Abstract
Existing evidence-based HIV risk reduction interventions have not been designed for implementation within clinical settings, such as methadone maintenance programs, where many high-risk drug users seek treatment services. We therefore systematically developed an adapted, significantly shortened, version of a comprehensive evidence-based intervention called the Community-friendly Health Recovery Program (CHRP) which has demonstrated preliminary evidence of efficacy in a feasibility/acceptability study already published. In a randomized controlled trial reported here, we tested the efficacy of the CHRP intervention among high-risk drug users newly enrolled in drug treatment at an inner-city methadone maintenance program. The CHRP intervention produced improvements in drug risk reduction knowledge as well as demonstrated sex- and drug-risk reduction skills. Support was found for the IMB model of health behavior change. Implications for future intervention research and practice are considered.
Keywords: HIV risk reduction, Behavioral intervention, Drug use, Intervention adaptation
Introduction
Despite a wide array of primary and secondary HIV prevention approaches, there has been a decade-long trend of approximately 50,000 new HIV diagnoses per year in the US alone [1]. Injection drug users (IDUs) are a target population—particularly in certain geographical areas such as the Northeastern US—as they represent a critical conduit for new HIV infections [2], which occur through preventable drug- and sex-related HIV risk behaviors. A range of evidence-based HIV risk reduction interventions (EBIs) are now widely available (http://www.effectiveinterventions.org), but few—if any—were designed for implementation within clinical settings, such as methadone maintenance programs, where high-risk drug users commonly seek treatment services.
Similar efforts to widely disseminate EBIs for the treatment of drug dependence have clearly shown that broad availability of efficacious interventions does not dramatically change the rate of implementation by clinicians in “real-world” clinical settings [3]. Thus, even though an intervention may have been developed with the intent for it to be ultimately implemented in clinical settings, this goal can be impeded by a host of factors that typically distinguish clinical settings from the research settings in which interventions are originally tested. Thus, in clinical settings, there tends to be less willingness to commit scarce resources toward delivering, monitoring, and evaluating complex and lengthy EBIs [4, 5].
In response to the unmet HIV prevention need in clinical settings, we have developed an adapted, significantly shortened, “community-friendly” version of a comprehensive EBI (Holistic Health Recovery Program, HHRP; http://www.effectiveinterventions.org) [2]. The adapted version, the Community-friendly Health Recovery Program (CHRP) [6] is based on the information–motivation–behavioral skills model of health behavior change [7, 8], which specifies that HIV prevention information, motivation, and behavioral skills are the fundamental determinants of HIV risk and HIV risk reduction behavior. From this perspective, HIV prevention information (i.e., knowledge) that is directly relevant to an individual’s practice of risk reduction behavior is a prerequisite for engaging in risk reduction action. Similarly, HIV prevention motivation to act on what one knows about HIV prevention—including personal motivation (attitudes about personally taking preventive actions) and social motivation (perceived social support/ social pressure to take preventive actions)—is an additional prerequisite for the initiating and maintaining HIV risk reduction behavior. Finally, HIV prevention behavioral skills for taking HIV preventive actions are a third prerequisite for engaging in HIV risk reduction behavior, and determine whether even a well-informed and well-motivated individual is able to skillfully initiate and maintain HIV risk reduction behavior.
The CHRP intervention previously demonstrated feasibility, acceptability, and preliminary evidence of efficacy in an initial within-subjects study among methadone maintenance patients in an inner-city drug treatment setting [6, 9]. In the randomized controlled trial (RCT) reported here, we tested the efficacy and theoretical underpinnings of the CHRP intervention among high risk drug users enrolled in drug treatment in the same inner-city methadone maintenance program. We hypothesized that patients assigned to the CHRP intervention condition would show greater drug- and sex-related HIV risk reduction outcomes compared with those in the control condition. We also hypothesized that—regardless of study condition—HIV risk reduction improvements over time would occur via pathways predicted by the information–motivation–behavioral skills (IMB) model of health behavior change [7, 8].
Methods
Participants
The study protocol was approved by the Investigational Review Board (IRB) at the University of Connecticut, the Human Investigation Committee at Yale University, and received board approval from the APT Foundation MMP, Inc. All participants were provided a verbal and written description of the study and were asked to sign an informed consent form prior to participation. Participants were reimbursed $50 per assessment for the time required to complete pre-intervention, post-intervention, and follow-up assessments and $10 per week for providing twice weekly urine samples and weekly assessments.
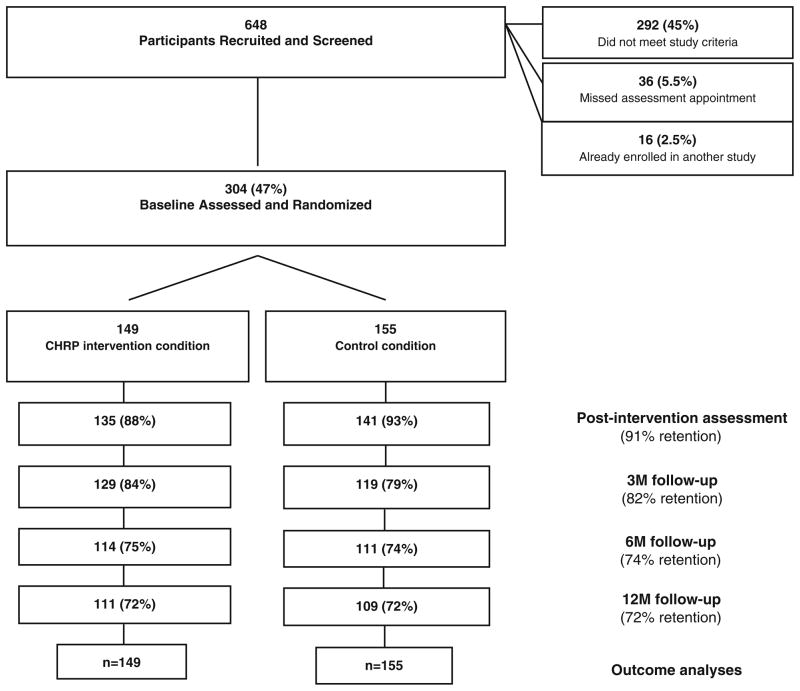
Participants were 304 HIV-negative opioid-dependent individuals (147 males) newly enrolled, though not necessarily first-time methadone patients, in a community-based methadone maintenance program who reported sex- or drug-related HIV transmission risk behavior (Fig. 1). Participants were randomly assigned to the CHRP experimental intervention condition (n = 149, 73 males) or the active control condition (n = 155, 74 males). A majority of the participants were Caucasian (74.7 %), never married (66.8 %), English-speaking (94.7 %), had a significant other (70.1 %), with a median age of 33, and with 12 yearsof education. Participants reported having a similar numbers of children (median = 1, p = 0.86) and had similar rates of living with their children (25.5 %, p = 0.40). There were no differences between groups in terms of demographics or participation rates (ps >0.31). On average, participants in the experimental group attended 3.3 of the 4 sessions while participants in the control condition attended 3.4 of the 4 sessions. The retention rate among participants was acceptable at post-intervention (91 %), 3-month (82 %), 6-month (74 %), and 12-month follow-up points (72 %), and there was no difference between conditions in terms of the dropout rate (p = 0.73).
Fig. 1.
Participant flow through phases of the study
Assessments
Using an audio computer assisted structured interview (ACASI) that has demonstrated sound psychometric properties in prior studies [6, 9] and controlled trials [10], participants were assessed at five standard time points (pre-intervention, post-intervention, 3-month follow-up, 6-month follow-up, and 12-month follow-up) using an event-level sex- and drug-related HIV risk behavior assessment approach (Tables 1, 2). Drug-risk behavior related items included how they used drugs, whether they used new syringes or cleaned syringes, and if so, how they cleaned them, and whether they shared syringes, rinse water, cooker, or cotton. Sex-risk behavior related items assessed whether they had used a male or female condom and, if not, whether it was due to abstinence from sexual activity. Participants’ HIV risk reduction behavioral skills were assessed as in prior randomized controlled trials [2, 11] by having participants demonstrate the steps necessary to properly clean a needle/syringe and the steps necessary to properly select and apply a male and female condom using replicas. Ratings of audio-taped demonstrations of these procedures by staff blind to treatment assignment have shown excellent inter-rater reliability in our prior trials (inter-rater reliability = 0.98) [11]. Using standardized skills rating forms [2, 11], each participant’s demonstrated needle cleaning and male and female condom application skills were blindly rated by a trained bachelor’s level research assistant under the supervision of a licensed clinical psychologist.
Table 1.
Drug risk reduction variables
| Construct | Item | α |
|---|---|---|
| Demonstrated IV skills: 18 steps (0–100 %) |
|
0.80 |
| Reported safer drug use (0–4) |
|
0.98 |
| Knowledge (0–1) | If an HIV positive person shares needles with another HIV positive person they don’t need to clean the needles | N/A |
| Personal motivation (1–5) | I plan to always use clean needles if I shoot up drugs, during the next three months | N/A |
| Self-efficacy (1–5) |
|
0.48 |
| Social motivation (1–5) | Most people who are important to me think I should always clean my needles before I share them with someone else during the next three months | N/A |
Table 2.
Sex risk reduction variables
| Construct | Item | α |
|---|---|---|
| Reported condom use (1–6) | In the past week, how much of the time did you use a condom or other latex protection when you had oral, anal, or vaginal sex | N/A |
| Demonstrated skills in using female condoms (0–100 %) |
|
0.65 |
| Demonstrated skills in using male condoms (0–100 %) |
|
0.53 |
| Knowledge (0–1) | If an HIV positive person only has sex with another HIV positive person, they don’t need to use condoms | N/A |
| Personal motivation (1–5) | Always using condoms during sexual intercourse during the next three months would be good | N/A |
| Self-efficacy (1–5) |
|
0.62 |
| Social motivation (1–5) | Most people who are important to me think I should always use condoms during sexual intercourse during the next three months | N/A |
Consistent with the IMB [7, 8] model of health behavior change, upon which the CHRP intervention is based, participants completed an assessment that covered the following domains: drug- and sex-related HIV-risk reduction knowledge (information component: e.g., “If an HIV+ person shared needles with another HIV+ person, they don’t need to clean the needles”; “If an HIV+ person only has sex with another HIV+ person, they don’t need to use a condom”), personal and social motivation to reduce HIV risk behavior (motivation component: e.g., “I plan to use condoms or other latex protection every time I have sex”; “Most people important to me think that is important for me to clean my needles”), self-efficacy about reducing HIV risk behavior (behavioral skills component: e.g., “How hard would it be for you to always clean your needles?”; “How hard would it be for you to always use condoms?”), and HIV risk behavior (Behavior component: e.g., Needle-sharing reported in the past week; Condom use reported in the past week). This assessment has been used in a randomized controlled trial of an evidence-based intervention [12] in order to expeditiously inform intervention clinicians about HIV-related information, motivation, and behavioral skills deficits among participants entering treatment.
Intervention and Control Conditions
The CHRP intervention is a systematically adapted—substantially abbreviated—form of an evidence-based intervention that was designed for optimal use within drug treatment settings. It is a manual-guided approach comprised of four 50-min group sessions that addresses sex-and drug-related HIV risks among opioid-dependent adults in treatment (Table 3). The sessions were provided by two trained bachelor’s level facilitators who delivered intervention content using cognitive remediation strategies (e.g., presenting material visually, verbally, and experientially) designed to accommodate the mild to moderate cognitive difficulties that are common among this population [12]. The manual-guided CHRP sessions were audio-taped and rated by a licensed clinical psychologist for interventionists’ competence and adherence to the manual, as in prior trials [13].
Table 3.
Outline of CHRP intervention content
| Group topic | Information, motivation, and skills addressed |
|---|---|
| Health care participation | Understanding HIV and your immune system, strategies for improving health, and developing a partnership with health care providers |
| Reducing the harm of injection drug use | Identifying drug-related HIV-risks, learning about proper needle cleaning, and managing cravings during needle cleaning |
| Harm reduction with latex | Identifying sex-related HIV risks, and learning about latex products and their correct use |
| Negotiating harm reduction with partners | Negotiating use of latex, communicating about sex-related HIV-risk, and eroticizing safer sexual practices |
The active control condition served as a time- and contact-matched support and orientation group for individuals entering the methadone maintenance program. The content—which was delivered by two different trained bachelor’s level individuals than the intervention condition groups—consisted of information regarding methadone program services and policies as well as general health care information relevant to opioid-dependent patients entering methadone therapy. There was no overlap between the content of the control condition and the intervention condition although the duration and meeting frequency was the same.
Procedures
All opioid-dependent patients entering a MMP were screened for participation within one week of receiving the first methadone dose. In the screening session, we determined whether the patient met study inclusion criteria, including: being 18 years of age; opioid-dependent and seeking methadone maintenance treatment; report drug- or sex-related HIV risk behavior in the past 6 months, able to read and understand the questionnaires, ACASI, and consent form in English; available for the duration of the study; not actively suicidal, homicidal, or psychotic.
Patients who met inclusion criteria, and who were willing to participate as subjects, were provided a description of the study and invited to provide informed consent, followed by a baseline assessment. Next, each participant was double-blindly randomized to one of the two treatment conditions (CHRP or control group) using a computerized “urn” randomization to ensure adequate representation of women and minorities. Following randomization, and depending upon study condition assignment, subjects were instructed to attend either the CHRP or control group weekly group sessions, both of which were conducted during routine treatment hours at the MMP. Group sessions for each condition were scheduled on different days and times in an effort to minimize contamination across conditions.
All participants continued to receive ‘standard of care’ methadone maintenance treatment regardless of assigned study condition, including: daily methadone (adhering to the clinic’s standard methadone dosing policies) and case management (maximum of 2 hours per month of individual face-to-face sessions with a drug treatment counselor). Following the 4-week intervention phase, and in the context of their routine MMP visits, we re-assessed each participant’s HIV risk behavior-related outcomes at post-intervention, and then again at 3-, 6-, and 12-month follow-up points (Fig. 1).
Results
Based on our first hypothesis, we compared participants in the intervention and control conditions to investigate the intervention effects. Because gender differences have been reported on HIV risk behavior outcomes, we adopted a 2 (Group: Intervention vs. Control) × 2 (Gender) × 5 (Time) mixed effects model to investigate the effects of the intervention.
Based on our second hypothesis, we conducted path analyses on all participants’ knowledge and social motivation responses at post-intervention, personal motivation responses at 3-months, self-efficacy responses at 6-months, and skills and reported behaviors at the 12-month follow-up point. This allowed us to examine the extent to which improvements in HIV risk reduction occurred through pathways predicted by the IMB model of health behavior change [7, 14]. In this case, our aim was to examine the associations among the theoretical variables over time—as opposed to examining intervention effects—as this could inform future intervention work in terms of what components to emphasize. Outcome data were included from the post-intervention point onward because any significant changes that occurred between pre- and post-intervention points would tend to weaken the magnitude of the associations between theoretical variables, and thus cloud these analyses. Outcomes for drug- and sex-related risk reduction domains are outlined separately below.
Information (0–1) and Motivation (1–5) Regarding Drug-Related HIV Risk Reduction
Participants in the intervention group showed improvements in their knowledge about safer drug use at the post-intervention point (from 0.96 to 1.00), whereas participants in the control group did not and these between group differences also reached significance over time, (F(4, 724) = 3.02, p = 0.017). All participants showed increasing social motivation (reaching the significant level from 4.69 at pretest to 4.88 at 3-month follow-up, F(4, 728) = 3.77 p = 0.005); all participants also showed increasing self-efficacy (reaching the significant level from 4.01 at pretest to 4.39 at post-intervention, F(4, 728) = 14.07, p = 0.000). In addition, females were generally found to demonstrate stronger self-efficacy when compared with their male counterparts (M = 4.46 vs. M = 4.17, F(1, 182) = 8.91, p = 0.003). No other effects were significant at the 0.05 p value level (Tables 4, 5 displays a summary of outcomes).
Table 4.
Summary of drug risk reduction outcome variables: means and standard errors
| Variables | Intervention group
|
Control group
|
Significant effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre- | Post- | 3-month | 6-month | 12-month | Pre- | Post- | 3-month | 6-month | 12-month | ||
| Demonstrated IV skills (0–100) | 40.60a (1.99) | 76.37e (2.03) | 69.59d (2.04) | 74.12e (1.97) | 72.61d,e (1.91) | 42.75a (2.09) | 53.11b (2.14) | 60.34c (2.15) | 63.12c (2.07) | 63.63c (2.01) | Group, Time, Group × Time, Time × Gender |
| Safer drug use (0–4) | 3.25a (0.10) | 3.78b (0.06) | 3.83b (0.05) | 3.84b (0.05) | 3.87b (0.05) | 3.39a (0.10) | 3.79b (0.06) | 3.81b (0.05) | 3.77b (0.06) | 3.85b (0.05) | Time |
| Knowledge (0–1) | 0.96a (0.02) | 1.00b (0.01) | 1.00b (0.01) | 0.96a (0.02) | 0.98a,b (0.02) | 1.00b (0.02) | 0.98a,b (0.01) | 0.96b (0.01) | 0.99a (0.02) | 0.96b (0.02) | Time × Group |
| Personal motivation (1–5) | 4.79a (0.05) | 4.91b (0.05) | 4.76a (0.07) | 4.87a,b (0.06) | 4.88a,b (0.04) | 4.85a,b (0.05) | 4.83a,b (0.05) | 4.86a,b (0.07) | 4.78a (0.06) | 4.92b (0.04) | Group × Gender |
| Self-efficacy (1–5) | 4.01a (0.10) | 4.41c (0.08) | 4.42c (0.08) | 4.22b (0.09) | 4.38b,c (0.08) | 4.00a (0.11) | 4.38b,c (0.09) | 4.36b,c (0.09) | 4.45c (0.10) | 4.52c (0.08) | Time, Gender |
| Social motivation (1–5) | 4.65a (0.08) | 4.77a,b (0.08) | 4.90b (0.04) | 4.73a (0.07) | 4.63a (0.09) | 4.72a (0.09) | 4.69a (0.08) | 4.85b (0.05) | 4.91b (0.07) | 4.75a,b (0.09) | Time |
Different letters indicate post-hoc test differences at p <0.05
Table 5.
Summary of sex risk reduction outcome variables: means and standard errors
| Variables | Intervention group
|
Control group
|
Significant effects | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre- | Post- | 3-month | 6-month | 12-month | Pre- | Post- | 3-month | 6-month | 12-month | ||
| Female condom skills (0–100) | 57.60a (1.69) | 82.54d (1.25) | 79.29c (1.38) | 83.03d (1.19) | 83.37d (1.19) | 59.38a (1.78) | 74.32b (1.31) | 79.07b.c (1.45) | 80.86c,d (1.26) | 82.19d (1.25) | Time, Group × Time |
| Male condom skills (0–100) | 71.63a (1.35) | 87.23c (1.40) | 85.01b,c (1.22) | 87.51c (1.04) | 86.70c (1.17) | 73.97a (1.42) | 82.71b (1.47) | 84.64b (1.28) | 87.64c (1.09) | 85.95c (1.24) | Time, Group × Time |
| Condom use (0–4) | 3.80a (0.24) | 3.61a (0.24) | 3.95a (0.24) | 3.65a (0.24) | 3.73a (0.24) | 3.56a (0.26) | 3.96a (0.26) | 3.75a (0.25) | 3.79a (0.25) | 3.83a (0.25) | None |
| Knowledge (0–1) | 0.87a (0.03) | 1.00b (0.02) | 0.97b (0.02) | 0.97b (0.02) | 0.98b (0.01) | 0.91a (0.03) | 0.95a (0.02) | 0.93a (0.02) | 0.95a (0.02) | 0.99b (0.01) | Time |
| Personal motivation (1–5) | 4.20a (0.09) | 4.41b (0.08) | 4.39b (0.08) | 4.40b (0.08) | 4.21a (0.09) | 4.26a (0.09) | 4.24a (0.08) | 4.24a (0.09) | 4.29a (0.08) | 4.22a (0.09) | Gender × Group |
| Self-efficacy (1–5) | 3.00a (0.12) | 2.99a (0.12) | 3.02a (0.12) | 3.16a (0.12) | 3.17a (0.12) | 3.09a (0.13) | 3.06a (0.13) | 3.14a (0.12) | 3.14a (0.12) | 3.20a (0.12) | Gender, Time × Gender × Group |
| Social motivation (1–5) | 4.67a,b (0.07) | 4.73a,b (0.07) | 4.73a,b (0.07) | 4.75b (0.07) | 4.57a (0.08) | 4.81b (0.08) | 4.75b (0.07) | 4.71a,b (0.07) | 4.77b (0.07) | 4.70a,b (0.08) | None |
Different letters indicate post-hoc test differences at p < 0.05
Demonstrated Drug Risk Reduction Skills (0–100 % Accuracy)
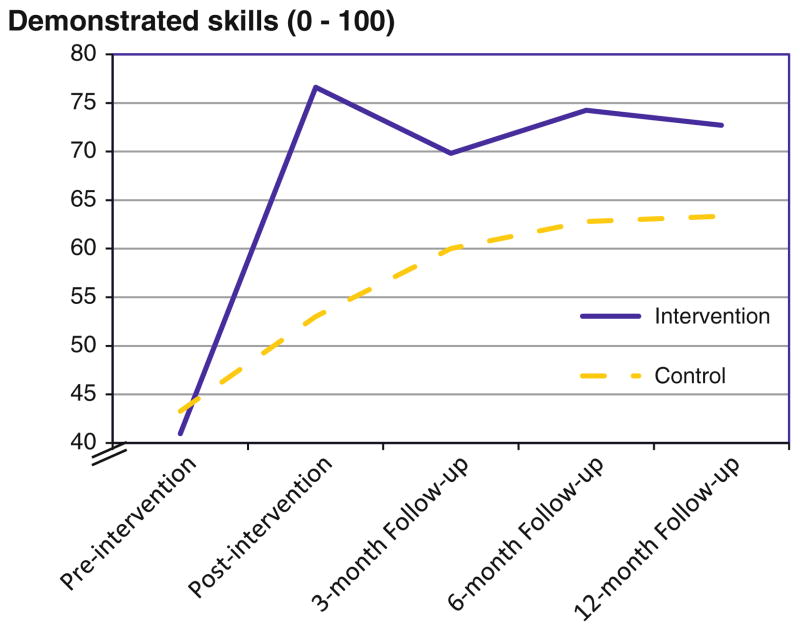
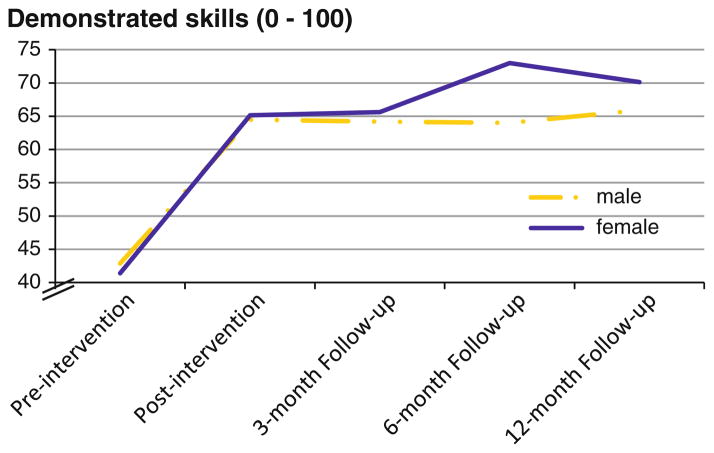
A Time effect indicated that all participants’ drug risk reduction skills improved over time, from 42.12 % at pre-to 64.82 % at post-intervention, and this effect remained stable at 12-months (68.02 %), F(4, 740) = 94.38, p < 0.001. There was also a Group effect in which participants in the intervention group showed greater skills versus those in the control group (66.87 % vs. 56.49 %), F(1, 185) = 25.99, p < 0.001). More importantly, there was a Group × Time interaction, F(4, 740) = 16.31, p < 0.001), with post hoc tests indicating that the intervention group demonstrated greater skill improvement versus the control group following the intervention (ps <0.01; Fig. 2). Interestingly, a Gender 9 Time interaction, F(4, 740) = 3.12, p < 0.05, showed that skills improvement among females tended to continue increasing over a longer time period following the intervention compared with males (Fig. 3). There was also a Gender × Group interaction on personal motivation, F(1, 181) = 4.11, p = 0.04; however, post hoc tests did not reveal robust differences.
Fig. 2.
Demonstrated drug risk reduction skills over time by group
Fig. 3.
Demonstrated drug risk reduction skills over time by gender
Reported Drug Risk Reduction Behavior (0–4 Scale)
There was a Time main effect, F(4, 740) = 42.06, p < 0.001. All participants showed improved drug risk reduction behavior at post-intervention (3.78 on a scale of 0–4, with higher scores indicating safer drug use) versus pre-intervention (3.32), and this improvement was sustained for all participants at 12-months (3.86). No other significant effects were observed.
Information (0–1) and Motivation (1–5) Regarding Sex-Related HIV Risk Reduction
All participants showed improvements in their knowledge from pretest (M = 0.89) to post-intervention (M = 0.98), regardless of which they were assigned to, F(4, 724) = 6.81, p <.001. There were also sporadic gender differences on personal motivation and self-efficacy. Across time, men were found to report stronger personal motivation than women in the intervention group (M = 4.45 vs. M = 4.20), but this was not the case in the control group (p = 0.36). Women were found to report greater self-efficacy than men (M = 3.37 vs. M = 2.82) (F(1, 182) = 16.11, p <0.001), and such gender differences were consistently observed in the control group but not in the intervention group (F(4, 728) = 2.45, p = 0.05). No other significant effects were observed.
Demonstrated Sex Risk Reduction Skills (0–100 Accuracy)
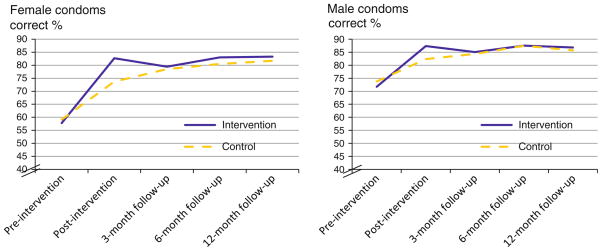
A Time effect showed that all participants improved in their skills at demonstrating the proper application of a female condom at post-intervention, F(4, 740) = 180.97, p <0.001. The Time effect was superseded by a Time × Group interaction, F(4, 740) = 6.87, p < 0.001, indicating that intervention group participants improved to a greater extent than did those in the control group in terms of properly applying the female condom (Fig. 4).
Fig. 4.
Demonstrated female and male condom application skills by group
Similarly, for the male condom demonstration skills, a Time effect indicated that all participants showed improvement at post-intervention, F(4, 740) = 73.50, p < 0.001. The Time effects were superseded by a Time × Group interaction effect which revealed that intervention group participants improved significantly more than those in the control group in terms of properly applying the male condom, F(4, 740) = 3.34, p = 0.01 (Fig. 4).
Reported Condom Use (1–6 Scale)
No significant effect was found on outcomes pertaining to reported changes in condom use behavior (ps >0.10).
The IMB Model of Health Behavior Change
Based on our secondary hypothesis, we conducted path analyses of our HIV risk reduction outcomes over time framed by the IMB model of health behavior change. Theoretical HIV risk reduction constructs were examined separately for drug- and sex-related outcomes in order to further explore the process leading to improved risk reduction behaviors among all study participants. In short, the IMB model predicts that individuals who have greater HIV knowledge, higher HIV motivation, and better HIV risk reduction skills—each of which is a pre-requisite to improved HIV risk reduction behavior—will be better equipped to engage in HIV risk reduction behavior compared to individuals who do not achieve such levels in those pre-requisite areas [7, 8]. Our longitudinal data provided a unique advantage for us to examine the causal relations among theoretical variables in the IMB model over time. Due to the relatively small sample size, we adopted a bootstrap analysis to provide greater power for mediation analyses with small sample sizes [15].
Drug Risk Reduction Theoretical Model
The path analysis of drug risk reduction outcomes that we used to examine the IMB model over all post-intervention assessment points resulted in good fitting indices (statistics in Fig. 5). The non-significant χ2 value and the good CFI (>0.95) and RMSEA values (<0.05) suggested that this model explains the data well. Aside from the influence of HIV knowledge (information component), all other paths provided the expected support for the IMB model. That is, greater social motivation at post-intervention predicted greater personal motivation (marginally) at the 3-month follow-up (motivation component). Greater personal motivation, in turn, predicted greater self-efficacy (behavioral skills component) at the 6-month follow-up (marginally). Greater levels of self-efficacy predicted improvement in reported drug risk reduction behavior at the 12-month follow-up. In addition to what the IMB model would predict, greater personal motivation at the 3-month follow-up predicted improved drug risk reduction behavior at the 12-month follow-up.
Fig. 5.
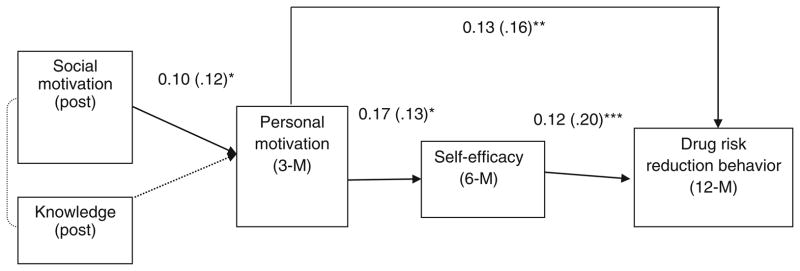
IMB model of drug risk reduction skills and behavior among all participants after the intervention onwards. The good fitting indices were: χ2(4) = 4.68, p = 0.32, CFI = 0.97, RMSEA = 0.025 (0.000, 0.098). Standardized coefficients in the parentheses. *p <0.05; **p <0.01; ***p <0.001
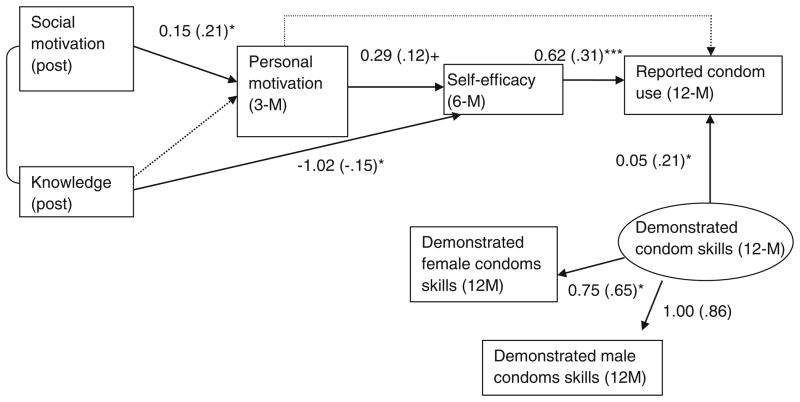
Sex Risk Reduction Theoretical Model
The path analysis of sex risk reduction outcomes that we used to examine the IMB model over all post-intervention assessment points also resulted in a good fitting model (statistics in Fig. 6), with a non-significant χ2 value and good CFI (>0.95) and RMSEA values (<0.05). Pathways were generally what would be predicted by the IMB model, aside from the pathways leading from HIV knowledge. Again, a higher level of social motivation at post-intervention predicted greater personal motivation at the 3-month follow-up (motivation component) which, in turn, predicted greater self-efficacy (behavioral skills component) at the 6-month follow-up. Finally, higher levels of self-efficacy (behavioral skills component) at the 6-month follow-up point predicted greater improvement in sex-risk reduction behavior at the 12-month follow-up.
Fig. 6.
IMB model of sex risk reduction skills and behavior among all participants after the intervention onwards. χ2(11) = 10.33, p = 0.50, CFI = 1.00, RMSEA = 0.00 (0.00, 0.07). Standardized coefficients in the parentheses. +p <0.10, *p <0.05; *** p <0.001
In addition to what is specified by the basic IMB model, our analysis indicated that higher levels of personal motivation at the 3-month follow-up point predicted greater condom use behavior at the 12-month follow-up and improvement in demonstrated condom skills at the 12-month follow-up predicted greater condom use behavior at that point. Surprisingly, increased knowledge at the post-intervention point predicted lower levels of self-efficacy at the 6-month follow-up.
Discussion
This RCT tested the efficacy of the CHRP, an adapted version of the comprehensive evidence-based HHRP [2]. Driven by the dearth of evidence-based interventions that are applicable to high-risk drug users in treatment, we designed the CHRP intervention for implementation in common clinical settings such as drug treatment programs.
The CHRP intervention generated significant improvement in participants’ objective knowledge regarding drug risk reduction. Moreover, the CHRP intervention produced pronounced enhancements to participants’ ability to demonstrate proper needle cleaning (drug risk reduction skills) as well as proper use of a male and female condom (sex risk reduction skills). These findings are particularly encouraging since they indicate that the CHRP intervention was able to improve participants’ capacity to actively engage in HIV risk reduction in addition to improving their knowledge about HIV.
Consistent with prior HIV risk reduction intervention studies conducted in the context of a methadone maintenance drug treatment program [2], no significant differences were found between intervention and control group participants with regard to drug-related risk behavior over the course of the study. Given that all study participants were newly enrolled in methadone maintenance, this finding is not surprising, and suggests the drug-related HIV risk reduction benefits of methadone maintenance treatment, as investigators asserted over a decade ago [16]. Similarly, consistent with findings from the RCT of the comprehensive version of HHRP [2], no intervention effects were found on changes in motivation, while significant intervention effects were found on demonstrated drug risk reduction skills. Given that all study participants were newly enrolled in drug treatment, they may have been sufficiently motivated to minimize HIV transmission risk, yet were not initially skillful at doing so at the time of enrollment in drug treatment. Thus, the CHRP intervention seems to have been particularly useful at addressing drug risk reduction skills deficits among newly enrolled methadone patients.
The pattern of outcomes was similar for sex-related HIV risk reduction except that no significant changes were found in sex-related HIV risk reduction knowledge. All participants showed similar levels of knowledge regarding sex-related HIV risk reduction as well as motivation. Significant improvements were found in demonstrated sex risk reduction skills, however, only among intervention group participants. Interestingly, the comprehensive version of HHRP [2] produced changes in motivation to use condoms but not sex risk reduction skills. Thus, the CHRP version—which was deliberately designed to emphasize risk reduction skill enhancement—seems to have exerted a sufficiently strong intervention effect with regard to such skills.
The path analyses were informative in terms of examining the IMB [7, 14] theoretical underpinnings upon which the CHRP intervention is based, and generally support the utility of this framework for future intervention design. Aside from the information (i.e., knowledge) construct, improvements in motivation and skills tended to be associated with subsequent improvements in HIV risk reduction behaviors. Interestingly, increased knowledge about safer sex was found to predict lower levels of self-efficacy at a later point in time. It is plausible that, in the process of gaining sex risk reduction knowledge, participants accurately recognized the need for cooperation from their partner(s) in order to incorporate condom use, and therefore lowered their expectations about successfully initiating such changes (i.e., lowered their sex-risk reduction self-efficacy). Indeed, prior research in a drug treatment setting [6] found that increases in participants’ sex-risk reduction knowledge were associated with lowered motivation to initiate condom use, as many participants may question their own ability to successfully negotiate condom use with their sexual partner(s) even when they achieve the requisite knowledge. This calls into question the relative utility of emphasizing HIV knowledge within interventions targeting this population. Indeed, meta-analytic findings of RCTs with high-risk drug users [6] indicate that interventions tend to be optimal when they emphasize skill development components, as does the CHRP intervention, and our path analyses lend further support to these findings.
Outcomes of this RCT underscore the importance of including multiple assessment methods to detect intervention outcomes. A range of HIV-risk related outcomes were measured using an ACASI assessment approach in an effort to limit participants’ reluctance to self-report embarrassing or socially undesirable behaviors [15–17]. Despite this effort, we did not detect changes in sex-risk behavior via ACASI self-report while—as in prior RCTs [2, 13]—we did detect changes in demonstrated sex- and drug-risk reduction skills using blindly-scored, objective measures. While we expected intervention effects across all measured HIV risk reduction outcomes, we also recognized the dangers of relying solely on self-report measures for such personally sensitive behavioral outcomes [18, 19], and therefore elected to implement multiple assessment methods. Based on the overall pattern of outcomes, our objective assessments appear more sensitive at detecting the specific changes targeted by the CHRP intervention. Because hands-on HIV risk reduction skills may be immediately transferrable to real world situations in which many high risk drug users find themselves, producing—and accurately detecting—such changes is crucial to HIV prevention efforts. Thus, the intervention effects pertaining to sex- and drug-related HIV risk reduction skills improvement were particularly promising.
Study Limitations and Future Directions
The limitations of this RCT should be acknowledged in order to properly frame the outcomes. Foremost, the study was conducted in the context of a methadone maintenance drug treatment facility among newly enrolled patients. The drug-risk reducing effects that methadone exerts on opioid use and related HIV risk behaviors [9, 16] are likely to have restricted our ability to detect intervention effects on the drug-related outcomes. Related to study context, we would not necessarily expect our outcomes to be generalizable to non-drug involved populations, non-treatment settings, or to persons not meeting our inclusion criteria including a willingness to participate. Future studies should be designed to test the efficacy of the CHRP intervention in other contexts and among other risk populations of interest.
Second, our assessment approach may have hampered our ability to precisely detect some of our outcomes. Due to participants’ reluctance to self-report socially undesirable behaviors [20], it is a common challenge to accurately capture data pertaining to sex- and drug-risk behaviors. Although we encouraged an open and honest response set through the use of a private ACASI assessment approach, it is possible that some of the self-reported outcomes were nevertheless impacted by participants’ reluctance to disclose HIV risk related behaviors. Use of single item measures for some constructs (e.g., HIV knowledge; condom use) may have also restricted our ability to detect significant changes over time. In addition, we found relatively low reliabilities for some constructs and suspect that these were influenced by the small number of self-reported items per construct [21, 22] as well as participant characteristics (e.g., low education level and low SES). In order to test the likelihood that the observed low reliabilities impacted our findings, however, we conducted analyses of variance with repeated measures (i.e., items of the examined construct) and found that the observed effects remained significant. That is, even when we did not treat the items as one coherent construct—and controlled for the family-wise error—the originally observed effects remained significant, which we found reassuring. In contrast to the weaknesses inherent in the self-reported measures, our objective measures (blindly rated demonstrations of sex- and drug-risk reduction skills) appear to have been robust at detecting changes over time. Thus, future intervention studies in this area should strongly consider also using a multiple methods assessment approach rather than relying solely on self-reported outcomes.
Considered as a whole, the outcomes of this RCT are encouraging and indicate the efficacy of the CHRP intervention, particularly with regard to HIV risk reduction skill enhancement for this target population. Moreover, because the CHRP intervention was deliberately developed and tested in the context of a common type of drug treatment setting, it is likely that it could be readily transportable to many similar drug-treatment settings in which high-risk drug users may be reached, and thus potentially convey significant individual and public health benefits. Future work in this area should be aimed at testing the CHRP intervention in other clinical settings (e.g., STD clinics, criminal justice settings) where large numbers of high risk drug-involved individuals may be readily reached.
Acknowledgments
Funding to support the design and conduct of this research and the preparation of this manuscript was provided by a National Institute on Drug Abuse (NIDA) Grants (R01-DA022122; K02DA033139) to Michael M. Copenhaver.
Contributor Information
Michael M. Copenhaver, Email: michael.copenhaver@uconn.edu, Department of Allied Health Sciences, University of Connecticut, 358 Mansfield Road, Box 2101, Storrs, CT 06269-2101, USA
I-Ching Lee, Department of Psychology, National Chengchi University, Taipei, Taiwan. Center for Mind, Brain, & Learning, National Chengchi University, Taipei, Taiwan.
Patrick Baldwin, APT Foundation, Inc., New Haven, CT, USA.
References
- 1.CDC. Estimated HIV incidence in the United States, 2007–2010. [Accessed 12 June 2013];HIV Surveillance Supplemental Report. 2012 http://www.cdc.gov/hiv/topics/surveillance/basic.htm#incidence.
- 2.Avants SK, Margolin A, Usubiaga MH, Doebrick C. Targeting HIV-related outcomes with intravenous drug users maintained on methadone: a randomized clinical trial of a harm reduction group therapy. J Subst Abuse Treat. 2004;26:67–78. doi: 10.1016/S0740-5472(03)00159-4. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Bridging the gap between practice and research: forging partnerships with community-based drug and alcohol treatment. Washington: National Academy Press; 1998. [PubMed] [Google Scholar]
- 4.Sholomskas DE, Syracuse-Siewert G, Rounsaville BJ, Ball SA, Nuro KF, Carroll M. We don’t train in vain: a dissemination trial of three strategies of training clinicians in cognitive-behavioral therapy. J Consult Clin Psychol. 2005;73(1):106–15. doi: 10.1037/0022-006X.73.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgenstern J, Morgan TJ, McCrady BS, Keller DS, Carroll KM. Manual-guided cognitive behavioral therapy training: a promising method for disseminating empirically supported substance abuse treatments to the practice community. Psychol Addict Behav. 2001;15:83–8. [PubMed] [Google Scholar]
- 6.Copenhaver MM, Lee IC, Margolin A. Successfully integrating an HIV risk reduction intervention into a Community-Based Substance Abuse Treatment Program. Am J Drug Alcohol Abuse. 2007;33:109–20. doi: 10.1080/00952990601087463. [DOI] [PubMed] [Google Scholar]
- 7.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992;111(3):455–74. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 8.Fisher JD, Amico KR, Fisher WA, Harman JJ. The information motivation-behavioral skills model of antiretroviral adherence and its applications. Curr HIV/AIDS Rep. 2008;5(4):193–203. doi: 10.1007/s11904-008-0028-y. [DOI] [PubMed] [Google Scholar]
- 9.Copenhaver MM, Lee IC. Optimizing a community-friendly HIV risk reduction Intervention for drug users in treatment: a structural equation modeling approach. J Urb Health. 2006;83(6):1132–42. doi: 10.1007/s11524-006-9090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher JD, Cornman DH, Osborn CY, Amico KR, Fisher WA, Friedland GA. Clinician-initiated HIV risk reduction intervention for HIV-positive persons: formative research, acceptability, and fidelity of the OPTIONS project. J Acquir Immune Defic Syndr. 2004;37(2):S78–87. doi: 10.1097/01.qai.0000140605.51640.5c. [DOI] [PubMed] [Google Scholar]
- 11.Margolin A, Avants SK, Warburton LA, Hawkins KA, Shi J. A randomized clinical trial of a manual-guided risk reduction intervention for HIV-positive injection drug users. Health Psy-chol. 2003;22(2):223–8. [PubMed] [Google Scholar]
- 12.Copenhaver M, Avants SK, Margolin A, Warburton LA. Intervening effectively with drug abusers infected with HIV: taking into account the potential for cognitive impairment. J Psychoact Drugs. 2003;35(2):209–18. doi: 10.1080/02791072.2003.10400002. [DOI] [PubMed] [Google Scholar]
- 13.Carroll KM, Nich C, Sifiy RL, Nuro KF, Frankforter TL, Ball SA, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictive disorders. J Drug Alcohol Depend. 2000;57:225–38. doi: 10.1016/s0376-8716(99)00049-6. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JD, Fisher WA, Shuper PA. The information–motivation–behavioral skills model of HIV preventive behavior. In: Di-Clemente RJ, Crosby RA, Kegler M, editors. Emerging theories in health promotion practice and research. San Francisco: Jossey-Bass; 2009. pp. 21–64. [Google Scholar]
- 15.Bryan A, Schmiege SJ, Broaddus MR. Mediational analysis in HIV/AIDS research: estimating multivariate path analytic models in a structural equation modeling framework. AIDS Behav. 2007;11:365–83. doi: 10.1007/s10461-006-9150-2. [DOI] [PubMed] [Google Scholar]
- 16.Metzger DS, Navaline H, Woody G. Drug abuse treatment as AIDS prevention. Public Health Rep. 1998;113:97–106. [PMC free article] [PubMed] [Google Scholar]
- 17.Kalichman SC, Stevenson LY. Psychological and social factors associated with histories of risk for human immunodeficiency virus infection among African-American inner-city women. J Women’s Health. 1997;6:209–17. doi: 10.1089/jwh.1997.6.209. [DOI] [PubMed] [Google Scholar]
- 18.Minnis AM, Muchini A, Shiboski S, Mwale M, Morrison C, Chipato T, Padian NS. Audio computer-assisted self-interviewing in reproductive health research: reliability assessment among women in Harare, Zimbabwe. Contraception. 2007;75(1):59–65. doi: 10.1016/j.contraception.2006.07.002. Retrived from, http://www.contraceptionjournal.org/ [DOI] [PubMed] [Google Scholar]
- 19.Brener ND, Billy JOG, Grady WR. Assessment of factors affecting the validity of self-reported health-rish behavior among adolescents: evidence from the scientific literature. J Adolesc Health. 2003;33:436–57. doi: 10.1016/s1054-139x(03)00052-1. [DOI] [PubMed] [Google Scholar]
- 20.Turner CF, Rogers SM, Hendershot TP, Miller HG, Thornberry JP. Improving representation of linguistic minorities in health surveys. Public Health Rep. 1996;111:276–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Peterson RA. A meta-analysis of Cronbach’s coefficient alpha. J Consum Res. 1994;21:381–91. [Google Scholar]
- 22.Peterson RA, Kim Y. On the relationship between coefficient alpha and composite reliability. J Appl Psychol. 2013;98:194–8. doi: 10.1037/a0030767. [DOI] [PubMed] [Google Scholar]