Abstract
The development of a vaccine against Streptococcus pneumoniae has been complicated by the existence of at least 90 antigenically distinct capsular serotypes. Common protein-based vaccines could represent the best strategy to prevent pneumococcal infections, regardless of serotype. In the present study, the immunoscreening of an S. pneumoniae genomic library allowed the identification of a novel immune protein target, BVH-3. We demonstrate that immunization of mice with BVH-3 elicits protective immunity against experimental sepsis and pneumonia. Sequence analysis revealed that the bvh-3 gene is highly conserved within the species. Since the BVH-3 protein shows homology at its amino-terminal end with other pneumococcal proteins, it was of interest to determine if protection was due to the homologous or to the protein-specific regions. Immunoprotection studies using recombinant BVH-3 and BVH-3-related protein fragments as antigens allowed the localization of surface-exposed and protective epitopes at the protein-specific carboxyl termini, thus establishing that BVH-3 is distinct from other previously reported protective protein antigens. Immunization with a chimeric protein comprising the carboxyl-terminal regions of BVH-3 and of a BVH-3-related protein improved the protection by targeting two surface pneumococcal components. Thus, BVH-3 and the chimeric protein hold strong promise as vaccine components to control pneumococcal disease.
Streptococcus pneumoniae, a gram-positive bacterium that expresses a polysaccharide capsule, is a leading cause of bacterial meningitis, sepsis, pneumonia, and otitis media in infants and young children (2, 12). Pneumococcal disease is also an important cause of morbidity and mortality in the elderly and immunocompromised or immunodeficient individuals (2, 11). Pneumococcal sepsis is associated with high mortality rates, even with the appropriate antibiotic treatment.
The bacterium is classified into 90 serotypes on the basis of the chemical compositions of the capsular polysaccharides, of which ∼20 are associated with nearly 90% of disease incidence worldwide (2). The capsule provides resistance to opsonophagocytic killing and is the best-defined virulence factor of S. pneumoniae (2). Capsular polysaccharides form the basis of the present vaccines, and the conventional vaccine for pneumococci, which replaced an earlier 14-valent vaccine, consists of a mixture of 23 different capsular polysaccharides (16). Although antibodies (Abs) to the capsular polysaccharides are highly protective, this class of antigens is poorly immunogenic in young children and induces poor memory responses at any age (28). The conjugation of a polysaccharide to a carrier protein can overcome the limitations associated with its T-cell-independent characteristics. A heptavalent conjugate vaccine was shown to be immunogenic and highly effective in preventing invasive disease in children (3). Nevertheless, important drawbacks of pneumococcal polysaccharide conjugate vaccines include the limited number of serotypes that can be included in the vaccine formulations, the serotype specificity of protection, and the technical complexity and high cost of vaccine production. Moreover, it was noted that the use of conjugate vaccines could favor, in a very short time span, an increase in nasopharyngeal carriage and ear infections by nonvaccine serotypes and was associated with true serotype replacement (6, 7, 15, 18, 19, 22). These observations have raised many questions as to how effective polysaccharide-protein conjugate vaccines could be in the long term.
Pneumococcal pneumonia is a serious problem among the elderly in industrialized countries and is associated with high death rates (8). The distribution patterns of disease-causing serotypes in pediatric and adult populations are different, and several serotypes not included in the heptavalent vaccine, or in the 9- or 11-valent conjugate vaccine formulations under investigation, cause a substantial proportion of disease in older children and adults (2, 11, 12). Furthermore, adults did not demonstrate booster responses to the pneumococcal conjugate vaccines (25, 27). Therefore, there is doubt that conjugate vaccines could reduce pneumococcal infections among adults.
An alternative vaccine approach is based on the identification of group-common, surface-exposed protective proteins. In previous reports, results have been presented demonstrating that highly conserved NspA (17) and Sip (5) proteins in encapsulated Neisseria meningitidis and group B streptococci, respectively, elicited protective immunity against lethal challenges with virulent bacteria. Several studies performed with animals demonstrated the ability of protein-based vaccines to protect against experimental pneumococcal disease (24). Protein vaccine candidates presently under investigation include the cell surface PspA, CbpA/PspC, and PsaA proteins and the cytoplasmic pneumolysin protein.
Previous reports (1, 31), including some from our laboratory (J. Hamel, N. Charland, I. Pineau, D. Martin, and B. R. Brodeur, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. E-65, 2001; J. Hamel, N. Charland, D. Martin, and B. R. Brodeur, Abstr. 3rd Int. Symp. Pneumo. Dis. 2002, abstr. 52B, 2002; J. Hamel, N. Charland, D. Martin, and B. R. Brodeur, Abstr. 102nd Gen. Meet. Am. Soc. Microbiol., abstr. E-39, 2002), described a family of similar but distinct proteins capable of conferring protection against lethal experimental S. pneumoniae infection. While we have named these proteins BVH-3 and BVH-11 (BVH-11, BVH-11-2, and BVH-11-3), this protein family was also designated Pht (for pneumococcal histidine triad; PhtA, -B, -D, and -E) (1) and Php (for S. pneumoniae histidine protein; PhpA, PhpB, and PhpC) (31) on the basis of a conserved histidine triad motif (HXXHXH) repeated several times in each of the identified proteins. The sequences of the bvh-11 genes from a given strain are closely related (>60% identity), while BVH-3 shows only ∼32% identity with the BVH-11 homologs (1; Hamel et al., Abstr. 3rd Int. Symp. Pneumo. Dis.). Immunization of mice with members of the BVH-11 family, i.e., PhtA (BVH-11-3), PhtB/PhpA (BVH-11), or PhtD (BVH-11-2), generated protection against S. pneumoniae (1, 31), while a truncated version of BVH-3 (PhtE) was shown to be ineffective (1).
Our findings provide the first evidence that BVH-3 elicits protective humoral immunity. Moreover, we demonstrate that the protective epitopes are located in the carboxyl-terminal region of BVH-3 and are exclusive to BVH-3. Furthermore, Abs specific to BVH-3 or BVH-11 aim at two distinct protective immune targets located on the surface of encapsulated pneumococci. These proteins are shown to be highly conserved throughout the species. Finally, a chimeric protein, comprising the carboxyl-terminal halves of both protein fused in frame, is shown to be capable of generating Abs that elicit superior protection.
MATERIALS AND METHODS
Bacterial strains and expression vectors.
The seven S. pneumoniae strains used in this study for amplification of bvh-3 and bvh-11 gene sequences were three capsular type 3 strains (WU2, A66, and P4241), the type 4 strain TIGR4, the type 6B strain SP64, the type 9V strain SP63, and the type 2-derived unencapsulated strain RX-1. The pneumococcal strains were obtained from M. G. Bergeron (Centre de Recherche en Infectiologie, Laval University, Sainte-Foy, Québec, Canada), D. E. Briles (University of Alabama at Birmingham, Birmingham), A. Camilli (Tufts University School of Medicine, Boston, Mass.), and the Laboratoire de Santé Publique du Québec (Sainte-Anne-de-Bellevue, Québec, Canada). S. pneumoniae was routinely grown overnight at 37°C with 8% CO2 on tryptic soy agar containing 5% (vol/vol) sheep blood or on chocolate agar plates. For challenge studies, frozen stocks from mouse-passaged WU2 and P4241 bacteria were grown in Todd-Hewitt broth enriched with 0.5% (wt/vol) yeast extract for 1 to 4 h at 37°C with 8% CO2 before the mice were infected. Another series of pneumococcal strains representing 14 different capsular serotypes or serogroups (serotypes or serogroups 1, 4, 5, 6A, 6B, 7, 9V, 11, 14, 15, 17, 18C, 19F, and 23F) were tested for the presence of BVH-3 and BVH-11 proteins by using Western blots.
Escherichia coli strain XL1 Blue MRF′ (Stratagene, La Jolla, Calif.) was infected with recombinant phages for the construction of the DNA library. Strain DH5α was used to amplify and stabilize the recombinant plasmid clones, and E. coli AD494 (DE3) and BL21 strains (Novagen, Madison, Wis.) were transformed with the recombinant pET (pET21 or pET32; Novagen) and pURV22.His (5) plasmids, respectively, for the expression of BVH-3 and BVH-11 recombinant proteins. Plasmid pURV22.His contains a kanamycin resistance cassette obtained from the plasmid vector pUC4K (Amersham BioSciences, Baie d'Urfé, Québec, Canada) and a λ PL promoter controlled by a thermolabile lambda repressor (9). E. coli was cultured in Luria-Bertani medium supplemented, when appropriate, with antibiotics.
Construction of the DNA library, molecular cloning of bvh-3 and bvh-11 genes, and protein expression.
A λZapII genomic library from S. pneumoniae strain SP64 (Stratagene) was constructed according to the manufacturer's recommendations. Genomic DNA from strain SP64 was isolated as previously described (14) with some modifications. The DNA was partially digested with 2.5 U of Tsp509I enzyme for 60 min at 65°C (New England Biolabs, Toronto, Canada). λZapII DNA was digested with EcoRI and dephosphorylated with calf alkaline phosphatase before the partially digested genomic DNA was ligated. The resulting recombinant phages were used to infect E. coli strain XL1 Blue MRF′, which was then plated onto Luria-Bertani agar plates. The plates were incubated for 4 h at 37°C for plaque formation. Nitrocellulose filters were gently applied to the surfaces of the plates for 3 h at 37°C to absorb the proteins produced by the recombinant viral clones. The filters were washed with phosphate-buffered saline (PBS) containing 0.02% (vol/vol) Tween and immunoblotted with human palatine tonsil exudates prepared from tissue obtained after routine tonsillectomies. The use of human tonsil tissue was approved by the Ethics Committee of the Centre Hospitalier de l'Université Laval. Positive plaques were purified, and the recombinant phagemids [pBluescript SK(−)] were excised using the Rapid Excision kit (Stratagene). Recombinant phagemids 3 and 11 containing 5,048- and 4,595-bp inserts, respectively, were selected for sequencing. The DNA sequences were analyzed for the presence of open reading frames (MacVector software [Oxford Molecular Ltd., Oxford, United Kingdom] and Vector NTI version 7.1 software [Informax Inc., Bethesda, Md.]) and compared to the available TIGR4 genomic sequence (30).
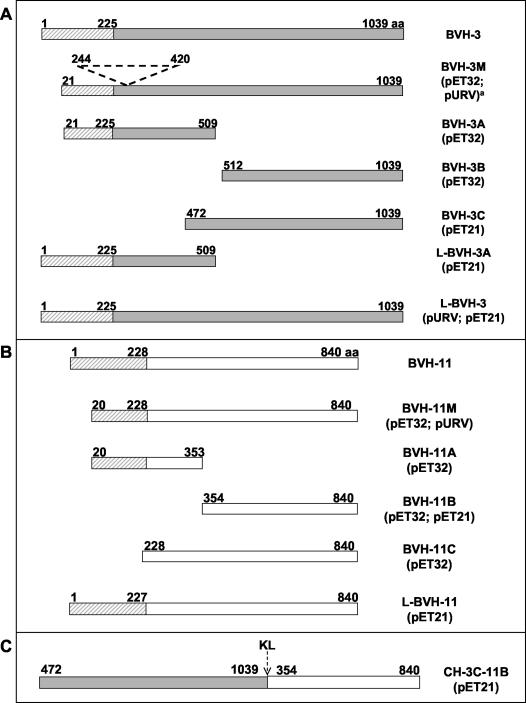
The BVH-3 and BVH-11 full-length or truncated gene products generated in this study are illustrated in Fig. 1. A detailed list of PCR primer sequences used for amplifications will be provided on request. The PCR products were purified from a 1% (wt/vol) agarose gel, digested, repurified with a QIAquick PCR purification kit (Qiagen, Mississauga, Ontario, Canada), and ligated to linearized plasmid expression vectors. In the first cloning experiments, the regions coding for L-BVH-3 (residues 1 to 1039), BVH-3M (residues 21 to 1039), L-BVH-11 (residues 1 to 840), and BVH-11M (residues 20 to 840) were amplified from strain SP64 genomic DNA. The amplified gene fragments bvh-3M and bvh-11M were initially cloned into the pSL301 (Invitrogen, Burlington, Ontario, Canada) or pCMV-GH (29) vector, and digested fragments from the recombinant plasmids were ligated to pET32 to create the coding sequences for N-terminal thioredoxin-histidine-tagged fusion proteins (Fig. 1). The bvh-3 gene region was also amplified from genomic DNA of strain SP63 to generate the BVH-3_SP63 protein. Gene cloning in pET21 and pURV22.His vectors was designed to express recombinant carboxyl- and amino-terminal thioredoxin-histidine-tagged fusion proteins, respectively. Construction of a chimeric gene coding for protein CH-3C-11B was as follows. Gene fragments corresponding to BVH-3C and BVH-11B truncates were amplified by PCR. The primers used had restriction sites allowing directional in-frame cloning of the amplified product into the digested pET21 plasmid vector. The pET21 vector containing bvh-3C was linearized with HindIII (corresponding to the 3′ region of the cloned gene fragment) and NotI restriction enzymes. The vector containing bvh-11B was digested with the same enzymes to excise the gene fragment. The latter was ligated in frame with a bvh-3C gene fragment to form the chimeric gene ch-3C-11B, with a linker composed of nucleotides coding for two residues (KL) added by the cloning site (Fig. 1C).
FIG. 1.
Schematic representation of BVH proteins and their respective truncated regions (A and B) and of the chimeric protein composed of BVH-3C and BVH-11B (C). The numbers indicate the first and last amino acid (aa) residues of each construct. The plasmids used for the cloning of the different gene regions are indicated in parentheses. M, mature proteins; L, potentially lipidated recombinant proteins; hatched rectangles, regions conserved between BVH-3 and BVH-11 proteins; shaded rectangles, BVH-3-specific region; open rectangles, BVH-11-specific regions. The dotted line indicates the amino acid residues missing in strain SP63 (gene cloned in pET32 only). The arrow indicates the two residues (HindIII restriction site) introduced by the cloning of each gene truncate to form the chimeric protein.
Overexpression of BVH-3 and BVH-11 gene products was achieved by incubating recombinant E. coli in the presence of IPTG (isopropyl-1-thio-β-d-galactoside) for pET vectors or heat induction at 40 to 42°C for the pURV22.His vector. The recombinant proteins were purified from the soluble cell fractions by affinity chromatography on a HiTrap metal chelation resin (Amersham BioSciences).
Nucleotide sequence computer analysis.
The nucleotide sequences of bvh-3 and bvh-11 genes were determined for seven S. pneumoniae strains by PCR amplification using the primer sets designed for the generation of the BVH-3 M and BVH-11 M proteins. Sequence homology searches were performed with gene and protein sequences using BLAST, and the sequences were compared by ClustalW alignment (MacVector and Vector NTI software).
Southern blot analysis.
Digoxigenin-labeled DNA probes were prepared by amplifying gene-specific regions of the bvh-3 and bvh-11 genes and labeling them using the PCR DIG Probe Synthesis kit (Roche, Laval, Québec, Canada). Hybridization and chemiluminescence detection conditions followed the manufacturer's instructions.
[3H]palmitic acid labeling of BVH proteins.
The palmitoylation of BVH-3 and BVH-11 was assessed by labeling RX-1 cells suspended in Dulbecco's modified Eagle's medium (Gibco, Burlington, Ontario, Canada) with [3H]palmitic acid (60 μCi/ml; Amersham BioSciences). The bacterial suspension was incubated overnight at 37°C and 8% CO2. Bacteria were harvested by centrifugation, suspended in 500 μl of RIPA buffer (1% [vol/vol] Nonidet P-40, 0.5% [wt/vol] deoxycholic acid, 10% [wt/vol] sodium dodecyl sulfate [SDS], 50 mM Tris-HCl, pH 8.0) and frozen overnight at −20°C. Thawed cells were then centrifuged, and native BVH proteins were immunoprecipitated from the supernatant by the addition of the BVH-3/BVH-11-cross-reactive monoclonal Ab 7G11 (unpublished data) and protein A-Sepharose (Amersham BioSciences) and incubated at 4°C for 1 h, followed by centrifugation. The pellet was then washed twice in washing buffer (0.01 M Tris-Cl, pH 8.0, 0.14 M NaCl, and 0.025% [wt/vol] NaN3) before being resuspended in sample buffer for SDS-polyacrylamide gel electrophoresis (PAGE). The pellet and the precipitation supernatant were separated by SDS-PAGE. The gel was transferred onto a nitrocellulose membrane, and an image was obtained by incubating the membrane in a film cassette with preflashed Hyperfilm-MP (Amersham BioSciences) at −70°C for 2 weeks. The presence of BVH-3 and BVH-11 proteins on the membrane was confirmed by immunoblotting the exposed membrane with specific Abs. A similar protocol was performed for recombinant E. coli overexpressing BVH-3 and BVH-11 proteins.
Immunization of animals and mouse infection.
All animal studies were approved by the Animal Care Committee of Laval University. Groups of BALB/c mice (Charles River Laboratories, St-Constant, Québec, Canada) were immunized subcutaneously two to three times at 3-week intervals with 25 μg of recombinant BVH-3 or BVH-11 antigens in the presence of 10 to 15 μg of Quil A adjuvant (Cedarlane, Hornby, Ontario, Canada). Mock-immunized animals received the adjuvant alone. Fourteen days following the last immunization, the mice were challenged with 106 CFU of strain WU2 intravenously (i.v.) or P4241 intranasally to induce sepsis or pneumonia, respectively. The challenge dose represents ∼103 times the 50% lethal dose and was verified by plating on agar. Deaths were recorded daily over a period of 14 days. Protection, defined as extension of life, was analyzed by the log rank test (Prism version 3.0; GraphPad, San Diego, Calif.) for statistical comparison of survival curves. P values of >0.05 were considered not significant.
New Zealand rabbits (Charles River Laboratories) were immunized as described above with 50 or 100 μg of recombinant antigen in the presence of 80 μg of Quil A. Blood was collected via ear bleeds before each immunization and 6 to 28 days after the third antigen dose was administered. Abs were purified from preimmune and post-dose-3 sera by ammonium sulfate precipitation and protein A chromatography, and the final volume was measured.
CBA/CAHN-xid mice (National Cancer Institute, Fredericton, Md.) were used for passive-protection studies because, in contrast to other mouse strains, sera from CBA/CAHN mice do not contain protective anti-phosphorylcholine Abs (4). These mice are extremely susceptible to pneumococcal infection, and <10 CFU of strain WU2 administered i.v. is sufficient for death. Volumes of 300 μl of purified rabbit Ab preparations (equivalent to 100 μl of crude sera) were passively administered to the mice by the intraperitoneal route 4 h before i.v. challenge with 150 CFU of strain WU2. For inhibition studies, Abs were incubated overnight at 4°C in the presence or absence of soluble protein competitor (the equivalent of 200 μl of sera was treated with 10 μg of proteins or left untreated) before passive-transfer experiments.
Monitoring of S. pneumoniae in blood and lung tissues during experimental pneumonia.
BALB/c mice immunized twice with BVH-3M, BVH-11M, CH-3C-11B, or adjuvant alone as described above were infected intranasally with 106 CFU of the virulent strain P4241. The animals were sacrificed on the second or third day postinfection. Blood was collected by cardiac puncture, and sterile PBS was injected through the right ventricle to empty the blood vessels of their content. The lungs were then collected and homogenized with a Potter homogenizer in 1 ml of PBS. Bacteria were quantified in blood and lung homogenates by plating 10-fold dilutions on agar plates. The results were expressed as CFU/100 μl, and the detection limit for blood and lung samples was 10 CFU/100 μl.
Western blot analysis.
Whole-cell lysates were prepared from S. pneumoniae strain SP64 and 14 other encapsulated pneumococcal strains by boiling cell suspensions containing SDS-PAGE sample buffer (0.2 M Tris-HCl [pH 6.8], 1% [wt/vol] SDS, 2% [vol/vol] mercaptoethanol, 10% [vol/vol] glycerol, 0.001% [wt/vol] bromophenol blue) for 5 min. After electrophoresis in 10% polyacrylamide gels, the proteins were electrotransferred onto nitrocellulose membranes for immunoblotting with rabbit and mouse sera diluted 1:500 and 1:400, respectively. Detection of antigens was performed by an indirect Ab immunoassay using peroxidase-labeled anti-rabbit (BioCan, Mississauga, Ontario, Canada) or anti-mouse (Invitogen) immunoglobulin (Ig) and o-dianisidine color substrate (Sigma-Aldrich, St. Louis, Mo.).
ELISA.
Enzyme-linked immunosorbent assays (ELISAs) were used to measure levels of serum Abs to recombinant proteins and heat-killed S. pneumoniae strain RX-1 cells. Falcon microtiter plates (VWR, Ville Mont-Royal, Québec, Canada) were coated overnight at room temperature with purified recombinant proteins diluted to 5 μg/ml in carbonate buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.6]). Heat-killed RX-1 cells were obtained by incubating bacterial suspensions for 20 min at 56°C. Cell pellets recovered after centrifugation were resuspended in PBS to an optical density at 490 nm (OD490) of 1.0, and 100 μl/well was added to ELISA plates. The plates were then incubated at 37°C overnight until they were dry.
The coated plates were blocked with 0.02% (vol/vol) Tween in PBS, and serum samples serially twofold diluted in 0.5% (wt/vol) skim milk in PBS were added in duplicate. Bound Ab was detected by incubation with alkaline-phosphatase-conjugated Abs to human, mouse, or rabbit Ig (Jackson ImmunoResearch Laboratories), followed by the p-nitrophenyl phosphate substrate (Sigma-Aldrich). Human and mouse Ab concentrations were calculated by interpolation on calibration curves generated for each plate, using wells coated with anti-Ig Abs (Kirkegaard & Perry Laboratories product; Mandel Scientific, Guelph, Ontario, Canada) in duplicate and known concentrations of purified human or mouse Ig (Sigma-Aldrich). The detection limits were 2 and 0.3 μg/ml for mouse and human Abs, respectively. Rabbit Ab titers are reported as the reciprocal serum dilution that gave an OD430-630 value of 0.1 above the background value obtained in wells incubated in the absence of primary Abs.
Ab binding to the surface of pneumococci.
The abilities of serum Abs to bind to the surface of live S. pneumoniae cells were determined using flow cytometry of the Ab-labeled encapsulated type 3 WU2 strain. S. pneumoniae was grown in broth to reach an OD600 of 0.26 (∼108 CFU/ml). Bacterial suspensions were aliquoted in 1-ml samples, and primary serum Abs were added for a final dilution of 1:100. The bacterial suspensions were incubated for 2 h at 4°C and washed twice before the addition of fluorescein isothiocyanate-conjugated goat Abs to mouse or rabbit Ig (Jackson ImmunoResearch Laboratories) for 1 h at room temperature. After two additional washes, the bacteria were fixed with 0.25% (vol/vol) formaldehyde for 18 h at 4°C. The fluorescence intensity of 10,000 cells per sample was analyzed by flow cytometry using FACScan (Beckman Coulter, Mississauga, Ontario, Canada). Inhibition flow cytometry studies were performed by incubating primary sera with competitor recombinant proteins overnight at 4°C as described above for passive-inhibition protection studies.
Human sera.
Sera from nine healthy adult volunteers with no known history of pneumococcal disease were tested by ELISA to define the levels of Abs reactive with BVH-3, BVH-11, and chimeric antigens.
Nucleotide sequence accession numbers.
GenBank accession numbers AX343070, AY278231, AY278232, AY278233, AY278234, AAK75121, and AX343078 correspond to the bvh-3 sequences of strains SP64, RX-1, P4241, WU2, A66, TIGR4, and SP63, respectively. Accession numbers AX343072, AY278235, AY278236, AY278237, AY278238, AAK75283, and AY439016, respectively, correspond to the bvh-11 sequences of the same strains. bvh-11-2 and bvh-11-3 sequences were submitted to the GenBank database (accession numbers AX343074, AY278239, AY278240, AY278241, AY278242, AY278243, and AY428576 correspond to bvh-11-2 of strains SP64, SP63, RX-1, P4241, WU2, and A66 and bvh-11-3 of strain SP64, respectively).
RESULTS
Characterization of the pneumococcal BVH proteins.
A pool of Abs from three people was used to screen a genomic expression library prepared with DNA from a type 6B clinical isolate. Two strongly reactive clones, identified as 3 and 11, were selected for further characterization. Western blot analysis of individual phage lysates revealed that human Abs detected protein bands of ∼130 and 100 kDa (not shown). Nucleotide sequencing of the DNA inserts revealed open reading frames of 3,120 and 2,523 bp coding for polypeptides of 1,039 (BVH-3) and 840 (BVH-11) amino acid residues, respectively. Both proteins contained a predicted signal sequence (LXXC) typical of bacterial lipoproteins, with a cysteine as a putative cleavage and lipid attachment site (13). The presence of a nontypical type I protease signal sequence (AYA^L, putative cleavage site) was also identified in BVH-3 (21). Both native (or recombinant) proteins were not labeled by [3H]palmitate, suggesting that neither is a lipoprotein (not shown). Interestingly, when the BVH-3 and BVH-11 sequences were compared to each other, significant homology was observed at the 5′ ends and amino termini of the genes and proteins, respectively, indicating that the two proteins are related (Fig. 2A). In fact, while the full-length protein sequences are 32% identical and 42% similar, the regions corresponding to the first 225 (BVH-3) and 228 (BVH-11) amino acid residues are 75% identical. On the other hand, the carboxyl-terminal ends (from residue 338 to the end) are 15% identical and 25% similar. Southern blot analysis of chromosomal DNAs from various S. pneumoniae isolates, with DNA probes spanning gene-specific regions of bvh-3 and bvh-11, revealed the presence of one bvh-3 gene copy and two bvh-11 gene copies (not shown). The two bvh-11 genes are very similar but are not identical and were arbitrarily designated bvh-11 and bvh-11-2. The bvh-11-3 gene was identified by screening the public pneumococcal genomic sequence (30) and was named by comparison with other BVH protein sequences. The three genes bvh-11, bvh-11-2, and bvh-11-3 were found in S. pneumoniae strain SP64. All BVH-11 proteins show homology with the BVH-3 protein at their amino termini.
FIG. 2.
Comparison of BVH-3, BVH-11, and BVH-11-2 predicted amino acid sequences from serotype 6B strain SP64. Underneath the alignment, a consensus line indicates identical (*) and conserved (.) amino acid residues, respectively. (A) Boldface residues represent the 177 amino acids missing from strain SP63. Histidine triad motifs found in the BVH-3A truncate are shaded. (B) boldface residues represent the BVH-11 and BVH-11-2 domain regions.
Pneumococcal BVH-3 and BVH-11 proteins are highly conserved throughout the species.
The genes encoding BVH-3 from seven pneumococcal strains representing five different serotypes were sequenced. Pairwise alignments of the deduced BVH-3 protein sequences revealed 99 to 100% identity for all strains (not shown), except for the serotype 9V SP63 isolate, for which a stretch of 177 amino acids at its N terminus was missing (Fig. 1A and 2A). Sequences from additional serogroup 9 strains were analyzed, and four out of the five strains evaluated had the same deletion. These results suggest that the four strains are members of a pneumococcal serogroup 9 clone. In agreement with this, strain ATCC 700671, a representative isolate of the Spain9V-3 clone (20), was also found to have the deletion.
Comparison of predicted BVH-11 and BVH-11-2 protein sequences from seven S. pneumoniae isolates revealed that these sequences were 73% identical and 82% similar. Interestingly, pairwise alignments demonstrated 79 to 100% identity (not shown). Although the sequences showed great similarity in their overall organization, variability was mostly restricted to the last 125 amino acids in the C termini, with this region constituting a domain (Fig. 2B). Two sequence types were observed. When domain sequences of each type were compared, 42% identity was obtained (Fig. 2B). Alternatively, when domain sequences belonging to the same group were compared, identity increased to 92%.
Immunization with pneumococcal BVH-3 and BVH-11 recombinant proteins induces surface-accessible Abs and protects against sepsis and pneumonia.
Mice immunized with the recombinant proteins BVH-3 M and BVH-11 M generated high levels of Ab, as detected by ELISA (Table 1). Western blots revealed that Abs raised to the recombinant proteins recognized S. pneumoniae antigens (Fig. 3, lanes A and E). The control thioredoxin fusion protein did not elicit a pneumococcus-specific immune response, as expected (Table 1 and Fig. 3, lane J). Cytofluorometry studies of live bacterial cells using mouse sera indicated that anti-BVH-3 and anti-BVH-11 Abs effectively labeled the surface of homologous and heterologous pneumococcal strains (Fig. 4A and data not shown). Immunization with the recombinant proteins BVH-3M and BVH-11M elicited complete protection against both sepsis and pneumonia models (P < 0.0001) (Fig. 4B and C). To monitor the presence of bacteria in blood and lung homogenates, some animals were sacrificed 2 or 3 days after intranasal challenge. Pneumococci were not detected in blood samples collected from any of the BVH-3- and BVH-11-vaccinated mice. In contrast, all mock-immunized control mice had positive hemocultures with values ranging from 3 × 105 to 2 × 107 CFU/ml. In addition, two control mice died from the infection. On the other hand, small numbers of pneumococci were detected in the lungs of mice vaccinated with either protein on day 2 postinfection, and no bacteria were detected in the lungs of 9 out of 10 immunized animals on day 3 postinfection (Table 2). In control mice, lung infection progressed, with a larger number of bacteria in the lungs on day 3 than on day 2 (Table 2). These results indicate that vaccination with either the BVH-3 or BVH-11 protein promoted the ability of mice to clear pneumococci from the lower respiratory tract and prevented septicemia and death.
TABLE 1.
ELISA Ab levels in sera from mice or rabbits immunized with protein BVH-3 or BVH-11 or their respective truncated regions or with the chimeric proteina
| Animal | Immunogen | ELISA Ab level with antigen
|
|||||
|---|---|---|---|---|---|---|---|
| BVH-3M | BVH-3C | BVH-11M | BVH-11B | Chimeric CH3-C-11B | RX-1 cells | ||
| Mouse | BVH-3M | 2,128 | 834 | 513 | 7 | 816 | 45 |
| BVH-3-SP63 | 1,853 | 753 | 386 | 5 | 736 | 17 | |
| BVH-3A | 2,867 | 44 | 567 | 11 | 22 | 18 | |
| BVH-3B | 1,527 | 1,197 | 17 | 3 | 1,662 | 27 | |
| BVH-11M | 828 | 37 | 2,491 | 1,705 | 2,993 | 97 | |
| BVH-11B | 10 | 33 | 3,016 | 3,816 | 5,431 | 113 | |
| Thioredoxin | 5 | <2 | 9 | <2 | 2 | 5 | |
| Rabbitb | BVH-3M | >128,000 | >128,000 | 32,000 | <100 | >128,000 | 32,000 |
| L-BVH-3A | >128,000 | 8,000 | >128,000 | 8,000 | 8,000 | 32,000 | |
| BVH-3C | >128,000 | >128,000 | 100 | 4,000 | >128,000 | 64,000 | |
| L-BVH-11 | >128,000 | 8,000 | >128,000 | >128,000 | >128,000 | >128,000 | |
| BVH-11B | 400 | 400 | >128,000 | >128,000 | >128,000 | 32,000 | |
| CH-3C-11B | >128,000 | >128,000 | >128,000 | >128,000 | >128,000 | >128,000 | |
Post-dose-3 sera were tested. Mouse Ab levels are expressed in micrograms per milliliter, while rabbit Ab levels are expressed as titers as defined in Materials and Methods.
All rabbit preimmune sera had anti-protein and anti-RX-1 Ab titer values of <100 and ≤800, respectively.
FIG. 3.
Immunoblot analyses of SP64 whole-cell lysates using mouse or rabbit Abs raised to BVH-3 M (lanes A and L), BVH-3M_SP63 (lane B), BVH-3A (lanes C and M), BVH-3B (lane D), BVH-3C (lane N), BVH-11 M (lanes E and O), BVH-11A (lane F), BVH-11B (lanes G and P), BVH-11C (lane H), CH-3C-11B (lanes I and Q), thioredoxin (lane J), and Quil A adjuvant (lane K).
FIG. 4.
Assessment of Ab responses and protection in mice immunized with recombinant BVH-3M or BVH-11M protein. (A) Flow cytometry analysis showing surface Ab binding to live heterologous strain WU2. Each square on the x axis represents units from 100 to 104, whereas the maximum cell number on each y axis is 64. (B and C) Protection elicited against heterologous challenges with strain WU2 in a sepsis model of infection (B) or with strain P4241 in a pneumonia model of infection (C).
TABLE 2.
Detection of pneumococci in lung homogenates of mice immunized with BVH-3M, BVH-11M, or the chimeric CH-3C-11B recombinant protein
| Immunogen | CFU/100 μl of lung homogenatesa
|
|
|---|---|---|
| Day 2 postinfection | Day 3 postinfection | |
| BVH-3M | 191 (10, 15, 150, 150, 630) | 24 (< 5, < 5, < 5, < 5, 100) |
| BVH-11M | 148 (10, 25, 70, 135, 500) | 4 (< 5, < 5, < 5, < 5, < 5) |
| CH-3C-11B | 56 (<5, <5, <5, <5, 260) | 159 (< 5, < 5, 20, 75, 690) |
| Quil A alone | 4.3 × 105 ([2.8, 3.2, 4.6, 7.0, 8.0] × 105) | 2.4 × 106 ([1.9, 2.2, 3.2] × 106)b |
Arithmetic mean of CFU values. CFU values for individual mice are indicated in parentheses. Values of 4 and 6.6 × 106 CFU/100 μl were assigned to samples without any colonies and to dead animals, respectively, to calculate arithmetic means.
In two mice from the Quil A group, death occurred between day 2 and day 3 postinfection.
Protective epitopes of the BVH-3 and BVH-11 proteins are located in their protein-specific, surface-accessible carboxyl-terminal regions.
Since homology between BVH-3 and BVH-11 proteins was observed, truncated versions of both proteins were produced to determine if the protective epitopes were to be found in the common or the protein-specific regions (Fig. 1). Mice and rabbits were immunized with the different truncated recombinant proteins, and immune responses were characterized by ELISA and Western blotting. Interestingly, immunization with the BVH-3M or BVH-3A construct induced BVH-11-cross-reactive Abs, due to the common region (Table 1 and Fig. 3, lanes C and M), as immunization with BVH-11M or BVH-11A (Table 1 and Fig. 3, lane O and faint BVH-3 bands in lanes E and F) did. On the other hand, the protein-specific truncates BVH-3B, -C, and -11B elicited protein-specific immune responses (Table 1 and Fig. 3, lanes D, G, N, and P). Surface accessibility studies with mouse and rabbit sera (Fig. 5A and B and 6B) indicated that epitopes of Abs raised to protein-specific truncates are more accessible than those reacting with Abs raised to the common region. Antiserum to BVH-3M of strain SP63, lacking an internal 177-amino-acid region, provided Ab surface binding as good as that of antiserum to BVH-3 without any deletion (Fig. 5A). Interestingly, accessibility was greater with Abs raised to the longest BVH-3-specific construct (BVH-3C), indicating that additional surface-accessible epitopes are located between amino acid residues 472 and 512. This is in agreement with results obtained with antisera to BVH3-A (ranging from amino acids 21 to 509) demonstrating some accessibility (Fig. 5A and 6A). In fact, the accessibility with Abs raised to BVH-3C is equivalent to that observed with Abs raised to the BVH-3M protein (Fig. 5A). In contrast, no accessibility was observed with Abs raised to BVH-11A, whereas surface accessibilities were equivalent with antiserum to BVH-11B, BVH-11C, or BVH-11M (Fig. 5A).
FIG. 5.
Assessment of Ab responses and protection in mice immunized with recombinant BVH-3 and BVH-11 antigens and chimeric CH-3C-11B protein or its respective counterparts. (A, B, and C) Flow cytometry analysis showing surface Ab binding to live pneumococci. Each square on the x axis represents units from 100 to 104, whereas the maximum cell number on each y axis is 64. (D, E, and F) Protection elicited against heterologous challenges with strain WU2 in a sepsis model of infection (D) or with strain P4241 in a pneumonia model of infection (E and F).
FIG. 6.
Assessment of rabbit Ab responses and Ab protective ability. (A and B) Flow cytometry analysis showing Ab surface binding to live pneumococci of rabbit Abs raised to recombinant BVH-3 or its truncates (A) or BVH-11 or its truncate, BVH-11B (B). (C) Inhibition flow cytometry using rabbit Abs raised to the chimeric CH-3C-11B protein with or without truncates or chimera competitors. Each square on the x axis represents units from 100 to 104, whereas the maximum cell number on each y axis is 64. (D) Passive protection elicited against heterologous challenges with strain WU2 in a sepsis model of infection. (E and F) Passive “inhibition” protection using recombinant truncates or chimeras as competitors.
Immunized mice were challenged using sepsis or pneumonia models. Protection occurred only when good surface Ab binding to live pneumococci was observed (Fig. 5B). Although the common and divergent regions generated equivalent levels of Abs reactive against native epitopes on RX-1-coated plates (Table 1), no protection was obtained when the common region (BVH-3A or BVH-11A) was used as the immunogen (Fig. 5B and data not shown). These results indicate that protective epitopes are found exclusively in BVH-3- and BVH-11-specific regions.
Passive transfer of protein-specific Abs confers protection.
In order to evaluate the role of Abs in protection, Abs purified from sera from immunized rabbits were transferred to naïve CBA/CAHN mice prior to a lethal challenge. As observed for active immunization, protected mice were those that received Abs reacting with the most surface-accessible pneumococcal epitopes. However, those that received Abs raised to the common region did not survive the challenge (P = 0.32; not significant) (Fig. 6D). To confirm the specificity of the induced responses, the purified Abs were mixed with different truncates before their transfer to mice. Mice that received Abs mixed with the amino-terminal half of BVH-3 (BVH-3A) were still protected (P = 0.0027), as opposed to those injected with Abs mixed with BVH-3C, which all succumbed (P = 0.13; not significant) (Fig. 6E).
Immunization with the chimeric BVH-3- BVH-11 protein targets two antigens at the bacterial surface and induces protection.
Our results suggested that the novel BVH-3 antigen could be combined with other protective BVH family members to aim at several targets on the pneumococcal surface, thus improving protection. To evaluate this possibility, a chimeric protein, CH-3C-11B, composed of the BVH-3- and BVH-11-specific regions BVH-3C and BVH-11B, was created. Abs elicited by CH-3B-11B recognized both BVH-3 and BVH-11 native pneumococcal epitopes (Fig. 3, lanes I and Q). Interestingly, better Ab surface labeling was obtained with Abs raised to the chimeric protein than with signals obtained with antisera to individual components (Fig. 5C). The specificity of the increased accessibility observed with anti-chimera Abs was confirmed by mixing purified rabbit Abs with individual components or the full-length chimeric protein. When individual truncates were used as inhibitors, accessibility was partially reduced, indicating that Abs specific to the other truncate were contributing to the surface labeling (Fig. 6C). When both components or the chimeric protein were mixed with anti-chimera sera, surface accessibility was no longer detected (Fig. 6C).
Immunization with the chimeric protein protected mice from a lethal pneumococcal challenge (P < 0.0001) (Fig. 5F). Passive transfer of anti-chimera Abs also prevented death (P < 0.0001) (Fig. 6D). When BVH-3C or BVH-11B competitors were added, protection corresponding to an individual component's contribution was observed (P < 0.0001) (Fig. 6F). However, the addition of soluble chimeric CH-3C-11B protein or a combination of BVH-3C and BVH-11B abolished the protection. These results imply that the efficacy of a vaccine composed of both protective regions could be superior to that of a one-component formulation.
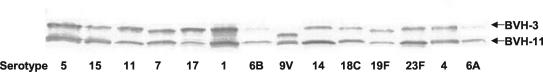
Detection of BVH-3 and BVH-11 across a panel of pneumococcal serotype or group strains.
Western blotting performed with rabbit immune sera raised against the chimeric recombinant protein CH-3C-11B demonstrated the ability of these Abs to cross-react with BVH-3 and BVH-11 in cell lysates from a broad selection of pneumococcal serotypes (Fig. 7). All tested strains showed a BVH-3 band at 130 kDa and a BVH-11 band at 100 kDa, except for the 9V strain, which possesses a BVH-3 protein missing a stretch of 177 amino acid residues and migrating at 117 kDa. When additional serogroup 9 strains were tested, four out of the five strains migrated at 117 kDa. These four strains correspond to those lacking the 177-amino-acid region, as indicated by sequence analysis, while the last strain possesses a full-length BVH-3 protein.
FIG. 7.
Immunoblot analysis of whole-cell lysates of representative strains of different serotypes using rabbit Abs raised to the chimeric CH-3C-11B protein.
Humans naturally produce Abs to BVH-3 and BVH-11 proteins.
Western blots, performed with sera obtained from healthy donors with no known history of pneumococcal disease, indicated that humans naturally respond to both BVH proteins (not shown). Since amino-terminal regions of BVH-3 and BVH-11 proteins show homology and bvh homologs were also found in GAS and GBS genome sequences, a panel of human sera was evaluated with different constructs in ELISA. The results obtained with truncates corresponding to the carboxyl termini of the proteins confirmed the presence of BVH-3- and BVH-11-specific Abs in all human sera tested (Table 3). In agreement with this, BVH-3- and BVH-11-monoreactive Abs were also detected in human sera in ELISA inhibition studies using BVH-3M and BVH-11M as inhibitors (data not shown).
TABLE 3.
Abs reactive with BVH-3, BVH-11, or their truncated regions or with the chimeric protein detected in human sera from healthy individuals
| Human donor | ELISA Ab level (μg/ml) detected against antigen:
|
||||
|---|---|---|---|---|---|
| BVH-3M | BVH-3C | BVH-11M | BVH-11B | Chimeric CH-3C-11B | |
| 1F | 221 | 10 | 226 | 182 | 253 |
| 2F | 39 | 12 | 50 | 28 | 38 |
| 3F | 19 | 2.6 | 24 | 15 | 21 |
| 4F | 82 | 6.2 | 130 | 58 | 99 |
| 5F | 36 | 4.4 | 37 | 15 | 22 |
| 6F | 23 | 5.8 | 31 | 19 | 21 |
| 7H | 80 | 5.1 | 111 | 66 | 73 |
| 8H | 60 | 7.8 | 79 | 54 | 72 |
| 9H | 117 | 12 | 152 | 91 | 100 |
DISCUSSION
In the present report, we demonstrated for the first time that BVH-3 confers protection against lethal infection with S. pneumoniae. As reported by others (1), BVH-3 shares common sequences with other pneumococcal proteins and could thus be considered a member of a protein family. The previous report, as well as those published by Zhang et al. (31) and others (Hamel et al., Abstr. 101st Gen. Meet. Am. Soc. Microbiol.; Hamel et al., Abstr. 3rd Int. Symp. Pneumo. Dis.), also demonstrated that other members of the family possess protective epitopes. However, no publication provided information on the exact locations of the protective epitopes or that S. pneumoniae actually simultaneously expresses BVH-3 and BVH-3-related proteins containing distinct protective epitopes. Our results revealed that the homologous amino region of BVH-3 elicits cross-reactive Abs that do not have protective activity despite relatively high Ab concentrations in serum samples from animals. We demonstrated that BVH-3 and BVH-11 (a BVH-3-related protein) protective epitopes are distinct and that the proteins constitute two distinct immune targets for protective Abs.
Ideally, protective-vaccine candidates should show no or minimal molecular and antigenic variation. Altogether, our sequence analyses revealed that the bvh-3 and bvh-11 genes encode proteins that are highly conserved among S. pneumoniae strains. Moreover, Western blot analysis revealed the presence of the proteins in all tested strains (Fig. 7). This contrasts with results obtained with PhtA, another BVH-3-related protein, which was not detected in some strains (1). The high degree of identity of protein-specific sequences containing the surface-exposed protective epitopes throughout representative strains of the species suggests that these molecules could elicit group-protective coverage. Indeed, immunization of mice with BVH-3 or BVH-11 conferred protection against experimental sepsis and pneumonia resulting from challenges with heterologous pneumococcal strains. Data from passive-immunization studies were consistent with those obtained from active-immunization experiments. Moreover, it was observed that protection conferred by rabbit anti-BVH-3 Abs was completely abolished by the addition of soluble BVH-3B (the carboxyl-terminal end) but not BVH-3A (the amino-terminal end), thus establishing the specificity of the protective Abs for epitopes located in the carboxyl half of BVH-3.
Since Ab-mediated opsonophagocytosis may be the major mechanism of protection against pneumococcal infection, the Abs detected by flow cytometry studies of intact cells would likely be the most biologically relevant. Indeed, our data demonstrated that there is good correlation between protection and the accessibility of epitopes. We observed that the C-terminal two-thirds of BVH-3 and half of BVH-11 were protective and surface exposed, while the first 200 amino acids residues were internal and nonprotective. Moreover, passive-protection assays by transfer of immune Abs clearly established that surface-labeling Abs are biologically linked to survival. Our results indicated that transfer of Abs alone could prevent experimental disease. This observation confirmed the role of anti-BVH-3 and anti-BVH-11 immunoglobulins as the major mechanism of protection. In addition, we demonstrated that a systemic immune response to these proteins could induce protection at a mucosal site, such as the lungs.
Since immune responses to BVH-3 and BVH-11 were both protective, it was reasoned that immunization with mixtures of these antigens would provide better protection against S. pneumoniae than the individual counterparts. It was demonstrated that, even though nonprotective, the common, inaccessible region is highly immunogenic. By combining in a vaccine molecule only the surface-accessible regions, the immune response is directed mainly to epitopes necessary for protection. Indeed, vaccination of animals with a chimeric gene product, corresponding to the surface-exposed protective regions of BVH-3 (i.e., BVH-3C) and BVH-11 (i.e., BVH-11B) fused in frame, conferred protection against lethal experimental infection and generated better immune responses. The superiority of the chimeric protein molecule over its BVH-3C and BVH-11B counterparts comes from the capacity of the chimera to induce Abs that recognize surface protective epitopes on two targets on pneumococci. This was demonstrated by several immunoassays including ELISA, Western blotting, and increased surface binding measured by flow cytometry. In addition, the use of competitor antigens to block the paratopes of the Abs clearly demonstrated that both the BVH-3C and BVH-11B vaccine regions are the targets of protective Abs and are thus the effectors of active protection. The chimeric approach has the advantage of focusing the immune response toward functional epitopes and limiting the total amount of protein contained in the vaccine. This could also facilitate the development of a vaccine, since only one recombinant protein needs to be produced and characterized.
The identification of BVH-3 and BVH-11 using human Abs, associated with the observation that human sera were reactive with both molecules, indicates their innate immunogenicity in the natural host. In agreement with these results, Adamou et al. (1) reported the development of Abs to PhtA and PhtD, two proteins distinct from but related to BVH-3 and BVH-11, during pneumococcal infection in humans. However, the fact that Abs to pneumococcal BVH-3 and BVH-11 recombinant proteins could recognize common epitopes, and that these epitopes are also detected in GBS (data not shown) and possibly in GAS, raises questions about antigenic stimulus associated with Ab production in humans. Interestingly, the Ab reactivity to full-length BVH-3 and BVH-11 is stronger than that observed to BVH-3C and BVH-11B truncates. Thus, it is possible that the common sequence shared by a variety of molecules produced by pneumococci, and possibly by other streptococci, is more abundantly and/or more frequently expressed and stimulates strong Ab responses. Our studies with truncates corresponding to BVH-3- or BVH-11-specific regions provide evidence for in vivo expression of both molecules in S. pneumoniae. Rapola et al. (26) reported that contact with pneumococci induces Abs to several pneumococcal proteins, including PsaA, pneumolysin, and PspA. Our results show that humans also naturally develop Abs to BVH-3 and BVH-11 protein epitopes. Further studies are needed to investigate the biological significance of these Abs.
Homology searches with protein fragment sequences corresponding to the carboxyl termini of the proteins (BVH-3C and BVH-11B) established the uniqueness of these sequences. Our flow cytometry data revealed that these regions comprise surface-exposed epitopes, while the common amino termini of BVH-3 and BVH-11 are not accessible to Abs. Genomic analyses indicating hydrophobic leader sequences predicted that these proteins reside on the bacterial-cell surface (1). We hypothesize that the proteins bind to the cytoplasmic membrane via the hydrophobic portion of the leader peptide sequence and not by a lipidation process of the amino end and that the homologous first 200 amino acid residues cross the peptidoglycan layer. This hypothesis was suggested by (i) the absence of [3H]palmitic acid labeling of BVH proteins and (ii) results obtained with the BVH-3 proteins of some serotype 9V strains. Although their BVH-3 protein is missing a stretch of 177 amino acids (residues 244 to 420), Abs raised to this truncated version were reactive with surface-accessible epitopes on strains of serotypes different from 9V. These results indicate that the deleted protein section is not involved in cell surface anchoring. In addition, accessibility results similar to those obtained with Abs raised to full-length BVH-3 show that the missing region does not contain accessible epitopes. In agreement with our findings, Adamou et al. reported that BVH-3 and BVH-3-related proteins were not detected in the Triton X-114 fraction phase from which lipoproteins are typically recovered (1).
The biological function of the BVH-3 protein is not known. The sequence homology among BVH-3 and BVH-3-related proteins suggests that the proteins might have similar functions through their homologous region and, at the same time, play distinct roles. A similar situation occurs with several pneumococcal proteins sharing a common repeated sequence, the choline-binding domain, which allows the proteins to bind to the phosphorylcholine moiety of the cell wall (10). Although these proteins are members of the choline-binding protein family, each molecule has a distinct role in the bacterial cell, such as autolysin (LytA), involved in cell division, or PspA, implicated in complement inactivation (10). It was speculated that the histidine triad motif, HXXHXH, of the BVH proteins may be involved in metal or nucleoside binding and that the binding of zinc ions by the histidine triad motifs would confer the functional conformation on the proteins (1, 23). It is noteworthy that protein functional analysis suggested that in situations where the bacteria face a zinc-restricted environment, the expression of the BVH protein would be induced and thus result in Streptococcus adhesion and invasion (23). Our studies highlighted the fact that histidine triad motifs present in BVH-3 and BVH-11 are not related to protection. Indeed, the truncated proteins BVH-3A and BVH-11A, possessing five and three of these triads, respectively, do not elicit surface-reactive Abs and are nonprotective antigens.
While protection is relative to a pathogen, it is very likely that a combination of preexisting protective Abs and a rapid secondary immune response will be sufficient to protect against S. pneumoniae. Surface proteins, like capsular antigens, could play an important role in protection through the attachment of Abs to the epitopes exposed on the pneumococcal cell surface and subsequent activation of the complement cascade. The protective immunity conferred by conserved protein antigens will have the advantages of being independent of capsular type, being T cell dependent, and eliciting an immunological memory. Continued studies of these proteins will provide new insights into the pathogenesis of these infections and protective immunity. Phase I clinical trials are being conducted to evaluate the safety and immunogenicity of BVH-3 and chimeric recombinant antigen vaccines in humans.
Acknowledgments
We thank Marie-Josée Fournier, Julie Dumont, Isabelle Lussier, Martine Harvey, Steve Labbé, and all our animal care facility technicians for their valuable assistance.
This research was financially supported by Shire Biologics Inc.
Editor: J. N. Weiser
REFERENCES
- 1.Adamou, J. E., J. H. Heinrichs, A. L. Erwin, W. Walsh, T. Gayle, M. Dormitzer, R. Dagan, Y. A. Brewah, P. Barren, R. Lathigra, S. Langermann, S. Koenig, and S. Johnson. 2001. Identification and characterization of a novel family of pneumococcal proteins that are protective against sepsis. Infect. Immun. 69:949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso De Velasco, E., A. F. Verheul, J. Verhoef, and H. Snippe. 1995. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol. Rev. 59:591-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 4.Briles, D. E., M. Nahm, K. Schroer, J. Davie, P. Baker, J. Kearney, and R. Barletta. 1981. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J. Exp. Med. 153:694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodeur, B. R., M. Boyer, I. Charlebois, J. Hamel, F. C. Couture, C. Rioux, and D. Martin. 2000. Identification of group B streptococcal Sip protein, which elicits cross-protective immunity. Infect. Immun. 68:5610-5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagan, R., N. Givon-Lavi, O. Zamir, M. Sikuler-Cohen, L. Guy, J. Janco, P. Yagupsky, and D. Fraser. 2002. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J. Infect. Dis. 185:927-936. [DOI] [PubMed] [Google Scholar]
- 7.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Käyhty, P. Karma, R. Kohberger, G. Siber, and P. H. Mäkelä. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 8.Feikin, D. R., A. Schuchat, M. Kolczak, N. L. Barrett, L. H. Harrison, L. Lefkowitz, A. McGeer, M. M. Farley, D. J. Vugia, C. Lexau, K. R. Stefonek, J. E. Patterson, and J. H. Jorgensen. 2000. Mortality from invasive pneumococcal pneumonia in the era of antibiotic resistance, 1995-1997. Am. J. Public Health 90:223-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.George, H. J., H. J. Watson, D. F. Harbrecht, and W. J. DeLorbe. 1987. A bacteriophage lambda cI857 cassette controls lambda PL expression vectors at physiologic temperature. Bio/Technology 5:600-603. [Google Scholar]
- 10.Gosink, K. K., E. R. Mann, C. Guglielmo, E. I. Tuomanen, and H. R. Masure. 2000. Role of novel choline binding proteins in virulence of Streptococcus pneumoniae. Infect. Immun. 68:5690-5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hausdorff, W. P., J. Bryant, C. Kloek, P. R. Paradiso, and G. Siber. 2000. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin. Infect. Dis. 30:122-140. [DOI] [PubMed] [Google Scholar]
- 12.Hausdorff, W. P., J. Bryant, P. R. Paradiso, and G. R. Siber. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100-121. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, S., and H. C. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 14.Jayarao, B. M., J. J. Dore, Jr., G. A. Baumbach, K. R. Matthews, and S. P. Oliver. 1991. Differentiation of Streptococcus uberis from Streptococcus parauberis by polymerase chain reaction and restriction fragment length polymorphism analysis of 16S ribosomal DNA. J. Clin. Microbiol. 29:2774-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilpi, T., H. Åhman, J. Jokinen, K. S. Lankinen, A. Palmu, H. Savolainen, M. Grönholm, M. Leinonen, T. Hovi, J. Eskola, H. Käyhty, N. Bohidar, J. C. Sadoff, P. H. Mäkelä, et al. 2003. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin. Infect. Dis. 37:1155-1164. [DOI] [PubMed] [Google Scholar]
- 16.Lee, L. H., C.-J. Lee, and C. E. Frasch. 2002. Development and evaluation of pneumococcal conjugate vaccines: clinical trials and control tests. Crit. Rev. Microbiol. 28:27-41. [DOI] [PubMed] [Google Scholar]
- 17.Martin, D., N. Cadieux, J. Hamel, and B. R. Brodeur. 1997. Highly conserved Neisseria meningitidis surface protein confers protection against experimental infection. J. Exp. Med. 185:1173-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mbelle, N., R. E. Huebner, A. D. Wasas, A. Kimura, I. Chang, and K. P. Klugman. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180:1171-1176. [DOI] [PubMed] [Google Scholar]
- 19.McEllistrem, M. C., J. Adams, E. O. Mason, and E. R. Wald. 2003. Epidemiology of acute otitis media caused by Streptococcus pneumoniae before and after licensure of the 7-valent pneumococcal protein conjugate vaccine. J. Infect. Dis. 188:1679-1684. [DOI] [PubMed] [Google Scholar]
- 20.McGee, L., L. McDougal, J. Zhou, B. G. Spratt, F. C. Tenover, R. George, R. Hakenbeck, W. Hryniewicz, J. C. Lefevre, A. Tomasz, and K. P. Klugman. 2001. Nomenclature of major antimicrobial-resistant clones of Streptococcus pneumoniae defined by the pneumococcal molecular epidemiology network. J. Clin. Microbiol. 39:2565-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nielsen, H., J. Engelbrecht, S. Brunak, and G. von Heijne. 1997. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 10:1-6. [DOI] [PubMed] [Google Scholar]
- 22.Obaro, S. K., R. A. Adegbola, W. A. Banya, and B. M. Greenwood. 1996. Carriage of pneumococci after pneumococcal vaccination. Lancet 348:271-272. [DOI] [PubMed] [Google Scholar]
- 23.Panina, E. M., A. A. Mironov, and M. S. Gelfand. 2003. Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc. Natl. Acad. Sci. USA 100:9912-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paton, J. C. 1998. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol. 6:85-87. [DOI] [PubMed] [Google Scholar]
- 25.Powers, D. C., E. L. Anderson, K. Lottenbach, and C. M. Mink. 1996. Reactogenicity and immunogenicity of a protein-conjugated pneumococcal oligosaccharide vaccine in older adults. J. Infect. Dis. 173:1014-1018. [DOI] [PubMed] [Google Scholar]
- 26.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Käyhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 27.Shelly, M. A., H. Jacoby, G. J. Riley, B. T. Graves, M. Pichichero, and J. J. Treanor. 1997. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect. Immun. 65:242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein, K. E. 1992. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J. Infect. Dis. 165:S49-S52. [DOI] [PubMed] [Google Scholar]
- 29.Tang, D. C., M. DeVit, and S. A. Johnston. 1992. Genetic immunization is a simple method for eliciting an immune response. Nature 356:152-154. [DOI] [PubMed] [Google Scholar]
- 30.Tettelin, H., K. E. Nelson, I. T. Paulsen, J. A. Eisen, T. D. Read, S. Peterson, J. Heidelberg, R. T. DeBoy, D. H. Haft, R. J. Dodson, A. S. Durkin, M. Gwinn, J. F. Kolonay, W. C. Nelson, J. D. Peterson, L. A. Umayam, O. White, S. L. Salzberg, M. R. Lewis, D. Radune, E. Holtzapple, H. Khouri, A. M. Wolf, T. R. Utterback, C. L. Hansen, L. A. McDonald, T. V. Feldblyum, S. Angiuoli, T. Dickinson, E. K. Hickey, I. E. Holt, B. J. Loftus, F. Yang, H. O. Smith, J. C. Venter, B. A. Dougherty, D. A. Morrison, S. K. Hollingshead, and C. M. Fraser. 2001. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science 293:498-506. [DOI] [PubMed] [Google Scholar]
- 31.Zhang, Y., A. W. Masi, V. Barniak, K. Mountzouros, M. K. Hostetter, and B. A. Green. 2001. Recombinant PhpA protein, a unique histidine motif-containing protein from Streptococcus pneumoniae, protects mice against intranasal pneumococcal challenge. Infect. Immun. 69:3827-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]