Abstract
To efficiently colonize and persist in the lower respiratory tract, bacteria must survive multiple host immune mechanisms. Bordetella bronchiseptica is a gram-negative respiratory pathogen that naturally infects mice and persists in the lower respiratory tract for up to 49 days postinoculation. In this work, we examined the effect of mutation of the pagP gene on the persistence of B. bronchiseptica in the lower respiratory tract of mice. The pagP gene encodes a palmitoyl transferase that is responsible for the addition of a palmitoyl group to the lipid A region of B. bronchiseptica lipopolysaccharide. Data presented here confirm that a B. bronchiseptica ΔpagP mutant demonstrates defective persistence in the lower respiratory tract of wild-type mice. We hypothesized that the defective persistence of the B. bronchiseptica ΔpagP mutant was due to an increased susceptibility of this mutant to a host immune response. In vivo data indicate that both B cells and the complement component C3 are required for the reduced bacterial numbers of the ΔpagP mutant on day 14 postinoculation. In addition, an in vitro complement killing assay demonstrated that B. bronchiseptica exhibits pagP-dependent resistance to antibody-mediated complement killing at low concentrations of immune serum. Taken together, these results suggest that pagP is required for B. bronchiseptica to resist antibody-mediated complement lysis during respiratory infection.
Lipopolysaccharide (LPS), the primary component of the outer leaflet of gram-negative bacteria, generally consists of a lipid A domain, a core oligosaccharide, and in many cases a repeating O-antigen polysaccharide. Immunologically, LPS is one of the most important gram-negative bacterial compounds, inducing a potent inflammatory response as well as providing protection for the bacteria against host immune responses (reviewed in reference 5). Modifications of LPS, including changes in lipid A structure, occur in many gram-negative species and may be a mechanism by which bacteria cope with changing environmental conditions and/or modify host responses (reviewed in reference 16).
The genus Bordetella presently consists of eight species, some of which are known to cause respiratory tract infections in a variety of animals and humans (14). Bordetella bronchiseptica infection results in respiratory disease in pigs, dogs, rabbits, rats, mice, and other nonhuman mammals, while the closely related species B. pertussis is the causative agent of whooping cough in humans (8, 10). The regulation of Bordetella virulence factors is primarily under the control of the BvgAS two-component regulatory system (12). In the Bvg+ phase, which is necessary and sufficient for respiratory tract infection, virulence factors such as adenylate cyclase toxin, filamentous hemagglutinin, pertactin, fimbriae, and a type III secretion system are expressed, while in the Bvg− phase, believed to be involved in environmental survival, these genes are suppressed and other genes, such as those for flagellin, are expressed.
In addition to the known virulence factors already mentioned, the wbm and pagP genes, whose expression results in LPS modifications, are also regulated by BvgAS, suggesting they may be involved in the infectious process (9, 17). The pagP gene encodes a palmitoyl transferase that is responsible for transferring a palmitate group to the lipid A region of LPS. Mutation of pagP homologues in Salmonella enterica serovar Typhimurium and Legionella pneumophila is associated with increased sensitivity to killing by cationic antimicrobial peptides (6, 18). This suggests that the lipid A acylation state may influence outer membrane permeability. In addition, changes in lipid A acylation have been shown to affect complement activation and opsonization, as well as TLR-4 recognition in vitro (1, 7, 13, 21). Despite these compelling data, the influence of lipid A acylation on in vivo host-pathogen interactions is not well understood.
The LPS structure of the wild-type RB50 strain of B. bronchiseptica is primarily hexa-acylated in the Bvg+ phase, while LPS from the ΔpagP mutant of this strain is penta-acylated in the Bvg+ phase due to the lack of a sixth palmitoyl group in the lipid A region. Mutation of the pagP gene results in a defect in the ability of B. bronchiseptica to persist in the lungs of wild-type mice (17). We hypothesize that a functional pagP gene protects B. bronchiseptica against host immune responses that are responsible for the early clearance of the ΔpagP mutant. Therefore, we investigated which host immune response(s) is required for the early clearance of the ΔpagP mutant from the lower respiratory tract of mice. Data presented here indicate that wild-type B. bronchiseptica persisted in the lungs of C57BL/6 mice up to day 49 postinoculation; however, a B. bronchiseptica ΔpagP mutant exhibited defective persistence beginning at day 7 postinoculation and was cleared from the lungs by day 28 postinoculation. The ΔpagP mutant demonstrated no defect in persistence in the lungs of B-cell-deficient (μMT) mice or mice deficient in the C3 component of the complement cascade (C3−/−) on day 14 postinoculation, indicating that B cells and complement are involved in the reduction of bacterial numbers of the ΔpagP mutant at this time point. Finally, B. bronchiseptica demonstrated pagP-dependent resistance to immune serum complement killing at low serum concentrations. These data suggest that increased lipid A acylation in B. bronchiseptica confers resistance to antibody-mediated complement lysis during infection.
MATERIALS AND METHODS
Bacteria.
The wild-type strain RB50 and the ΔpagP mutant of B. bronchiseptica have been described previously (4, 17). Bacteria were maintained on Bordet-Gengou agar (Difco) containing 7.5% sheep blood and either streptomycin (20 μg/ml) for RB50 or streptomycin (20 μg/ml) plus erythromycin (10 μg/ml) for the RB50 ΔpagP strain. For experiments, bacteria were inoculated into Stainer-Scholte broth at optical densities of 0.1 or lower and grown to mid-log phase at 37°C on a roller drum.
Animal experiments.
C57BL/6 and μMT mice were obtained from Jackson Laboratories, C3−/− mice were obtained from Rick Wetzel of Baylor University, and FcγR−/− mice were obtained from Taconic Laboratories. Mice lightly sedated with isoflurane (Abbott Laboratories) were inoculated intranasally by pipetting 50 μl of phosphate-buffered saline (PBS) containing 5 × 105 CFU of bacteria onto the tip of the external nares. Groups of four animals were sacrificed on days 3, 7, 14, 28, 49, and 70 postinoculation or as indicated, and the nasal cavity, trachea, and lungs were excised. Bacterial numbers in the respiratory tract were quantified by homogenization of each tissue in PBS followed by plating onto Bordet-Gengou blood agar containing streptomycin (20 μg/ml). Colonies were enumerated after 2 days of growth at 37°C. All animal experiments were carried out in accordance with institutional guidelines.
Antibodies.
Titers of anti-Bordetella antibody in sera collected on day 28 postinoculation were determined by enzyme-linked immunosorbent assay (ELISA) with polyvalent anti-mouse secondary antibodies as previously described (4). Specific classes and isotypes of antibodies were determined by using appropriate anti-mouse secondary antibodies (Southern Biotechnology Associates and Pharmingen). Titers were calculated by the endpoint method using naïve mouse serum as the negative control.
Serum killing assays.
Complement killing assays were performed with rabbit serum purchased from Covance Laboratories. Immune serum was collected from a Bordetella-positive rabbit and determined to have significant titers of RB50-specific antibodies by ELISA as previously described. Lungs were collected on day 14 postinoculation from μMT mice inoculated with either RB50 or RB50 ΔpagP. The lung samples were diluted to obtain a final concentration of approximately 100 CFU/μl. Differing concentrations of serum were diluted in PBS and added to 5 μl of diluted lung sample to a total sample volume of 50 μl. After a 1-h incubation at 37°C followed by a 5-min incubation on ice, the entire 50-μl sample was plated onto Bordet-Gengou blood agar containing streptomycin (20 μg/ml). Colonies were enumerated after a 2-day incubation at 37°C. To determine whether the killing in this assay requires Ca+ and Mg+, which are required for complement activity, Ca+ and Mg+ were chelated by the addition of EDTA to a final concentration of 10 mM and the assay was performed as described above (19). Finally, to ensure that other serum components do not contribute to killing during this assay, naïve rabbit serum, which lacks B. bronchiseptica-specific antibodies but retains a functional complement system, was used and the assay was performed as described above.
Statistics.
Statistical significance was determined using Student's t test, with P values of less than or equal to 0.05 being considered significant.
RESULTS
pagP is required for the persistence of B. bronchiseptica in the lower respiratory tract of wild-type mice.
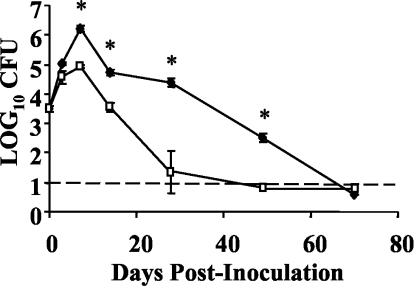
Previous work has indicated that a functional pagP gene is required for the persistence of B. bronchiseptica after day 14 postinoculation in the lungs of wild-type BALB/c mice (17). To determine whether this is also true in C57BL/6 mice, groups of four C57BL/6 mice were intranasally inoculated with approximately 5 × 105 CFU of either the wild-type or ΔpagP mutant strain of B. bronchiseptica in a total volume of 50 μl in PBS, a regimen shown to deliver bacteria throughout the respiratory tract (8). Bacterial numbers in the lungs were measured on days 0, 3, 7, 14, 28, and 70 postinoculation (Fig. 1). The ΔpagP mutant was recovered from the lungs at approximately 1/10 the levels of wild-type B. bronchiseptica on days 7 and 14 postinoculation. In addition, the ΔpagP mutant was cleared from the lungs by day 28 postinoculation while wild-type B. bronchiseptica was not cleared until day 70 postinoculation. The kinetics of persistence of the ΔpagP mutant in C57BL/6 mice follows a pattern similar to that previously described for BALB/c mice with the exceptions that in BALB/c mice the numbers of the ΔpagP mutant bacteria in the lungs were indistinguishable from those of wild-type B. bronchiseptica on day 7 postinoculation and the mutant was cleared from BALB/c lungs by day 14 postinoculation. Despite these minor differences, both experiments indicate that pagP is required for the persistence of B. bronchiseptica in the lungs of wild-type mice.
FIG. 1.
Time course of bacterial numbers in the lungs of C57BL/6 mice. Groups of four C57BL/6 mice were intranasally inoculated with 5 × 105 CFU of wild-type B. bronchiseptica (⧫) or the B. bronchiseptica ΔpagP mutant (□). Bacterial numbers in the lungs were enumerated on days 3, 7, 14, 28, 49, and 70 postinoculation. The time course was replicated twice. The dashed line indicates the limit of detection. Results are means ± standard errors (SE). *, P ≤ 0.05.
B cells may be required for the reduced numbers of B. bronchiseptica ΔpagP mutant bacteria in the lungs on days 14 and 28 postinoculation.
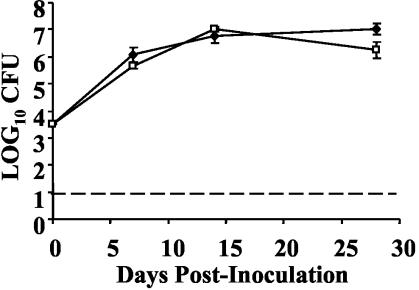
As the defect of the ΔpagP mutant is most prominent after day 14 postinoculation, it was hypothesized that this mutant is more susceptible to an aspect of the host-adaptive immune response. B cells are required for the clearance of wild-type B. bronchiseptica from the respiratory tract of mice, so we sought to determine whether B cells are required for the reduced persistence of the ΔpagP mutant (11). Groups of five μMT mice were intranasally inoculated with approximately 5 × 105 CFU of either the wild-type or ΔpagP mutant strain of B. bronchiseptica, and bacterial numbers in the lungs on days 7, 14, and 28 postinoculation were determined. In contrast to those recovered from C57BL/6 mice, in which the ΔpagP mutant had a significant defect in persistence on days 7, 14, and 28 postinoculation, the ΔpagP mutant was recovered from the lungs of μMT mice in numbers similar to those of wild-type B. bronchiseptica (Fig. 2). These data suggest that the early clearance of the ΔpagP mutant may require B cells.
FIG. 2.
B cells may be required for the reduced numbers of B. bronchiseptica ΔpagP. Groups of five C57BL/6 and μMT mice were intranasally inoculated with 5 × 105 CFU of wild-type B. bronchiseptica (⧫) or B. bronchiseptica ΔpagP (□). Bacterial numbers in the lungs were enumerated on days 7, 14, and 28 postinoculation. The time course was replicated twice. Results are means ± SE. The dashed line indicates the limit of detection. *, P ≤ 0.05.
B. bronchiseptica ΔpagP does not elicit higher serum antibody titers than wild-type B. bronchiseptica.
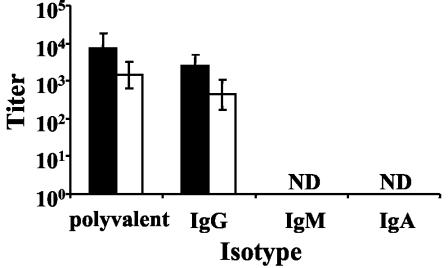
One of the primary functions of B cells is the production of antibodies, and previous work in our laboratory has indicated that B. bronchiseptica is susceptible to clearance by antibodies (15). This, coupled with data indicating that B cells are required for the reduced numbers of ΔpagP mutant bacteria on days 14 and 28, led to the hypothesis that the ΔpagP mutant may induce higher antibody titers than wild-type B. bronchiseptica, thus speeding its clearance from the lungs. Antibody titers in serum collected on day 28 postinoculation were determined by ELISA and indicate that the ΔpagP mutant and wild-type B. bronchiseptica strains elicited similar antibody titers (Fig. 3). This suggests that the early clearance of the ΔpagP mutant is not due to increased antibody titers.
FIG. 3.
Serum antibody isotype titers from C57BL/6 mice at 28 days postinoculation. Serum was collected from groups of three C57BL/6 mice on day 28 postinoculation with 5 × 105 CFU of wild-type B. bronchiseptica (black columns) or B. bronchiseptica ΔpagP (white columns). Antibody isotype titers were measured by ELISA. Titers were measured from two independent replicates. Results are means ± SE. ND, not detected.
C3 but not FcγRs or IgA is required for the reduced numbers of B. bronchiseptica ΔpagP bacteria on day 14 postinoculation.
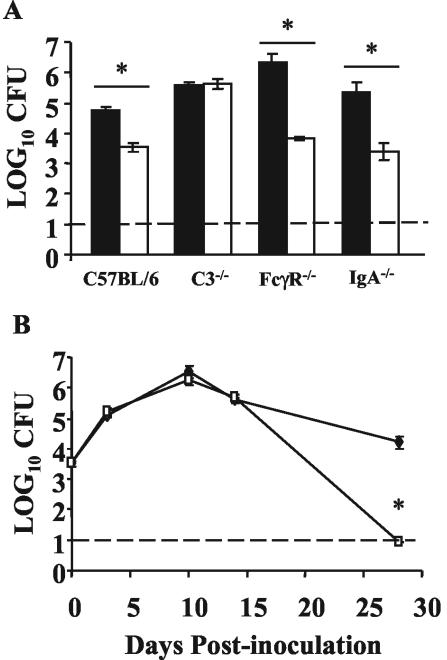
Since B cells are required for the reduced numbers of ΔpagP mutant bacteria in the lungs on day 14 postinoculation but the ΔpagP mutant did not elicit higher antibody titers, we hypothesized that pagP may be required for resistance to an antibody-mediated mechanism of clearance. Antibodies can clear bacteria through several mechanisms, including classical pathway complement lysis, opsonization, and phagocytosis via FcγRs or neutralization. To determine whether C3, FcγRs, or immunoglobulin A (IgA) is required for the reduced numbers of ΔpagP mutant bacteria in the lungs, C3−/−, FcγR−/−, and IgA−/− mice were inoculated with 5 × 105 CFU of the wild-type or ΔpagP strain of B. bronchiseptica and bacterial numbers were measured in the lungs on day 14 postinoculation (Fig. 4A). These data indicate that a functional complement system is required for the reduced numbers of ΔpagP mutant bacteria on day 14 postinoculation but that FcγRs and IgA are not.
FIG. 4.
Bacterial numbers in immunocompromised mice. (A) Groups of four C57BL/6, C3−/−, FcγR−/−, and IgA−/− mice were intranasally inoculated with 5 × 105 CFU of wild-type B. bronchiseptica (black columns) or B. bronchiseptica ΔpagP (white columns). Bacterial numbers in the lungs were enumerated on day 14 postinoculation. (B) Groups of four C3−/− mice were inoculated with 5 × 105 CFU of wild-type B. bronchiseptica (⧫) or B. bronchiseptica ΔpagP (□) as described for panel A, and bacteria in the lungs were enumerated on days 3, 10, 14, and 28 postinoculation. The time course was replicated twice. The dashed line indicates the limit of detection. Results are means ± SE. *, P ≤ 0.05 for the indicated comparisons.
To further explore the requirement for complement in the clearance of the ΔpagP mutant, C3−/− mice were inoculated with 5 × 105 CFU of either the wild-type or ΔpagP mutant strain of B. bronchiseptica, as described above, and bacterial numbers in the lungs on days 3, 10, 14, and 28 postinoculation were determined (Fig. 4B). Bacterial numbers in the lungs of these mice indicate that in the absence of C3, the ΔpagP mutant persisted at levels similar to those of wild-type B. bronchiseptica up to day 14 postinoculation, suggesting that the reduction in numbers of ΔpagP mutant bacteria in the lungs up to this time point is dependent on functional C3. However, on day 28 postinoculation the ΔpagP mutant was cleared from both C3−/− and wild-type mice, suggesting that C3 is not required for the clearance of the ΔpagP mutant at later time points. Taken together, these data suggest that expression of pagP provides protection against complement-mediated responses in the lungs during the first 2 weeks of infection; however, clearance of the mutant by day 28 occurs via a complement-independent mechanism.
pagP is required for resistance to antibody-mediated complement lysis.
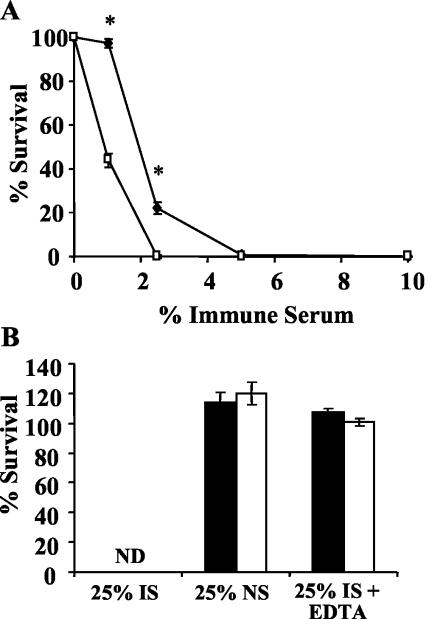
Since B cells and C3 are required for the reduced numbers of ΔpagP mutant bacteria on day 14 postinoculation, we hypothesized that pagP may be required for resistance to antibody-mediated complement lysis in vivo. To examine this hypothesis, the susceptibilities of wild-type and ΔpagP mutant strains of B. bronchiseptica to complement killing by immune serum were tested. μMT mice were used to exclude the possibility that endogenous antibodies may affect complement-mediated lysis. Mice of this strain were also used because the numbers of wild-type and ΔpagP mutant strains of B. bronchiseptica in their lungs are similar on day 14 postinoculation, which removes the possibility that different concentrations of other lung homogenate components may affect killing in this assay. These mice were inoculated with approximately 5 × 105 CFU of either the wild-type or ΔpagP mutant strain of B. bronchiseptica, the lungs were excised on day 14 postinoculation, and lung homogenate was diluted to the appropriate concentration of bacteria for the immune serum killing assay. The diluted lung homogenate was then incubated at 37°C for 1 h with various concentrations of rabbit serum containing antibodies to B. bronchiseptica, and survival was measured by colony enumeration as described above. At immune serum concentrations of 1 and 2.5%, the ΔpagP mutant was significantly more susceptible to antibody-mediated complement lysis than wild-type B. bronchiseptica (Fig. 5A).
FIG. 5.
Bacterial resistance to killing via immune serum complement lysis. (A) Wild-type B. bronchiseptica (⧫) or B. bronchiseptica ΔpagP (□) was recovered from the lungs of μMT mice on day 14 postinoculation and incubated for 1 h with various concentrations of rabbit immune serum, and survival was measured. The experiment was replicated twice. (B) Wild-type B. bronchiseptica (black columns) or B. bronchiseptica ΔpagP (white columns) was recovered from the lungs of μMT mice on day 14 postinoculation and incubated for 1 h with 25% immune serum (IS), 25% naïve serum (NS), or 25% immune serum chelated with 10 mM EDTA, and survival was measured. ND, not detected. The experiment was replicated twice. Results are means ± SE. *, P ≤ 0.05.
To further assess whether the killing in this assay was due to complement or other serum components, naïve serum or EDTA chelations were added to the complement killing assay. With a final concentration of 25% immune serum, both the wild-type and ΔpagP strains of B. bronchiseptica were completely killed (Fig. 5B). To examine the role of complement in this assay, EDTA was used to chelate divalent cations, which are required for the function of the classical and alternative pathways of complement (19). The addition of 10 mM EDTA to the 25% immune serum reaction mixture resulted in 100% survival of both wild-type B. bronchiseptica and the ΔpagP mutant (Fig. 5B). These data suggest that the killing in this assay was due to complement-mediated lysis; however, we cannot rule out the possibility that other serum components which require divalent cations are responsible for killing. To assess the role of antibodies as well as to rule out the possibility that other serum components may result in bacterial killing of wild-type and ΔpagP mutant strains of B. bronchiseptica, serum from a naïve rabbit was used. The incubation of the wild-type and ΔpagP mutant strains of B. bronchiseptica with 25% naive serum resulted in over 100% survival, indicating that other serum components do not play a role in bacterial killing (Fig. 5B). In addition, these data suggest that antibodies are required for the killing of the wild-type and ΔpagP mutant strains of B. bronchiseptica. Taken together, the data from these serum killing assays suggest that the ΔpagP mutant is more susceptible than wild-type B. bronchiseptica to antibody-mediated complement lysis.
DISCUSSION
Under normal conditions, the lower respiratory tract is a sterile environment. During infection, bacteria reaching this site elicit responses from multiple cell types that are resident in or recruited to the lungs. The fact that some bacteria can persist in spite of the variety of immune responses intended to eliminate them implies that they have evolved systems for defense against these host clearance mechanisms. Since wild-type B. bronchiseptica persists in the lower respiratory tract of mice for up to 49 days postinoculation, this model provides an excellent opportunity for the study of bacterial resistance mechanisms in the context of a natural host-pathogen interaction. In this study, the use of a bacterial mutant coupled with immunocompromised mouse strains allowed us to examine the role of a single bacterial gene in protection against host immune responses in the lower respiratory tract.
The pagP gene encodes a palmitoyl transferase which is responsible for the addition of a palmitoyl group to the lipid A region of B. bronchiseptica LPS (17). In this work, we investigated whether early clearance of a ΔpagP mutant from the lungs of wild-type mice was the result of increased susceptibility to a host response normally induced by B. bronchiseptica. As the primary outer membrane structure, LPS can provide an important initial barrier for gram-negative bacteria against host immune responses. However, LPS detection, which is mediated through a variety of receptors, can also initiate host immune responses. Changes in LPS structure, as exhibited when B. bronchiseptica modulates to the Bvg+ phase and expresses pagP, may provide these bacteria with mechanisms to alter and/or protect against the elicited immune response.
To elucidate the specific role of pagP, we investigated which host immune mechanisms are required for the early clearance of the ΔpagP mutant. By looking at bacterial numbers in the lungs on day 14 postinoculation, it was found that B cells and C3, but not FcγR or IgA, are required for the reduced numbers of ΔpagP mutant bacteria. In vitro data indicated that the ΔpagP mutant is more susceptible than wild-type B. bronchiseptica to antibody-mediated complement lysis when recovered on day 14 postinoculation. This strongly suggests that pagP has a role in resistance of the bacteria to antibody-mediated complement lysis.
The data presented here support the results of previous work with Salmonella, Yersinia, and Haemophilus species which indicated that decreased acylation of LPS/lipooligosaccharide results in increased susceptibility to lysis by various mechanisms (2, 6, 20). This, coupled with data indicating that decreased LPS acylation results in increased membrane permeability in phospholipid bilayers, suggests a model in which the addition of an acyl group to the lipid A region increases the rigidity of the membrane, thus decreasing the susceptibility to lysis by various immune mechanisms (21).
In addition to supporting previous work regarding the role of lipid A acylation in gram-negative bacterial species, the data presented here also provide some insight into the resistance mechanisms that allow B. bronchiseptica to persist in the lower respiratory tract. A prior study from our laboratory has indicated that expression of the wbm locus, which directs expression of the O antigen of LPS, protects B. bronchiseptica from complement lysis in the absence of antibodies (3). Therefore, it is not surprising that modification of another portion of the LPS molecule, namely lipid A, is also involved in protection of B. bronchiseptica from complement lysis. Data from C3−/− mice indicate that C3 is not required to clear wild-type B. bronchiseptica from the lower respiratory tract (15). The data presented in this work suggest that C3 may not be required for the clearance of B. bronchiseptica because the pagP and wbm genes protect B. bronchiseptica against complement lysis during infection.
These findings also provide intriguing information regarding the role of antibodies in the clearance of B. bronchiseptica from the lower respiratory tract of mice. During the course of infection, wild-type B. bronchiseptica persists in this site several weeks after the detection of anti-B. bronchiseptica antibodies in the serum (11). The data regarding resistance to immune serum complement lysis suggest that the persistence of B. bronchiseptica after the production of antibodies may result from bacterial resistance mechanisms such as pagP-dependent lipid A palmitoylation.
This work strongly suggests that pagP plays a role in the resistance of B. bronchiseptica to antibody-mediated complement lysis on day 14 postinoculation. Interestingly, our data also indicate a second mechanism for the clearance of the ΔpagP mutant on day 28 postinoculation. Analyses of bacterial numbers in C3−/− mice indicate that this factor is not required for the clearance of the ΔpagP mutant from the lungs on day 28 postinoculation (Fig. 4B). This suggests that while complement is required for the reduced numbers of ΔpagP mutant bacteria on day 14 postinoculation, the complete clearance of this mutant by day 28 postinoculation occurs via a complement-independent mechanism. Given the complexity of host-pathogen interactions and the importance of LPS to gram-negative bacterial species, it is not surprising that there are multiple mechanisms responsible for the clearance of the ΔpagP mutant. Investigation of these additional immune mechanisms may lead to a better understanding of the role of pagP and lipid A palmitoylation in B. bronchiseptica respiratory infection.
Acknowledgments
This work was funded by USDA grant 2002-35204-11684 (E.T.H.), NIH grant 5-RO1-A1053075-02 (E.T.H.), and The Wellcome Trust programme grant 054588 (A.P. and D.J.M.).
We thank Kelly Elder, Girish Kirimanjeswara, and Paul Mann for critical reading of the manuscript.
Editor: D. L. Burns
REFERENCES
- 1.Backhed, F., S. Normark, E. K. Schweda, S. Oscarson, and A. Richter-Dahlfors. 2003. Structural requirements for TLR4-mediated LPS signalling: a biological role for LPS modifications. Microbes Infect. 5:1057-1063. [DOI] [PubMed] [Google Scholar]
- 2.Bengoechea, J. A., K. Brandenburg, M. D. Arraiza, U. Seydel, M. Skurnik, and I. Moriyón. 2003. Pathogenic Yersinia enterocolitica strains increase the outer membrane permeability in response to environmental stimuli by modulating lipopolysaccharide fluidity and lipid A structure. Infect. Immun. 71:2014-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns, V. C., E. J. Pishko, A. Preston, D. J. Maskell, and E. T. Harvill. 2003. Role of Bordetella O antigen in respiratory tract infection. Infect. Immun. 71:86-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erridge, C., E. Bennett-Guerrero, and I. R. Poxton. 2002. Structure and function of lipopolysaccharides. Microbes Infect. 4:837-851. [DOI] [PubMed] [Google Scholar]
- 6.Guo, L., K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett, and S. I. Miller. 1998. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell 95:189-198. [DOI] [PubMed] [Google Scholar]
- 7.Hajjar, A. M., R. K. Ernst, J. H. Tsai, C. B. Wilson, and S. I. Miller. 2002. Human Toll-like receptor 4 recognizes host-specific LPS modifications. Nat. Immunol. 3:354-359. [DOI] [PubMed] [Google Scholar]
- 8.Harvill, E. T., P. A. Cotter, and J. F. Miller. 1999. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect. Immun. 67:6109-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvill, E. T., A. Preston, P. A. Cotter, A. G. Allen, D. J. Maskell, and J. F. Miller. 2000. Multiple roles for Bordetella lipopolysaccharide molecules during respiratory tract infection. Infect. Immun. 68:6720-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heininger, U., K. Stehr, S. Schmitt-Grohe, C. Lorenz, R. Rost, P. D. Christenson, M. Uberall, and J. D. Cherry. 1994. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr. Infect. Dis. J. 13:306-309. [DOI] [PubMed] [Google Scholar]
- 11.Kirimanjeswara, G. S., P. B. Mann, and E. T. Harvill. 2003. Role of antibodies in immunity to Bordetella infections. Infect. Immun. 71:1719-1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattoo, S., A. K. Foreman-Wykert, P. A. Cotter, and J. F. Miller. 2001. Mechanisms of Bordetella pathogenesis. Front. Biosci. 6:E168-E186. [DOI] [PubMed] [Google Scholar]
- 13.Mey, A., D. Ponard, M. Colomb, G. Normier, H. Binz, and J. P. Revillard. 1994. Acylation of the lipid A region of a Klebsiella pneumoniae LPS controls the alternative pathway activation of human complement. Mol. Immunol. 31:1239-1246. [DOI] [PubMed] [Google Scholar]
- 14.Musser, J. M., E. L. Hewlett, M. S. Peppler, and R. K. Selander. 1986. Genetic diversity and relationships in populations of Bordetella spp. J. Bacteriol. 166:230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pishko, E. J., G. S. Kirimanjeswara, M. R. Pilione, L. Gopinathan, M. J. Kennett, and E. T. Harvill. 2004. Antibody-mediated bacterial clearance from the lower respiratory tract of mice requires complement component C3. Eur. J. Immunol. 34:184-193. [DOI] [PubMed] [Google Scholar]
- 16.Preston, A., and D. J. Maskell. 2002. Molecular genetics and role in infection of environmentally regulated lipopolysaccharide expression. Int. J. Med. Microbiol. 292:7-15. [DOI] [PubMed] [Google Scholar]
- 17.Preston, A., E. Maxim, E. Toland, E. J. Pishko, E. T. Harvill, M. Caroff, and D. J. Maskell. 2003. Bordetella bronchiseptica PagP is a Bvg-regulated lipid A palmitoyl transferase that is required for persistent colonization of the mouse respiratory tract. Mol. Microbiol. 48:725-736. [DOI] [PubMed] [Google Scholar]
- 18.Robey, M., W. O'Connell, and N. P. Cianciotto. 2001. Identification of Legionella pneumophila rcp, a pagP-like gene that confers resistance to cationic antimicrobial peptides and promotes intracellular infection. Infect. Immun. 69:4276-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandberg, A. L., and A. G. Osler. 1971. Dual pathways of complement interaction with guinea pig immunoglobulins. J. Immunol. 107:1268-1273. [PubMed] [Google Scholar]
- 20.Starner, T. D., W. E. Swords, M. A. Apicella, and P. B. McCray, Jr. 2002. Susceptibility of nontypeable Haemophilus influenzae to human β-defensins is influenced by lipooligosaccharide acylation. Infect. Immun. 70:5287-5289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiese, A., P. Grunewald, K. J. Schaper, and U. Seydel. 2001. Influence of acyl chain fluidity on the lipopolysaccharide-induced activation of complement. J. Endotoxin Res. 7:147-155. [PubMed] [Google Scholar]