Abstract
Background
The interplay between androgen and Hedgehog (Hh) signaling pathways may be associated with prostate cancer progression and resistance to therapy.
Methods
Tissue microarrays from prostatectomy specimens were derived from 53 patients treated preoperatively with androgen ablation (AA) with or without chemotherapy, and from 26 stage- and grade-matched controls. A previously characterized androgen-regulated human prostate cancer xenograft was used to conduct parallel murine studies. Expression of markers of interest was determined on both untreated and castrated tumors.
Results
Four-month exposure to AA or AA with chemotherapy led to a uniform increase in Hh signaling as compared to controls, paired with an inverse trend of androgen receptor (AR) and CYP17 expression in clinically derived specimens. Changes in the expression profiles of Hh signaling were observed in the epithelium and stroma, in response to genotoxic stress of androgen ablation and chemotherapy. A reduced expression of KI67and increased bcl2 expression was observed in the malignant epithelial compartment.
Conclusion
To our knowledge, this is the first clinical evidence that Hh signaling is induced by AA or the combination of AA and chemotherapy and, by inference, contributes to castrate-resistant progression of prostate cancer as supported by parallel human and murine studies. These data are in agreement with previous reports that implicate Hh signaling in castrate-resistant progression of prostate cancer. Based on these findings, we are pursuing parallel clinical and murine investigations to determine if Hh signaling inhibition combined with AA will be more effective than AA alone.
Keywords: Prostate cancer, preoperative treatment, Hedgehog signaling, resistance to treatment, androgen ablation
Introduction
Advanced prostate cancer is characterized by a near-universal initial response to androgen ablation (AA), which is then followed by disease progression. To develop rational combination therapies and thus improve AA efficacy, we have integrated findings from murine and clinical studies that may better define future research. The data we present here build on the evidence that androgen and Hedgehog (Hh) signaling are linked to prostate carcinogenesis and resistance to therapy. With the results of our clinical study in patients with high-grade or locally advanced prostate cancers and through experimental observations, we hope to frame planned investigation of potentially lethal prostate cancers.
We have given research priority to elucidating the mechanism(s) of castrate-resistant progression, and have adopted two broad perspectives: (1) activation of alternative androgen signaling leading to castrate-resistant progression, and (2) activation of compensatory survival pathways leading to progression despite AA. Recent reports have provided strong clinical and laboratory support for the hypothesis that continued androgen signaling contributes to castrate-resistant progression [1, 2, 3]. In a complementary line of research, we and others are investigating the role of molecular pathways that contribute to resistance to AA and likely to the castrate-resistant progression of prostate cancer.
Hedgehog signaling, a stromal-epithelial–interacting pathway, has been implicated in prostate cancer progression [4, 5]. In this project, we used the preoperative model for high-risk disease to determine whether Hh signaling is induced by AA in a pattern consistent with castrate-resistant progression of human prostate cancer. The parallel murine experiments were undertaken to gain confidence in the findings of the clinical study and to validate a model system for use in future functional studies. The integration of the findings from the companion murine and clinical studies will be used to guide the further development of combination therapy for advanced prostate cancer.
Materials and Methods
A. Clinical Study
Patients
This prospective phase II study of preoperative AA included 64 men with histologically confirmed prostate adenocarcinoma with no evidence of regional or distant metastases. Patients were eligible for study enrollment if they had clinical stage T1c-T2c disease with a Gleason score of ≥7 on their initial biopsy or if they had clinical stage T3 disease. Fifty-three patients were eligible, and all patients gave their written informed consent to participate in this phase II study, which had been approved by the institutional review board of The University of Texas M. D. Anderson Cancer Center.
Study design
Eligible patients were randomized at a 1:1 ratio to receive either AA or AA with chemotherapy. The KAVE chemotherapy regimen was administered to patients in the latter group (AA with chemotherapy) in two 8-week cycles and consisted of oral ketoconazole with hydrocortisone replacement, starting simultaneously with the first dose of doxorubicin (Adriamycin; given in weeks 1, 3, and 5). In weeks 2, 4, and 6, intravenous vinblastine was administered, followed by a week of oral estramustine treatment. Androgen ablation consisted of LHRH agonist administration every 4 or 12 weeks. This treatment regimen was continued for at least 16 weeks unless evidence of disease progression was seen, followed by radical prostatectomy. The 53 eligible study patients underwent radical prostatectomy, and 26 had received AA and 27 AA+KAVE.
B. Tissue-Based Studies
Tissue microarrays
Tissue microarrays were constructed from available specimens from radical prostatectomies performed on 26 patients who had been pretreated with AA, 27 patients pretreated with AA + KAVE (the chemotherapy group), and 26 untreated patients (controls) who were matched for pathologic stage and Gleason score at the time of surgery (Table I). From the individual radical prostatectomy specimens, we selected areas representing all histologic tumor patterns present. Each case was represented by a median of 36 0.6 mm-diameter cores (range, 18–90 cores) with 3,178 cores obtained. Expression of 10 biomarkers was assessed by immunohistochemical staining in the tumor microenvironment. These included sonic hedgehog (Shh), gli1, gli2, smoothened (Smo), AR, CYP17, AKT, pAKT, VEGF, bcl2, and p53. Antibodies and staining methods are shown in Supplementary Table S1. A Dako Autostainer (Dako North America, Inc., Carpinteria, CA) and standard 3,3-diaminobenzidine were used.
Table I.
Preoperative Characteristics of Patients*
| Control | Androgen ablation | Androgen ablation with chemotherapy (KAVE) | |
|---|---|---|---|
| Clinical stage | |||
| T2a | 9 | 8 | 7 |
| T2b | 14 | 17 | 14 |
| T3 | 3 | 1 | 6 |
| Biopsy 7 | 10 | 9 | 10 |
| Gleason Score (GS) At least 1 biopsy GS≥8 | 16 | 17 | 17 |
| Median PSA (ng/dl)(range) | 8(2.2-38.6) | 8 (2.2-130.8) | 11 (0.7-205) |
| PSA>10 ng/dl | 10 | 10 | 12 |
| PSA<10 ng/dl | 16 | 16 | 15 |
Untreated and treated patients were matched for clinical stage, Gleason score at biopsy, and prostate-specific antigen (PSA) levels at diagnosis.
Biomarker analyses
Images of each biomarker in each core of the tissue microarray were acquired by using a BLISS imaging system (Bacus Laboratories, Inc., Lombard, IL). We used an 11-point system to assess involvement (i.e., percentage of tumor cells exhibiting detectable staining): 0 = no staining to <1% stained; 10 = 1–10% of cells stained; 20 = 11–20%; 30 = 21–30%; 40 = 31–40%; 50 = 41–50%; 60 = 51–60%; 70 = 61–70%; 80 = 71–80%; 90 = 81–90%; and 100 = >90% stained. Intensity of the staining was scored by using a 3-point categorical system: 1 = low, 2 = intermediate, and 3 = high. Subcellular localization of biomarker staining and the predominant histologic type of tumor in each core were noted.
Statistical methods and analyses
Characteristics of the biomarkers in the samples were determined by standard descriptive statistics for continuous variables (Fisher's t test) or tabulations for categorical variables (Fisher's exact test). Univariate analyses were used to compare differences in biomarker expression between the control and chemotherapy groups. We used recursive-partitioning procedures and Fisher's discriminant analysis to identify the different biomarkers among the three groups and the optimal cut-off points for those biomarkers. To incorporate multiple observations (cores) from an individual patient, mixed-effects models were fitted to allow estimates of variability within and among patients. The analysis was based on the involvement score (extent of staining) alone, which was treated as a continuous variable. All reported P values are two-sided at a significance level of 5%. Analyses were performed with SAS for Windows (1999–2000) (Release 8.1; SAS Institute, Inc., Cary, NC) and S-PLUS 2000 (1988–2000) (Professional Release 3; Data Analysis Products Division, Insightful Corporation, Seattle, WA) software.
Parallel xenograft studies: cancer cell line MDA PCA 2b
The cancer cell line MDA PCA 2b was derived from the bone metastasis of a patient with castrate-resistant prostate adenocarcinoma [6]. This cell line is androgen-responsive, expresses androgen receptor, prostate-specific antigen (PSA), and prostate acid phosphatase (PAP), and is capable of developing tumors after subcutaneous or intraprostatic injection.
For non-castrate tumor analysis, we subcutaneously injected mice with 8 × 106 cells and then harvested the first-passage cells 24 weeks later. Passage-three cells were used for further analysis. For castrate tumor analysis, frozen tumors of 1- to 1.5-cm3 size that had grown in castrate mice were transplanted into normal mice. Passage-three cells were used for analysis in this study.
RNA isolation
Total RNA was extracted from fresh-frozen OCT blocks by using an RNeasy Mini Kit (Qiagen Inc., Valencia, CA) according to manufacturer's instructions. Genomic DNA was eliminated by a DNase on-column treatment with an RNase-free DNase set (Qiagen). Total RNA was eluted in RNase-free water, and the concentration was calculated by determining absorbance at 260 nm (Ultraspec 2000; Pharmacia Biotech, Uppsala, Sweden). The samples were diluted in sterile water to a working stock concentration of 4 μg/μL, and 100 μl aliquots were stored at –80°C until use.
Reverse-transcription and quantitative real-time PCR (qRT-PCR)
One microgram of total RNA was reverse-transcripted to single-stranded cDNA (Invitrogen Corp., Carlsbad, CA) according to the manufacturer's instructions. Amplifications of qRT-PCR were performed in Optical 96-well plates (Applied Biosystems, Inc., Foster City, CA). All standards and samples were run in triplicate using an MX3000P analyzer (Agilent Technologies, Inc., Santa Clara, CA), and the mean result was obtained for use in further calculations. The transcript level of glyceraldehyde-3-phosphatase dehydrogenase (GAPDH) was assayed simultaneously as an internal control to normalize transcript levels of genes of interest. Cycle conditions were set as follows: initial template denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 15 seconds, and combined primer annealing-elongation at 60°C for 15 seconds. Baseline and threshold values were determined using MXPro QPCR software. We transformed raw cycle threshold (Ct) values to quantities on the basis of the comparative Ct method. Epithelial and stromal expression of components could be discriminated by human- and mouse-specific primers, respectively (Supplemental Table S2).
Results
Between December 1997 and March 2001, 64 patients (median age, 60 years; range, 43–71) were enrolled in the phase II study. Radical prostatectomy specimens from 53 patients who underwent radical prostatectomy were available for tissue microarray construction. Twenty-seven untreated control specimens used for tissue-based studies were matched for clinical stage, Gleason score at biopsy, and PSA level (>10 v ≤10 ng/mL) (Table I).
A. Human Tissue-Based Studies: Effect of AA With or Without Chemotherapy on Locally Advanced Prostate Cancer
We assessed the expression of four main components of the Hh signaling pathway: gli2, gli1, Smo, and the Shh ligand. Hh signaling in the residual tumor epithelium and adjacent stroma of samples treated with AA with or without chemotherapy was higher than in controls, as indicated by the following characteristics.
Nuclear expression of the transcription factors gli2 and gli1, hallmarks of Hh signaling activation [7, 8], was significantly higher in treated tumor epithelium and adjacent stroma than in control specimens. When transcription factor expression was present in the control specimens, it was localized primarily in the cytoplasm and less so in the nucleus, in contrast with the predominant nuclear expression in the treated epithelium and stroma (Table II). Expression of both the ligand Shh and the Hh signaling intermediate Smo was significantly higher in treated tumor epithelium and adjacent stroma than it was in the control specimens (Table II and Fig. 1A, Fig. 1B and Fig. 1C). Treated samples also showed evidence of homogeneous expression overall, as evidenced by the lower standard deviations within and between samples (Table II).
Table II.
Expression of Hedgehog and Androgen Signaling Components in the Tumor Microenvironment
| Mean control (sd) | Mean AA (sd) | Mean CH (sd) | P value Control vs AA | P value Control vs CH | |
|---|---|---|---|---|---|
| Gli2 Epithelium | 39.6 (20.7) | 79.6 (17.2) | 85.5 (11.9) | <0.0001 | <0.0001 |
| Gli2 Stroma | 22.9 (15.2) | 43.3 (17.9) | 54.7 (15.4) | <0.0001 | <0.0001 |
| Gli1 Epithelium | 45.7 (22.9) | 57.7 (25.9) | 76.3 (21.8) | 0.084 | <0.0001 |
| Gli1 Stroma | 11.3 (10.8) | 27.1 (12.5) | 25.3 (9.8) | 0.0001 | 0.0003 |
| Smoothened Epithelium | 68.9 (18.2) | 81.1 (14.2) | 83.5 (8.9) | 0.0047 | 0.0008 |
| Smoothened Stroma | 16.1 (11.2) | 21.6 (10.7) | 28.2 (13.9) | 0.118 | 0.0011 |
| Shh Epithelium | 49.9 (24.8) | 61.3 (19.2) | 62.6 (22.4) | 0.085 | 0.05 |
| Shh Stroma | 4.6 (6.5) | 12.8 (13.5) | 19.4 (13.9) | 0.0203 | 0.0001 |
| AR Epithelium | 59.4 (24.2) | 47.4 (22.2) | 47.8 (21.1) | 0.077 | 0.087 |
| AR Stroma | 6.7 (11.6) | 8.6 (10.2) | 10.1 (8.2) | 0.522 | 0.253 |
| CYP17 Epithelium | 82.77 | 64.55 | 80.54 | 0.015 | 0.755 |
| CYP17 Stroma | 49.08 | 38.24 | 37.25 | 0.074 | 0.047 |
Hedgehog signaling in the residual tumor epithelium and adjacent stroma of samples from patients treated with AA or AA with chemotherapy was higher than in the control group. This was indicated by the difference in nuclear expression of the transcription factors gli2 and gli1, and expression of Hh signaling intermediate smoothened and the ligand sonic hedgehog.
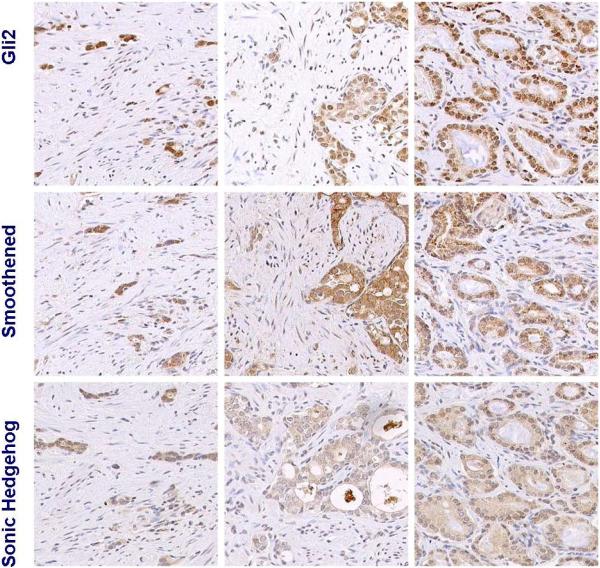
Figure 1a. Hedgehog signaling in untreated control tumors.
Representative images of 3 different untreated tumors. Active Hh signaling is heterogeneous and limited compared to that of treated tumors (Figure 1b-c) as illustrated by the expression of Gli2 and smoothened.
Figure 1b. Increased Hh signaling in residual tumors following AA.
Representative images of 3 different radical prostatectomy specimens with varied extent of residual tumor. Expression of all components of Hh signaling assessed (gli2, smoothened, and sonic hedgehog) is higher than in untreated controls (Figure 1a). Hedgehog signaling is active both in the residual tumor epithelium and stroma as indicated by the nuclear expression of the transcription factor gli2. Three cases are included to represent heterogeneity in histology.
Figure 1c.
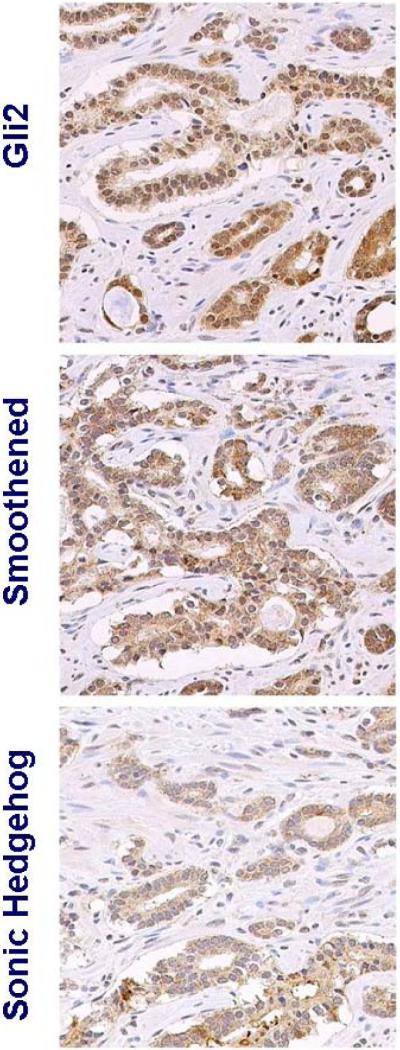
Increased Hh signaling in residual tumor following AA and KAVE chemotherapy.
Active Hh signaling was parallel in the tumor epithelium and adjacent stroma (Table III) as indicated by the mean nuclear expression of gli2 (0.78 by Pearson's correlation; p<0.001). Paracrine Hh signaling is further supported by the correlation identified between Shh ligand stromal expression and the pathway component expression in the tumor epithelium.
Table III.
Pearson's Correlation Table
| Gli2. Epithelium | Gli2 Stroma | Gli1 Epithelium | Gli1 Stroma | Smoothened Epithelium | Smoothened Stroma | Shh Epithelium | Shh Stroma | |
|---|---|---|---|---|---|---|---|---|
| Gli2. Epithelium | 1 | 0.789 | 0.349 | 0.485 | 0.516 | 0.359 | 0.349 | 0.545 |
| Gli2 Stroma | 0.789 | 1 | 0.305 | 0.52 | 0.443 | 0.601 | 0.305 | 0.731 |
| Gli1 Epithelium | 0.485 | 0.52 | 1 | 0.393 | 0.479 | 0.418 | 0.306 | 0.505 |
| Gli1 Stroma | 0.290 | 0.363 | 0.362 | 1 | 0.286 | 0.381 | 0.362 | 0.455 |
| Smoothened Epithelium | 0.516 | 0.443 | 0.496 | 0.418 | 1 | 0.449 | 0.496 | 0.359 |
| Smoothened Stroma | 0.359 | 0.601 | 0.172 | 0.286 | 0.449 | 1 | 0.172 | 0.552 |
| Shh Epithelium | 0.349 | 0.305 | 1 | 0.306 | 0.496 | 0.172 | 1 | 0.333 |
| Shh Stroma | 0.545 | 0.731 | 0.505 | 0.455 | 0.359 | 0.552 | 0.333 | 1 |
Active Hh signaling was parallel in the tumor epithelium and adjacent stroma, as primarily indicated by the increased correlation (0.78) in gli2 expression. The paracrine activity of the pathway is supported by correlation of the Shh ligand stromal expression and pathway component expression in the tumor epithelium.
Treated tumor epithelium exhibited moderately reduced androgen receptor and CYP17 expression relative to that in control tissue following this limited 16-week AA period. An interesting observation was a trend toward increased androgen receptor expression in the stroma (Table II and Fig. 2). Finally, angiogenic factor VEGF expression, also identified as an Hh downstream target, increased following treatment.
Figure 2. Androgen receptor expression.
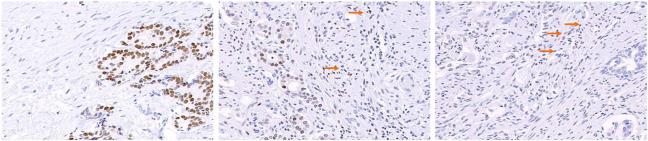
There was a trend for lower AR expression in the tumor epithelium following AA (middle panel) and chemotherapy (right panel) for 16 weeks versus untreated control specimens (left panel). Interestingly when this occurred, adjacent stroma exhibited an increase in AR expression (arrows).
We assessed by nuclear Ki67 expression, the effect of treatment on proliferation AKT and phospho-AKT, bcl2, and p53 and found a decrease (Table IV). Bcl2, as previously reported, was induced as a result of chemotherapy and not AA alone. While AKT expression increased as a result of treatment, phospho-AKT expression was particularly reduced following AA hormonal therapy alone. Finally, p53 expression was not affected by treatment.
Table IV.
Expression of other markers of interest in the tumor microenvironment*
| mean.Control | Mean ChemoHormonal | mean AA | Standard Deviation.btw.pts | Standard Deviation Within patients | p.value: CH vs Ctrl | p.value: AA vs Ctrl | |
|---|---|---|---|---|---|---|---|
| pAKT.epithelium.NUCL. | 35.86 | 85.17 | 58.51 | 21.62 | 29.02 | <0.001 | 0.002 |
| pAKT.epithelium.CYTOPL. | 85.33 | 80.90 | 70.71 | 17.39 | 20.03 | 0.417 | 0.009 |
| pAKT.stroma.NUCL | 8.15 | 14.70 | 5.67 | 8.05 | 8.85 | 0.011 | 0.327 |
| pAKT.stroma.CYTOPL. | 0.73 | 2.03 | 1.05 | 2.01 | 3.39 | 0.066 | 0.651 |
| AKT.epithelium.NUCL. | 85.12 | 95.81 | 94.21 | 9.00 | 16.46 | 0.001 | 0.006 |
| AKT.epithelium.CYTOPL. | 80.33 | 88.86 | 86.45 | 14.90 | 21.55 | 0.082 | 0.221 |
| AKT.stroma.NUCL. | 22.22 | 23.11 | 15.89 | 13.32 | 13.25 | 0.824 | 0.125 |
| AKT.stroma.CYTOPL. | 10.27 | 15.15 | 11.86 | 5.50 | 8.89 | 0.01 | 0.404 |
| bcl2.epithelium. | 3.72 | 13.46 | 7.78 | 14.73 | 12.70 | 0.027 | 0.358 |
| bcl2 stroma. | 5.10 | 7.25 | 9.24 | 4.95 | 6.67 | 0.174 | 0.011 |
| p53 epithelium. | 28.94 | 16.73 | 10.98 | 21.91 | 17.48 | 0.058 | 0.006 |
| VEGF.epithelium. | 66.25 | 86.52 | 71.37 | 21.52 | 23.74 | 0.003 | 0.439 |
| VEGF.stroma. | 16.15 | 36.12 | 36.35 | 17.63 | 13.51 | <0.001 | <0.001 |
Markers of interest included AKT, phosphor AKT, bcl2, p53 and VEGF
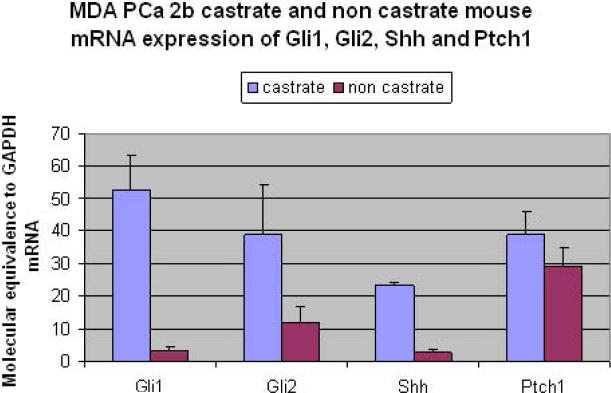
B. Parallel Murine Tissue-Based Studies: Effect of AA on MDAPCA2b Xenograft Model
Increased Hh signaling was observed at message and protein level in the tumor microenvironment of castrate but not non-castrate xenograft models. Stromal expression of gli1 and Shh, assessed by mouse gene-specific primers, was significantly higher in the castrate xenograft model by 16- and 8-fold, respectively, and expression of gli2 and Patched1 was also elevated (Fig. 3). Tumor epithelial expression of gli1, assessed by human gene-specific primers, was also significantly greater in castrate tumors than in non-castrate tumors. Immunohistochemical testing confirmed an increase in nuclear gli2 expression in the tumor epithelium analogous to that observed in the human study (data not shown).
Figure 3. Hedgehog signaling expression in human xenograft model.
An increase is shown in stromal mRNA expression of Hh signaling components following castration (blue bars) in a castrate-responsive tumor xenograft as compared to non castrate (red bars). These include the transcription factors gli1, gli2 and the ligand Shh.
Discussion
The clinical and experimental findings we report illustrate an increased expression of Hh following castration in murine and clinical prostate cancers, consistent with the hypothesis that the interplay between Hh and androgen receptor signaling is implicated in prostate cancer progression and resistance to therapy. The findings lend further support to the view that Hh signaling should be prioritized as a therapy target in combination with AA for select patients with prostate cancer. The preoperative platform we used for high-risk prostate cancers allows us to determine the molecular consequences of an intervention [9].
The parallel murine investigation was conducted in an androgen-regulated human prostate cancer xenograft that shares important properties with human castrate-resistant prostate cancer. The similarities and differences between the murine and clinical studies will be used to frame future studies.
It is speculated that prostate cancer usurps pathways implicated in the normal development and function of the prostate and bone during progression [10, 11]. Hedgehog signaling is a stromal-epithelial–interacting pathway that cooperates with androgen signaling in the normal development and function of the prostate and bone [12]. Of particular relevance is the observation that organ development defects in Hh ligand knock out mice can be rescued in the presence of androgens in utero [12]. Additionally, Hh pathway inhibition blocks epithelial regeneration in androgen-ablated rodent ventral prostate on androgen supplementation [4]. The evidence to support the interplay between Hh and androgen signaling in prostate development and regeneration is of particular relevance to prostate cancer, given the need to identify molecular mechanisms involved in castrate-resistant progression of the disease [4, 5].
Heterogeneous expression of Hh signaling components was found in approximately 40% of the untreated tumors, indicative of a baseline level of pathway activation in a significant portion of locally advanced prostate cancers. Homogeneous, increased expression of Hh signaling components was observed as a result of AA or AA with chemotherapy (Fig. 1B and Fig. 1C). Nuclear localization of transcription factors gli1 and gli2, hallmarks of pathway activation, was abundant in the tumor microenvironment after treatment. The ratio of Smo to Patched expression is important for normal development. A limitation of our study is that we were unable to determine the expression ratio of Smo to Patched which is a reported measure of hedgehog signaling activity, given the lack of antibodies to Patched suitable for human or murine tissue immunohistochemical analysis [13]. Nevertheless, overall, we observed not only increased but also homogeneous Hh signaling, as evidenced by the reduction in standard deviation between and within samples after treatment. Taken together, these observations provide strong supportive data for linking AA to Hh signaling in advanced prostate cancers. Our findings are consistent with recently published experimental observations [14].
The short-term exposure to AA also affected the epithelial compartment of the cancer. Consistent with previous reports, AA and chemotherapy resulted in decreased epithelial proliferation and induction of bcl2. The expression of stromal and epithelial Hh signaling in treated and control specimens supports the hypothesis that Hh signaling is implicated in prostate development, function, and carcinogenesis as well as the more aggressive phenotype of the disease [15, 16, 17]. Our findings suggest that Hh signaling may also be a determinant of resistance to AA. Given that all the study patients receive AA, it is difficult to determine the added effect of chemotherapy on Hh signaling. Further studies will be required to assess the role of Hh signaling in chemotherapy resistance [18].
The effects of AA on MDA PCA 2b xenografts mirrored aspects of the clinical study to the extent that species-specific limitations allow. A robust and homogeneous increase in Hh expression in the tumor microenvironment was seen after castration. We conclude that MDA PCa 2b shares important aspects of human prostate cancer and therefore can be used to inform future clinical study.
The potential relevance of our findings is based on the assumption that observations made after short-term exposure to AA will identify pathways with a role in therapy resistance. To our surprise, we did not detect a significant increase in androgen receptor expression, as reported in metastatic castrate-resistant prostate cancer [19]. This suggests that there is variability in the time to modulation of pathways implicated in castrate-resistant prostate cancer progression. The temporal heterogeneity in modulation of signaling pathways may affect the optimum use of molecular targeted therapies in combination. The findings suggest that both initial combination and sequential application of microenvironment targeted therapies should be considered [20]. The results also highlight the potential value of parallel murine studies. The parallel detailed exploration of time course is feasible in murine models and can provide valuable insight.
Conclusions
Our observations support the hypothesis that Hh signaling is implicated in resistance to anti-androgen therapy and provide rationale for a combination therapy. This is based on the data reported and our past experience establishing feasibility of Hh signaling inhibition in humans [9]. We have initiated a preoperative study combining a Smo inhibitor with AA in patients with locally advanced prostate cancer to explore the therapeutic relevance of our results. The findings we report are but a portion of our broad effort to elucidate the stromal-epithelial–interacting signaling networks implicated in prostate cancer progression. The parallel conduct of human and murine studies will be critical to our efforts to develop combinatorial microenvironment-targeting therapy for prostate cancer.
Supplementary Material
Acknowledgements
We thank Vassiliki Tzelepi at the University of Texas, M.D. Anderson Cancer Center for her assistance in immunohistochemical evaluation and analysis and Nora M. Navone at the University of Texas, M.D. Anderson Cancer Center for providing xenograft model MDA PCA 2b.
Supported by : Young Investigator Award, Prostate Cancer Foundation (Dr. Efstathiou) and National Cancer Institute grants CA84964, CA90270, NIEHSES07784, and 5 P30 CA016672-35.
Footnotes
Data Presented at the Annual Meeting of the American Society for Clinical Oncology, Chicago, 2007
References
- 1.Attard G, Reid AH, Olmos D, de Bono JS. Antitumor activity with CYP17 blockade indicates that castration-resistant prostate cancer frequently remains hormone driven. Cancer Res. 2009;69(12):4937–4940. doi: 10.1158/0008-5472.CAN-08-4531. [DOI] [PubMed] [Google Scholar]
- 2.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431(7009):707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez P, Hernández AM, Stecca B, Kahler AJ, DeGueme AM, Barrett A, Beyna M, Datta MW, Datta S, Ruiz i Altaba A. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci USA. 2004;101(34):12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navone NM, Olive M, Ozen M, Davis R, Troncoso P, Tu SM, Johnston D, Pollack A, von Eschenbach AC, Logothetis CJ. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res. 1997;3(12 Pt 1):2493–2500. [PubMed] [Google Scholar]
- 7.Riobo NA, Manning DR. Pathways of signal transduction employed by vertebrate Hedgehogs. Biochem J. 2007;403(3):369–379. doi: 10.1042/BJ20061723. [DOI] [PubMed] [Google Scholar]
- 8.Thiyagarajan S, Bhatia N, Reagan-Shaw S, Cozma D, Thomas Tikhonenko A, Ahmad N, Spiegelman VS. Role of GLI2 transcription factor in growth and tumorigenicity of prostate cells. Cancer Res. 2007;67(22):10642–10646. doi: 10.1158/0008-5472.CAN-07-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efstathiou E, Troncoso P, Wen S, Do KA, Pettaway CA, Pisters LL, McDonnell TJ, Logothetis CJ. Initial modulation of the tumor microenvironment accounts for thalidomide activity in prostate cancer. Clin Cancer Res. 2007;13(4):1224–1231. doi: 10.1158/1078-0432.CCR-06-1938. [DOI] [PubMed] [Google Scholar]
- 10.Logothetis CJ, Navone NM, Lin SH. Understanding the biology of bone metastases: key to the effective treatment of prostate cancer. Clin Cancer Res. 2008;14(6):1599–1602. doi: 10.1158/1078-0432.CCR-07-4603. [DOI] [PubMed] [Google Scholar]
- 11.Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J Clin Oncol. 2005;23(32):8232–8241. doi: 10.1200/JCO.2005.03.0841. [DOI] [PubMed] [Google Scholar]
- 12.Berman DM, Desai N, Wang X, Karhadkar SS, Reynon M, Abate-Shen C, Beachy PA, Shen MM. Roles for Hedgehog signaling in androgen production and prostate ductal morphogenesis. Dev Biol. 2004;267(2):387–398. doi: 10.1016/j.ydbio.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Casali A, Struhl G. Reading the Hedgehog morphogen gradient by measuring the ratio of bound to unbound Patched protein. Nature. 2004;431(7004):76–80. doi: 10.1038/nature02835. [DOI] [PubMed] [Google Scholar]
- 14.Chen M, Tanner M, Levine AC, Levina E, Ohouo P, Buttyan R. Androgenic regulation of hedgehog signaling pathway components in prostate cancer cells. Cell Cycle. 2009;8(1):149–157. doi: 10.4161/cc.8.1.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, Nannini-Pepe M, Kotkow K, Marsters JC, Rubin LL, de Sauvage FJ. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455(7211):406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz i Altaba A. Therapeutic inhibition of Hedgehog-GLI signaling in cancer: epithelial, stromal, or stem cell targets? Cancer Cell. 2008;14(4):281–283. doi: 10.1016/j.ccr.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Karlou M, Lu JF, Wu G, Maity S, Tzelepi V, Navone NM, Hoang A, Logothetis CJ, Efstathiou E. Hedgehog signaling inhibition by the small molecule smoothened inhibitor GDC-0449 in the bone forming prostate cancer xenograft MDA PCa 118b. Prostate. 2012 doi: 10.1002/pros.22517. doi: 10.1002/pros.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narita S, So A, Ettinger S, Hayashi N, Muramaki M, Fazli L, Kim Y, Gleave ME. GLI2 knockdown using an antisense oligonucleotide induces apoptosis and chemosensitizes cells to paclitaxel in androgen-independent prostate cancer. Clin Cancer Res. 2008;14(18):5769–5777. doi: 10.1158/1078-0432.CCR-07-4282. [DOI] [PubMed] [Google Scholar]
- 19.Linja MJ, Savinainen KJ, Saramäki OR, Tammela TL, Vessela RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61(9):3550–3555. [PubMed] [Google Scholar]
- 20.Efstathiou E, Logothetis CJ. A new therapy paradigm for prostate cancer founded on clinical observations. Clin Cancer Res. 2010;16(4):1100–1117. doi: 10.1158/1078-0432.CCR-09-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.