Abstract
Although oncologic efficacy is the primary goal of radical prostatectomy, preserving potency and continence is also important, given the indolent clinical course of most prostate cancers. In order to preserve and recover postoperative potency and continence after radical prostatectomy, a detailed understanding of the pelvic anatomy is necessary to recognize the optimal nerve-sparing plane and to minimize injury to the neurovascular bundles. Therefore, we reviewed the most recent findings from neuroanatomic studies of the prostate and adjacent tissues, some of which are contrary to the established consensus on pelvic anatomy. We also described the functional outcomes of radical prostatectomies following improved anatomical understanding and development of surgical techniques for preserving the neurovascular bundles.
Keywords: Prostatic neoplasms, Neurovascular bundle, Fascial anatomy, Penile erection, Urinary incontinence
INTRODUCTION
Prostate cancer is the most common cancer among men, and approximately 2.8 million men are estimated to have a history of prostate cancer in the United States [1]. Currently, prostate cancer can be detected in patients because its association with high prostate specific antigen levels, thus allowing early diagnosis and prolonging survival after diagnosis [2]. This, in turn, has increased the number of candidates for radical prostatectomy, with the intention to cure prostate cancer while minimizing the risk of urinary incontinence and erectile dysfunction [3].
Neuroanatomy of the prostate is important owing to its relationship with postoperative functions of continence and potency. Initially, Walsh’s anatomic nerve-sparing technique in 1982 was based on the idea that the neurovascular bundles (NVBs) are situated posterolaterally and symmetrically to the prostate in the space defined by the levator fascia, prostatic fascia, and Denonvilliers’ fascia [4]. In the past few decades, several anatomic studies have provided deeper insight into the neuroanatomy of the prostate and adjacent tissue, which formed the basis for ensuring good oncologic and functional outcomes after radical prostatectomy. This article summarizes the most recent findings from neuroanatomic studies, some of which are contrary to the established consensus on pelvic anatomy. We also described the functional outcomes of radical prostatectomies following improved anatomical understanding and development of surgical techniques for preserving the NVBs.
EXPANSION OF NEUROANATOMIC STUDIES
In 1982, Walsh and Donker [4] introduced the nerve-sparing radical prostatectomy procedure to preserving cavernous nerves situated posterolaterally and symmetrically to the prostate. This technique has inspired greater acceptance of the surgical approach for prostate cancer therapy and came to be used globally. Since then, however, there has been an ongoing debate about the course of these cavernous nerves [5–8] (Table 1). The precise relationship of the NVBs and cavernous nerves to Denonvillier’s fascia has been questioned by Kourambas et al. [5]. Costello et al. [9] expanded on Walsh’s initial efforts by using cadaver models to further detail the precise anatomy of the NVBs because of their close relation with the prostate and seminal vesicles (Fig. 1). They identified 3 functional components of the NVBs. The posterior and posterolateral component runs within Denonvillier’s fascia and the pararectal fascia and innervates the rectum. A second component in the lateral NVB supplies the levator ani. The cavernosal nerves and prostatic neurovascular supply, the third component originally described by Walsh and Donker [4], lie along the posterolateral surface. The organization of these nerve bundles is rather disordered at the base of the prostate and at the seminal vesicles, further showing the complexity of the NVBs and the challenges of performing a technically sound nerve-sparing procedure [9]. Takenaka et al. [6] confirmed that branches of the hypogastric nerve and pelvic splanchnic nerve are likely to interdigitate at multiple levels, showing spray-like arrangement without clear bundle formation (Fig. 2). In addition, Lunacek et al. [7] demonstrated that the cavernous nerves running along the prostate are displaced more anteriorly and disperse along the convex surface of the prostatic capsule (like a “curtain”) during the growth of the prostate. From these anatomical findings, they proposed a “curtain dissection” technique, in which the incision of the periprostatic fascia and dissection of the NVBs is far more anterior than previously described. Furthermore, Menon et al. [8] described a technique for preserving the lateral prostatic fascia containing NVBs, the “Veil of Aphrodite.” On the basis of these studies, the high anterior release, “Veil of Aphrodite,” or “Superveil” technique have been developed for preserving the maximum number of nerve fibers [10–12].
Table 1.
Expansion of neuroanatomic studies: historical aspects
| Study | Concept | |
|---|---|---|
| Introduction of NVB | Walsh and Donker [4] | NVB posterolateral side of the prostate |
| Era of nerve sparing | Kourambas et al. [5] | Scattered nerves throughout the Denonvilliers’ fascia, including medi-ally towards the midline |
| Costello et al. [9] | Three functional components of the NVBs | |
| Takenaka et al. [6] | Spray-like arrangement of nerves without clear bundle formation | |
| Era of wider nerve sparing | Lunacek et al. [7] | “Curtain dissection”: dispersion of cavernous nerves along the prostatic capsule |
| Menon et al. [8] | “Veil of Aphrodite”: lateral prostatic fascia containing NVBs |
NVB, neurovascular bundle.
Fig. 1.
Posterior view of the neurovascular bundle and prostate. The entire posterior surface of the prostate is covered by nerve fibers with fewer fibers at the 6-o’clock position. Reproduced from Costello et al. BJU Int 2004;94:1071-6, with permission of Wiley-Blackwell [9].
Fig. 2.
Fresh cadaver dissections to identify neurovascular bundle (NVB) (left lateral view). Caudal branches (arrows) of pelvic splanchnic nerve (PSN) appeared to join NVB at levels inferior to bladder-prostate junction (asterisks). BL, bladder; DPN, dorsal penile nerves; PP, pelvic plexus; PR, prostate; R, PSN rectal branches. Reproduced from Takenaka et al. J Urol 2004;172:1032–5, with permission of Elsevier B.V. [6].
DISTRIBUTION OF PERIPROSTATIC NERVES
Recent anatomic studies have shown the variable degrees of periprostatic nerves both in the dorsolateral and ventrolateral positions [13–16]. Eichelberg et al. [13] illustrated that, while most periprostatic nerves were found posterolaterally as initially described, a significant portion of the nerves (21.5–28.5%) were located on the anterior surface. Similarly, Lee et al. [16] investigated the pattern of distribution of nerves surrounding the prostate by analyzing specimens from non–nerve-sparing radical prostatectomies (Fig. 3). Significant proportions (19.9–22.8%) of the total nerves were located on the anterior side of the prostate. NVBs with a relatively round, bundle-like formation were observed in approximately half the cases; in other cases, NVBs were more widely spread as they extended anteriorly.
Fig. 3.
(A) Neurovascular bundle (NVB) localized to the postero-lateral aspect of the prostate (S-100 stain). (B) NVB in the formation are relatively more spread to the anterior side of the prostate (S-100 stain). Reproduced from Lee et al. Urology 2008;72: 878–81, with permission of Elsevier B.V. [16].
In a study using whole-mount sections of non–nerve-sparing radical prostatectomies, Ganzer et al. [14] used novel computerized planimetry software to characterize the topographical anatomy of periprostatic and capsular nerves [15]. The percentage of total nerve surface area was highest dorsolaterally (84.1%, 75.1%, and 74.5% at the base, middle, and apex, respectively), but this finding was variable. Up to 39.9% of nerve surface area was found ventrolaterally with up to 45.5% in the dorsal position. However, the dilemma is a product of growing evidence on anatomic distribution NVBs without any clear understanding of their role in the physiology of erectile function. Since the presence of periprostatic nerve fibers was proven not to be involved in erection, Kaiho et al. [17] provided evidence to confirm the role of these fibers using electrophysiologic testing. Although the largest amplitudes of pressure responses were induced by stimulation at the 5-o’clock position, electrical stimulation at all positions of the midprostate (between 1- and 5- o’clock) evoked the cavernosal pressure responses in all patients.
Although the existence of ventrolateral periprostatic nerves has been confirmed, detailed knowledge of the type of nerve fibers innervating the prostate is important in understanding the pathophysiology and functional consequences. Alsaid et al. [18] demonstrated the location and type of nerve fibers within the NVBs and provided a three-dimensional representation of their structural relationship in male fetus. The three-dimensional reconstruction illustrated that nerve fibers were derived from the inferior hypogastric plexus, providing cholinergic, adrenergic, and sensory innervation to seminal vesicles, vas deferens, prostate, and urethral sphincter in a fanlike formation. However, in their cadaver study, Costello et al. [19] reported that functionally significant parasympathetic nerve fibers accounted for 4%, 5%, and 6.8% of the nerves located on the anterolateral aspect of the prostate at the base, mid, and apex, respectively. Ganzer et al. [20] recently confirmed this finding using topographic distribution of periprostatic nerves, including immunohistochemical differentiation of proerectile parasympathetic from sympathetic nerves. They found that parasympathetic nerves were dispersed at the base and were mainly located dorsolaterally at the apex, with 14.6% above the horizontal line at the base and only 1.5% at the apex. Thus, no consensus has been reached on the anatomic evidence for supporting high anterior incision in the lateral prostatic fascia in order to spare the cavernous nerve fibers.
FASCIAL ANATOMY OF THE PROSTATE
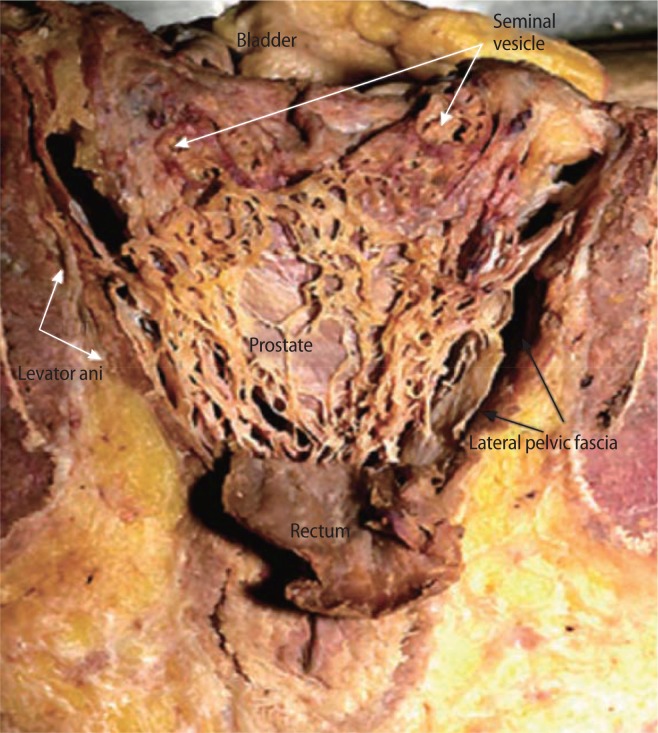
The fascial anatomy near the prostate is not well understood anatomically, and many urologists have not reached a consensus on its nomenclature (Fig. 4). The endopelvic fascia comprises of multilayered connective tissue that encases and supports the prostate and bladder and provides adherence to the pubic bone by the puboprostatic ligaments. The parietal and visceral components of the endopelvic fascia are fused along the pelvic sidewall at the lateral aspect of the prostate and bladder. This fusion is often recognizable as a whitish line and is named the fascial tendinous arch of the pelvis [21]. The prostatic fascia directly covers the prostate, forming an intrafascial plane between this fascia and the prostate capsule. The levator ani fascia is immediately exterior to the prostatic fascia and serves as the boundary for an interfascial plane. After the endopelvic fascia is opened laterally to the fascial tendinous arch and the levator ani muscle is deflected laterally, the outermost fascial layer on the lateral surface of the prostate, the levator ani fascia, is observed [22]. Both the levator ani fascia and prostatic fascia constitute periprostatic fascia for the operating surgeon. The posterior surface of the prostate and the seminal vesicles are closely covered by a continuous layer of the posterior prostatic fascia and seminal vesicles fascia, known as Denonvillier’s fascia. Dissection along these avascular planes preserves the NVBs, as the majority of the NVBs are thought to run between the anterior extension of Denonvillier’s fascia and the levator ani fascia. A thorough understanding of these planes is crucial for performing an anatomic dissection, while avoiding mechanical and thermal injury to the NVBs.
Fig. 4.
Schematic of prostate and periprostatic fascias at mid-prostate with three different dissection planes demonstrated (intrafascial, interfascial, and extrafascial). VEF ant.-lat., visceral endopelvic fascia anterior-lateral; PEF, parietal endopelvic fascia; C, capsule of prostate; LAF post.-lat., levator ani fascia posterior-lateral; PF, prostatic fascia. Reproduced from Walz et al. Eur Urol 2010;57:179–92, with permission of Elsevier B.V. [43].
DEVELOPMENT OF NERVE-SPARING TECHNIQUES
Several techniques have been proposed to optimize the preservation of erectile function on the basis of the anatomic principles summarized above. In particular, the intraoperative magnification offered by robotic surgical systems enables identification and preservation of periprostatic fascial planes that have nerve fibers [23].
Interfascial dissection of NVBs involves a dissection lateral to the prostatic fascia at the anterolateral and posterolateral aspects of the prostate, combined with a dissection medial to the NVB at the 5-o’clock and 7-o’clock positions or the 2-o’clock and 10-o’clock positions of the prostate in axial section [24,25]. Depending on individual anatomic variations, the NVBs might be more prone to partial resection with this technique. According to the experience gained from intrafascial nerve-sparing prostatectomy, Stolzenburg et al. [26,27] emphasized the importance of the dissection depth for preserving NVBs. The intrafascial technique is a dissection that follows a plane on the prostate capsule, remaining medial to the prostatic fascia at the anterolateral and posterolateral aspect of the prostate and anterior to Denonvillier’s fascia.
Tewari et al. [28] studied the neuroanatomy of the pelvic erectile nerves as relevant to robotic radical prostatectomy. They grouped important neural structures into the proximal neurovascular plate (PNP), the predominant NVB (PNB), and the accessory neural pathways (ANPs). The PNP, located lateral to the bladder neck, seminal vesicles, and branches of the inferior vesical vessels, processes and relays erectogenic neural signals. The PNB is the classical bundle that carries neural impulses to the cavernosal tissue, and ANPs are the putative accessory neural pathways around the prostate, other than the PNB, that might be additional conduits for neural impulses. These authors described a hammock-like distribution of the nerves on which the prostate rests, showing that the NVB is more of a network of multiple fine dispersed nerves than a distinct structure. Because the classical nerve-sparing approach will sacrifice most of the proximal and posterior extensions of the neurovascular tissue, the neural zones around the prostate have important implications in robotic radical prostatectomy. They proposed a novel risk-stratified nerve-sparing approach for determining the degree of nerve sparing based on the observation of venous distribution over the prostate and periprostatic fascial planes [29]. They reported that patients with greater degrees of nerve-sparing had higher rates of intercourse and return to baseline sexual function [29], and early return of urinary continence without compromising oncologic safety [30].
Similarly, Schatloff et al. [31] described a nerve-sparing grading system based on the arterial periprostatic distribution on the posterolateral aspect of the prostate. The landmark artery, which could be either a prostatic or a capsular artery, is located approximately 2–3 mm outside the capsule and can be used as a visual cue to delineate the extension of the resection of the NVBs. They independently graded nerve sparing on either sides (1, no nerve sparing; 2, <50% nerve sparing; 3, 50% nerve sparing; 4, 75% nerve sparing; 5, ≥95% nerve sparing), and found that the side-specific positive surgical margin rate according to the nerve-sparing score were 3.6% for grade 5, 7.5% for grade 4, 16.7% for grade 3, 5.7% for grade 2, and 0% for grade 1.
CLINICAL OUTCOMES
The aforementioned studies have improved anatomical understanding, development of surgical techniques for preserving periprostatic nerves, and functional outcomes, simultaneously preserving the oncological goals after radical prostatectomy. Potency rates after radical prostatectomy are influenced by numerous factors including baseline characteristics, nerve-sparing extension and techniques, and definition of potency. A recent meta-analysis revealed a progressive increase in potency rates with follow-up after radical prostatectomy [32]. Different modifications of the initial nerve-sparing technique have been described, which reflected improvements in anatomic understanding. Ahlering et al. [33] described a cautery-free nerve-sparing procedure that significantly improved early return of potency (47% vs. 8.3%, P<0.001). Menon et al. [8,10] described “Veil of Aphrodite” or “superveil” technique in which the prostatic fascia is dissected to the prostatic surface, and the periprostatic tissue is released in a relatively avascular plane. With the “superveil” technique, 94% of men who attempted sexual intercourse were successful at 6–18 months after radical prostatectomy. Tewari’s risk stratified approach to athermal, traction-free nerve sparing reported that increased nerve sparing corresponds to increased percentages of patients with postoperative recovery of potency [34]. In their study, patients who underwent nerve-sparing grade 1 had a potency rate of 92.4% with a positive surgical margin rate of 10.5%.
The role of NVB preservation for urinary control is particularly controversial. Recent studies, however, have shown a relationship between the urinary continence recovery and nerve sparing. Choi et al. [35] reported that bilateral nerve-sparing prostatectomy improved postoperative urinary functions and was associated with improved continence at 4 months (47.2% vs. 26.7%, P=0.043), but not at 12 or 24 months. Similarly, Ko et al. [36] demonstrated that the probability of continence recovery within 3 months was significantly higher for the partial nerve-sparing and bilateral nerve-sparing groups with a shorter time to recovery of continence, compared with the non–nerve-sparing group. Gandaglia et al. [37] reported that preoperative erectile function should be considered in predicting urinary continence after bilateral nerve-sparing radical prostatectomy. Since erectile function depends on systemic vascular status [38], it may also represent a marker of pelvic vascular disease, which may subsequently affect the status of the external urinary sphincter. In their study, patients who were fully potent before surgery had a higher probability of urinary continence recovery than patients with any degree of preoperative erectile dysfunction.
FUTURE DIRECTIONS
There are several novel techniques for improving the efficacy of a nerve-sparing procedure during radical prostatectomy without sacrificing any degree of cancer control. Multiphoton microscopy for real-time tissue imaging of the prostate and periprostatic neural tissue obtains high-resolution images of the prostate capsule, underlying acini, and individual cells outlining the glands at varying magnifications [39]. Tewari et al. [40] reported that multiphoton microscopy of freshly excised, unprocessed, and unstained tissue can identify all relevant prostatic and periprostatic structures and also pathological changes that were validated in pathologic examinations. Moreover, to aid the identification and preservation of the NVBs, numerous imaging modalities, including optical coherence tomography [41] and fluorescent peptides [42] are currently under investigation for assessing possible roles in the development of a more individualized anatomic nerve-sparing radical prostatectomy. These technologies, as well as accurate knowledge of the neuroanatomy of the prostate, will reveal the course of the nerves and sites of nerve branching otherwise not grossly visible during radical prostatectomy.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–41. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–83. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 4.Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492–7. doi: 10.1016/s0022-5347(17)53012-8. [DOI] [PubMed] [Google Scholar]
- 5.Kourambas J, Angus DG, Hosking P, Chou ST. A histological study of Denonvilliers’ fascia and its relationship to the neurovascular bundle. Br J Urol. 1998;82:408–10. doi: 10.1046/j.1464-410x.1998.00749.x. [DOI] [PubMed] [Google Scholar]
- 6.Takenaka A, Murakami G, Soga H, Han SH, Arai Y, Fujisawa M. Anatomical analysis of the neurovascular bundle supplying penile cavernous tissue to ensure a reliable nerve graft after radical prostatectomy. J Urol. 2004;172:1032–5. doi: 10.1097/01.ju.0000135648.33110.df. [DOI] [PubMed] [Google Scholar]
- 7.Lunacek A, Schwentner C, Fritsch H, Bartsch G, Strasser H. Anatomical radical retropubic prostatectomy: ‘curtain dissection’ of the neurovascular bundle. BJU Int. 2005;95:1226–31. doi: 10.1111/j.1464-410X.2005.05510.x. [DOI] [PubMed] [Google Scholar]
- 8.Menon M, Hemal AK, VIP Team Vattikuti Institute prostatectomy: a technique of robotic radical prostatectomy: experience in more than 1000 cases. J Endourol. 2004;18:611–9. doi: 10.1089/end.2004.18.611. [DOI] [PubMed] [Google Scholar]
- 9.Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int. 2004;94:1071–6. doi: 10.1111/j.1464-410X.2004.05106.x. [DOI] [PubMed] [Google Scholar]
- 10.Menon M, Shrivastava A, Bhandari M, Satyanarayana R, Siva S, Agarwal PK. Vattikuti Institute prostatectomy: technical modifications in 2009. Eur Urol. 2009;56:89–96. doi: 10.1016/j.eururo.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 11.Menon M, Kaul S, Bhandari A, Shrivastava A, Tewari A, Hemal A. Potency following robotic radical prostatectomy: a questionnaire based analysis of outcomes after conventional nerve sparing and prostatic fascia sparing techniques. J Urol. 2005;174:2291–6. doi: 10.1097/01.ju.0000181825.54480.eb. [DOI] [PubMed] [Google Scholar]
- 12.Montorsi F, Salonia A, Suardi N, Gallina A, Zanni G, Briganti A, et al. Improving the preservation of the urethral sphincter and neurovascular bundles during open radical retropubic prostatectomy. Eur Urol. 2005;48:938–45. doi: 10.1016/j.eururo.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Eichelberg C, Erbersdobler A, Michl U, Schlomm T, Salomon G, Graefen M, et al. Nerve distribution along the prostatic capsule. Eur Urol. 2007;51:105–10. doi: 10.1016/j.eururo.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Ganzer R, Blana A, Gaumann A, Stolzenburg JU, Rabenalt R, Bach T, et al. Topographical anatomy of periprostatic and capsular nerves: quantification and computerised planimetry. Eur Urol. 2008;54:353–60. doi: 10.1016/j.eururo.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Ganzer R, Blana A, Stolzenburg JU, Rabenalt R, Fritsche HM, Wieland WF, et al. Nerve quantification and computerized planimetry to evaluate periprostatic nerve distribution-does size matter? Urology. 2009;74:398–403. doi: 10.1016/j.urology.2008.12.076. [DOI] [PubMed] [Google Scholar]
- 16.Lee SB, Hong SK, Choe G, Lee SE. Periprostatic distribution of nerves in specimens from non-nerve-sparing radical retropubic prostatectomy. Urology. 2008;72:878–81. doi: 10.1016/j.urology.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 17.Kaiho Y, Nakagawa H, Saito H, Ito A, Ishidoya S, Saito S, et al. Nerves at the ventral prostatic capsule contribute to erectile function: initial electrophysiological assessment in humans. Eur Urol. 2009;55:148–54. doi: 10.1016/j.eururo.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Alsaid B, Karam I, Bessede T, Abdlsamad I, Uhl JF, Delmas V, et al. Tridimensional computer-assisted anatomic dissection of posterolateral prostatic neurovascular bundles. Eur Urol. 2010;58:281–7. doi: 10.1016/j.eururo.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Costello AJ, Dowdle BW, Namdarian B, Pedersen J, Murphy DG. Immunohistochemical study of the cavernous nerves in the periprostatic region. BJU Int. 2011;107:1210–5. doi: 10.1111/j.1464-410X.2010.09711.x. [DOI] [PubMed] [Google Scholar]
- 20.Ganzer R, Stolzenburg JU, Wieland WF, Brundl J. Anatomic study of periprostatic nerve distribution: immunohistochemical differentiation of parasympathetic and sympathetic nerve fibres. Eur Urol. 2012;62:1150–6. doi: 10.1016/j.eururo.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 21.Myers RP. Practical surgical anatomy for radical prostatectomy. Urol Clin North Am. 2001;28:473–90. doi: 10.1016/s0094-0143(05)70156-7. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen ME, Schaeffer EM, Marschke P, Walsh PC. High anterior release of the levator fascia improves sexual function following open radical retropubic prostatectomy. J Urol. 2008;180:2557–64. doi: 10.1016/j.juro.2008.08.047. [DOI] [PubMed] [Google Scholar]
- 23.Ficarra V, Cavalleri S, Novara G, Aragona M, Artibani W. Evidence from robot-assisted laparoscopic radical prostatectomy: a systematic review. Eur Urol. 2007;51:45–55. doi: 10.1016/j.eururo.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Zorn KC, Gofrit ON, Orvieto MA, Mikhail AA, Zagaja GP, Shalhav AL. Robotic-assisted laparoscopic prostatectomy: functional and pathologic outcomes with interfascial nerve preservation. Eur Urol. 2007;51:755–62. doi: 10.1016/j.eururo.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 25.Barré C. Open radical retropubic prostatectomy. Eur Urol. 2007;52:71–80. doi: 10.1016/j.eururo.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 26.Stolzenburg JU, Rabenalt R, Tannapfel A, Liatsikos EN. Intrafascial nerve-sparing endoscopic extraperitoneal radical prostatectomy. Urology. 2006;67:17–21. doi: 10.1016/j.urology.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 27.Stolzenburg JU, Rabenalt R, Do M, Schwalenberg T, Winkler M, Dietel A, et al. Intrafascial nerve-sparing endoscopic extraperitoneal radical prostatectomy. Eur Urol. 2008;53:931–40. doi: 10.1016/j.eururo.2007.11.047. [DOI] [PubMed] [Google Scholar]
- 28.Tewari A, Takenaka A, Mtui E, Horninger W, Peschel R, Bartsch G, et al. The proximal neurovascular plate and the tri-zonal neural architecture around the prostate gland: importance in the athermal robotic technique of nerve-sparing prostatectomy. BJU Int. 2006;98:314–23. doi: 10.1111/j.1464-410X.2006.06266.x. [DOI] [PubMed] [Google Scholar]
- 29.Tewari AK, Srivastava A, Huang MW, Robinson BD, Shevchuk MM, Durand M, et al. Anatomical grades of nerve sparing: a risk-stratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP) BJU Int. 2011;108(6 Pt 2):984–92. doi: 10.1111/j.1464-410X.2011.10565.x. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava A, Chopra S, Pham A, Sooriakumaran P, Durand M, Chughtai B, et al. Effect of a risk-stratified grade of nerve-sparing technique on early return of continence after robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2013;63:438–44. doi: 10.1016/j.eururo.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Schatloff O, Chauhan S, Sivaraman A, Kameh D, Palmer KJ, Patel VR. Anatomic grading of nerve sparing during robot-assisted radical prostatectomy. Eur Urol. 2012;61:796–802. doi: 10.1016/j.eururo.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 32.Ficarra V, Novara G, Ahlering TE, Costello A, Eastham JA, Graefen M, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418–30. doi: 10.1016/j.eururo.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 33.Ahlering TE, Skarecky D, Borin J. Impact of cautery versus cautery-free preservation of neurovascular bundles on early return of potency. J Endourol. 2006;20:586–9. doi: 10.1089/end.2006.20.586. [DOI] [PubMed] [Google Scholar]
- 34.Tewari AK, Ali A, Metgud S, Theckumparampil N, Srivastava A, Khani F, et al. Functional outcomes following robotic pros-tatectomy using athermal, traction free risk-stratified grades of nerve sparing. World J Urol. 2013;31:471–80. doi: 10.1007/s00345-012-1018-7. [DOI] [PubMed] [Google Scholar]
- 35.Choi WW, Freire MP, Soukup JR, Yin L, Lipsitz SR, Carvas F, et al. Nerve-sparing technique and urinary control after robot-assisted laparoscopic prostatectomy. World J Urol. 2011;29:21–7. doi: 10.1007/s00345-010-0601-z. [DOI] [PubMed] [Google Scholar]
- 36.Ko YH, Coelho RF, Chauhan S, Sivaraman A, Schatloff O, Cheon J, et al. Factors affecting return of continence 3 months after robot-assisted radical prostatectomy: analysis from a large, prospective data by a single surgeon. J Urol. 2012;187:190–4. doi: 10.1016/j.juro.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 37.Gandaglia G, Suardi N, Gallina A, Capitanio U, Abdollah F, Salonia A, et al. Preoperative erectile function represents a significant predictor of postoperative urinary continence recovery in patients treated with bilateral nerve sparing radical prostatectomy. J Urol. 2012;187:569–74. doi: 10.1016/j.juro.2011.10.034. [DOI] [PubMed] [Google Scholar]
- 38.Montorsi P, Ravagnani PM, Galli S, Salonia A, Briganti A, Werba JP, et al. Association between erectile dysfunction and coronary artery disease: Matching the right target with the right test in the right patient. Eur Urol. 2006;50:721–31. doi: 10.1016/j.eururo.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 39.Yadav R, Mukherjee S, Hermen M, Tan G, Maxfield FR, Webb WW, et al. Multiphoton microscopy of prostate and periprostatic neural tissue: a promising imaging technique for improving nerve-sparing prostatectomy. J Endourol. 2009;23:861–7. doi: 10.1089/end.2009.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tewari AK, Shevchuk MM, Sterling J, Grover S, Herman M, Yadav R, et al. Multiphoton microscopy for structure identification in human prostate and periprostatic tissue: implications in prostate cancer surgery. BJU Int. 2011;108:1421–9. doi: 10.1111/j.1464-410X.2011.10169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rais-Bahrami S, Levinson AW, Fried NM, Lagoda GA, Hristov A, Chuang Y, et al. Optical coherence tomography of cavernous nerves: a step toward real-time intraoperative imaging during nerve-sparing radical prostatectomy. Urology. 2008;72:198–204. doi: 10.1016/j.urology.2007.11.084. [DOI] [PubMed] [Google Scholar]
- 42.Whitney MA, Crisp JL, Nguyen LT, Friedman B, Gross LA, Steinbach P, et al. Fluorescent peptides highlight peripheral nerves during surgery in mice. Nat Biotechnol. 2011;29:352–6. doi: 10.1038/nbt.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walz J, Burnett AL, Costello AJ, Eastham JA, Graefen M, Guillonneau B, et al. A critical analysis of the current knowledge of surgical anatomy related to optimization of cancer control and preservation of continence and erection in candidates for radical prostatectomy. Eur Urol. 2010;57:179–92. doi: 10.1016/j.eururo.2009.11.009. [DOI] [PubMed] [Google Scholar]