Abstract
Purpose
Reconstruction of grasp is a high priority for tetraplegic patients. Restoration of finger flexion by surgical activation of flexor digitorum profundus can result in roll-up finger flexion, interphalangeal (IP) joint before metacarpophalangeal (MCP) joint flexion, which can be improved by restoring intrinsic function. This study compares grasp kinematics between 2 intrinsic balancing procedures—Zancolli-llasso and House.
Methods
The intrinsic muscles of 12 cadaver hands were reconstructed by either the Zancolli-lasso or the House procedure (n=6 each) and tested by deforming FDP with a motor to simulate hand closure. Results were compared to 5 control hands. All 17 hands were studied by video analysis. Kinematics were characterized by the order of MCP joint and IP joint flexion. Optimal grasp was defined as the maximal fingertip-to-palm distance during the arc of finger closure.
Results
Kinematics differed between the 2 procedures. The Zancolli-lasso reconstructed hands flexed first in the IP joints, and then in MCP joints, resembling an unreconstructed intrinsic-minus hand while the House reconstructed hands flexed first in MCP joints and then in the IP jointss, resembling an intrinsic-activated hand. Maximal fingertip-to-palm distance did not differ significantly between the 2 procedures, and both showed improvement over unreconstructed controls.
Discussion
Both intrinsic balancing techniques improved grasp. Only the House procedure restored hand kinematics approximating those of an intrinsic-activated hand. Improvement in fingertip-to-palm distance in Zancolli-lasso hands resulted primarily from the initial resting MCP joint flexion of 40°. We therefore advocate the more physiologic House procedure for restoration of intrinsic function in tetraplegic patients.
Clinical Relevance
This study provides a rationale for advocacy of 1 reconstructive procedure over another.
Keywords: House procedure, intrinsic balancing, reconstructive hand surgery, tetraplegia, Zancolli-Lasso procedure
Introduction
Spinal cord injury is a life-altering event for any patient. When tetraplegia results, the loss of functional independence can be devastating. Worldwide, the incidence of spinal cord injury is 10 to 80 per million individuals each year, with roughly one third of those injuries resulting in tetraplegia.1 In a survey of tetraplegic patients, 49% ranked reconstitution of hand/arm function as their number one priority in rehabilitation, with no other priority surpassing 13%.2 Despite this fact, surgical reconstructions of the upper extremity remain underperformed as a whole. In the United States. Despite over 100,000 citizens living with tetraplegia, less than 400 upper extremity reconstructive procedures are performed each year.3
Finger flexion can often be restored by tendon transfer to activate the flexor digitorum, profundus (FDP). ENREF 94,5 However, this transfer may result in roll-up finger flexion— interphalangeal (IP) joint before metacarpophalangeal (MCP joint) flexion—because most tetraplegic patients lack intrinsic intrinsic muscle function. While the extrinsic flexors provide concurrent flexion of MCPand IP joints, the intrinsic muscles couple MCP joint flexion with IP joint extension.6,7 This coupling delays IP flexion relative to MCP joint flexion, allowing the hand to wrap around larger objects. Thus, this sweeping “intrinsic-activated” grasp is considered much more functional.
The goal of this study was to compare 2 surgical techniques for intrinsic reconstruction—the Zancolli-lasso procedure and the House procedure.8-10 We performed these surgeries on cadaveric hands, comparing their effects on grasp to unreconstructed control hands and to each other. Hands were characterized both by their kinematics and their functional capacity.
Materials and methods
Modified House reconstructed hands
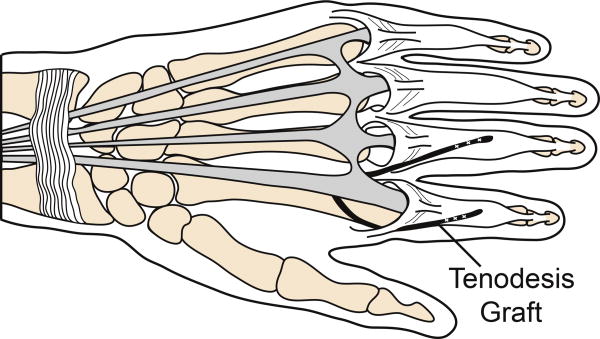
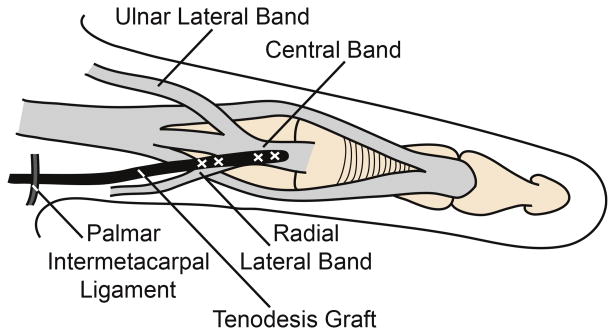
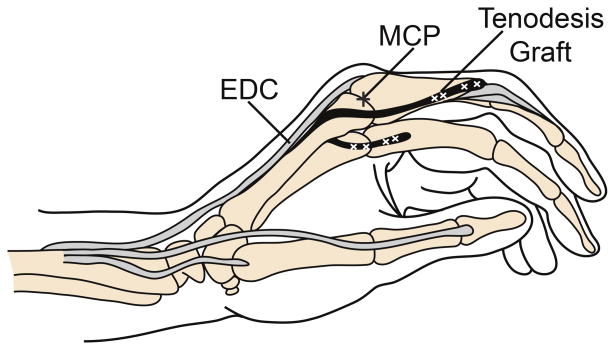
Six fresh-frozen hands (6 female; average age 86 years; range 84 – 89 years) were reconstructed using the House procedure (Fig. 1).9-11 Briefly, flexor digitorum superficialis (FDS) tendons were harvested and were split longitudinally into 2 equally sized grafts. Incisions were made dorsally over the extensor apparatus of each digit. The graft was sutured to the radial, lateral and central bands of the index finger, passed proximally beneath the insertion of the first dorsal interosseus such that it remained palmar to the MCP joint axis of rotation, directed under the extensor digitorum communis index and extensor indicis proprius tendons proximal to the MCP joint, passed distally through the lumbrical tunnel of the middle finger palmar to the intermetacarpal ligament, and sutured to the radial lateral and central bands of the middle finger with 3-0 suture with the MCP joint held in a flexed position. A second graft was sutured in an identical fashion to the radial lateral bands of the ring and small fingers.
Figure 1.
House procedure. (A) Dorsal view of tenodesis with FDS tendon graft (indicated in black) sutured into the extensor apparatus of the index and middle fingers. (B) Dorsal view of tendon graft (black) sutured into the radial lateral and central bands of the extensor apparatus after passing anterior to the intermetacarpal ligament. (C) Lateral view of the House tenodesis, demonstrating the tendon graft (black) passing anterior to the MCP joint (indicated by plus sign) and inserting into the extensor apparatus dorsal to the IP joints. Diagrams drawn with reference to previously published figures.9,11
After reconstruction, hands were amputated at the radiocarpal joint. The thumb was amputated at the MCP joint so as not to obscure video recording. FDP tendons were identified proximal to the carpal tunnel, and the ends individually sutured proximally with 2-0 suture. The palmar carpal ligament remained intact. The extensor digitorum communis tendonswere sutured individually at the level of the wrist with 2-0 suture. Due to the scarce contribution of extensor digitorum communis to the extensor apparatus of the small finger in most hands, the extensor digiti minimi tendon was the substituted recipient of the graft.
Zancolli-lasso reconstructed hands
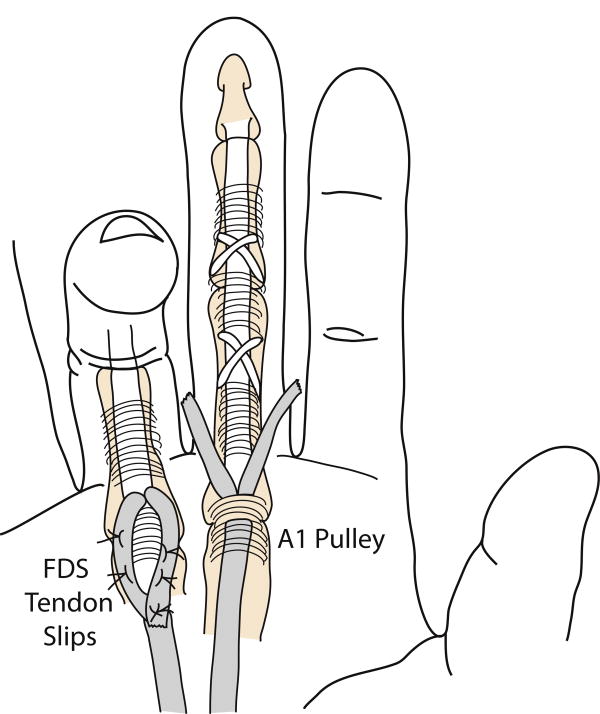
Another 6 fresh-frozen hands (6 female; average age 87 years; range 80 – 94 years) were reconstructed using the Zancolli-lasso procedure (Fig. 2).8,10,12 Briefly, incisions were made over the palmar aspect of each MCP joint. Each FDS tendon was identified and cut distal to the A1 pulley. The proximal end was sutured to itself proximally with 3-0 suture, thus lassoing the A1 pulley. The MCP joint was held in a flexed position during this tightening, and all fingers were balanced relative to each other to secure a normal cascade. After reconstruction, hands were dissected and prepared similarly to the House hands. Additionally, FDS tendons were identified proximal to the carpal tunnel and sutured en masse at their ends with 2 suture. All reconstructions were performed by an experienced hand surgeon (JF).
Figure 2.
Zancolli-lasso procedure demonstrating cutting of the FDS tendon slips distal to the A1 pulley (middle finger) and subsequent suturing of these slips down to the FDS tendon proximal to the A1 pulley (ring finger). Diagrams created with reference to previously published, figures.12
Sample preparation, mechanical testing, and kinematic analysis
Reconstructed hands were tested and analyzed in a similar fashion to the 5 control hands previously reported. Briefly, Kirschner wires were drilled into metacarpals and phalanges to serve as markers, and hands were positioned palm-up with FDP sutures affixed to a dual-mode servomotor (Aurora Scientific, Model 310, Aurora Inc, Ontario, Canada) pulling to an excursion of 50 mm at a rate of 5 mm/sec. The extensor digitorum communis was statically loaded with 50 g to simulate passive resistance of this tendon and muscle. For Zancolli-lasso reconstructed hands, each FDS tendon was fixed via its suture proximally to simulate the origin of this muscle at the elbow. This provided pre-flexion of MCP joint to 40 degrees, simulating the average postoperative positioning in these patients due to the passive tension along the FDS muscle and tendon. Upon activation of FDP and further flexion of MCP joints, these FDS sutures went slack, providing no additional active load. For comparison, we also characterized the Zancolli-lasso hands with MCP joint pre-flexion of 0, 20, 60, and 80 degrees. Finger movement was video-captured from the radial side of the hand and digitized in Matlab (MathWorks, Inc., Torrance, CA). To define the relative order of joint flexion, the excursion of maximal angular change was determined for each joint, and these were compared for the different joints and for the different procedures. Additionally, the maximal vertical distance from the fingertip to the palm (in the sagittal plane) was measured for each finger in each hand during flexion. These distances were normalized to the size of the hands based on x-ray measurements (Faxitron Specimen Radiography System, Tucson, AZ). All results were expressed as mean ± standard error of the mean.
Statistical analysis
The 2 primary end points were joint kinematics and fingertip-to-palm distance. These were compared between reconstruction procedures by analysis of variance with repeated measures. Statistical significance (α) was set at 0.05. Two-way analysis of variance compared maximal fingertip-to-palm distance of each procedure and explored interaction with finger (index, middle, ring, small). Two-way analysis of variance was also used to compare angular changes at MCP, PIP, and DIP joints as a function of procedure. In cases where results were not significant, post hoc power analysis was performed.
Results
Kinematics
At rest, prior to FDP activation with the motor, the House tenodesis produced 6 ± 9, −1 ± 1, and 10 ± 3 degrees of flexion at the MCP, PIP, and DIP joints, respectively (mean across all hands and fingers ± SEM). The Zancolli-lasso produced 40 ± 6, 2 ± 7, and 6 ± 3 degrees of resting flexion at the MCP, PIP, and DIP joints, respectively, with the elevated resting flexion at MCP joint resulting from our proximal fixation of FDS.
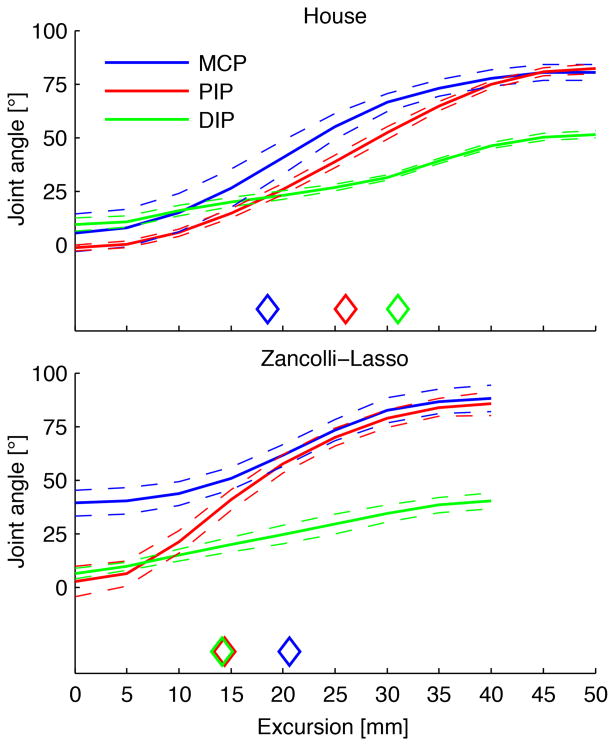
Kinematics were characterized by the order of angular change of MCP, PIP, and DIP joints (Fig. 3). These differed between the 2 reconstructive procedures (P < 0.001). With the House procedure, maximal angular change occurred first in the MCP joint (at 19 ± 2mm of FDP excursion) and then in the PIP joint (26 ± 1) and DIP joint (31 ± 3). Conversely, with the Zancolli-lasso procedure, maximal angular change occurred first in PIP joint (14 ± 2) and DIP joint (14 ± 2) and then in the MCP joint (21 ± 1) joint.
Figure 3.
Joint angles of the MCP, PIP, and DIP joints as a function of FDP excursion during finger flexion for House and Zancolli-lasso reconstructed hands. Note that for House hands, MCP joint flexion precedes IP joint flexion (see diamonds), whereas for Zancolli hands, IP joint flexion precedes MCP joint flexion. Means (—) and standard errors (--) are shown over all hands (n = 6 for each) and all fingers (index, middle, ring, and small). ◊ = excursion of FDP tendon where the greatest change of joint movement occurred for each joint.
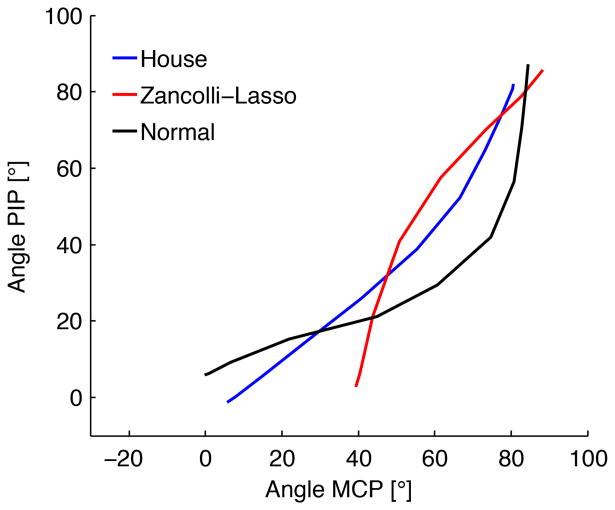
For comparison, in the intrinsic-unloaded control hands, maximal change occurred first at PIP joints (10 ± 2 mm of FDP excursion) and DIP joints (27 ± 7) and then at the MCP joints (31 ± 4). For intrinsic-loaded (500 g) control hands, maximal change occurred first at MCP joints (19 ± 2), and then at PIP joints (35 ± 3) and DIP joints (45 ± 1) (Intrinsic hand muscle function I: creating a functional grasp. Manuscript submitted for publication). Thus, the MCP joint-first flexion of House more closely approximated the active/loaded intrinsic condition of the control hands compared to the IP joint-first flexion of Zancolli-lasso (Fig. 4).
Figure 4.
Order of joint flexion as represented by MCP joint vs PIP angle during hand closure. House and Zancolli-lasso reconstructed hands (n = 6 each) are shown, along with normal control hands (intrinsic-loaded with 500 g, n = 5). Normal and House reconstructed hands display MCP joint-first flexion, whereas Zancolli-lasso reconstructed hands display PIP-first flexion.
Maximal fingertip-to-palm distance
Maximal fingertip-to-palm distances are displayed in Table 1. No significant difference was found in maximal fingertip-to-palm distance between the Zancolli-lasso and House procedures. Each procedure produced significant or near-significant improvement compared to the unreconstructed control hands. As such, reconstruction in both cases represented an improvement over the intrinsic-inactivated scenario. Post hoc analysis revealed a power of 0.8 to show any difference in maximal fingertip-to-palm distance > 5 mm between the 2 procedures, and a power of 0.99 for any difference > 10 mm. For comparison, the difference between intrinsic-unloaded and fully loaded (500 g) control hands was 20 mm.
Table 1. Maximal fingertip-to-palm distances.
| Finger | Significance | ||||||
|---|---|---|---|---|---|---|---|
| Procedure | Index | Middle | Ring | Small | Z vs H | Z vs C | H vs C |
| Zancolli-Lasso (Z) | 70 ± 5 | 74 ± 4 | 74 ± 2 | 58 ± 2 | 0.416 | ||
| House (H) | 67 ± 3 | 74 ± 3 | 70 ± 2 | 53 ± 2 | 0.038 | ||
| Control (C)* | 59 ± 3 | 67 ± 4 | 62 ± 4 | 47 ± 3 | 0.061 | ||
| Intrinsic-activated* | 78 ± 3 | 87 ± 3 | 83 ± 4 | 69 ± 3 | |||
Maximal fingertip-to-palm distance of each finger for reconstructed and unreconstructed control hands, mean over all hands ± SEM, mm. Significance based upon 2-way analysis of variance, expressed as P value. Values for previously reported intrinsically activated hands are included for reference, normalized by size to hands in the current study. Zancolli-Lasso, Z. House, H. Unreconstructed control, C.
Arnet U, Muzykewicz DA, Fridén J, Lieber RL. Intrinsic hand muscle function I: creating a functional grasp. Manuscript submitted for publication.
As expected, maximal fingertip-to-palm distance depended on finger type (P < 0.001); for example, the middle fingers consistently showed greater maximal distances than did the small fingers. However, there was no interaction between finger type and reconstructive procedure (p = 0.744). This result was thus simply a result of the different sizes of the fingers.
Discussion
Intrinsic balancing is paramount for optimal grasp in reconstructive hand surgery for the tetraplegic patient. The purpose of this study was to compare 2 commonly used intrinsic balancing procedures with respect to creating functional grasp. We evaluated functional grasp based on 2 specific criteria of restoring normal order of joint flexion during grasp, beginning with MCP joint and proceeding to PIP and DIP joints and creating maximum fingertip-to-palm proximity.
The 2 reconstructive procedures differed significantly with respect to their ability to restore the normal order of joint flexion. Specifically, the Zancolli-lasso reconstructed hands flexed IP joints first and then the MCP joints, resembling an unreconstructed, intrinsic-minus hand. Conversely, the House reconstructed hands flexed MCP joints first and then the IP joints, resembling an intrinsic-plus hand. Thus, kinematically, the House reconstructed hands better approximated normal intrinsic function. In these hands, activation of FDP resulted in IP joint flexion, which tensioned the extensor apparatus. This tensioning the House tenodesis across the MCP joint resulted in earlier flexion.
With respect to our second criterion, fingertip-to-palm distance, both reconstructive procedures showed improvements compared to control, while no difference was seen between the 2 procedures (Table 1). Because the Zancolli-lasso procedure could not mimic intrinsic-activated finger kinematics, the improvement in fingertip-to-palm distance over the unreconstructed hand was mainly caused by the initial MCP joint flexion of 40 degrees that resulted from the reconstruction. So, while grasp in the House hand was more physiologic, both, reconstructive procedures optimized hand closure when evaluated based on maximal fingertip-to-palm closure. This measure provides a surrogate for how successfully hands will grasp objects. Further studies are needed to determine the validity of this surrogate and to compare the effects of these procedures on finger extension and hand opening.
Both the Zancolli-lasso and House procedures are designed to promote MCP joint flexion and optimize grasp.8,9 The Zancolli-lasso procedure accomplishes this by affixing the FDS to itself around the A1 pulley. As the FDS is paralyzed in most tetraplegic patients, this procedure relies on passive FDS tension to hold the hand in a position of fixed MCP joint flexion at rest. The House procedure also provides a tenodesis palmar to the MCP joint; but by inserting into the extensor apparatus, it also provides an extension force with respect to the IP joints. Thus, it effectively couples MCP joint flexion with IP joint extension, mimicking intrinsic function. Additional fundamental differences between the 2 procedures exist. Palmar incisions and dissection in the Zancolli-lasso hands are much more extensive than in the House hands. Further, the tenodesis in the Zancolli procedure results in a fixed resting flexion of MCP joint.
While no difference has previously been shown between the 2 procedures in terms of grip strength or activities of daily living,10,13 these technical differences have functional implications. For example, because MCP joint flexion is mechanically linked to PIP jointextension in the House procedure, this creates a more open hand during grasp, which we would expect to allow opening around larger objects, such as gripping the drive ring of the manual wheelchair. This effect is seen in Fig. 3, where, for the House procedure, flexion proceeds in the order MCP joint, PIP joint, and DIP joint, whereas for the Zancolli, flexion proceeds in the order DIP joint, PIP joint, and MCP joint (see diamonds in Fig. 3). Further studies are warranted to compare finger extension and hand opening in these 2 procedures.
Several limitations exist for this experiment. First, finger flexion was measured with the hands in a palm up position. This assured that the fingers began in a fully extended position. However, this also meant that gravity may have assisted in IP joint extension until MCP joint flexion reached 90 degrees. While the House procedure inherently provides some IP joint extension regardless of gravity, the Zancolli-lasso does not. Therefore, this experimental, condition may have benefitted the lasso hands in terms of artificially delaying IP joint flexion and optimizing tip-to-palm distance due to gravity. Additionally, we chose a starting MCP joint flexion angle of 40 degrees with the Zancolli reconstructions. In our clinical experience, this best estimates the average postoperative scenario. However, we also tested our Zancolli-lasso hands at starting MCP joint flexion angles of 0, 20, 60, and 80 degrees. Kinematics (IP joint-first flexion) were identical in all scenarios (Supplemental Fig. S1), whereas maximal fingertip-to-palm closure increased with increasing degrees of MCP joint pre-flexion, as expected (Supplemental Table 1). Finally, because all hands were amputated at the level of the radiocarpal joint, we were unable to assess the effect of wrist position on tenodesis efficacy.
Reconstructive hand surgery is an important tool in the physician's skill set to optimize function and quality of life for the tetraplegic patient. Our findings suggest that both the Zancolli-lasso and House procedures provide patients with a functionally larger grasp pattern than observed in hands with FDP transfers alone. However, only the House procedure restores hand kinematics resembling those of an intrinsic-activated hand.
Supplementary Material
SUPPLEMENTAL FIGURE S1: Joint angles of the MCP, PIP, and DIP joints as a function of FDP excursion during grasp for the Zancolli-lasso reconstructed hands with varying degrees of MCP joint pre-flexion. Note that IP joint flexion precedes MCP joint flexion (see diamonds) for all conditions. Means (—) and standard errors (- -) are shown over all hands (n = 6) and all fingers (index, middle, ring, and small). ◊ = excursion of FDP tendon where the greatest change of joint movement occurred for each joint.
Acknowledgments
The authors wish to thank Lindsey Earp for her hand illustrations, as well as individuals who donate their bodies and tissues for the advancement of education and research.
Supported by: Supported in part by an NIH R24 grant (HD050837) and a grant from the American Foundation for Surgery of the Hand.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wyndaele M, Wyndaele JJ. Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey. Spinal Cord. 2006;44(9):523–529. doi: 10.1038/sj.sc.3101893. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD. Targeting recovery: priorities of the spinal cord injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 3.Curtin CM, Gater DR, Chung KC. Upper extremity reconstruction in the tetraplegic population, a national epidemiologic study. J Hand Surg Am. 2005;30(1):94–99. doi: 10.1016/j.jhsa.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Ejeskar A. Reconstruction of grip function in tetraplegia. In: Friden J, editor. Tendon transfers in reconstructive hand surgery. London, UK: Taylor & Francis Group; 2005. pp. 103–119. [Google Scholar]
- 5.Sammer DM, Chung KC. Tendon transfers part II: transfers for ulnar nerve palsy and median nerve palsy. Plast Reconstr Surg. 2009;124(3):212e–2213. doi: 10.1097/PRS.0b013e3181b037c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Backhouse K, Catton W. An experimental study of the functions of the lumbrical muscles in the human hand. J Anat. 1954;88(2):133–141. [PMC free article] [PubMed] [Google Scholar]
- 7.Dell PC, Sforzo CR. Ulnar intrinsic anatomy and dysfunction. J Hand Ther. 2005;18(2):198–207. doi: 10.1197/j.jht.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Zancolli E. Correccion de la “garra” digital por paralysis intrinseca. La operacion del ‘lazo’. Acta Ortop Latinoam. 1974;1:65–71. [Google Scholar]
- 9.House JH, Shannon MA. Restoration of strong grasp and lateral pinch in tetraplegia: a comparison of two methods of thumb control in each patient. J Hand Surg Am. 1985;10(1):22–29. doi: 10.1016/s0363-5023(85)80243-4. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy CK, House JH, Heest AV, Kawiecki JA, Dahl A, Hanson D. Intrinsic balancing in reconstruction of the tetraplegic hand. J Hand Surg Am. 1997;22(4):596–604. doi: 10.1016/S0363-5023(97)80115-3. [DOI] [PubMed] [Google Scholar]
- 11.Peljovich AE, Keith MW. Tendon transfer for hand reconstruction in spinal cord injury. In: Van Heest A, Goldfarb CA, editors. American Society for Surgery of the Hand. Tendon transfer surgery of the upper extremity: a master skills publication; Chicago, IL: 2012. pp. 121–143. [Google Scholar]
- 12.Goldfarb CA, Stern PJ. Low ulnar nerve palsy. Journal of the American Society for Surgery of the Hand. 2003;3(1):14–26. [Google Scholar]
- 13.Ejeskar A, Dahlgren A, Friden J. Clinical and radiographic evaluation of surgical reconstruction of finger flexion in tetraplegia. J Hand Surg Am. 2005;30(4):842–849. doi: 10.1016/j.jhsa.2005.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE S1: Joint angles of the MCP, PIP, and DIP joints as a function of FDP excursion during grasp for the Zancolli-lasso reconstructed hands with varying degrees of MCP joint pre-flexion. Note that IP joint flexion precedes MCP joint flexion (see diamonds) for all conditions. Means (—) and standard errors (- -) are shown over all hands (n = 6) and all fingers (index, middle, ring, and small). ◊ = excursion of FDP tendon where the greatest change of joint movement occurred for each joint.