Abstract
Macrophages provide the first line of defense against invading pathogens. The aim of this study was to determine the role of macrophages during infection with group A streptococci (Streptococcus pyogenes) in mice. Here, we report that resident macrophages can efficiently take up and kill S. pyogenes during in vivo infection, as demonstrated by immunofluorescence and electron microscopy, as well as colony counts. To evaluate the contribution of macrophages to the resolution of experimental infection with S. pyogenes, we compared the susceptibility of BALB/c mice rendered macrophage deficient by treatment with carrageenan with that of intact mice. The results show that depletion of macrophages enhanced the susceptibility of BALB/c mice to S. pyogenes infection, as evidenced by 100% mortality of macrophage-depleted mice compared to 90% survival of nondepleted control animals. The in vivo depletion of macrophages strongly enhanced bacterial loads in the blood and systemic organs. Resistance to S. pyogenes can be restored in macrophage-depleted mice by adoptive transfer of purified macrophages. The in vivo blocking of the macrophage phagocytic function by treatment with gadolinium III chloride also resulted in enhanced susceptibility to S. pyogenes. Interestingly, depletion of macrophages prior to or during the first 24 h of infection decreased survival dramatically; in contrast, no mortality was observed in infected nondepleted animals or mice depleted after 48 h of infection. These results emphasize the important contribution of macrophages to the early control of S. pyogenes infection.
Group A streptococci are important human pathogens which cause a variety of pyogenic infections that can be mild, such as pharyngitis, impetigo, and erysipelas, to extremely severe, such as cellulites, necrotizing fasciitis, septicemia, pneumonia, meningitis, and streptococcal toxic shock syndrome (7). Since the late 1980s, a marked increase in the incidence of streptococcal toxic shock syndrome, bacteremia, and severe, invasive skin and soft tissue infections (the “flesh-eating” bacterium) have been reported around the world (5, 10, 14, 22, 30). The resurgence of severe streptococcal diseases has renewed interest in understanding the pathogeneses of these infections and in the elucidation of the host defense mechanisms involved in these processes. The identification of key immune components involved in bacterial clearance might provide new means of prevention and management of severe streptococcal diseases.
The primary line of innate defense against most bacterial pathogens consists of resident macrophages, which reside in tissues, and polymorphonuclear neutrophils (PMNs), which migrate from the blood to the site of infection (2, 21). Most studies of the innate host response to Streptococcus pyogenes have focused on the role of PMNs. S. pyogenes has developed a broad array of virulence functions directed at circumventing the phagocytic activities of these cells (8, 17, 35, 36). Thus, the surface-associated M protein confers resistance to phagocytosis by inhibiting activation of the alternative complement pathway (8). The antiphagocytic capacity of the hyaluronic acid capsule has also been demonstrated (35). The streptococcal C5a peptidase interferes with the recruitment of PMNs to the site of infection by inactivating the chemotactic component C5a of the complement system (36) and the recently identified Mac protein that inhibits professional phagocyte function by binding to FcγRIIIB on the surfaces of PMNs (17).
However, little is known about the factors governing the very first interactions of S. pyogenes and the host, before PMNs begin to accumulate in the site of infection. The nature of these interactions might be critical for dictating the fate of the infection: either bacterial clearance or spread from a local infection to progressive invasive disease.
Macrophages play an essential role in the innate immune response against invading pathogens (11). Thus, activated macrophages kill microorganisms by phagocytosis, become efficient antigen-presenting cells, and produce mediators, such as cytokines and growth factors, that trigger local inflammation (3, 15, 23, 26). However, the potential role of macrophages in the pathogenesis of streptococcal infections has received little attention.
In this study, we have examined the interactions between S. pyogenes and mouse resident peritoneal macrophages following in vivo infection. Our study reveals that resident macrophages can efficiently phagocytose and kill S. pyogenes in the absence of opsonic antibodies.
The role of macrophages in the host defense against S. pyogenes was also addressed by using a previously described experimental mouse model of streptococcal infection (9, 18). For this purpose, mice were either depleted of macrophages by treatment with carrageenan or rendered deficient in macrophage functional activities by treatment with gadolinium III chloride (GdIIICl) and subsequently infected with an inoculum of S. pyogenes which is normally sublethal and causes little illness in normal BALB/c mice (18). The relative importance of macrophages in the onset of systemic streptococcal infection was assessed by studying the effects of macrophage deficiency on survival and bacterial clearance.
MATERIALS AND METHODS
Bacteria.
S. pyogenes strain A20 (M type 23) is a human isolate obtained from the German Culture Collection (DSM 2071). Stocks were maintained at −70°C and were routinely cultured at 37°C in Todd-Hewitt broth (Oxoid, Basingstoke, United Kingdom) supplemented with 1% yeast extract for ∼6 h. Bacteria were collected in mid-log phase, washed twice with sterile phosphate-buffered saline (PBS), and diluted to the required inoculum, and the number of viable bacteria was determined by counting CFU after dilution and plating in blood agar plates (GIBCO, Paisley, United Kingdom) containing 5% sheep blood. When required, S. pyogenes was labeled green with carboxyfluorescein (Molecular Probes, Göttingen, Germany). A suspension of 5 × 108 bacteria was centrifuged, resuspended in 1 ml of Hanks balanced salt solution containing 0.2 mg of carboxyfluorescein/ml, and incubated for 30 min at 4°C in the dark. After incubation, the labeled bacteria were washed several times to remove unbound dye.
Mice.
Female BALB/c mice were purchased from Harlan-Winkelmann (Borchen, Germany) and used in experiments when they were between 8 and 10 weeks of age. They were housed in microisolator cages and given food and water ad libitum. All studies were approved by the appropriate authorities.
Intraperitoneal infection of mice.
The BALB/c mice were intraperitoneally infected with the appropriate dose of S. pyogenes and euthanized 1 h thereafter, and their peritoneums were lavaged with sterile PBS. The lavage samples were then centrifuged, and the cell pellets were resuspended in the corresponding solution for either microscopy or flow cytometry analysis.
Flow cytometry detection of phagocytosed bacteria.
Phycoerythrin (PE)-conjugated anti-F4/80 antibodies (Pharmingen, San Diego, Calif.) were used to label peritoneal macrophages present in the lavage washes from mice infected with S. pyogenes or from uninfected control mice. Briefly, peritoneal cells were incubated with purified anti-CD32 antibodies to block the Fc receptor, followed by PE-conjugated anti-mouse F4/80 antibodies. After incubation for 30 min at 4°C, the cells were washed, and flow-cytometric analysis was performed with a FACScan (Becton Dickinson, San Jose, Calif.). Macrophages were gated according to their expression of F4/80 antigen (FL2). Association of green-labeled streptococci with macrophages is expressed as the increase in green fluorescence of macrophages (FL1).
Immunofluorescence and light microscopy.
Lavage fluid samples obtained from mice intraperitoneally infected with 5 × 107 CFU of S. pyogenes were centrifuged, and the cell pellets were washed twice with sterile PBS and resuspended in complete Dulbecco's modified Eagle's medium (GIBCO) supplemented with 10% heat-inactivated fetal calf serum, 100 μg of gentamicin/ml, 5 × 10−5 M 2-mercaptoethanol, and 1 mM l-glutamine. The cell suspensions were applied to sterile coverslips and incubated at 37°C for 2 h to allow the cells to adhere. After incubation, the coverslips were rinsed to remove unbound cells, and adherent cells were fixed with 3.7% formaldehyde. For double-immunofluorescence staining, extracellular bacteria were stained with polyclonal rabbit anti-S. pyogenes antibodies, followed by Alexa green-conjugated goat anti-rabbit antibodies (Sigma, Deisenhofen, Germany). After several washes, the cells were permeabilized by 0.025% Triton X-100 in PBS and washed again, and intracellular bacteria were stained by anti-S. pyogenes antibodies, followed by Alexa red-conjugated goat anti-rabbit antibodies (Sigma). The fluorescence images were obtained with a confocal laser scanning microscope (Bio-Rad, Hercules, Calif.).
Macrophage-killing assay.
The ability of macrophages to kill S. pyogenes was determined by performing colony counts. Mice were intraperitoneally infected with 5 × 107 CFU of S. pyogenes and euthanized after 1 h, and their peritoneums were washed with sterile PBS. The peritoneal cells were resuspended in complete Dulbecco's modified Eagle's medium, seeded at 5 × 106/well in a flat-bottomed 48-well microtiter plate (Nunc, Wiesbaden, Germany), and incubated for 2 h at 37°C in a 5% CO2 atmosphere. Subsequently, nonadherent cells were removed by washing the plates twice with warm PBS, and adherent cells were incubated further in the presence of 100 μg of gentamicin/ml. At different incubation times, macrophages were detached from the plate by treatment with 100 μl of 0.25% trypsin supplemented with 0.02% EDTA (GIBCO) and then lysed by the addition of 400 μl of Triton X-100 (0.025% in H2O). Appropriate dilutions were plated on blood agar to determine the numbers of viable bacteria.
Electron microscopy.
For scanning electron microscopy, samples were fixed with 5% formaldehyde and 2% glutaraldehyde in cacodylate buffer (0.1 M cacodylate, 0.01 M CaCl2, 0.01 M MgCl2, pH 6.9) for 1 h on ice and washed in TE buffer (20 mM Tris, 1 mM EDTA, pH 7.0). Dehydration was performed with a graded series of acetone, critical-point dried with CO2, and sputter coated with gold before examination in a Zeiss field emission scanning electron microscope (DSM982 Gemini) at 5 kV using the Everhart-Thornley SE detector and the in-lens secondary electron (SE) detector in a 50:50 ratio.
For transmission analysis, samples were fixed as described above and washed with cacodylate buffer, followed by fixation with 1% aqueous osmium tetroxide for 1 h at room temperature. The samples were then dehydrated in a graded series of acetone and embedded in an epoxy resin following Spurr's formula (29). Ultrathin sections were cut with glass knives and counterstained with 4% aqueous uranyl acetate for 2 min and with lead citrate for 5 min before examination in a Zeiss EM910 transmission electron microscope at an acceleration voltage of 80 kV.
Intravenous infection of mice.
Mice were inoculated with 105 CFU of S. pyogenes in 0.2 ml of PBS via a lateral tail vein. For kinetic studies, infected mice were euthanized by CO2 asphyxiation, and the bacteria were enumerated at progressive times in the organs of infected mice by preparing homogenates and plating 10-fold serial dilutions on blood agar. Colonies were counted after 24 h of incubation at 37°C. Viable bacterial counts were also determined in the blood of infected mice by collecting blood samples from the tail vein at different times postinoculation and plating serial dilutions in blood agar.
Depletion of macrophages by treatment with carrageenan.
Depletion of macrophages by using carrageenan was performed as previously described (28, 33, 38). Briefly, mice were treated intraperitoneally with 1 mg of carrageenan (type IVλ; Sigma) either prior to bacterial challenge or at specific times after bacterial inoculation. Control mice received 200 μl of sterile PBS. The depletion of macrophages was verified by flow-cytometric analysis of spleen cells after double staining them with fluorescein isothiocyanate (FITC)-conjugated anti-mouse Mac-1 and biotin-conjugated anti-mouse F480 antibodies, followed by streptavidin-PE (Pharmingen).
FACScan analysis.
Approximately 5 × 105 cells were incubated in staining buffer (PBS supplemented with 2% fetal calf serum and 0.1% sodium azide) with the desired antibody or combination of antibodies for 30 min at 4°C. After washes, the cells were analyzed on a FACScan (Becton Dickinson, Erembodegem-Aalst, Belgium). The monoclonal antibodies used were FITC-conjugated anti-CD4, PE-conjugated anti-CD8, PE-conjugated anti-Gr-1, FITC-conjugated anti-CD19, FITC-conjugated anti-Mac-1, and biotin-conjugated anti-mouse F480 (Pharmingen). When biotin-conjugated antibodies were used, the cells were washed and incubated for 30 min at 4°C with streptavidin-PE.
Reconstitution of carrageenan-treated mice with purified macrophages.
Reconstitution experiments were performed as previously described (6). Briefly, resident macrophages were harvested from donor mice by peritoneal lavage and purified by negative selection using a cocktail of antibodies (anti-B220, anti-CD4, anti-CD8, anti-DX5, and anti-Gr-1) and MiniMACS magnetic beads (Miltenyi Biotec GmbH, Bergisch-Gladbach, Germany) according to the instructions of the manufacturer. Cell preparations contained >95% macrophages; 2 × 106 macrophages were injected intravenously after 2 days of carrageenan treatment, and the animals were infected the next day.
GdIIICl treatment.
Treatment with GdIIICl has been shown to inactivate macrophages by suppressing phagocytic and inflammatory responses (1, 12, 25, 27). GdIIICl was dissolved in H2O, and 10 mg/kg of body weight was injected via the tail vein 30 and 6 h prior to bacterial challenge in a total volume of 200 μl. Control animals were injected with 200 μl of PBS.
Lancefield bactericidal assay.
A modified version of the classical Lancefield bactericidal assay was used (16). Briefly, S. pyogenes was grown until mid-log phase, washed, and adjusted to 105 bacteria/ml in PBS. Fifty microliters of bacterial suspension (5 × 103 CFU) was mixed with 450 μl of fresh heparinized blood obtained from untreated or carrageenan-treated mice and rotated at 37°C. Viable counts after 3 h of incubation were determined by plating diluted samples on blood agar.
Statistical analysis.
Statistical significance between paired samples was determined by Student's t test.
RESULTS
In vivo phagocytosis of S. pyogenes by resident peritoneal macrophages.
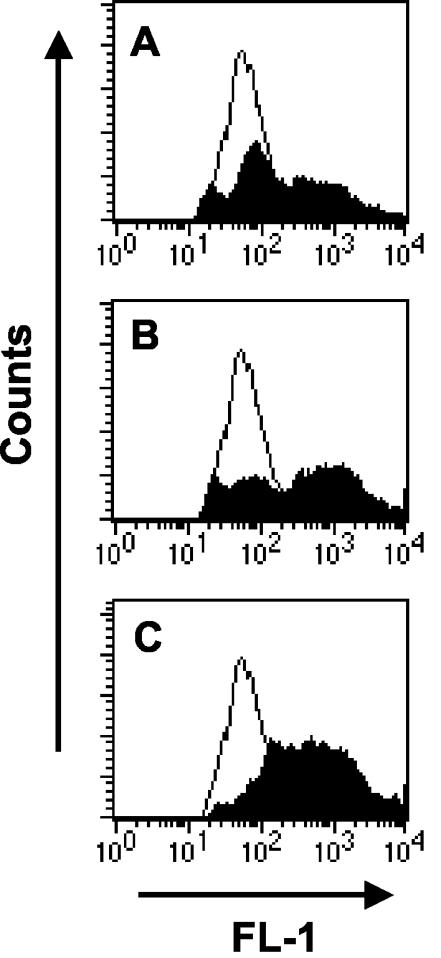
To determine the ability of resident macrophages to take up S. pyogenes during in vivo infection, mice were injected intraperitoneally with increasing numbers (107, 5 × 107, or 108 CFU) of green-labeled S. pyogenes cells or PBS as a control, and peritoneal washes were collected after 1 h. Peritoneal cells were stained with PE-conjugated anti-F4/80 antibodies, and the proportion of F4/80+ cells with green fluorescence above background levels was determined by flow cytometry. As shown in Fig. 1, phagocytosis took place in a dose-dependent manner. Upon inoculation with 107 CFU, 42% ± 5% of macrophages became associated with green-labeled S. pyogenes, as evidenced by an increase in their mean fluorescent intensity (MFI) from 317 ± 22 (uninfected macrophages) to 1,006 ± 94 (Fig. 1A). The inoculum dependence of the association is demonstrated by the higher phagocytosis rate observed when the inoculum dose was increased. Thus, inoculation of 5 × 107 CFU resulted in 59% ± 7% of macrophages associated with bacteria and an MFI of 1,392 ± 92 (Fig. 1B), and inoculation with 108 CFU resulted in 70% ± 6% phagocytosis and an MFI of 1,216 ± 58 (Fig. 1C).
FIG. 1.
In vivo uptake of S. pyogenes by peritoneal macrophages. Mice were intraperitoneally inoculated with either 107 (A), 5 × 107 (B), or 108 (C) CFU of green-labeled S. pyogenes (solid histograms) or PBS (open histograms) and subjected to peritoneal lavage, and peritoneal macrophages were labeled with PE-conjugated anti-F4/80 antibodies. Flow cytometry analysis was performed by gating the F4/80+ population, and the association of green-labeled streptococci with macrophages was expressed as the increase in green fluorescence of macrophages (FL-1). One representative experiment out of three is shown.
To confirm that the association of S. pyogenes with macrophages was not an artifact of the bacterial labeling procedure, the in vivo phagocytosis of unlabeled S. pyogenes by peritoneal macrophages was then determined by fluorescence (Fig. 2) and electron microscopy (Fig. 3).
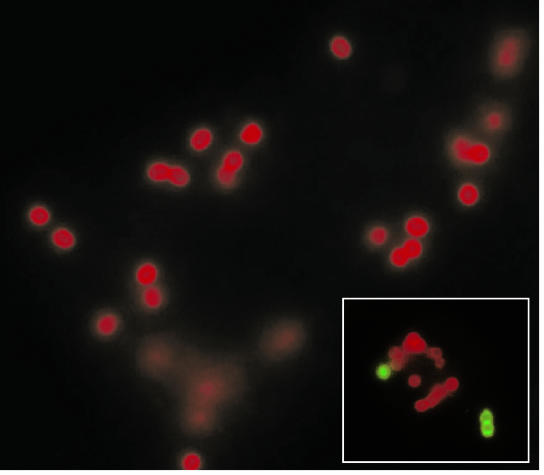
FIG. 2.
Localization of S. pyogenes in peritoneal macrophages after in vivo infection. Peritoneal macrophages were isolated from mice intraperitoneally inoculated with S. pyogenes and processed for double-immunofluorescence staining. The extracellular bacteria stained green, whereas the intracellular bacteria stained red. Magnification, ×3,000. (Inset) A macrophage containing both attached (green) and internalized (red) microorganisms. Magnification, ×2,500.
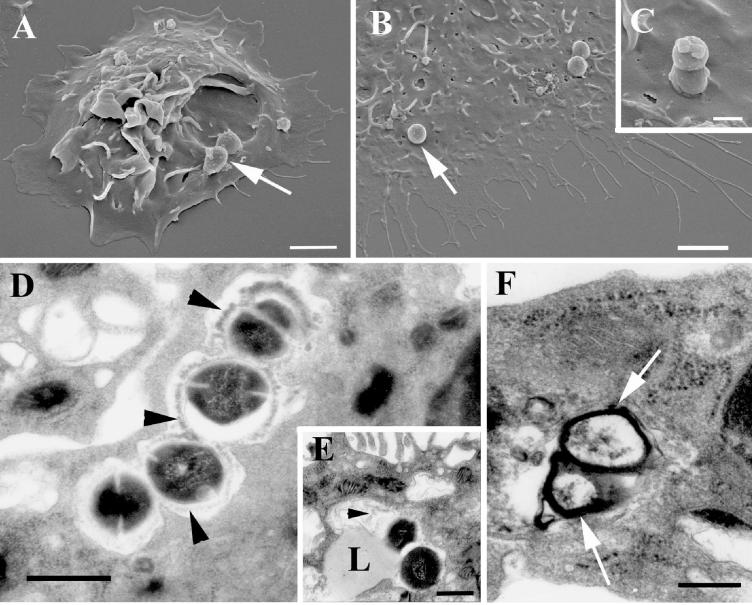
FIG. 3.
Electron microscopy examination of S. pyogenes-infected peritoneal macrophages. (A to C) Scanning electron microscopy revealed streptococci (arrows) attached to macrophages (A and B) and microorganisms in the process of being internalized (C). Ultrathin sections of infected macrophages show degradation of intracellular streptococci. (D) The capsular structure (arrowheads) is being detached from the surface of the bacterium and undergoing degradation. (E) Fusion of a lysosome (L) with the phagosome. Again, degraded capsular material (arrowhead) can be identified in the phagolysosome. (F) This process leads to a complete degradation of the microorganism. Only the debris of the cell wall (arrows) can be found inside the phagolysosome. Bars, 2 (A and B), 0.5 (C), and 1 (D to F) μm.
For double immune fluorescence studies, extracellular bacteria were labeled green and intracellular bacteria were labeled red. As shown in Fig. 2, most of the microorganisms displayed red fluorescence, indicating that S. pyogenes was very efficiently internalized by macrophages. Control experiments with nonpermeabilized cells were devoid of red-fluorescing bacteria, corroborating the intracellular location of these microorganisms. Occasionally, extracellular bacteria (green) attached to the surfaces of the phagocytic cells could be observed (Fig. 2, inset).
Scanning electron microscopic examination of in vivo-infected macrophages revealed the presence of microorganisms attached to (Fig. 3A and B) and in the process of being phagocytosed by (Fig. 3C) resident macrophages. Ultrathin sections of infected macrophages displayed in Fig. 3D show that S. pyogenes resides intracellularly within phagosomes, where the bacteria undergo progressive degradation. Also evident in Fig. 3D is the fact that the degradation process of streptococci starts with the detachment of the capsule from the bacterial cell wall and the degradation of capsular material. Next, lysosomes fuse with the streptococcus-containing phagosome (Fig. 3E), resulting in the complete degradation of the microorganism. Eventually, only debris of the cell wall of the streptococci can be found within the phagolysosome (Fig. 3F).
The ability of macrophages to kill S. pyogenes was further confirmed by measuring the number of bacteria surviving at increasing times after phagocytosis. Briefly, after 1 h of intraperitoneal infection, peritoneal macrophages were obtained from infected mice, and any remaining live extracellular bacteria were killed by incubation in the presence of 100 μg of gentamicin/ml. After 2 and 4 h of incubation, intracellular bacteria were released and plated on blood agar. A sevenfold reduction in the number of bacteria per well was observed between 2 and 4 h of incubation (4.75 ± 0.3 CFU after 2 h and 3.92 ± 0.22 CFU after 4 h of incubation), corroborating the ability of macrophages to kill phagocytosed S. pyogenes.
Depletion of macrophages significantly increased susceptibility of BALB/c mice to S. pyogenes infection.
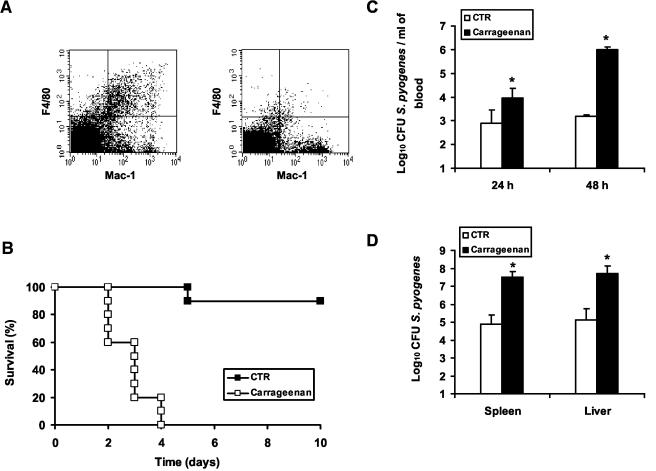
To determine the role of macrophages in host resistance to S. pyogenes, BALB/c mice were treated with carrageenan prior to bacterial challenge. Previous investigators reported successful depletion of resident macrophages by treatment with carrageenan (28, 33, 38). The efficacy of the depletion (>90%) was determined by assessing the percentage of macrophages (Mac-1-F4/80 double-positive cells) in the spleens of untreated (6.7% ± 0.4%) or carrageenan-treated (0.7% ± 0.5%) mice using flow cytometry (Fig. 4A). The results shown in Fig. 4B clearly demonstrate that mice depleted of macrophages were highly susceptible to infection with S. pyogenes, showing significantly reduced survival (100% mortality on day 4 postinfection) compared with controls (90% survival). Consistent with the loss of macrophages, carrageenan-treated mice have significantly higher bacterial loads in the blood (Fig. 4C) and in the liver and spleen (Fig. 4D) than control mice. These results indicate that macrophages are important effector cells for controlling infection with S. pyogenes.
FIG. 4.
Impaired immune defense against S. pyogenes in macrophage-depleted mice. BALB/c mice were depleted of macrophages by treatment with carrageenan and then challenged intravenously with 105 CFU of S. pyogenes. Control mice were injected with PBS. (A) Flow cytometric analysis of spleen cells isolated from control (left) or carrageenan-treated (right) animals, showing the efficiency of depletion. The dot plot in each upper right quadrant shows the F4/80 and Mac-1 profile for macrophages. (B) Survival curves of macrophage-depleted (□) and control (CTR) (▪) mice. Each group contained 10 mice. (C) Bacteremias of macrophage-depleted (solid bars) and control (open bars) mice 24 and 48 h postinoculation (n = 5 mice/group). (D) Bacterial loads in livers and spleens of macrophage-depleted (solid bars) and control (open bars) mice 48 h postinfection (n = 5 mice/group). (*, P < 0.05). One representative experiment out of four is shown. The error bars indicate standard deviations.
In addition to depletion of macrophages, treatment with carrageenan resulted in increased numbers of PMNs in the blood and also in the spleen. To exclude the possibility that these increased numbers of PMNs could have some influence on the observed susceptibility of carrageenan-treated mice, a modified bactericidal Lancefield assay was performed using untreated mice as controls. Streptococci exhibited comparable levels of resistance to phagocytosis in blood from carrageenan-treated and untreated mice (152% ± 74% survival in blood from carrageenan-treated mice and 144% ± 58% survival in blood from untreated mice). Treatment with carrageenan did not affect spleen B cells (49.3% ± 0.6% before and 49% ± 1.7% after carrageenan treatment), CD4+ T cells (20.8% ± 1.03% before and 19.7% ± 1.5% after carrageenan treatment), or CD8+ T cells (8.34% ± 1.03% before and 7.95% ± 0.5% after carrageenan treatment).
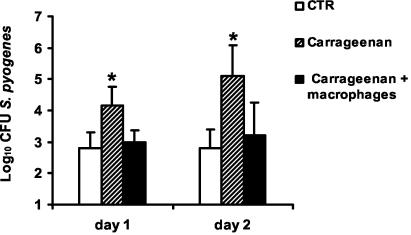
Further confirmation that the loss of resistance of carrageenan-treated BALB/c mice was due to the depletion of macrophages and not to the effect of the drug on other cell populations was provided by repopulation of carrageenan-treated mice with purified resident peritoneal macrophages and then infection with S. pyogenes. After repopulation, 80 to 90% of carrageenan-treated mice recover their capacity to control S. pyogenes infection, as demonstrated by their ability to survive infection and the significantly lower levels of bacteremia than in nonrepopulated mice (P < 0.05) (Fig. 5).
FIG. 5.
Restoration of anti-S. pyogenes resistance by reconstitution of carrageenan-treated mice with resident macrophages. Mice were treated with carrageenan, and one group was reconstituted with resident macrophages (solid bars) or injected with PBS (hatched bars) and then infected with S. pyogenes. Untreated mice were used as controls (CTR) (open bars). Bacteremia was determined after 24 and 48 h of bacterial inoculation. The bars represent the mean plus standard deviation of four mice per group. Statistical significance based on a comparison of carrageenan-treated and macrophage-reconstituted carrageenan-treated mice is indicated by an asterisk (P < 0.05). One representative experiment out of three is shown.
Increased susceptibility to group A streptococcus infection by blocking the functional phagocytosis of macrophages after treatment with GdIIICl.
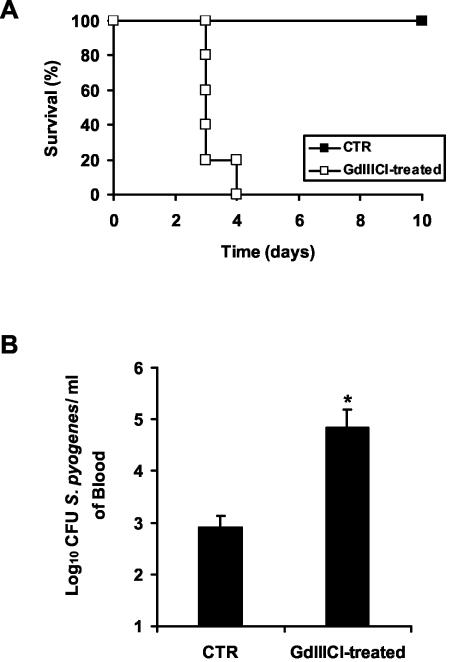
To further demonstrate that phagocytosis of S. pyogenes by macrophages was critical for the resolution of infection, BALB/c mice were treated with GdIIICl prior to bacterial challenge. Intravenous injections of GdIIICl have been shown to inhibit phagocytosis of macrophages without affecting viability (1, 12, 25, 27). Thus, the percentage of splenic macrophages did not change significantly after GdIIICl treatment (7.2% ± 0.2% for untreated mice and 7.05% ± 0.3% for GdIIICl-treated mice). There is no evidence that GdIIICl has any direct toxic effects on other cell populations. As seen with macrophage-depleted mice, treatment with GdIIICl resulted in a dramatic decrease in survival (Fig. 6A), concomitant with a remarkable increase in bacterial loads (Fig. 6B). These data indicate the requirement for an intact phagocytic activity of macrophages for bacterial clearance in this model of infection.
FIG. 6.
Effect of blocking macrophage phagocytic function by treatment with GdIIICl on survival of mice intravenously infected with 105 CFU of S. pyogenes (A) or on bacteremia 48 h postinfection (B). Control (CTR) mice were treated with PBS. Each bar represents the mean plus standard error of the mean of five mice per group (*, P < 0.05). One representative experiment out of four is shown.
The effect of macrophage depletion on resistance to S. pyogenes infection is only evidenced when depletion is performed prior to or during the first 24 h of infection.
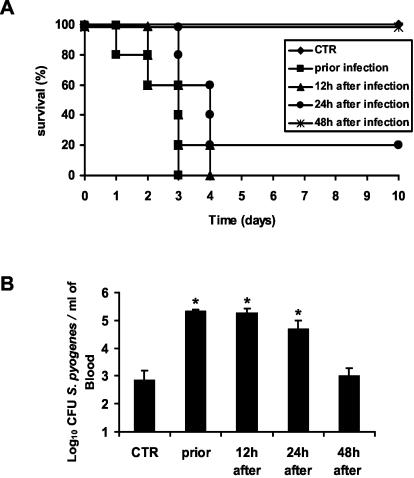
To determine the roles of resident macrophages at different stages of infection with S. pyogenes, BALB/c mice were treated with carrageenan either 24 h prior to or at 12, 24, and 48 h after bacterial inoculation. As seen in Fig. 7A, survival of macrophage-depleted animals decreased substantially when macrophage depletion was performed prior to or during the first 24 h of infection (100% lethality was noted by day 4 postinoculation in the groups treated with carrageenan prior to infection or after 12 h of infection, and 80% mortality was noted in the group depleted 24 h after bacterial inoculation). In contrast, the survival of infected mice was not affected after depletion of macrophages 48 h postinfection, and 100% of the mice survived after the 10 days of follow up. Macrophage depletion prior to or during the first 24 h of infection resulted in dramatic increases in bacterial loads in the blood compared to those of control mice (Fig. 7B). Bacteremia in mice depleted of resident macrophages after 48 h postinfection did not differ significantly from that in nondepleted mice. These findings suggest that resident macrophages might play an important role in the initial bacterial clearance during the onset of streptococcal infection.
FIG. 7.
Effect of macrophage depletion either prior to infection or 12, 24, or 48 h after intravenous inoculation on resistance against S. pyogenes. Depletion of macrophages at each time point was performed by treatment with carrageenan or PBS for control (CTR) mice. (A) Survival times of macrophage-depleted and control mice monitored over a period of 10 days. Each group contained 10 mice. (B) Bacteremias of macrophage-depleted and control mice 48 h after bacterial challenge. CFU are expressed as mean values plus standard deviations of five mice per group (*, P < 0.05). One representative experiment out of five is shown.
DISCUSSION
It has been shown that BALB/c mice are very efficient at controlling infection with S. pyogenes (9, 18). The resolution of streptococcal infection in these mice was dependent on innate immune mechanisms, as shown by the capacity of immunodeficient SCID BALB/c mice to clear infection (18). It was also shown that resistance to S. pyogenes in BALB/c mice was expressed very early during the infection process (18), suggesting the participation of resident immune mechanisms in these events. In this study, we sought to elucidate the contribution of macrophages to resistance expressed by BALB/c mice to infection with S. pyogenes.
Resident macrophages constitute the primary line of innate defense against most bacterial pathogens (2, 21). Macrophages are found in all body tissues, and they function as sentinels capable of rapidly recognizing and internalizing bacterial pathogens, and they also recruit other immune cells to the site of infection by the production of chemokines and acute-phase proteins (3, 11, 15, 23, 26). In this study, we sought to elucidate the contribution of macrophages to resistance expressed by BALB/c mice to infection with S. pyogenes.
The critical role of macrophages during infection with S. pyogenes was demonstrated here by the increased susceptibility of macrophage-depleted mice. Macrophage-depleted mice were unable to recover from a sublethal dose of S. pyogenes, with 100% of the macrophage-depleted animals dead by day 4 postinoculation, whereas 90% of the control animals survived infection. This increased mortality was accompanied by enhanced bacterial loads in the blood and systemic organs. Interestingly, increased susceptibility to infection was observed only when depletion of macrophages was performed prior to or during the first 24 h postinoculation, which indicates that macrophages might be required for resistance against S. pyogenes during the very early phase of the infection.
The possibility that macrophages may play an important role in clearing streptococci was previously suggested by Ji and coworkers (13). In that study, a mutant strain of S. pyogenes deficient in C5a peptidase was found to be more efficiently eliminated than the wild-type strain in spite of attracting higher number of PMNs to the site of infection and being resistant to the phagocytic activity of these cells in vitro (13).
The mechanisms underlying the protective function of macrophages seem to be dependent on the ability of these phagocytic cells to efficiently take up and kill S. pyogenes, as demonstrated by (i) double-immunofluorescence microscopy showing that most of the microorganisms associated with macrophages were localized intracellularly, (ii) survival assays showing that macrophages are capable of killing S. pyogenes at early stages of ingestion, and (iii) electron microscopy studies showing intraphagosomal destruction of streptococcal microorganisms.
The role of macrophages in host defense has been shown to be particularly important early in the host-pathogen encounter, when specific immunity has not yet developed. Several pathogens express surface domains that are recognized by macrophages in the absence of specific antibodies (24). These interactions have been shown to play an important role in the phagocytosis of Listeria monocytogenes, Pseudomonas aeruginosa, Neisseria meningitidis, group B streptococci, and mycobacteria, among others (4, 20, 31, 32).
Two principal mechanisms of phagocytosis have been described, opsonin-dependent phagocytosis (37) and opsonin-independent phagocytosis (24). In opsonin-dependent phagocytosis, immunoglobulins or complement molecules bind to microorganisms, thereby promoting ingestion via Fcγ or complement receptor on phagocytic cells (37). In contrast, in opsonin-independent phagocytosis, ligands on the surfaces of microorganisms are directly recognized by receptors on the surfaces of phagocytes (24). Which of these mechanisms is preferentially used by resident mouse macrophages to take up S. pyogenes during early infection is under investigation.
In addition to a direct killing activity, macrophages can indirectly contribute to control of streptococcal infections by promoting the influx of other inflammatory cells into the focus of infection. The capacity of macrophages to promote chemotaxis in response to S. pyogenes is supported by several in vitro studies showing the ability of the microorganism to activate transcription factors involved in cytokine signaling and to induce the expression of chemokines in human macrophages (19, 34). The cooperation between macrophages and PMNs may be important for the final outcome of the infection.
High variability in the capacities of genetically different mouse strains to resolve S. pyogenes infection has been also reported (9, 18). Although the specific host characteristics that contribute to the observed differences in the resolution of streptococcal infection are still unknown, the inability to eliminate bacteria, leading to an overproduction of inflammatory mediators, was the main mechanism underlying the more susceptible phenotype (9). The possibility that host genetic factors may determine the capacity of resident macrophages to reduce the number of bacteria during early infection should be addressed in further studies.
In summary, the results obtained in this study clearly indicate that macrophages are central determinants of the course of infection caused by S. pyogenes. Further studies are required to identify the bacterial determinants and their counterpart receptors on macrophages involved in phagocytosis and/or the release of inflammatory mediators.
Acknowledgments
This work was supported, in part, by the Deutsche Forschungsgemeinschaft (DFG) grant 2407/1 to M.R. and, in part, by the DFG grants GU 482/2-1 and GU 482/2-2.
Editor: D. L. Burns
REFERENCES
- 1.Adding, L. C., G. L. Bannenberg, and L. E. Gustafsson. 2001. Basic experimental studies and clinical aspects of gadolinium salts and chelates. Cardiovasc. Drug Rev. 19:41-56. [DOI] [PubMed] [Google Scholar]
- 2.Aderem, A. 2003. Phagocytosis and the inflammatory response. J. Infect. Dis. 187(Suppl. 2):S340-S345. [DOI] [PubMed] [Google Scholar]
- 3.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. [DOI] [PubMed] [Google Scholar]
- 4.Albanyan, E. A., and M. S. Edwards. 2000. Lectin site interaction with capsular polysaccharide mediates nonimmune phagocytosis of type III group B streptococci. Infect. Immun. 68:5794-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cone, L. A., D. R. Woodard, P. M. Schlievert, and G. S. Tomory. 1987. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N. Engl. J. Med. 317:146-149. [DOI] [PubMed] [Google Scholar]
- 6.Debrick, J. E., P. A. Campbell, and U. D. Staerz. 1991. Macrophages as accessory cells for class I MHC-restricted immune responses. J. Immunol. 147:2846-2851. [PubMed] [Google Scholar]
- 7.Efstratiou, A. 2000. Group A streptococci in the 1990s. J. Antimicrob. Chemother. 45(Suppl.):3-12. [DOI] [PubMed] [Google Scholar]
- 8.Fischetti, V. A. 1989. Streptococcal M protein: molecular design and biological behaviour. Clin. Microbiol. Rev. 2:285-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldmann, O., G. S. Chhatwal, and E. Medina. 2003. Immune mechanisms underlying host susceptibility to infection with group A streptococci. J. Infect. Dis. 187:854-861. [DOI] [PubMed] [Google Scholar]
- 10.Holm, S. E. 1996. Invasive group A streptococcal infections. N. Engl. J. Med. 335:590-591. [DOI] [PubMed] [Google Scholar]
- 11.Hume, D. A., L. L. Ross, S. R. Himes, R. T. Sasmono, C. A. Wells, and T. Ravasi. 2002. The mononuclear phagocyte system revisited. J. Leukoc. Biol. 72:621-627. [PubMed] [Google Scholar]
- 12.Jadus, M. R., C. C. Williams, M. D. Avina, M. Ly, S. Kim, Y. Liu, R. Narasaki, C. A. Lowell, and H. T. Wepsic. 1998. Macrophages kill T9 glioma tumor cells bearing the membrane isoform of macrophage colony stimulating factor through a phagocytosis-dependent pathway. J. Immunol. 160:361-368. [PubMed] [Google Scholar]
- 13.Ji, Y., L. McLandsborough, A. Kondagunta, and P. P. Cleary. 1996. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect. Immun. 64:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan, E. L. 1991. The resurgence of group A streptococcal infections and their sequelae. Eur. J. Clin. Microbiol. Infect. Dis. 10:55-57. [DOI] [PubMed] [Google Scholar]
- 15.Keshav, S., L. P. Chung, and S. Gordon. 1990. Macrophage products in inflammation. Diagn. Microbiol. Infect. Dis. 13:439-447. [DOI] [PubMed] [Google Scholar]
- 16.Lancefield, R. C. 1962. Current knowledge of type-specific M antigens of group A streptococci. J. Immunol. 89:307-313. [PubMed] [Google Scholar]
- 17.Lei, B., F. R. DeLeo, N. P. Hoe, M. R. Graham, S. M. Mackie, R. L. Cole, M. Liu, H. R. Hill, D. E. Low, M. J. Federle, J. R. Scott, and J. M. Musser. 2001. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat. Med. 7:1298-1305. [DOI] [PubMed] [Google Scholar]
- 18.Medina, E., O. Goldmann, M. Rohde, A. Lengeling, and G. S. Chhatwal. 2001. Genetic control of susceptibility to group A streptococcal infection in mice. J. Infect. Dis. 184:846-852. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen, M., A. Lehtonen, I. Julkunen, and S. Matikainen. 2000. Lactobacilli and Streptococci activate NF-kappa B and STAT signaling pathways in human macrophages. J. Immunol. 164:3733-3740. [DOI] [PubMed] [Google Scholar]
- 20.Mork, T., and R. E. Hancock. Mechanisms of nonopsonic phagocytosis of Pseudomonas aeruginosa. Infect. Immun. 61:3287-3293. [DOI] [PMC free article] [PubMed]
- 21.Muller, W. A. 2003. Leukocyte-endothelial-cell interactions in leukocyte transmigration and the inflammatory response. Trends Immunol. 24:327-334. [DOI] [PubMed] [Google Scholar]
- 22.Musher, D. M., R. J. Hamill, C. E. Wright, J. E. Clarridge, and C. M. Ashton. 1996. Trends in bacteremic infection due to Streptococcus pyogenes (group A streptococcus), 1986-1995. Emerg. Infect. Dis. 2:54-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nathan, C. F. 1987. Secretory products of macrophages. J. Clin. Investig. 79:319-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ofek, I., J. Goldhar, Y. Keisari, and N. Sharon. 1995. Nonopsonic phagocytosis of microorganisms. Annu. Rev. Microbiol. 49:239-276. [DOI] [PubMed] [Google Scholar]
- 25.Roland, C. R., Y. Nakafusa, and M. W. Flye. 1999. Gadolinium chloride inhibits lipopolysaccharide-induced mortality and in vivo prostaglandin E2 release by splenic macrophages. J. Gastrointest. Surg. 3:301-307. [DOI] [PubMed] [Google Scholar]
- 26.Sansonetti, P. 2001. Phagocytosis of bacterial pathogens: implications in the host response. Semin. Immunol. 13:381-390. [DOI] [PubMed] [Google Scholar]
- 27.Shi, J., Y. Kokubo, and K. Wake. 1998. Expression of P-selectin on hepatic endothelia and platelets promoting neutrophil removal by liver macrophages. Blood 92:520-528. [PubMed] [Google Scholar]
- 28.Sosroseno, W., and E. Herminajeng. 2002. The role of macrophages in the induction of murine immune response to Actinobacillus actinomycetemcomitans. J. Med. Microbiol. 51:581-588. [DOI] [PubMed] [Google Scholar]
- 29.Spurr, A. R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31-43. [DOI] [PubMed] [Google Scholar]
- 30.Stevens, D. L., et al. 1989. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N. Engl. J. Med. 321:1-7. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki, H., Y. Kurihara, M. Takeya, N. Kamada, M. Kataoka, K. Jishage, O. Ueda, H. Sakaguchi, T. Higashi, T. Suzuki, Y. Takashima, Y. Kawabe, O. Cynshi, Y. Wada, M. Honda, H. Kurihara, H. Aburatani, T. Doi, A. Matsumoto, S. Azuma, T. Noda, Y. Toyoda, H. Itakura, Y. Yazaki, T. Kodama, et al. 1997. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 386:292-296. [DOI] [PubMed] [Google Scholar]
- 32.Thomas, C. A., Y. Li, T. Kodama, H. Suzuki, S. C. Silverstein, and J. El Khoury. 2000. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J. Exp. Med. 191:147-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udono, H., D. L. Levey, and P. K. Srivastava. 1994. Cellular requirements for tumor-specific immunity elicited by heat shock proteins: tumor rejection antigen gp96 primes CD8+ T cells in vivo. Proc. Natl. Acad. Sci. USA 91:3077-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veckman, V., M. Miettinen, S. Matikainen, R. Lande, E. Giacomini, E. M. Coccia, and I. Julkunen. 2003. Lactobacilli and streptococci induce inflammatory chemokine production in human macrophages that stimulates Th1 cell chemotaxis. J. Leukoc. Biol. 74:395-402. [DOI] [PubMed] [Google Scholar]
- 35.Wessels, M. R., A. E. Moses, J. B. Goldberg, and T. J. DiCesare. 1991. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc. Natl. Acad. Sci. USA 88:8317-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wexler, D. E., D. E. Chenoweth, and P. P. Cleary. 1985. Mechanism of action of the group A streptococcal C5a inactivator. Proc. Natl. Acad. Sci. USA 82:8144-8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright, S. D., and S. C. Silverstein. 1986. Overview: the function of receptors in phagocytosis, p. 41.1-41.14. In D. M. Weir (ed.), Cellular immunology. Blackwell Scientific Publications, Oxford, United Kingdom.2879638
- 38.Yamazaki, K., T. Nguyen, and E. R. Podack. 1999. Cutting edge: tumor secreted heat shock-fusion protein elicits CD8 cells for rejection. J. Immunol. 163:5178-5182. [PubMed] [Google Scholar]