Abstract
Purpose
Regaining hand function has been identified as the highest priority for persons with tetraplegia. In many patients, finger flexion can be restored with a tendon transfer of extensor carpiradialis longus to flexor digitorum profundus (FDP). In the absence of intrinsic function, this results in a roll-up finger movement, which tends to push large objects out of grasp. To enable patients to grasp objects of varying sizes, a functional grasp is required that has a larger excursion of fingertip-to-palm distance than can be supplied without intrinsic function. The aim of this study was to quantify the role of intrinsic muscle force in creating a functional grasp.
Methods
Finger kinematics during grasp were measured on 5 cadaveric hands. To simulate finger flexion, the FDP was activated by a motor while intrinsic muscles were loaded at various levels (0, 125, 250, 375 or 500g). Finger movement was characterized by the order of metacarpophalangeal, proximal interphalangeal, and distal interphalangeal joint flexion and by the maximal fingertip-to-palm distance during finger closure.
Results
Without any intrinsic muscle contribution (0g load), FDP activation resulted in flexion of all 3 joints, whereby flexion began at the proximal interphalangeal joint, followed by distal interphalangeal joint and then metacarpophalangeal joint. With increasing intrinsic muscle load, finger flexion was initiated at the metacarpophalangeal joint, followed by the proximal interphalangeal and distal interphalangeal joints. This altered joint flexion order resulted in a larger maximal fingertip-to-palm distance during finger flexion. The difference between the 2 extreme conditions (0g vs. 500g of intrinsic muscle load) was 19mm.
Discussion
These findings demonstrate that simultaneous activation of the FDP and the intrinsic muscles results in an apparently more functional hand closing compared to FDP activation alone because of altered kinematics and larger fingertip-to-palm distances.
Clinical Relevance
These findings suggest that intrinsic muscle balancing during reconstruction of grasp in tetraplegic patients may improve function.
Keywords: grasp, hand, intrinsic muscles, tendon transfer, tetraplegia
Introduction
Regaining arm and hand function has been identified as the highest priority for persons with tetraplegia.1,2 Recovering even partial function can have an enormous impact on independence, which enhances quality of life.3 Restoration of finger flexion is an achievable goal in reconstructive surgery of many tetraplegic hands. It can be performed if both the extensor carpi radialis longus and brevis are fully innervated, in which case the extensor carpi radialis longus is transferred to the flexor digitorum profundus (FDP),4 which enables patients to grasp and hold objects. However, by restoring FDP function only, fingertips coming into full flexion approach the bases of the fingers rather than the center of the palm.5 This happens because finger flexion begins at the distal interphalangeal (DIP) joint, and fingers curl into flexion rather than following a large arc, which would provide a broad sweeping movement.6 The roll-up finger flexion tends to push large objects out of grasp5 and is therefore considered less functional in daily life.
In normal hand function, the intrinsic muscles, both the lumbricals and interosseus muscles balance finger movement7 and create this broad sweeping movement. Besides abducting and adducting the fingers, they are responsible for coupling metacarpophalangeal joint (MCP) flexion with interphalangeal joints (IP) extension.8,9 For 2-dimensional finger movement during grasp, the lumbricals and the interossei provide the same function, as shown by Leijnse et al.10 Since their function is redundant in the sagittal plane, we refer to them as 1 entity, the intrinsics. Intrinsic function is so important that even when patients have neither functional intrinsic muscle nor a sufficient number of transferable muscles to reconstruct them with an active tendon transfer, passive tenodeses are used to substitute for intrinsic muscle function.11,12
We characterize functional hand movement for tetraplegic persons as a large fingertip-to-palm distance during flexion because this enables them to grasp objects of varying sizes and shapes necessary for activities of daily life. The exact contribution of the intrinsic muscles to this function is incompletely understood. Therefore, the purpose of this study was to quantify the role of intrinsic muscle force in creating a functional grasp. We hypothesized that increasing intrinsic muscle contribution would result in a more functional grasp.
Materials and Methods
Sample preparation
Five fresh-frozen hands were used for the experiment (3 male, 2 female; average age 75 years; range 59 – 88 years). Hands had been amputated at the level of the radiocarpal joint. For each hand, dissection and data collection were performed on the same day. During preparation and testing, hands were maintained at room temperature, and tendons were kept moist with Ringer solution.
The thumb was amputated at the MCP joint to permit clear video recording. The volar skin was excised distally to the level of the middle phalanx, and the palmar aponeurosis was resected. The FDP tendons were identified at the level of the carpal tunnel and the ends individually sutured proximally with 2-0 suture. The palmar carpal ligament remained intact. Each lumbrical was identified by its origin on the FDP and insertion into the radial lateral band. For our purposes, intrinsic muscle insertion was defined as the tendinous insertion of the lumbricals into the radial lateral bands and were tagged with 2-0 suture. The lumbrical and interossei origins were left intact. All volar tendons and affixed sutures were then passed proximally through the carpal tunnel. Dorsal skin was excised entirely. Tendons of the extensor digitorum communis (EDC) were sutured individually with 2-0 suture at the level of the wrist. Due to the scarce contribution of EDC to the extensor apparatus of the small finger in most hands, the extensor digiti quinti tendon was sutured for this digit.
Kirschner wires (1.1 mm) were drilled into each metacarpal and phalanx dorsally to mark motion. The angle between the wire and its respective bone was measured for later analysis. To facilitate data collection (i.e. prevent markers from obscuring one another), each intrinsic muscle loading condition was performed once with wires in the index and ring fingers and once with wires in the middle and small fingers. The order was randomized. Two Schanz pins were drilled into the base of the third metacarpal dorsally to affix and stabilize the hand during experimentation.
Mechanical testing
The hand was positioned palm-up with fingers fully extended (Fig. 1). The FDP sutures were attached to a single dual-mode servo-motor (Aurora Scientific, Model 310, Aurora Inc., Ontario, Canada). To approximate passive resistance, EDC sutures were affixed to a 50g mass, which was allowed to move via a pulley and created a fixed resistance. Intrinsic muscle sutures were affixed via a pulley to 0, 125, 250, 375 and 500g masses, which were also allowed to move. The order in which these intrinsic loads were trialed in each hand was randomized. Five hundred grams represented the maximal weight that could be attached to the radial lateral bands so that all hands were starting with an extended MCP joint and approximates the maximum tetanic tension of the intrinsic muscle.13
Figure 1. Experimental setup. The FDP tendons are moved by the motor while the intrinsic muscles and EDC are subjected to fixed loads.

For each experimental condition, the motor pulled the FDP tendon 50mm in a linear deformation pattern and at a velocity of 5mm/s. This excursion was determined to be sufficient to create a closed fist. A video camera was positioned on the radial side of the hand perpendicular to the plane of movement and at a fixed distance to record marker movement during FDP excursion.
Following all trials, each hand was x-rayed (Faxitron Specimen Radiography System, Tucson, AZ, USA) to determine bone lengths. Films were digitized (Bio-Rad GS-800 calibrated densitometer, Hercules, CA) and loaded into Adobe Photoshop CS6 (Adobe, Inc., San Jose, CA). Metacarpals and phalanges were measured for each hand and calibrated by a 100mm marker included in each x-ray image (Table 1).
Table 1.
Mean (standard deviation) bone length of all subjects (n=5).
| Bone length[mm] | Metacarpal | Proximal | Middle | Distal |
|---|---|---|---|---|
| Index finger | 70.2 (4.3) | 39.0 (3.9) | 22.2 (3.0) | 15.0 (2.5) |
| Middle finger | 68.0 (4.7) | 44.4 (4.2) | 27.3 (3.6) | 15.9 (2.3) |
| Ring finger | 60.5 (5.1) | 42.3 (3.8) | 26.8 (2.8) | 16.8 (2.9) |
| Small finger | 54.4 (5.2) | 33.2 (3.6) | 19.1 (2.7) | 17.8 (1.2) |
Kinematic analysis
The 2-dimensional position of the markers attached to each finger was digitized in Matlab (The MathWorks Inc., Natick, Massachusetts, USA) at a frame rate of 1Hz. The vectors of the bones and the resulting joint angles of the MCP, proximal interphalangeal (PIP), and distal interphalangeal (DIP) joints were calculated from the marker vectors and their relative angles to the bones. To define the relative order of joint flexion, the time point of maximal angular change was determined for each joint, and these were compared for the different joints and for the different experimental conditions. Other methods of assessing these kinematics, such as joint angle at 50% excursion and time to reach 50% total angular change, were trialed, and results mirrored those presented here.
To calculate the 2-dimensional position of each joint, the magnitude of the bone vectors was scaled to the measured bone length. The vertical distance from the fingertip to the palm (sagittal plane) was calculated for each finger in each hand.
Statistical analysis
Two-way repeated measures analysis of variance was performed to evaluate the effect of the intrinsic muscle load on grasp capacity (maximal fingertip-to-palm distance). Within-subject factors were intrinsic muscle load (0, 125, 250, 375 or 500g) and finger (index, middle, ring, or small finger). To analyze the effect of intrinsic muscle load on finger kinematics (order of joint flexion as defined above) a 3-way repeated measures analysis of variance was performed. The within-subject factors were intrinsic muscle load, finger, and joint (MCP, PIP, or DIP joint). Level of significance (α) was set to P < 0.05. Bonferroni post hoc tests adjusted for multiple comparisons were conducted to identify intrinsic muscle loading conditions significantly different from one other.
Results
Increasing intrinsic muscle load resulted in a qualitatively different finger movement compared to no intrinsic muscle load (Fig. 2 and supplemental video). With no intrinsic muscle load, fingers moved in a roll-up motion with the PIP and DIP joints flexing early. With increasing load, the IP joints flexed later relative to the MCP and fingers moved without early digital roll-up.
Figure 2.
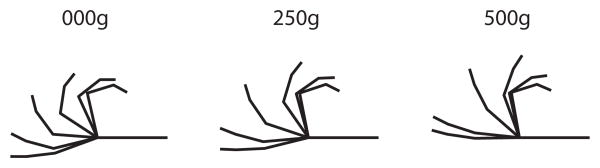
Graphical illustration of a digitized middle finger at a frame rate of 0.5Hz for intrinsic muscle load conditions of 0g, 250g and 500g. See also supplemental video. Note the greater flexion of the PIP and DIP joints for the 0g intrinsic muscle load conditions.
Loading intrinsic muscle muscles altered the closing cascade of the fingers (Fig. 3), especially the order of flexion of MCP and PIP joints. With no intrinsic muscle load, the PIP joint flexed first, followed by the DIP and MCP joints (see colored diamonds, Fig. 3). The same movement pattern was found for an intrinsic muscle load of 125g, except that the MCP joint flexed before the DIP joint. With an intrinsic muscle load of 250g, the MCP and PIP joints flexed similarly, and the maximal angular change occurred approximately at the same time (Fig. 3, diamonds). With a load of 375g and above on the intrinsic muscle, the MCP joint flexed first followed by the PIP joint. Under these conditions, the DIP joint flexed after the other joints. Statistical analysis revealed that there was a significant difference in the order of joint movement (quantified by the excursion where maximal angular change occurred) between intrinsic muscle load conditions (p = 0.005) and a significant interaction between intrinsic muscle load and joint (P < 0.001). There was no difference in the order of joint movement between fingers (p = 0.190), and there was no finger × joint (p = 0.358) or intrinsic muscle load × finger × joint (p = 0.882) interaction. Bonferroni post hoc tests did not reveal significant differences between individual comparisons for interaction between intrinsic muscle load and joint, the outcome of most clinical relevance. This indicates that even though there is a main effect of load, sample sizes may have been too small to demonstrate specific paired differences.
Figure 3.
Angle of MCP, PIP or DIP joint relative to FDP tendon excursion. Mean (—) and standard error (--) were calculated over all hands (n=5) and all fingers (index, middle, ring, and small). ◊ = excursion of FDP tendon where the greatest joint angle change occurred for the different finger joints.
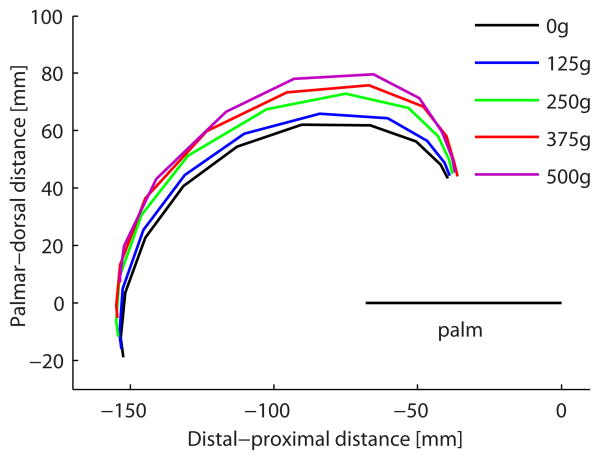
Increasing intrinsic muscle load altered the maximal fingertip-to-palm distance during finger flexion. Lower load conditions resulted in a roll-up finger flexion whereby the fingertips followed a lower arc over the palm, whereas increasing load allowed fingertips to follow a higher arc (Fig. 4A). This resulted in a significant difference in the maximal distance between fingertip and palm between the conditions (P < 0.001, Table 2). For example, the mean maximal distance of the middle finger increased from 64mm (0g) to 82mm (500g). Bonferroni post hoc tests revealed significant differences only between the 0g and 125g conditions (p = 0.036) owing to the lower SEMs at these conditions, though many other pairs trended towards significance. The difference in fingertip arc was related to the order of joint flexion which is seen by plotting MCP joint vs. PIP joint angle (Fig. 4B). As intrinsic muscle load increased, MCP joint angle changed first, resulting in a more horizontal initial excursion of the trace.
Figure 4.
(A) Path of the tip of the middle finger during flexion relative to the plane of the palm for all intrinsic muscle load conditions. Mean calculated across hands (n=5). Note larger arcs with increasing intrinsic muscle load. (B) MCP joint angle plotted against PIP joint angle for intrinsic muscle load conditions to illustrate the interaction between these joint motions as a function of intrinsic muscle load. Means were calculated over all hands (n=5) and all fingers (index, middle, ring, and small). Note the more horizontal initial excursion with increasing intrinsic muscle load due to larger initial MCP flexion.
Table 2. Maximal distance between fingertip and palm.
| Finger | Significance | ||||||
|---|---|---|---|---|---|---|---|
| Load | Index | Middle | Ring | Small | Load | Finger | Load*Finger |
| 0g | 54 ± 3 | 64 ± 4 | 61 ± 4 | 43 ± 2 | |||
| 125g | 60 ± 2 | 68 ± 4 | 64 ± 5 | 48 ± 3 | |||
| 250g | 66 ± 2 | 75 ± 3 | 72 ± 4 | 55 ± 3 | < 0.001 | < 0.001 | 0.804 |
| 375g | 70 ± 3 | 80 ± 3 | 77 ± 4 | 60 ± 4 | |||
| 500g | 72 ± 3 | 82 ± 2 | 80 ± 4 | 63 ± 3 | |||
Maximal distance between fingertip and palm (mean over all hands ± SEM, mm) and results of the statistical analysis. Post hoc test load indicates the INT load conditions where significant differences were found. P-values refer to the three p-values obtained from two-way ANOVA of the data using load and finger as grouping factors.
Discussion
This study demonstrated the kinematic influence and potential for functional advantage of providing intrinsic muscle function after paralysis. Increasing intrinsic muscle contribution increased the distance between fingertip and palm during finger flexion, resulting in a theoretically more functional grasp (Fig. 4A). The difference between the 2 extreme conditions for the middle finger (0g vs. 500g of intrinsic muscle load) was 19mm, which represents a large difference in finger movement when considering activities of daily life. This can determine whether objects can be grasped and lifted independently or whether assistance will be required. For example, grasping a standard beverage can, which has a diameter of 66mm, may theoretically not be possible if a person does not have active intrinsic muscle function. This study showed that if grasp is powered by FDP alone, the middle finger will not be able to grasp the can and will simply push the can away. The results showed that grasping the can with the middle finger is theoretically only possible if the intrinsic muscles are loaded with at least 250g. Of course, actual grasp results from an interplay of all 4 fingers, and as a result of the different size of each finger, there was a significant difference in fingertip-to-palm distance among fingers (index, middle, ring or small finger). Further study is required to determine whether this theoretical advantage bears true, and, if so, whether it is clinically relevant.
The difference in fingertip-to-palm distance with increasing intrinsic muscle load described above resulted from the altered kinematics of joint flexion. In the absence of intrinsic muscle loading, FDP activation resulted in flexion beginning at the PIP joint, followed by the DIP joint and then the MCP joint (Fig. 3, diamonds represent maximum angular change for each joint). These results support the findings of Kamper et al., who analyzed whether it is biomechanically feasible for extrinsic flexors to initiate concurrent flexion at all 3 finger joints.14 Their results demonstrated that shortening of the extrinsic flexors resulted in simultaneous flexion of all 3 joints. The rates of change of the DIP and PIP joint angles were greater than that of MCP joint.
Even though the intrinsic muscle are not necessary to produce finger flexion, they are important to mediate finger flexion. With higher intrinsic muscle loads, MCP joint flexion starts earlier, followed by IP joint flexion. This can be explained in part by the contribution of the intrinsic muscle to the MCP joint flexion moment. Recent studies showed that the intrinsic muscles produce a considerable fraction of the MCP flexion moment. Depending on the finger, the following contributions of the intrinsic muscle to MCP flexion moment have been found: index 12_ENREF_15 - 26%, middle 5 - 8%, ring 2 - 13% and small 15 - 28%._ENREF_1715-17
Intrinsic muscle activation contributes not only to earlier MCP joint flexion but also to the delay in IP joint flexion. While analyzing the contribution of the intrinsic and extrinsic muscles to the extension of the DIP joint, Murai et al. found an almost equal contribution of these muscles.18 At the highest intrinsic muscle load condition of the present study, the IP joints flexed with a delay (Fig. 3; Fig 4B). While the MCP joint was already fully flexed and at its terminal position, the IP joints were still flexing. Thus, the intrinsic muscle-mediated coupling of MCP joint flexion to IP joint extension modulated the FDP-mediated IP joint flexion. This resulted in the largest observed fingertip-to-palm distance (Fig. 4A). A similar idea was presented by Landsmeer et al. who studied the mechanisms of finger control based on electromyographic measurements.19 Starting with all joints extended, subjects were asked to flex the IP joints first and subsequently the MCP joint. This movement resulted in a measurable electrical signal from the FDP and EDC, but not the intrinsic muscles. Conversely, when moving from the same starting position but flexing the MCP joint alone without IP joint flexion, only the EDC and intrinsic muscles were activated while the FDP remained silent.19
In normal, everyday finger movement, hand muscles work together and either sequentially or simultaneously activate, depending on the task. In this study, these task-dependent motor control patterns were not addressed since the muscles were either active or not during the entire excursion. Though this may not represent intact hand function, it does reflect the situation of tetraplegic patients after surgical reconstruction of finger flexion. Depending on the lesion level, finger flexion can be restored by tendon transfer; however, most patients do not have a sufficient number of transferable muscles to also reconstruct the intrinsic muscles by a tendon transfer. In these patients, passive intrinsic muscle balancing is accomplished by tenodesis. Two such procedures, the Zancolli-Lasso and House reconstructions, are compared in part II of this study (Intrinsic hand muscle function II: kinematic comparison of two reconstructive procedures. Manuscript submitted for publication). In the future, nerve transfers may also play a larger role in direct reactivation of the intrinsic muscles.
Limitations of this study include the small sample size and the age of the tested hands. While degenerative muscle changes may have been present in our study (age range 59-88 years), finger movement was generated by the external forces of the motor and weights, thus muscular changes were not relevant. Another limitation was the fact that the kinematic analysis was performed in 2-dimensions. The majority of finger movement during grasp occurred in this sagittal plane, however, there was some out-of-plane motion as well. Specifically, the small finger curled towards the middle of the palm during grasp, which was not considered. Since neither the kinematic nor the fingertip-to-palm results were dependent on the small finger, we concluded that 2-dimensional analysis adequately captured the grasping motion. The choice of 50g of fixed resistance applied to EDC was necessarily arbitrary. The exact magnitude of this force is unknown and could vary throughout finger flexion. However, we do not believe that this would have biased our results. Finally, this study used constant intrinsic muscle loads and therefore cannot provide information regarding appropriate timing of intrinsic muscle activation.
Our results illustrate the importance of intrinsic muscle balancing during reconstruction of grasp in tetraplegic patients. The theoretical improvement of grasp with increasing fingertip-to-palm distances should be verified experimentally, and the kinematic outcome of different reconstruction techniques should be compared with respect to functional grasp.
Supplementary Material
Acknowledgments
The authors wish to thank individuals who donate their bodies and tissues for the advancement of education and research.
Supported by: This work was supported in part by NIH R24 grant (HD050837) and a Resident Research Grant from the American Foundation for Surgery of the Hand. No benefits in any form have been received or will be received related directly or indirectly to the subject of this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 2.Snoek GJ, MJ IJ, Hermens HJ, Maxwell D, Biering-Sorensen F. Survey of the needs of patients with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Spinal Cord. 2004;42(9):526–532. doi: 10.1038/sj.sc.3101638. [DOI] [PubMed] [Google Scholar]
- 3.Wuolle KS, Bryden AM, Peckham PH, Murray PK, Keith M. Satisfaction with upper-extremity surgery in individuals with tetraplegia. Arch Phys Med Rehabil. 2003;84(8):1145–1149. doi: 10.1016/s0003-9993(03)00292-2. [DOI] [PubMed] [Google Scholar]
- 4.Fridén J. Tendon Transfers in Reconstructive Hand Surgery. Taylor & Francis; 2005. [Google Scholar]
- 5.Hentz VR, Leclerc C. Surgical Rehabilitation of the Upper Limb in Tetraplegia. Philadelphia: WB Saunders; 2002. [Google Scholar]
- 6.McCarthy CK, House JH, Van Heest A, et al. Intrinsic balancing in reconstruction of the tetraplegic hand. J Hand Surg Am. 1997;22(4):596–604. doi: 10.1016/S0363-5023(97)80115-3. [DOI] [PubMed] [Google Scholar]
- 7.Liss FE. The interosseous muscles: the foundation of hand function. Hand Clin. 2012;28(1):9–12. doi: 10.1016/j.hcl.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Backhouse KM, Catton WT. An experimental study of the functions of the lumbrical muscles in the human hand. J Anat. 1954;88(2):133–141. [PMC free article] [PubMed] [Google Scholar]
- 9.Ranney DA, Wells RP, Dowling J. Lumbrical function: interaction of lumbrical contraction with the elasticity of the extrinsic finger muscles and its effect on metacarpophalangeal equilibrium. J Hand Surg Am. 1987;12(4):566–575. doi: 10.1016/s0363-5023(87)80210-1. [DOI] [PubMed] [Google Scholar]
- 10.Leijnse JN, Kalker JJ. A two-dimensional kinematic model of the lumbrical in the human finger. J Biomech. 1995;28(3):237–249. doi: 10.1016/0021-9290(94)00070-k. [DOI] [PubMed] [Google Scholar]
- 11.House JH, Shannon MA. Restoration of strong grasp and lateral pinch in tetraplegia: a comparison of two methods of thumb control in each patient. J Hand Surg Am. 1985;10(1):22–29. doi: 10.1016/s0363-5023(85)80243-4. [DOI] [PubMed] [Google Scholar]
- 12.Zancolli E. Surgery for the quadriplegic hand with active, strong wrist extension preserved. A study of 97 cases. Clin Orthop Relat Res. 1975;(112):101–113. [PubMed] [Google Scholar]
- 13.Jacobson MD, Raab R, Fazeli BM, et al. Architectural design of the human intrinsic hand muscles. J Hand Surg Am. 1992;17(5):804–809. doi: 10.1016/0363-5023(92)90446-v. [DOI] [PubMed] [Google Scholar]
- 14.Kamper DG, George Hornby T, Rymer WZ. Extrinsic flexor muscles generate concurrent flexion of all three finger joints. J Biomech. 2002;35(12):1581–1589. doi: 10.1016/s0021-9290(02)00229-4. [DOI] [PubMed] [Google Scholar]
- 15.Koh S, Buford WL, Jr, Andersen CR, Viegas SF. Intrinsic muscle contribution to the metacarpophalangeal joint flexion moment of the middle, ring, and small fingers. J Hand Surg Am. 2006;31(7):1111–1117. doi: 10.1016/j.jhsa.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 16.Buford WL, Jr, Koh S, Andersen CR, Viegas SF. Analysis of intrinsic-extrinsic muscle function through interactive 3-dimensional kinematic simulation and cadaver studies. J Hand Surg Am. 2005;30(6):1267–1275. doi: 10.1016/j.jhsa.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 17.Li ZM, Zatsiorsky VM, Latash ML. Contribution of the extrinsic and intrinsic hand muscles to the moments in finger joints. Clin Biomech (Bristol, Avon) 2000;15(3):203–211. doi: 10.1016/s0268-0033(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 18.Murai S, Tanaka T, Aoki M. Combinatorial roles of extrinsic and intrinsic muscles in extension strength of the distal interphalangeal joint. J Orthop Res. 2012;30(6):893–896. doi: 10.1002/jor.22021. [DOI] [PubMed] [Google Scholar]
- 19.Landsmeer JM, Long C. The mechanism of finger control, based on electromyograms and location analysis. Acta Anat (Basel) 1965;60(3):330–347. doi: 10.1159/000142668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.