Benard, V., B. P. Bohl, and G. M. Bokoch. 1999. Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274:13198-13204. [DOI] [PubMed] [Google Scholar]
- 3.Bendayan, M. 1984. Concentration of amylase along its secretory pathway in the pancreatic acinar cell as revealed by high resolution immunocytochemistry. Histochem. J. 16:85-108. [DOI] [PubMed] [Google Scholar]
- 4.Bonnin, A., A. Lapillonne, T. Petrella, J. Lopez, C. Chaponnier, G. Gabbiani, S. Robine, and J. F. Dubremetz. 1999. Immunodetection of the microvillous cytoskeleton molecules villin and ezrin in the parasitophorous vacuole wall of Cryptosporidium parvum (Protozoa: Apicomplexa). Eur. J. Cell Biol. 78:794-801. [DOI] [PubMed] [Google Scholar]
- 5.Campbell, A. T., L. J. Robertson, and H. V. Smith. 1992. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl. Environ. Microbiol. 58:3488-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carabeo, R. A., S. S. Grieshaber, E. Fischer, and T. Hackstadt. 2002. Chlamydia trachomatis induces remodeling of the actin cytoskeleton during attachment and entry into HeLa cells. Infect. Immun. 70:3793-3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, L. M., S. Hobbie, and J. E. Galan. 1996. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science 274:2115-2118. [DOI] [PubMed] [Google Scholar]
- 8.Chen, X. M., S. A. Levine, E. Krueger, M. A. McNiven, D. M. Jefferson, M. Mahle, and N. F. LaRusso. 1998. Cryptosporidium parvum is cytopathic for cultured human biliary epithelial via an apoptotic mechanism. Hepatology 28:906-913. [DOI] [PubMed] [Google Scholar]
- 9.Chen, X. M., and N. F. LaRusso. 2000. Mechanisms of attachment and internalization of Cryptosporidium parvum to biliary and intestinal epithelial cells. Gastroenterology 118:368-379. [DOI] [PubMed] [Google Scholar]
- 10.Chen, X. M., J. S. Keithly, C. V. Paya, and N. F. LaRusso. 2002. Cryptosporidiosis. N. Engl. J. Med. 346:1723-1731. [DOI] [PubMed] [Google Scholar]
- 11.Chen, X. M., B. Q. Huang, P. L. Splinter, H. Cao, G. Zhu, M. A. McNiven, and N. F. LaRusso. 2003. Cryptosporidium parvum invasion of biliary epithelia requires host cell tyrosine phosphorylation of cortactin via c-Src. Gastroenterology 125:216-228. [DOI] [PubMed] [Google Scholar]
- 12.Clark, D. P. 1999. New insights in human cryptosporidiosis. Clin. Microbiol. Rev. 12:554-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dluzewski, A. R., and C. R. Garcia. 1996. Inhibition of invasion and intraerythrocytic development of Plasmodium falciparum by kinase inhibitors. Experientia 52:621-623. [DOI] [PubMed] [Google Scholar]
- 14.Dobrowolski, J. M., and L. D. Sibley. 1996. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell 34:933-939. [DOI] [PubMed] [Google Scholar]
- 15.Dumenil, G., P. Sansonetti, and G. Tran Van Nhieu. 2000. Src trrosine kinase activity down-regulates Rho-dependent responses during Shigella entry into epithelial cells and stress fibre formation. J. Cell Sci. 113:71-80. [DOI] [PubMed] [Google Scholar]
- 16.Elliott, D. A., and D. P. Clark. 2000. Cryptosporidium parvum induces host cell actin accumulation at the host-parasite interface. Infect. Immun. 68:2315-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott, D. A., D. J. Coleman, M. A. Lane, R. C. May, L. M. Machesky, and D. P. Clark. 2001. Cryptosporidium parvum infection requires host cell actin polymerization. Infect. Immun. 69:5940-5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forney, J. R., D. B. DeWald, S. Yang, C. A. Speer, and M. C. Healey. 1999. A role for host phosphoinositide 3-kinase and cytoskeletal remodeling during Cryptosporidium parvum infection. Infect. Immun. 67:844-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu, Y., and J. E. Galan. 1999. A Salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401:293-297. [DOI] [PubMed] [Google Scholar]
- 20.Godbold, G. D., and B. J. Mann. 1998. Involvement of the actin cytoskeleton and p21rho-family GTPases in the pathogenesis of the human protozoan parasite Entamoeba histolytica. Braz. J. Med. Biol. Res. 31:1049-1058. [DOI] [PubMed] [Google Scholar]
- 21.Grubman, S. A., R. D. Perrone, D. W. Lee, S. L. Murray, L. C. Rogers, L. I. Wolkoff, A. E. Mulberg, V. Cherington, and D. M. Jefferson. 1994. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am. J. Physiol. 266:G1060-G1070. [DOI] [PubMed] [Google Scholar]
- 22.Gruenheid, S., and B. B. Finlay. 2003. Microbial pathogenesis and cytoskeletal function. Nature 422:775-781. [DOI] [PubMed] [Google Scholar]
- 23.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 24.Hardt, W. D., L. M. Chen, K. E. Schuebel, X. R. Bustelo, and J. E. Galan. 1998. S. typhimurium encodes an activator of Rho GTPases that induces membrane ruffling and nuclear responses in host cells. Cell 93:815-826. [DOI] [PubMed] [Google Scholar]
- 25.Hayward, R. D., and V. Koronakis. 1999. Direct nucleation and bundling of actin by the SipC protein of invasive Salmonella. EMBO J. 18:4926-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hecht, G. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VII. Enteropathogenic Escherichia coli: physiological alterations from an extracellular position. Am. J. Physiol. 281:G1-G7. [DOI] [PubMed] [Google Scholar]
- 27.Higgs, H. N., L. Blanchoin, and T. D. Pollard. 1999. Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization. Biochemistry 38:15212-15222. [DOI] [PubMed] [Google Scholar]
- 28.Kraynov, V. S., C. Chamberlain, G. M. Bokoch, M. A. Schwartz, S. Slabaugh, and K. M. Hahn. 2000. Localized Rac activation dynamics visualized in living cells. Science 290:333-337. [DOI] [PubMed] [Google Scholar]
- 29.Krueger, E. W., J. D. Orth, H. Cao, and M. A. McNiven. 2003. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol. Biol. Cell 14:1085-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchand, J. B., D. A. Kaiser, T. D. Pollard, and H. N. Higgs. 2001. Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex. Nat. Cell Biol. 3:76-82. [DOI] [PubMed] [Google Scholar]
- 31.Martinez, J. J., and S. J. Hultgren. 2002. Requirement of Rho-family GTPases in the invasion of Type 1-piliated uropathogenic Escherichia coli. Cell. Microbiol. 4:19-28. [DOI] [PubMed] [Google Scholar]
- 32.McDonald, V., R. Stables, D. C. Warhurst, M. R. Barer, D. A. Blewett, H. D. Chapman, G. M. Connolly, P. L. Chiodini, and K. P. McAdam. 1990. In vitro cultivation of Cryptosporidium parvum and screening for anticryptosporidial drugs. Antimicrob. Agents Chemother. 34:1498-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meacci, E., V. Vasta, J. P. Moorman, D. A. Bobak, P. Bruni, V. Moss, and M. Vaughan. 1999. Effect of Rho and ADP-ribosylation factor GTPases on phospholipase D activity in intact human adenocarcinoma A549 cells. J. Biol. Chem. 274:18605-18612. [DOI] [PubMed] [Google Scholar]
- 34.Nobes, C. D., and A. Hall. 1995. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81:53-62. [DOI] [PubMed] [Google Scholar]
- 35.O'Donoghue, P. J. 1995. Cryptosporidium and cryptosporidiosis in man and animals. Int. J. Parasitol. 25:139-195. [DOI] [PubMed] [Google Scholar]
- 36.Ren, X-D., W. B. Kiosses, and M. A. Schwartz. 1999. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18:578-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohatgi, R., L. Ma, H. Miki, M. Lopez, T. Kirchhausen, T. Takenawa, M. W. Kirschner. 1999. The interaction between N-WASP and the Arp2/3 complex links Cdc42-dependent signals to actin assembly. Cell 97:221-231. [DOI] [PubMed] [Google Scholar]
- 38.Self, A. J., and A. Hall. 1995. Purification of recombinant Rho/Rac/G25K from Escherichia coli. Methods Enzymol. 256:3-10. [DOI] [PubMed] [Google Scholar]
- 39.Thomas, A., T. Giesler, and E. White. 2000. p53 mediates bcl-2 phosphorylation and apoptosis via activation of the Cdc42/JNK1 pathway. Oncogene 19:5259-5269. [DOI] [PubMed] [Google Scholar]
- 40.Trushin, S. A., N. K. Pennington, E. M. Carmona, S. Asin, D. N. Savoy, D. D. Billadeau, and P. V. Paya. 2003. Protein kinase Cα (PKCα) acts upstream of PKCθ to activate IκB kinase and NF-κB in T lymphocytes. Mol. Cell. Biol. 23:7068-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzipori, S., and J. K. Griffiths. 1998. Natural history and biology of Cryptosporidium parvum. Adv. Parasitol. 40:5-36. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida, S., E. Katayama, A. Kuwae, H. Mimuro, T. Suzuki, and C. Sasakawa. 2002. Shigella deliver an effector protein to trigger host microtubule destabilization, which promotes Rac1 activity and efficient bacterial internalization. EMBO J. 21:2923-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong, L., H. G. Lu, S. N. Moreno, and R. Docampo. 1998. Tyrosine phosphate hydrolysis of host proteins by Trypanosoma cruzi is linked to cell invasion. FEMS Microbiol. Lett. 161:15-20. [DOI] [PubMed] [Google Scholar]