Abstract
Objective
To perform a comprehensive meta-analysis of task-based functional MRI studies of Attention-Deficit/Hyperactivity Disorder (ADHD).
Method
PubMed, Ovid, EMBASE, Web of Science, ERIC, CINHAL, and NeuroSynth were searched for studies published through 06/30/2011. Significant differences in activation of brain regions between individuals with ADHD and comparisons were detected using activation likelihood estimation meta-analysis (p<0.05, corrected). Dysfunctional regions in ADHD were related to seven reference neuronal systems. We performed a set of meta-analyses focused on age groups (children; adults), clinical characteristics (history of stimulant treatment; presence of psychiatric comorbidities), and specific neuropsychological tasks (inhibition; working memory; vigilance/attention).
Results
Fifty-five studies were included (39 in children, 16 in adults). In children, hypoactivation in ADHD vs. comparisons was found mostly in systems involved in executive functions (frontoparietal network) and attention (ventral attentional network). Significant hyperactivation in ADHD vs. comparisons was observed predominantly within the default, ventral attention, and somatomotor networks. In adults, ADHD-related hypoactivation was predominant in the frontoparietal system, while ADHD-related hyperactivation was present in the visual, dorsal attention, and default networks. Significant ADHD-related dysfunction largely reflected task features and was detected even in the absence of comorbid mental disorders or history of stimulant treatment.
Conclusions
A growing literature provides evidence of ADHD-related dysfunction within multiple neuronal systems involved in higher-level cognitive functions but also in sensorimotor processes, including the visual system, and in the default network. This meta-analytic evidence extends early models of ADHD pathophysiology focused on prefrontal-striatal circuits.
Introduction
Attention-Deficit/Hyperactivity Disorder (ADHD) is one of the most common childhood-onset psychiatric conditions, with an estimated worldwide-pooled prevalence exceeding 5% in children (1). Impairing ADHD symptoms persist into adulthood in as many as 65% of cases (2). Despite a voluminous literature (3), ADHD pathophysiology remains incompletely understood. To gain insight into the neural correlates of ADHD, Dickstein et al. (4) conducted a quantitative meta-analysis of 16 functional magnetic resonance imaging (fMRI) studies published up to February 2006. They found evidence suggesting significant neuronal hypoactivation in ADHD vs. comparisons mostly in fronto-striatal and parietal regions. A substantial number of studies included in Dickstein et al. (4) assessed response inhibition, reflecting the influence of a neuropsychological theory positing inhibitory dysfunction as the core deficit in ADHD (5) and potentially contributing to the particular dysfunctional regions identified in ADHD.
The ADHD fMRI literature has grown substantially since, and neuropsychological paradigms beyond response inhibition have been more frequently investigated (6). In addition, the field has shifted to reporting “between-group” (i.e., ADHD vs. comparisons) contrasts instead of relying on qualitative comparisons of “within-group” results, as was common in the early literature. Finally, from a theoretical perspective, ADHD is increasingly thought to reflect altered connectivity within and among several neural networks, rather than abnormalities of discrete, isolated brain regions (7;8).
Accordingly, we present an updated meta-analytic review of the ADHD fMRI literature. We included pertinent task-based fMRI studies reporting “between-group” contrasts regardless of the type of task examined. We conducted a set of meta-analyses focusing on clinically relevant issues that could now be addressed with greater precision than in the previous meta-analysis (4). In particular, the larger number of available studies allowed us to explore possible ADHD-related dysfunctions in relation to specific age groups (children and adolescents; adults), clinical characteristics (history of stimulant treatment; presence of comorbid psychiatric disorders), or neuropsychological paradigms (inhibitory control; working memory; vigilance/attention).
Based on the perspective that ADHD is a disorder reflecting dysfunction of large-scale neuronal systems (7), we interpreted abnormally activated brain regions from our meta-analysis as dysfunctional nodes of large-scale networks described in the current neuroscience literature. As reference, we used a set of functional networks recently derived from a large dataset of resting-state functional imaging (9). As proposed in a recent qualitative review (7), we hypothesized ADHD-related dysfunctions in networks involved not only in higher-level cognitive/behavioral functions, such as the frontoparietal, dorsal attention, and default networks, but also in sensorimotor processes, including somatomotor and visual networks. Consistent with qualitative reviews of fMRI studies in ADHD (10;11), we expected ADHD-related dysfunctions: 1) to differ in adults compared to children; 2) to be present regardless of comorbid psychiatric disorders or history of stimulant treatment; and 3) to differ according to the specific neuropsychological task examined.
Methods
Search strategy
We searched the following databases: PubMed, Ovid (including PsycINFO and Ovid MEDLINE®), EMBASE, Web of Science (SCI-EXPANDED, SSCI, A&HCI), ERIC, CINHAL, and “NeuroSynth” (12). Details of the search strategy are reported in Supplemental Material A1.
Study Eligibility Criteria
Inclusion criteria were: 1) diagnosis of ADHD according to DSM-IV(-TR) or ICD-10; 2) presence of a typically developing comparison group; 3) data reported as 3-D coordinates in stereotactic space; and 4) between-group contrasts.
Studies were excluded if they: 1) used a neuroimaging method other than fMRI; 2) included participants with ADHD symptoms but without a formal diagnosis of ADHD; 3) assessed the effect of medication without reporting fMRI data at baseline or after wash-out; 4) reported only within-group contrasts; 5) conducted a priori region-of-interest analyses (as these violate the assumption that the likelihood of locating activated foci is equal at every voxel under the null hypothesis); 6) reported only deactivations (this occurred in only one study (13), which was thus not comparable to the others); or 7) included adults with ADHD in partial remission, since it has not been established if the neuronal correlates of individuals with ADHD in partial remission are similar to those with the full syndrome.
Data Extraction
Two authors (SC, CC) independently searched the literature, examined the retrieved papers, extracted and crosschecked data. Initial disagreements on 7 of 2287 screened papers were resolved by consensus. The data extracted were: demographic information; ADHD diagnostic criteria and subtype; psychiatric comorbidities; medication status; 3-D coordinates; tasks; and contrasts.
Meta-analytic Technique
We conducted an activation likelihood estimation meta-analysis using GingerALE version 2.1.1 (14). Activation likelihood estimation allows the detection of quantitative interstudy consistencies in activation by generating maps of activation likelihood estimates. In fMRI studies, the precise localization of specific activation coordinates is limited by substantial intersubject anatomical variability. Within studies, this is imperfectly addressed by Gaussian smoothing. Accordingly, activation foci are best considered as localization probability distributions centered at the reported coordinates. Based on this logic, in activation likelihood estimation meta-analysis, foci are first transformed into probability distributions using three-dimensional Gaussian functions with width expressed in millimeters at half the maximum value (referred to as full-width-half-maximum). Second, a whole-brain map is created by assigning each voxel a value equal to the probability that at least one of the activation points will be found within the voxel. This value is referred to as the activation likelihood estimation for each voxel. Third, to differentiate the voxels within the map that represent signal (i.e., nonrandom clustering of foci) from those that represent noise (i.e., random clustering), activation likelihood estimation values are compared to a null hypothesis distribution generated by permutation analysis (14).
For the present meta-analysis, coordinates reported in Talairach space were transformed to Montreal Neurological Institute coordinates using the icbm2tal (Lancaster) transformation (15). Moreover, since nearby coordinates cannot be assigned unequivocally to different regions, when coordinates associated with multiple contrasts from the same task were less than 12mm apart, we excluded all but one (e.g., for a set of 4 coordinates within 12mm of each other, all but the 4th peak were excluded). Statistical significance was determined using a permutation test (5000 permutations) of randomly generated foci, corrected for multiple comparisons. Per Eickhoff et al. (16), full-width-half-maximum was calculated based on the number of participants in each study. Final activation likelihood estimation maps were thresholded at p< 0.05 using false discovery rate with an extent threshold greater than 200-mm3 (GingerALE default) and overlaid onto the Montreal Neurological Institute 152 template. As recommended (17), anatomical labels were assigned after direct examination of anatomy (18).
We performed focused meta-analyses of studies contrasting: 1) children (<18 years) with ADHD and comparisons across all tasks; 2) adults (≥18 years) with ADHD and comparisons across all tasks; 3) all stimulant-naïve participants (regardless of age) with ADHD and comparisons (studies were only included in this sub-analysis if all participants were stimulant-naïve); 4) all comorbidity-free individuals with ADHD and comparisons (regardless of age); all individuals with ADHD and comparisons (children and adults) in: 5) inhibition tasks; 6) working memory tasks; and 7) vigilance/attention tasks. As shown in Table 1, the number of retrieved studies with relevant foci was insufficient to perform separate meta-analyses of studies assessing: 1) paradigms other than inhibition, working memory, or vigilance/attention; 2) individual tasks in children and adults, separately; 3) individual paradigms in participants who were stimulant-naïve or without psychiatric comorbidities; and 4) ADHD > Comparisons for working memory or vigilance/attention tasks. Additionally, for comparability with the previous meta-analysis (4), we also performed a meta-analysis across all pertinent studies reporting results of between-group contrasts, regardless of participant characteristics and specific paradigm tested (“omnibus” meta-analysis).
Table 1.
Characteristics of included studies. Legend for abbreviations, acronyms and symbols used in the table is provided in the footnote.
| Ref.* | ADHD1 | Comparisons2 | ADHD Type | Comorb. | Stimul. | Withd. | Task(s) | Contrast(s) | # Foci | Correction for multiple comparisons** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | M | F | Age M | Age SD | n | M | F | Age M | Age SD | |||||||||
| Banich (1) | 23 | 14 | 9 | 20 | 1.7 | 23 | 13 | 10 | 19 | 0.9 | All: Co | No | N=20: lifetime N=14: current |
24 h | Color word stroop (variant) | 6 contrasts included in the meta-analysis§ | 20 (tot) | Cluster-wise protection; voxel level threshold: p = 0.01 (AFNI AlphaSim) |
| Bayerl (2) | 30 | 16 | 14 | 31 | 0.6 | 30 | 16 | 14 | 31 | 8.4 | All: Co | No | All: naïve | N/A | 2-back working memory | C > A | 1 | Cluster-size thresholding (50 voxels); voxel level: uncorrected p = 0.001; (SPM99) |
| Booth (3) | 12 | 8 | 4 | 9.4 | 1.2 | 12 | 7 | 5 | 9.3 | 12 | N=8: Co N=4: I |
No | All: current | 48 h | Go/No-Go Selective attention | C >A C > A |
17 3 |
Cluster-size thresholding (10 voxels); voxel level: uncorrected p < 0.001 (SPM99) |
| Braet (4) | 20 | 17 | 3 | 14 | 2.1 | 38 | 31 | 7 | 13 | 1.9 | NS | NS | Some (NS): current | 24 h | SART (Go/No-Go) | C > A: Successful inhibition | 5 | NS for second-level analysis (AFNI) |
| Cao (5) | 12 | 12 | 0 | 13 | 1.7 | 13 | 13 | 0 | 13 | 1.2 | N=7: Co N=5: I |
N=5: ODD N= 2: CD |
N=3: current | 2 wks | Cued target detection | C > A: Intrinsic alerting effect C > A: Phasic alerting effect C > A: Alerting effect |
3 4 3 |
Cluster-size thresholding (10 voxels); voxel level: uncorrected p < 0.001 (SPM2) |
| Cerullo (6) | 10 | 7 | 3 | 14 | 2 | 13 | 7 | 6 | 15 | 1.9 | NS | N=2: ODD N=2: Tics |
N=8: current | Day of the scan | CPT-X | C > A A > C |
1 2 |
Cluster-size thresholding (137 voxels); voxel-level: uncorrected p < 0.05; (AFNI) |
| Cubillo (7) | 11 | 11 | 0 | 29 | 1 | 14 | 14 | 0 | 28 | 2 | N=6: Co N=2: HI N=3: I |
N=8§§ | All: naïve | N/A | Tracking Stop | C > A: Successful stop-go trials C > A: Unsuccessful stop-go trials C > A: Switch task |
2 1 2 |
Cluster-mass threshold; voxel-wide significance: p < 0.05; cluster threshold: p<0.01 (XBAM) |
| Cubillo (8) | 11 | 11 | 0 | 29 | 1 | 15 | 15 | 0 | 28 | 3 | N=6: Co N=2: HI N=3: I |
N=8§§ | All: naïve | N/A | Simon task | C > A: Incongruent-Congruent C > A: Oddball-Congruent |
1 1 |
Cluster-mass threshold; voxel-wide significance: p < 0.05; cluster threshold: p < 0.01 (XBAM) |
| Cubillo (9) | 11 | 11 | 0 | 29 | 1 | 15 | 15 | 0 | 28 | 3 | N=6: Co N=2: HI N=3: I |
N=8§§ | All: naïve | N/A | Rewarded CPT | C > A: Effect of attention C > A: Effect of reward |
3 2 |
Cluster-mass threshold; voxel-wide significance: p < 0.05; cluster threshold: 0.01 (XBAM) |
| Dibbets (10) | 16 | 16 | 0 | 29 | 6.4 | 13 | 13 | 0 | 29 | 6.4 | All: Co | No | N=14: current | 24 h | Modified Go/No-Go | A > C: Overall activation | 1 | Cluster-size thresholding (3 voxels); voxel-level: p < 0.05; (SPM2) |
| Dibbets (11) | 15 | 15 | 0 | 29 | 6.2 | 14 | 14 | 0 | 29 | 6.4 | All: Co | No | N=14: current | 24 h | Switch | C > A A > C |
6 6 |
Cluster-level thresholding (1000 iterations; p < 0.05) (Brainvoyager QX) |
| Dillo (12) | 15 | 11 | 4 | 21–42 | N/A | 15 | 11 | 4 | 21–46 | N/A | N=5: Co N=7: I N=3: HI |
No | Some (NS): current | 3 wks | Go/No-Go | A > C | 2 | N/S (SPM2) |
| Durston (13) | 7 | 6 | 1 | 8.5 | 1.6 | 7 | 6 | 1 | 8.7 | 1.5 | N=4: Co N=3: I |
ODD or CD (NS) | All: current | 24 h | Go/No-Go | C > A: No-Go > Go A > C: No-Go > Go |
1 8 |
Cluster-size thresholding (3 voxels); voxel-level: p < 0.05; (NeuroImaging Software) |
| Durston (14) Sample 1 | 10 | 8 | 2 | 12 | 2.6 | 10 | 8 | 2 | 12 | 2.1 | N=5: Co N=2: I N=3: HI |
N=4: ODD | N=5: current | 24 h | Variant Go/No-Go | C > A: Unexpected stimulus-Unexpected time | 1 | Cluster-size thresholding (5 voxels); voxel-level: p < 0.05; (SPM2) |
| Durston (14) Sample 2 | 12 | 12 | 0 | 15 | 2.3 | 12 | 12 | 0 | 15 | 2.1 | N=9: Co N=3: HI |
N=4: ODD | N=9: current | 24 h | Variant Go/No-Go | C > A: Expected stimulus-Unexpected time C > A: Unexpected stimulus-Expected time |
1 2 |
Cluster-size thresholding (5 voxels); voxel-level: p < 0.05; (SPM2) |
| Hale (15) | 10 | 9 | 1 | 35 | 8.1 | 10 | 9 | 1 | 27 | 4.1 | N=3: Co N=7: I |
N=1: GAD+ SoP+SP N=1: SP |
N=4: current | 2 wks | WAIS-Forward/Backward digit span | C > A: Backward A > C: Forward A > C: Backward |
5 7 3 |
Cluster-size thresholding (25 voxels); voxel-level: uncorrected p < 0.001; (SPM2) |
| Karch (16) | 8 | 7 | 1 | 38 | 7.8 | 8 | 7 | 1 | 38 | 6.6 | All: Co | No | All: naïve | N/A | Auditory Go/No-Go (modified) | C > A | 7 | Cluster-size thresholding; uncorrected p < 0.001; threshold: 10 voxels (BrainVoyager) |
| Kobel (17) | 14 | 14 | 0 | 10 | 1.3 | 12 | 12 | 0 | 11 | 1.6 | N=9: Co N=5: I |
N=5: ODD-CD, N=4: GAD | All: current | 24 h | N-back | C > A: averaged 2-and 3-back | 5 | Family-wise error correction (p < 0.05); cluster size: 10 voxels (SPM 5) |
| Kobel (18) | 14 | 14 | 0 | 10 | 1.3 | 12 | 12 | 0 | 11 | 1.6 | N=9: Co N=5: I |
N=5: ODD/CD N=4: GAD |
All: current | No | N-back | C > A: Activation in 3-back task | 1 | Correction applied but type of correction NS (SPM 5) |
| Konrad (19) | 16 | 16 | 0 | 10 | 1.9 | 16 | 16 | 0 | 10 | 1.3 | N=9: Co N=6: I N=1: HI |
N=5: ODD N=3: AD |
All: naïve | N/A | Attention Network Test (modified) | C > A: Alerting C > A: Exec. Control A > C: Alerting A > C: Reorienting A > C: Exec. control |
1 2 1 3 1 |
Cluster-size thresholding (10 voxels); uncorrected p < 0.001; (SPM2) |
| Kooistra (20) | 10 | 10 | 0 | 22 | NS | 10 | 10 | 0 | 22 | NS | NS | N/A | No after age 16 y | N/A | Go/No-Go | C > A: Go A > C: Go |
16 2 |
Corrected cluster significance threshold: p= 0.01 (FSL) |
| Krauel (21) | 12 | 12 | 0 | 15 | 0.7 | 12 | 12 | 0 | 15 | 1.3 | N=6: Co N=5: I N=1: HI |
N=3:ODD N=2: CD |
N=5: current | 24 h | Recognition memory | C > A: Neutral A > C: Emotional A > C: Neutral |
1 4 4 |
Cluster-size thresholding (10 voxels); voxel-level: uncorrected p < 0.001; (SPM99) |
| Mostofsky (22) | 11 | 8 | 3 | 10 | 1.2 | 11 | 8 | 3 | 10 | 1.4 | N=9: Co N=2: I |
No | N=8: current | 24 h | Sequential finger tapping | C > A | 2 | Cluster-size thresholding (84 voxels); voxel-level: uncorrected p < 0.001; (SPM99) |
| Passarotti (23) | 15 | 12 | 3 | 13 | 2.6 | 14 | 7 | 7 | 14 | 2.4 | All: C | No | N=5: current | Over a 3-week period | Emotional valence stroop | C > A: Negative > neutral C > A: Positive > neutral words A > C: Negative > neutral words A > C: Positive > neutral words |
2 2 4 5 |
Contiguity threshold (uncorrected p < 0.01); experiment-wise Type 1: p < 0.02 (corrected) (AFNI AlphaSim) |
| Passarotti (24) | 14 | 9 | 5 | 13 | 2.3 | 19 | 9 | 10 | 14 | 3.1 | All: Co | No | N/S | At least 4 days | Affective 2-back working memory | C > A: Angry > neutral faces A > C: Angry > neutral faces A > C: Happy > neutral faces |
13 1 12 |
Contiguity threshold (uncorrected p < 0.01); experiment-wise Type 1: p < 0.02 (corrected) (AFNI AlphaSim) |
| Passarotti (25) | 11 | 6 | 5 | 13 | 2.7 | 15 | 7 | 8 | 14 | 3.1 | All: Co | No | N=6: current | 3 wks | Response Inhibition | C > A A > C |
7 3 |
Contiguity threshold (uncorrected p < 0.01); experiment-wise Type 1: p < 0.02 (corrected) (AFNI AlphaSim) |
| Prehen-Kristensen (26) | 12 | NS | NS | 13 | 1.8 | 12 | NS | NS | 14 | 2 | N=12: Co; N= 2: I | N=5: ODD | All: current | 48 h | Delayed-match-to-sample paradigm | C > A | 15 | Cluster-size thresholding (5 voxels); voxel-level: p < 0.05; (SPM5) |
| Rubia (27) | 7 | 7 | 0 | 16 | NS | 9 | 9 | 0 | 15 | NS | All: Co | CD (NS) | (NS) | 1 wk | Stop Delay |
C > A C > A |
4 2 |
Permutation; Voxel-wise probability Type I error: 0.05 |
| Rubia (28) | 16 | 16 | 0 | 13 | 2.1 | 21 | 21 | 0 | 14 | 1.6 | All: Co | N=5: CD | All: naïve | N/A | Stop | C > A: Successful > unsuccessful inhibition C > A: Unsuccessful inhibition > baseline go |
2 2 |
Cluster level difference; < 1 false activated cluster at p < 0.05 (voxel comparison); p < 0.01 (cluster comparison) |
| Rubia (29) | 17 | 17 | 0 | 14 | 2 | 18 | 18 | 0 | 13 | 2 | All: Co | No | All: naïve | N/A | Oddball | C > A: Successful oddball > standard C >A: Standard > oddball |
3 2 |
Cluster level difference; < 1 false activated cluster at p < 0.05 (voxel comparison); p < 0.01 (cluster comparison) |
| Rubia (30) | 20 | 20 | 0 | 13 | 1.5 | 20 | 20 | 0 | 14 | 2 | All: Co | No | All: naïve | N/A | Tracking stop | C > A: Successful > failed stop C > A: Failed stop > go C > A: Go > stop |
1 1 1 |
Cluster level difference; < 1 false activated cluster at p < 0.05 (voxel comparison); p < 0.03 (cluster comparison) |
| Rubia (31) | 18 | 18 | 0 | 13 | 1 | 16 | 16 | 0 | 13 | 3 | All: Co | No | All: naïve | N/A | Rewarded CPT | C > A: Effect of attention C > A: Effect of reward A > C: Effect of attention |
2 1 9 |
Cluster level difference; < 1 false activated cluster at p < 0.05 (voxel comparison); p < 0.01 (cluster comparison) |
| Rubia (32) | 20 | 20 | 0 | 13 | 1.4 | 20 | 20 | 0 | 14 | 1.9 | All: Co | No | All: naïve | N/A | Simon | C > A: Incongruent > oddball C > A: Oddball > congruent |
2 2 |
Cluster level difference; < 1 false activated cluster at p < 0.05 for both voxel comparison and cluster comparison |
| Rubia (33) | 13 | 13 | 0 | 13 | 1.4 | 13 | 13 | 0 | 13 | 1.8 | All: Co | N=1: CD | All: naïve | N/A | Rewarded CPT | C > A: Attention C > A: Reward A > C: Reward |
13 2 4 |
Cluster level difference; < 1 false activated cluster at p < 0.05 (voxel comparison); p < 0.02 (cluster comparison) (XBAM) |
| Rubia (34) Study 1 |
10 | 10 | 0 | 14 | 2 | 10 | 10 | 0 | 15 | 4 | All: Co | N=1: CD | N= 4: naïve; N= 6: current | 36 h (N=6) | Delay discounting | C > A | 6 | Cluster level difference; < 1 false activated cluster at p < 0.05 (voxel comparison); p < 0.006 (cluster comparison) |
| Rubia (34) Study 2 |
12 | 12 | 0 | 13 | 1 | 12 | 12 | 0 | 13 | 1 | All: Co | N=1: CD | All: naïve | N/A | Time discrimin. | C > A A > C |
1 2 |
Cluster level difference; < 1 false activated cluster at p < 0.05 for voxel comparison and p < 0.006 for cluster comparison |
| Rubia (35) | 14 | 14 | 0 | 13 | 1.1 | 20 | 20 | 0 | 14 | 1.9 | All: Co | No | All: naïve | N/A | Switch | C > A | 2 | Cluster level difference; < 1 false activated cluster at p < 0.05 for both voxel and cluster comparison (XBAM) |
| Rubia (36) | 18 | 18 | 0 | 14 | 1.1 | 20 | 20 | 0 | 15 | 1.1 | All: Co | No | All: naïve | N/A | Visual tracking stop Meiran Switch |
C > A: Successful-stop C > A: Failed-stop C > A |
1 2 3 |
Cluster level difference; < 1 false activated cluster at p < 0.05 for voxel comparison and p < 0.003 for cluster comparison (XBAM) |
| Rubia (37) | 12 | 12 | 0 | 13 | 1 | 13 | 13 | 0 | 13 | 1 | All: Co | No | All: naïve | N/A | Visual tracking stop | C > A: Successful inhibition C > A: Inhibition failure |
13 13 |
Threshold-Free Cluster Enhancement (p < 0.05) |
| Rubia (38) | 12 | 12 | 0 | 13 | 1 | 13 | 13 | 0 | 13 | 1 | All: Co | No | All: naïve | N/A | Simon | C > A: Simon > oddball condition | 4 | Cluster level difference; < 1 false activated cluster at p < 0.05 for voxel comparison and p < 0.01 for cluster comparison (XBAM) |
| Rubia (39) | 18 | 18 | 0 | 14 | 2 | 20 | 20 | 0 | 16 | 1 | All: Co | No | All: naïve | N/A | Simon | C > A: Oddball > congruent C > A: Incongruent > oddball |
2 2 |
Cluster level difference; < 1 false activated cluster at p < 0.05 for voxel comparison and p < 0.01 for cluster comparison (XBAM) |
| Schulz (40) | 5† | 5 | 0 | 18 | 1.3 | 5 | 5 | 0 | 18 | 1.4 | N=1: Co N=3: I N=1: HI |
N=1: CD | N=4: treated†† | N/A | Go/No-Go | C > A: Correct No-Go > correct GO A > C: Correct No-Go > correct GO |
2 3 |
Cluster-size thresholding (120 voxels); voxel level: uncorrected p < 0.01 (SPM99) |
| Sheridan (41) | 10 | 0 | 10 | 15 | 2 | 10 | 0 | 10 | 15 | 1.3 | N=6: Co N=4: I |
N=2: ODD N=2: SP |
N=5:‡ N=2: current |
24 h | Delay match-to-sample | C > A: Activation at high load period | 2 | Gaussian field theory; p= 0.05 corrected at cluster level (fmristat program) |
| Silk (42) | 12 | 12 | 0 | 11 | 1.5 | 12 | 12 | 0 | 11 | 1.5 | All: Co | No | All: naïve | N/A | Raven’s Standard Progressive Matrices | C > A | 45 | Clusters of voxels (z > 2.33) with cluster level p corr < 0.05; (FSL) |
| Silk (43) | 7 | 7 | 0 | 14 | 1.8 | 7 | 7 | 0 | 15 | 1.8 | All: Co | No | All: naïve | N/A | Mental rotation | C > A A > C |
8 4 |
Voxel level p (uncorr) < 0.001; cluster level p corr < 0.05 |
| Smith (44) | 17 14 |
17 14 |
0 0 |
13 13 |
2 1.8 |
18 27 |
18 27 |
0 0 |
14 14 |
2 1.8 |
All: Co | N=5: CD | All: naïve | N/A | Go/No-Go Switch |
C > A: Successful No-Go stimuli C> A: Switch |
1 9 |
Cluster level difference; < 1 false activated cluster at p < 0.05 (voxel comparison) and p < 0.01 (cluster comparison) |
| Smith (45) | 21 | 21 | 0 | 13 | 1.6 | 17 | 17 | 0 | 14 | 2.1 | All: Co | N=3: CD/ODD | All: naïve | N/A | Time discrimination | C > A: Time discrimination > temporal order judgment | 2 | Cluster level difference; < 1 false activated cluster at p < 0.05 (voxel comparison) and p < 0.01 (cluster comparison) (XBAM) |
| Spinelli (46) | 13 | 9 | 4 | 11 | 1.4 | 17 | 8 | 9 | 11 | 1.2 | N=10: Co; N=3: | N=3: ODD N=1: SP |
N=2: current | 24 h | Go/No-Go | A > C: Pre-Error vs. Pre-Correct Inhibition | 2 | Spatial extent cluster size threshold to achieve a corrected statistical threshold of p= 0.05 (SPM5) |
| Spinelli (47) | 13 | 9 | 4 | 11 | 1.4 | 17 | 8 | 9 | 11 | 1.2 | N=10: Co; N=3: | N=3: ODD N=1: SP |
N=2: current | 24 h | Go/No-Go | A > C: Post-error versus post-correct inhibition trials | 9 | Spatial extent cluster size threshold to achieve a corrected statistical threshold of p= 0.05 (SPM5) |
| Strohle (48) | 10 | 10 | 0 | 32 | 8.1 | 10 | 10 | 0 | 32 | 9.9 | N=4: Co N=4:I N=2: HI |
No | All: naïve | N/A | Monetary Incentive Delay | C > A: Anticipation of gain > non gain A > C: outcome of gain > non gain |
1 7 |
p < 0.05 False Discovery Rate corrected (SPM2) |
| Tamm (49) | 10 | 10 | 0 | 16 | 1.4 | 12 | 12 | 0 | 16 | 0.8 | All: Co | No | N=5 current | 18 h | Modified Go/No-Go | C > A: “A-B “ contrast A > C: “A-B” contrast |
1 1 |
Deactivation mask (Z= 1.67; p < 0.05) (SPM99) |
| Tamm (50) | 14 | 14 | 0 | 14–18 | N/A | 12 | 12 | 0 | 14–18 | N/A | All: Co | No | N=5: current | 18 h | Oddball | C > A: activation during the oddball event | 4 | NS |
| Valera (51) | 20 | 12 | 8 | 34 | 11.8 | 20 | 12 | 8 | 33 | 11 | NS | No | N=10: lifetime | 24 h | Variant of visual N-back test | C > A: 2-back minus X-task contrast | 2 | p = 0.001 (uncorrected) with extent threshold determined by Gaussian random field theory (SPM99) |
| Valera (52) | 21 | 15 | 6 | 34 | 10.1 | 19 | 12 | 7 | 33 | 11 | N=5: Co N=12: I |
No | N=9: current | 24 h | Paced and unpaced finger tapping | C > A: Paced finger tapping C > A: Unpaced finger tapping |
24 17 |
p < 0.05, family-wise error corrected, with extent threshold of 20 contiguous voxels (SPM5) |
| Valera (53) | 44 | 23 | 21 | 37 | 11 | 49 | 23 | 26 | 33 | 10 | N=13 Co N=17: I N=1: HI |
No | N=18: current | 24 h | 2-back | C > A: 2-back visual memory task | 2 | p < 0.05, corrected (Gaussian random field theory) (SPM2) |
| Vance (54) | 12 | 12 | 0 | 11 | 1.5 | 12 | 12 | 0 | 10 | 1.3 | All: Co | No | All: naïve | N/A | Mental rotation | C > A | 4 | Cluster level threshold p corrected < 0.05 (FSL) |
| Wolf (55) | 13 | 13 | 0 | 22 | 4.4 | 12 | 12 | 0 | 22 | 4.7 | N=9: Co N=2: I N=2: HI |
No | All: lifetime | N=6: At least 6 wks; N=6: 3 days |
Cognitive Activation | C > A: Increasing working memory load | 5 | p < 0.05 corrected using False Discovery Rate (SPM5) |
Total number of ADHD participants (After removing complete overlap but including partial overlap of participants across studies from the same research groups): 741;
Total number of comparisons (After removing complete overlap but including partial overlap of participants across studies from the same research groups): 801.
Abbreviations:
M: Males; F: Females; Age M: mean age; Age SD: standard deviation of age; Comorb.: Psychiatric comorbidities; Stimul.: Treatment with psychostimulants; Withd.: Withdrawal from psychostimulants.
Acronyms (in alphabetical order):
A= ADHD; AD: Anxiety Disorders; AFNI: Analysis of Functional NeuroImages; C= Comparisons; CD: Conduct Disorder; Co: ADHD combined type; FSL: FMRIB Software Library; GAD: Generalized Anxiety Disorder; HI: ADHD hyperactive-impulsive type; I: ADHD inattentive type; MD: Mood Disorders; ND: Nicotine Dependence; NS: Not Specified; ODD: Oppositional Defiant Disorder; SoP: Social Phobia; SP: Specific Phobia; SPM: Statistical Parametric Mapping; SUD: Substance Use Disorders; XBAM: X Activation Brain Maps.
Number in parenthesis after first author refers to reference # provided in Supplemental Material A3;
The software used for the analyses is provided in parenthesis when indicated in the paper;
C > A: Blocked activity-congruent > fixation baseline, C > A: Blocked activity-incongruent > fixation baseline, C > A: Blocked activity-neutral > fixation baseline, A > C: Blocked activity-congruent >fixation baseline, A > C: Blocked activity-incongruent > fixation baseline, A > C: Blocked activity-neutral > fixation baseline;
N=1: AD; N=3: MD; N=1: CD; N=2: SUD; N=1: ND;
Only individuals with persistent ADHD (N=5) were included in the analysis; remitters (N=5) were excluded;
stopped 6 months before the study;
stopped 1 year before the study.
To test if results of the focused meta-analyses differed statistically, we performed subtraction analyses using the Contrast Studies procedure in GingerALE 2.1.1. We compared: “Adults with ADHD” vs. “Children with ADHD”; “Stimulant-naïve individuals” vs. “Stimulant-treated individuals”; and “Participants with comorbid mental disorders” vs. “Comorbidity-free participants” in the contrasts “Comparisons > ADHD” and “ADHD > Comparisons”. For “All participants, inhibition tasks” vs. “All participants, working memory tasks,” “All participants, inhibition tasks” vs. “All participants, vigilance/attention tasks,” and “All participants, working memory tasks” vs. “All participants, vigilance/attention tasks” we could only examine the contrast “Comparisons > ADHD.”
ALE Results in Relation to Neuronal Networks
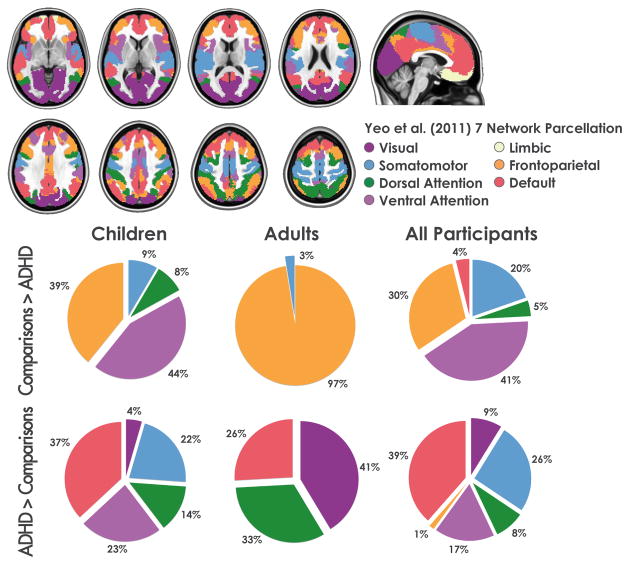
We related the ADHD-hypo and hyperactivated regions from our meta-analysis to the seven reference networks defined by Yeo et al. on the basis of a data-driven analysis of resting state functional imaging data collected from 1000 participants (9). Those seven robustly replicable networks, which are limited to cortical regions, include the frontoparietal, dorsal and ventral attentional, somatomotor, visual, limbic, and default networks. We first determined the network within which each voxel of the ADHD-related hypo-or hyperactivated regions was located, by computing the number of significant voxels from the Comparisons > ADHD and ADHD > Comparisons contrasts that overlapped each of the network masks (downloaded from http://surfer.nmr.mgh.harvard.edu/fswiki/CorticalParcellation_Yeo2011). We then performed a χ2 contrasting the proportions of hypo-and hyperactivated voxels in the seven networks.
Results
Studies Included in the Meta-analysis
Figure 1 shows the search results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (19). Details of included/excluded studies are provided in Supplemental Material A2. The search yielded 55 eligible studies. Sixteen included adults (age ≥18 years) with ADHD; 39 assessed children (<18 years-old). Further characteristics of included studies are summarized in Table 1. While we endeavored to count individuals from the same sample only once, the total number of participants reported in Table 1 (741 with ADHD and 801 comparisons) is approximate, since some research groups reported results with partially overlapping participants. References of the included studies are provided in Supplemental Material A3.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) (19) flowchart reporting the search strategy and retrieved studies.
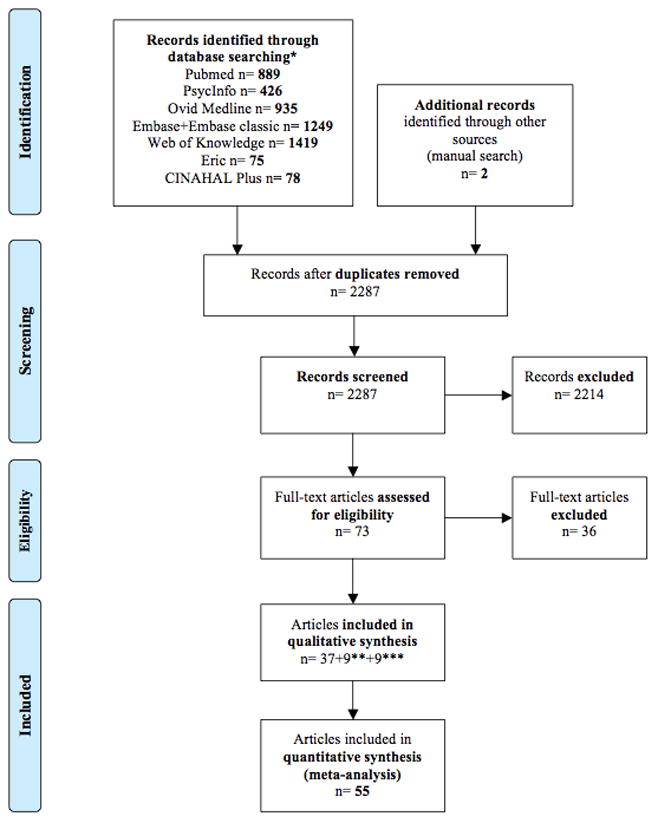
* Up to January 27, 2011 ** From updated search (June 30, 2011) *** From Dickstein et al. (4).
ALE Results
The meta-analysis focused on children (Table 2 and Figure 2) revealed significant ADHD-related hypoactivation in bilateral frontal, right parietal, and right temporal regions, as well as in bilateral putamen. ADHD-related hyperactivation was found in the right angular gyrus/middle occipital gyrus, posterior cingulate cortex, and midcingulate cortex. In the meta-analysis restricted to adults (Table 2 and Figure 2), a different pattern emerged. Adults with ADHD showed significant hypoactivation relative to comparisons in the right central sulcus/precentral gyrus and middle frontal gyrus. ADHD-related hyperactivation was found in a region with a peak in the right angular gyrus/middle occipital gyrus.
Table 2.
Regions exhibiting significantly greater activation in comparisons relative to individuals with ADHD and vice-versa in the meta-analyses focused on children or adults across all tasks. Brodmann areas (BA) are indicated in parenthesis when identifiable. When located unambiguously in a cortical region, the anatomic label is followed in parenthesis, in italics, by the neural network corresponding to the maximum activation likelihood estimation value, from the seven reference neuronal networks identified by Yeo et al. (9).
| Cluster # | Volume (mm^3) | Weighted Center* | Extrema Value | Maximum ALE value* | Anatomical Label | ||||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | x | y | z | ||||
| Comparisons > Participants with ADHD | |||||||||
|
Children, all tasks Number of foci= 241; Number of experiments= 35; Total number of subjects= 958** | |||||||||
| 1 | 1864 | −0.35 | 15.56 | 48.61 | 0.0211 | 0 | 16 | 54 | Medial Superior Frontal Gyrus/Supplementary Motor Area (BA6) R, L *** (Frontoparietal/Ventral Attention) |
| 0.0186 | 0 | 14 | 44 | Paracingulate Gyrus (BA 32) R, L (Ventral Attention) | |||||
| 2 | 688 | 31.42 | 0.19 | 3.33 | 0.0148 | 32 | 0 | 4 | Putamen R |
| 3 | 464 | −21.92 | 2.37 | 4.1 | 0.0119 | −20 | 4 | 4 | Putamen L |
| 4 | 424 | 33.7 | −27.47 | 50.75 | 0.0136 | 34 | −28 | 52 | Postcentral Gyrus R (Somatomotor) |
| 5 | 400 | 42.23 | 9.52 | 29.36 | 0.0134 | 42 | 10 | 30 | Inferior Frontal Sulcus; Inferior Precentral Sulcus R (Frontoparietal) |
| 6 | 384 | 57.29 | −25 | 28.42 | 0.0145 | 58 | −26 | 28 | Inferior Parietal Lobule (Supramarginal Gyrus; BA40) R (Ventral Attention) |
| 7 | 368 | 55.66 | 12.64 | −6.64 | 0.0136 | 56 | 12 | −6 | Superior Temporal Gyrus (BA 22) R (Somatomotor) |
| 8 | 280 | −41.26 | 31.46 | 23.46 | 0.0131 | −42 | 32 | 24 | Middle Frontal Gyrus (BA 9/46) L (Frontoparietal) |
| 9 | 248 | 47.46 | 25.85 | 31.84 | 0.0115 | 48 | 26 | 32 | Middle Frontal Gyrus (BA 9/46) R (Frontoparietal) |
|
Adults, all tasks Number of foci= 81; Number of experiments= 12; Total number of subjects= 414** | |||||||||
| 1 | 400 | 21.7 | −27.31 | 50.84 | 0.0106 | 22 | −28 | 50 | Central Sulcus/Precentral Gyrus R |
| 2 | 232 | 34.38 | 56.96 | 13.62 | 0.0103 | 34 | 56 | 14 | Middle Frontal Gyrus (BA 10) R (Frontoparietal) |
| Participants with ADHD > Comparisons | |||||||||
|
Children, all tasks Number of foci= 80; Number of experiments= 17; Total number of subjects=431** | |||||||||
| 1 | 680 | 39.72 | −58.51 | 18.51 | 0.0135 | 40 | −58 | 20 | Angular Gyrus; Middle Occipital Gyrus R |
| 0.0079 | 40 | −66 | 14 | Angular Gyrus; Middle Occipital Gyrus R (Visual) | |||||
| 2 | 400 | 15.02 | −50.94 | 31.01 | 0.0101 | 16 | −52 | 32 | Posterior Cingulate Cortex; Subparietal Sulcus R |
| 3 | 392 | 31.22 | −7.23 | 16.7 | 0.0107 | 32 | −8 | 16 | White Matter R (sub-operculum) |
| 4 | 352 | 4.67 | −13.72 | 42.61 | 0.0101 | 4 | −14 | 42 | Midcingulate Cortex R (Ventral Attention) |
|
Adults, all tasks Number of foci= 49; Number of experiments= 8; Total number of subjects= 226 ** | |||||||||
| 1 | 872 | 40.39 | −58.08 | 16.91 | 0.0152 | 40 | −58 | 18 | Angular Gyrus; Middle Occipital Gyrus R |
Montreal Neurological Institute coordinates;
After removing complete overlap but including partial overlap of participants across studies from the same research groups;
R: Right; L: Left. ALE: Activation likelihood estimation.
Figure 2. Regions exhibiting significantly greater activation in comparisons relative to individuals with ADHD (upper panel) and in individuals with ADHD relative to comparisons (lower panel). The figure reports results for meta-analyses focused on adults or children and for the omnibus meta-analysis.
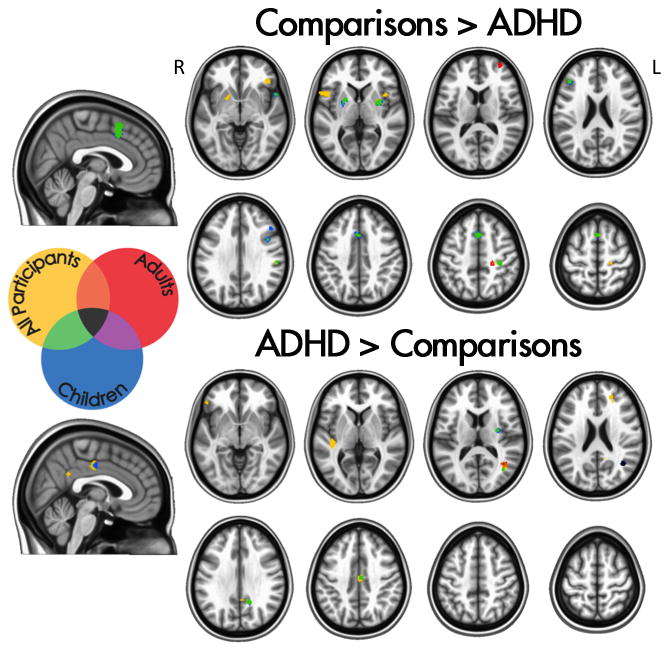
R: Right; L: Left. “All participants” refers to the omnibus meta-analysis.
In stimulant-naïve participants (Supplemental Table S1 and Figure S1) ADHD-related hypoactivation emerged in several bilateral frontal regions, right superior temporal gyrus, right posterior cingulate cortex, right postcentral gyrus, bilateral putamen, and right thalamus. Only one significant cluster of ADHD-related hyperactivation was found, with a peak located within the right superior longitudinal fasciculus underlying the insula.
When considering comorbidity-free participants (Supplemental Table S1 and Figure S1), ADHD-related hypoactivated regions were located in bilateral frontal, bilateral putamen, right superior temporal gyrus, and right occipital pole. ADHD-related hyperactivation was found in left inferior frontal gyrus, left Heschl’s gyrus, and several right posterior regions.
In analyses limited to specific tasks (Supplemental Table S1), ADHD-related hypoactivation in studies examining inhibition paradigms included several frontal regions bilaterally, as well as the right superior temporal gyrus, the left inferior occipital gyrus, the right thalamus, and mid-brain. The ADHD > Comparisons contrast yielded two regions with peaks in right deep parieto-occipital cortex and right intermediate frontal sulcus. The analysis restricted to working memory tasks revealed significant ADHD-related hypoactivation in left inferior frontal gyrus/anterior insula and in right middle frontal gyrus. In vigilance/attention tasks, we found significant ADHD-related hypoactivation only in right paracingulate gyrus.
The omnibus meta-analysis (Supplemental Table S2 and Figure 2) largely recapitulated the results of the meta-analysis focused on children, with additional ADHD-related hypoactivations in the inferior frontal gyri, right central sulcus and right posterior parietal lobe and hyperactivations in Heschl’s gyrus as well as additional loci in inferior frontal gyri.
When we performed planned subtraction analyses (adults vs. children; stimulant-naïve vs. stimulant-treated; comorbid participants vs. comorbidity-free; and comparisons among specific tasks) no significant differences were found that survived whole-brain false discovery rate correction. However, with an exploratory threshold at p<0.05, uncorrected, we observed differences in the following analyses: “Adults with ADHD” vs. “Children with ADHD” for “Comparisons > ADHD, all tasks”; “Stimulant-naïve individuals” vs. “Stimulant-treated individuals” for “Comparisons > ADHD, all tasks” and “ADHD > Comparisons, all tasks”; “Comorbid participants” vs. “Participants without comorbidity” for “Comparisons > ADHD, all tasks” and “ADHD > Comparisons, all tasks” (Supplemental Table S3).
Results in Relation to Neuronal Networks
Figure 3 shows the number of voxels located within each of the seven networks described by Yeo et al. (9), expressed as a percentage of the total number of significant voxels. In children, ADHD-related hypoactivation was predominantly located within the ventral attention (44%) and frontoparietal (39%) networks, whereas hyperactivation was predominant within the default (37%), ventral attention (23%) and somatomotor networks (22%). The overall distribution of hypo-and hyperactivations by network differed significantly (χ2(5) >100, p<0.0001). In adults, voxels hypoactivated in ADHD were almost exclusively located in the frontoparietal network (97%), whereas hyperactivated voxels were found in the visual (41%), dorsal attention (33%), and default (26%) networks. The overall distribution of hypo- and hyperactivations by network differed significantly (χ2(5) >100, p<0.0001). Results for the omnibus meta-analyses largely overlapped those for the meta-analysis focused on children (Figure 3, Supplemental Table S2). Considering comorbidity-free participants (Supplemental Figure S2), voxels hypoactivated in ADHD were predominantly in the ventral attention (30%), frontoparietal (29%) and default (21%) networks, while most of the ADHD-related hyperactivation was in the default (44%) and somatomotor (26%) networks. In medication-naïve participants, voxels hypoactivated in ADHD were mostly in the frontoparietal (27%), default (24%), and ventral attention (18%) networks. Considering the opposite contrast (ADHD > comparisons), few voxels were hyperactivated, all in the somatomotor system, which accounts for the unanimity reported in Supplemental Figure S2.
Figure 3. Regions of ADHD-related hypo- or hyperactivation in relation to Yeo et al. seven seven networks (9) for meta-analyses focused on adults or children and for the omnibus meta-analysis.
R: Right; L: Left. Number of significant voxels in the contrast “Comparisons > ADHD”: children= 3320; adults= 272; omnibus= 6024. Number of significant voxels in the contrast “ADHD > Comparisons”: children= 888; adults= 464; omnibus= 2720.
Given the lack of data for the contrast “ADHD > Comparisons” for most of the specific tasks, we do not report assignment to the canonical networks for the meta-analyses restricted to specific tasks.
Discussion
The ADHD fMRI literature has grown substantially since an initial meta-analysis examined 16 studies (4). Using stringent selection criteria, we included 55 papers in meta-analyses focused on children or adults, as well as on clinical characteristics (previous history of stimulant treatment; presence of comorbid psychiatric disorders) or specific neuropsychological constructs.
Abnormally activated regions in ADHD vs. comparisons can be interpreted within a systems neuroscience perspective, i.e., as dysfunctional nodes within large-scale neuronal networks (7). While the number of definable neural networks can vary substantially, we selected the seven networks identified by Yeo et al. (9) as being heuristically appropriate for the study of ADHD. These include the frontoparietal, dorsal and ventral attention, sensorimotor, visual, limbic, and default networks.
Meta-analysis focused on children
Brain regions hypoactivated in ADHD vs. comparisons were prevalent in the frontoparietal and ventral attention networks (Figure 3). The canonical frontoparietal network (9) includes the lateral frontal pole, dorsal anterior cingulate, dorsolateral anterior prefrontal cortex, lateral cerebellum, anterior insula, and inferior parietal lobe. This network supports goal-directed executive processes and guides decision-making by integrating information from the external world with internally-elaborated representations (20). Deficiencies in performing goal-directed executive processes have been considered pivotal in early theoretical models of ADHD (5).
The ventral attention network and its interplay with the dorsal attention network have been an increased focus in cognitive neuroscience (20), although their potential dysfunction in ADHD has been considered infrequently. The ventral attention network includes the temporoparietal junction, the supramarginal gyrus, frontal operculum and anterior insula; the dorsal system is anchored in the intraparietal sulcus and the frontal eye fields (20). While the dorsal attention network underpins the selection of sensory stimuli based on internal goals or personal expectations, the ventral network supports attentional reorienting to salient and behaviorally relevant external stimuli (20). A recent preliminary study reported deficient ventral attention network engagement in adults with ADHD, suggesting that this may underlie an ADHD-related deficit in adaptive switching to external salient stimuli (21). This, along with our finding of ventral attention network hypoactivation, is in line with the theoretical framework proposed by Nigg and Casey (6), which emphasizes that learning when and in which contexts to expect an event is critical for planning and maintaining appropriate behaviors. In their model, difficulties in detecting regularities or irregularities in the environment lead to problems in modulating behaviors according to changes of the environment, which manifest as ADHD symptoms. We speculate that hypoactivation in the ventral attention network underpins ADHD-related deficits in detecting regularities and irregularities in the environment. We also found hyperactivation of regions in the ventral attention network. Since the suppression of this network is necessary to prevent shifts of attention to irrelevant objects (22), its hyperactivation might underpin distractibility, one of the cardinal symptoms of ADHD.
We note that the dorsal attention system was relatively underrepresented among ADHD-related hypoactivated regions, although hypofunction of this system has been proposed in ADHD (7). In part, our results may reflect the substantial prevalence of inhibition-related tasks which are subserved predominantly by the ventral, rather than the dorsal attention network (20).
We also identified peaks of ADHD-related hypoactivation in the right somatomotor system and in the putamen bilaterally. Together with the cluster of hypoactivation in medial superior frontal gyrus/supplementary motor area, these regions are consistent with abnormal function of the pyramidal motor system, which would be expected in ADHD given the salience of motoric hyperactivity. Remarkably, inter-individual differences in locomotor activity have rarely been examined in ADHD in relation to neuroimaging measures. Using transcranial magnetic stimulation, robustly abnormal intracortical inhibition has been reported in the motor system in ADHD (23).
Besides hypoactivation, we also observed several regions of ADHD-related hyperactivation, predominantly in the default network. This network underlies self-referential cognitive processes that are typically suppressed during the performance of externally-oriented attentionally demanding tasks (24). Spontaneous activity fluctuations within the default network tend to be anti-correlated with fluctuations in “task positive” networks (i.e., networks that are activated during active tasks), such as the frontoparietal and dorsal attention networks (24). According to the “default-mode hypothesis” of ADHD (24), the default network might be inadequately regulated by other task-active systems, and might consequently intrude on or disrupt ongoing cognitive performance, contributing to spontaneous fluctuations in attention that characterize ADHD. The studies whose coordinates contributed to the hyperactivated default network clusters in our meta-analysis did not systematically report whether this hyperactivation reflected weaker task-related deactivation in ADHD, relative to comparisons, or stronger activation in ADHD relative to comparisons. However, amelioration of inadequate default network deactivation in ADHD by methylphenidate was recently reported by two separate groups (13;25).
We also observed ADHD-related hyperactivation in the somatomotor and visual systems. This is in line with the hypothesis that individuals with ADHD compensate for impaired function in prefrontal and anterior cingulate cortex by overreliance (relative to comparisons) on brain regions associated with visual, spatial, or motoric processing (26).
Meta-analysis focused on adults
The meta-analysis restricted to adults yielded fewer regional group differences compared to that in children. This may be accounted for by the smaller number of retained studies (n=16) and consequently lower statistical power. Almost all the hypoactivated voxels were found in the frontoparietal system, which is consistent with the persistence of executive dysfunction in adults with ADHD (27). Hypoactivation in the somatomotor system was less prominent in adults than in children, in line with clinical observations that motoric hyperactivity decreases with age (28). In the visual and dorsal attention systems, adults with ADHD exhibited proportionally more hyperactivation than children, suggesting the hypothesis that these networks may carry the bulk of the compensatory load in adults (26).
Meta-analysis focused on stimulant-naïve participants
Although early imaging studies of ADHD were confounded by prior stimulant treatment history (29), recent meta-analytic evidence has confirmed that brain structural changes are present in stimulant-naïve individuals with ADHD (30) and suggested that treatment with stimulants may even normalize structural abnormalities (31). Here, we extend those observations by finding significant differences between stimulant-naïve ADHD individuals and comparisons, which indicates that ADHD neuronal dysfunctions are also not likely accounted for by previous stimulant treatment.
The thalamus, despite its central location in multiple brain circuits, has been generally overlooked in the ADHD neuroimaging literature (32). We found thalamic hypoactivation in stimulant-naïve ADHD individuals and also in the meta-analysis of inhibition tasks. Given the role of the thalamus in alertness/arousal via thalamo-cortical projections (33), thalamic abnormalities in ADHD may be related to arousal dysmodulation, which has long been considered a core component of ADHD pathophysiology (6).
Meta-analysis focused on comorbidity-free participants
The pattern of ADHD-related hypo or hyperactivation in comorbidity-free participants generally overlapped with that found in the meta-analyses restricted to children or inhibition tasks. One exception was the inclusion of the default network among hypoactivated regions although, as in the other meta-analyses, it was proportionally more represented among the hyperactivated regions.
Meta-analyses focused on specific tasks
The regions found in analyses limited to inhibition tasks generally overlapped with those reported in the meta-analysis focused on children, which is not surprising since inhibition tasks were the most represented paradigm among those analyzed in children. In the analysis focused on studies of vigilance/attention, only a cluster in the ventral attention system emerged as significant. The limited number of retained studies on vigilance/attention may have limited the chance to detect other significant regions. Similarly, a region in the frontoparietal network, which is involved in executive functions such as working memory, was significantly hypoactivated in ADHD in the working memory analysis.
Omnibus meta-analysis
Besides analyses limited to children or adults, we performed an omnibus meta-analysis in which all ages and tasks were combined, as done previously by Dickstein et al. (4). The omnibus results largely overlapped those of the meta-analysis focused on children, which was expected since 70% of the included studies were conducted in children. Still, the larger number of studies and greater statistical power allowed us to detect a substantially larger number of significantly different voxels than in the sum of the two age-limited meta-analyses (see Figure 3). This yielded additional putatively dysfunctional regions in ADHD, including superior frontal gyrus/supplementary motor area, putamen, and superior temporal gyrus, that had not emerged in the previous meta-analysis (4).
Subtraction analyses
Despite specificities in each focused meta-analysis, subtraction analyses corrected for multiple comparisons showed that age, clinical characteristics, or type of task did not moderate our results. However, these negative results must be interpreted cautiously. Although definitive standards for statistical power in subtraction analyses do not exist, the informal consensus in the GingerALE users forum (14) is that between-analyses subtractions with fewer than 10 studies provide inadequate statistical power. Therefore, while the number of studies was sufficient for focused meta-analyses within subgroups, we were likely underpowered to carry out reliable subtraction analyses across these subgroups. Indeed, when we relaxed the statistical threshold by considering non-corrected results, we did detect differences in some subtraction analyses. Those results are not discussed further as they likely contain too many type I errors. However, we report them in Supplemental Table S3 since they may be useful for comparison with future analyses from this growing literature.
General overview
ADHD is increasingly being conceived as a disorder underpinned by dysfunctions in multiple large-scale brain networks (7;8). This perspective facilitates identification of several broad themes that emerged across our multiple meta-analytic instances. These include hypoactivation in the frontoparietal executive control network, putamen, and ventral attention network, which is consistent with the classical model of ADHD as a disorder of deficient fronto-striatal activation (34). However, we also detected substantial hyperactivation, particularly in the default network and visual circuits, which supports a model of ADHD based on the interrelationships among neural networks. Together with more recent reports (13;25), our results of default network hyperactivation are consistent with the hypothesis that the inconsistency that characterizes many individuals with ADHD results from faulty inter-regulation between the default network and “task-positive” circuits such as the frontoparietal, ventral or dorsal attention networks (7). The next generation of functional imaging studies should examine the temporal dependencies between behavioral indices of attentional lapses (e.g., episodically prolonged response times) in relation to their brain imaging correlates, with the goal of capturing the deviations in the interplay between the default and “task-positive” networks.
Our results also provide meta-analytic support to views positing ADHD as a disorder characterized not only by functional deficiencies but also by possible compensatory mechanisms, such as hyperactivation in visual regions. Such putative compensatory mechanisms can be difficult to observe through clinical measures alone, but become evident through neuroimaging (35). Awareness and documentation of brain compensatory mechanisms may eventually yield a clinical benefit from neuroimaging. This would be analogous to the use of neurocognitive assessments to identify particular strengths to best formulate a comprehensive clinical treatment plan.
We failed to support involvement of regions related to motivation and emotion, such as ventral striatum (36), orbitofrontal region, or amygdala/hippocampus (37), despite increasing recognition of motivational/emotional dysfunction in models of ADHD (38). We cannot rule out type II error as three studies (39–41), specifically assessing reward, and two (42;43) focusing on emotional processing, did not meet our inclusion criteria. In addition, the orbitofrontal cortex and the medial temporal lobes are challenging brain regions to examine with fMRI because of susceptibility artifact and signal dropout. We also note the lack of apparent involvement of the cerebellum, which has been implicated in ADHD by multiple volumetric and functional studies and by theoretical models emphasizing the role of the cerebellar dysfunctions in contributing to deficits in monitoring the frequency and timing of events (6). In our meta-analysis, several peaks in the cerebellum did not reach our cluster size threshold for significance. We suspect that high inter-subject variability in cerebellar geometry relative to stereotaxic space mitigated the emergence of cerebellar findings across studies.
Limitations
Our results should be considered in light of several limitations. The first relates to selection criteria. To minimize confounding factors such as differences in diagnostic procedures or analytical approaches, we excluded about half the screened studies. Still, the studies included were heterogeneous, for example with respect to the method used to correct for multiple comparisons. This is notable since activation likelihood estimation does not take into account inter-study differences in statistical thresholds. Second, separate meta-analyses could not be performed by ADHD subtype or sex, since separate results for males and females and ADHD subtypes are not usually reported in the literature. This is unfortunate since patterns of fMRI activation in ADHD can differ by sex (44) and, possibly, subtype (45). Third, the meta-analytic approach we adopted allows a quantitative summary of positive results but cannot take into account negative findings. Effect sizes in fMRI may be confounded by many factors, such as movement covariates, and there is no agreement on how they should be handled. Thus, activation likelihood estimation should be considered a summary of the spatial distribution of positive results, rather than a true meta-analysis. Fourth, it was generally not possible to determine the extent to which overlapping participants were reported in more than one study. Thus, the total number of participants represents an upper bound. The open sharing of fMRI data (e.g., via www.OpenfMRI.org) should obviate this problem in the future. Finally, fMRI data are fundamentally limited. Besides only indirectly reflecting neuronal activity (46), fMRI data cannot define an absolute or quantitative baseline state of activation (47), as they always depend on the differences in signal between two conditions. Positron emission tomography provides absolute quantification [e.g., (48;49)], but current ethical constraints limit its application to adults.
Conclusions
Moving beyond models of ADHD focused on a limited set of brain regions, the maturing fMRI literature in ADHD reveals dysfunctions in regions belonging to multiple neuronal networks involved in higher-level cognitive or sensorimotor functions. Results were not ascribable to stimulant treatment history or presence of comorbidities. The systems neuroscience perspective we adopted is in line with the NIH Research Domain Criteria framework (50), which conceptualizes mental disorders in terms of dysfunctions of brain circuits to inform future nosological systems beyond a symptoms-based approach. Future work aiming at understanding the interplay among large-scale neural networks and their links to ADHD symptom dimensions should advance the goal of illuminating the pathophysiology of this common and vexing disorder.
Supplementary Material
Footnote: R: Right; L: Left.
Footnote: For the contrast ADHD >Comparisons, in stimulant-naïve, as shown in Supplemental Table S1, only one significant cluster was detected.
Footnote: *Montreal Neurological Institute coordinates; **After removing complete overlap but including partial overlap of participants across studies from the same research groups; *** R: Right; L: Left. ALE: Activation likelihood estimation.
Footnote: *Montreal Neurological Institute coordinates; **After removing complete overlap but including partial overlap of participants across studies from the same research groups; *** R: Right; L: Left. ALE: Activation likelihood estimation.
Footnote: *Montreal Neurological Institute coordinates; **After removing complete overlap but including partial overlap of participants across studies from the same research groups; *** R: Right; L: Left.
Acknowledgments
Grant support:
Dr. Cortese is currently supported by a grant from the European Commission (‘Marie Curie’ grant for Career Development, Outgoing International Fellowship, POIF-253103). This work was also supported by NIH grants MH083246, MH081218, HD065282, and K23M087770.
Footnotes
Potential conflicts of interest:
Dr. Cortese received financial support to attend medical meetings from Eli Lilly and Company (2007–9) and Shire Pharmaceuticals (2009–10), and was a co-investigator in studies sponsored by GlaxoSmithKline (2006), Eli Lilly and Company (2007–8), and Genopharm (2008). He served as a consultant for Shire Pharmaceuticals (2009–10). Dr. Cortese has no current relationships with pharmaceutical companies. The other co-authors report no competing interests.
Location of work:
Phyllis Green and Randolph Cowen Institute for Pediatric Neuroscience, Child Study Center of the NYU Langone Medical Center, New York, NY, USA.
References
- 1.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- 2.Mannuzza S, Klein RG, Moulton JL., III Persistence of Attention-Deficit/Hyperactivity Disorder into adulthood: what have we learned from the prospective follow-up studies? J Atten Disord. 2003;7:93–100. doi: 10.1177/108705470300700203. [DOI] [PubMed] [Google Scholar]
- 3.Wolraich M. Attention deficit hyperactivity disorder: The most studied and yet most controversial diagnosis. Ment Retard Devel Disabil Res Rev. 1999;5:163–168. [Google Scholar]
- 4.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 5.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 6.Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17:785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 7.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends Cogn Sci. 2012;16:17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makris N, Biederman J, Monuteaux MC, Seidman LJ. Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci. 2009;31:36–49. doi: 10.1159/000207492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cubillo A, Rubia K. Structural and functional brain imaging in adult attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2010;10:603–620. doi: 10.1586/ern.10.4. [DOI] [PubMed] [Google Scholar]
- 11.Paloyelis Y, Mehta MA, Kuntsi J, Asherson P. Functional MRI in ADHD: a systematic literature review. Expert Rev Neurother. 2007;7:1337–1356. doi: 10.1586/14737175.7.10.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. [Last access: 06/30/2011]; http://www.neurosynth.org/
- 13.Peterson BS, Potenza MN, Wang Z, Zhu H, Martin A, Marsh R, Plessen KJ, Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166:1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. [Last access: 12/01/2011]; http://www.brainmap.org/ale/
- 15.Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devlin JT, Poldrack RA. In praise of tedious anatomy. Neuroimage. 2007;37:1033–1041. doi: 10.1016/j.neuroimage.2006.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. 2010;53:1–15. doi: 10.1016/j.neuroimage.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helenius P, Laasonen M, Hokkanen L, Paetau R, Niemivirta M. Impaired engagement of the ventral attentional pathway in ADHD. Neuropsychologia. 2011;49:1889–1896. doi: 10.1016/j.neuropsychologia.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76:615–621. doi: 10.1212/WNL.0b013e31820c2ebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 25.Liddle EB, Hollis C, Batty MJ, Groom MJ, Totman JJ, Liotti M, Scerif G, Liddle PF. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiatry. 2011;52:761–71. doi: 10.1111/j.1469-7610.2010.02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fassbender C, Schweitzer JB. Is there evidence for neural compensation in attention deficit hyperactivity disorder? A review of the functional neuroimaging literature. Clin Psychol Rev. 2006;26:445–465. doi: 10.1016/j.cpr.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seidman LJ. Neuropsychological functioning in people with ADHD across the lifespan. Clin Psychol Rev. 2006;26:466–485. doi: 10.1016/j.cpr.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Biederman J, Mick E, Faraone SV. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. Am J Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. [DOI] [PubMed] [Google Scholar]
- 29.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- 30.Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatr Scand. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- 31.Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. Am J Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- 32.Bush G, Valera EM, Seidman LJ. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 33.Platt B, Riedel G. The cholinergic system, EEG and sleep. Behav Brain Res. 2011;221:499–504. doi: 10.1016/j.bbr.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 34.Castellanos FX. Toward a pathophysiology of attention-deficit/hyperactivity disorder. Clin Pediatr (Phila) 1997;36:381–393. doi: 10.1177/000992289703600702. [DOI] [PubMed] [Google Scholar]
- 35.Rubia K. The dynamic approach to neurodevelopmental psychiatric disorders: use of fMRI combined with neuropsychology to elucidate the dynamics of psychiatric disorders, exemplified in ADHD and schizophrenia. Behav Brain Res. 2002;130:47–56. doi: 10.1016/s0166-4328(01)00437-5. [DOI] [PubMed] [Google Scholar]
- 36.Carmona S, Proal E, Hoekzema EA, Gispert JD, Picado M, Moreno I, Soliva JC, Bielsa A, Rovira M, Hilferty J, Bulbena A, Casas M, Tobena A, Villaroya O. Ventro-striatal reductions underpin symptoms of hyperactivity and impulsivity in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;66:972–977. doi: 10.1016/j.biopsych.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, Martin L, Durkin K, Blair C, Royal J, Hugdahl K, Peterson BS. Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2006;63:795–807. doi: 10.1001/archpsyc.63.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonuga-Barke EJ. Editorial: ADHD as a reinforcement disorder - moving from general effects to identifying (six) specific models to test. J Child Psychol Psychiatry. 2011;52:917–918. doi: 10.1111/j.1469-7610.2011.02444.x. [DOI] [PubMed] [Google Scholar]
- 39.Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, Fallgater AJ, Gron G. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 41.Stoy M, Schlagenhauf F, Schlochtermeier L, Wrase J, Knutson B, Lehmkuhl U, Huss M, Heinz A, Ströhle A. Reward processing in male adults with childhood ADHD-a comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology (Berl) 2011;215:467–481. doi: 10.1007/s00213-011-2166-y. [DOI] [PubMed] [Google Scholar]
- 42.Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, Thomas LA, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlochtermeier L, Stoy M, Schlagenhauf F, Wrase J, Park SQ, Friedel E, Huss M, Lehmkuhl U, Heinz A, Strohle A. Childhood methylphenidate treatment of ADHD and response to affective stimuli. Eur Neuropsychopharmacol. 2011;21:646–654. doi: 10.1016/j.euroneuro.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 44.Valera EM, Brown A, Biederman J, Faraone SV, Makris N, Monuteaux MC, Whitfield-Gabrieli S, Vitulano M, Schiller M, Seidman LJ. Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am J Psychiatry. 2010;167:86–94. doi: 10.1176/appi.ajp.2009.09020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Solanto MV, Schulz KP, Fan J, Tang CY, Newcorn JH. Event-related FMRI of inhibitory control in the predominantly inattentive and combined subtypes of ADHD. J Neuroimaging. 2009;19:205–212. doi: 10.1111/j.1552-6569.2008.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 47.Gusnard DA, Raichle ME, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 48.Ernst M, Liebenauer LL, King AC, Fitzgerald GA, Cohen RM, Zametkin AJ. Reduced brain metabolism in hyperactive girls. J Am Acad Child Adolesc Psychiatry. 1994;33:858–868. doi: 10.1097/00004583-199407000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Zametkin AJ, Nordahl TE, Gross M, King AC, Semple WE, Rumsey J, Hamburger S, Cohen RM. Cerebral glucose metabolism in adults with hyperactivity of childhood onset. N Engl J Med. 1990;323:1361–1366. doi: 10.1056/NEJM199011153232001. [DOI] [PubMed] [Google Scholar]
- 50.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Footnote: R: Right; L: Left.
Footnote: For the contrast ADHD >Comparisons, in stimulant-naïve, as shown in Supplemental Table S1, only one significant cluster was detected.
Footnote: *Montreal Neurological Institute coordinates; **After removing complete overlap but including partial overlap of participants across studies from the same research groups; *** R: Right; L: Left. ALE: Activation likelihood estimation.
Footnote: *Montreal Neurological Institute coordinates; **After removing complete overlap but including partial overlap of participants across studies from the same research groups; *** R: Right; L: Left. ALE: Activation likelihood estimation.
Footnote: *Montreal Neurological Institute coordinates; **After removing complete overlap but including partial overlap of participants across studies from the same research groups; *** R: Right; L: Left.