Abstract
Alternate modalities for the treatment of Mycobacterium tuberculosis are needed due to the rise in numbers of immunosuppressed individuals at risk for serious disease and the increasing prevalence of multidrug-resistant isolates. Interleukin-12 (IL-12) has been shown to improve immune responses against M. tuberculosis infection in both humans and mice. Previous studies using high-dose IL-12 in various disease models reported a paradoxical immunosuppression. We demonstrate here that exogenous administration of IL-12 for 8 weeks after an aerosolized low dose of M. tuberculosis results in increased survival and decreased pulmonary bacterial loads for CD4-T-cell-deficient mice, most likely due to an early increase in gamma interferon. IL-12 treatment did not impair or enhance the ability of the wild-type mice to control infection, as measured by bacterial numbers. Two novel findings are reported here regarding exogenous IL-12 therapy for M. tuberculosis infections: (i) IL-12 treatment resulted in decreased numbers of immune cells and reduced frequencies of lymphocytes (CD8+, CD4+, and NK cells) in the lungs of infected mice and (ii) IL-12 therapy reduced the pathology of M. tuberculosis-infected lungs, as granulomas were smaller and less numerous. These studies support an immunoregulatory role for IL-12 in tuberculosis.
Mycobacterium tuberculosis remains a significant cause of morbidity and mortality throughout the world. Worldwide, tuberculosis (TB) causes 2 to 3 million deaths annually. In addition, one-third of the world's population is infected. The cornerstone of controlling tuberculosis remains antimycobacterial therapy. Given the increase in numbers of immunosuppressed individuals (including human immunodeficiency virus [HIV]-positive individuals) at risk for infection and the rising prevalence of multidrug-resistant isolates, other treatment modalities for TB, such as immunomodulators, are becoming more attractive.
Interleukin-12 (IL-12) is a heterodimeric cytokine (p70) consisting of subunits p35 and p40. This cytokine is produced by dendritic cells (DCs) and phagocytes after microbial or cytokine stimulation (47). The activation of T cells and NK cells increases the production of the IL-12 receptor (beta 1 and beta 2), which explains why IL-12 can direct the proliferation and activation of T lymphocytes, NK cells, and NKT cells and can induce both gamma interferon (IFN-γ) and increased cytotoxic activity (47). The IL-12 receptor beta 2 subunit is expressed mainly on cells of the Th1 T-cell subpopulation, which also explains the responsiveness of these cells to IL-12 (47).
IL-12 is an excellent inducer of IFN-γ and can form synergy with other cytokines (notably IL-2, IL-18, and IL-27) to further increase IFN-γ production. IFN-γ is essential for the immune response against M. tuberculosis (11, 23, 34). In studies using a neutralizing antibody against IL-12 (9) or IL-12 knockout mice (p35−/−, p40−/−, or double knockouts) (7, 8), the lack of IL-12 caused a decrease in the IFN-γ response and an increased susceptibility of mice to M. tuberculosis infection. The administration of exogenous IL-12 to susceptible mice improved the survival rate and reduced bacterial numbers in mice infected intravenously with M. tuberculosis, although the effect was more obvious in BALB/c mice than in C57BL/6 mice (9, 12).
Given its numerous functions, IL-12 attracted interest as an adjuvant for the treatment of cancer, autoimmune syndromes, and infectious diseases, particularly for immunosuppressed hosts. Exogenous IL-12 has been shown to promote the clearance of Mycobacterium avium in murine models of infection (10, 24, 45). IL-12 may be useful for HIV therapy or its complications (6, 21, 22) and was used successfully in a patient with refractory extrapulmonary TB after an initial failure with antituberculous drugs and IFN-γ (17).
The adverse effects of IL-12 are also documented. IL-12 has a narrow therapeutic window, mainly because of its induction of toxic levels of IFN-γ. Oncology trials using IL-12 in humans resulted in side effects of oligemia, nausea, hepatic dysfunction, and even death (30). Mice given IL-12 displayed pulmonary congestion and hepatic dysfunction after chronic use (3). Interestingly, an increasing number of articles have reported suppressive effects of IL-12 on the immune response (1, 15, 26-28, 32, 33, 37, 39).
The experiments described here document the effects of IL-12 therapy on mice infected acutely with M. tuberculosis via the aerosol route. CD4−/− mice had improved outcomes after 8 weeks of IL-12 administration, characterized by reduced bacterial numbers and less inflammation in the lungs. These CD4−/− mice may have benefited from an early boost in IFN-γ. IL-12 administration to wild-type (WT) mice also resulted in fewer bacterial cells in the lungs and improved pathologies, with no detrimental effect on bacterial numbers. The findings of decreased pathology and reduced immune cell numbers during IL-12 therapy for M. tuberculosis infection are reported here for the first time. These data suggest that IL-12 may have a beneficial effect on the pathology associated with TB and provide evidence for an immunoregulatory role for IL-12.
MATERIALS AND METHODS
Mice.
C57BL/6 mice and CD4−/− (C57BL/6 background) breeding pairs were purchased from Jackson Laboratory (Bar Harbor, Maine). CD4−/− mice were bred in the specific-pathogen-free facility at the University of Pittsburgh Biotechnology Center. When they were 8 to 12 weeks of age, mice were infected and used in experiments. All mice were regularly monitored for the presence of murine pathogens by serological and histological examinations of sentinel mice. C57BL/6 mice were used as the source of bone-marrow-derived DCs.
Bacteria and infections.
M. tuberculosis (Erdman strain; Trudeau Institute, Saranac Lake, N.Y.) was passaged through mice, grown in culture once, and frozen in aliquots. Before infection, an aliquot was thawed, diluted in phosphate-buffered saline (PBS)-0.05% Tween 80, and sonicated for 20 s in a cup-horn sonicator. For aerosol infections, 106 CFU/ml were placed into a nebulizer and mice were exposed to M. tuberculosis for 20 min, followed by 5 min of air, using a nose-only exposure unit (InTox Products, Albuquerque, N.Mex.). This procedure delivered 50 to 100 CFU to the lungs of each mouse. The actual number of CFU delivered for each aerosol infection was determined by plating whole lung homogenates from two mice onto 7H10 agar plates (Difco Laboratories, Detroit, Mich.) at 1 day postinfection. At various time points postinfection, three to four mice per group were sacrificed for studies. There were three concurrent experiments with CD4−/− and C57BL/6 mice and one experiment with C57BL/6 mice alone.
For infections with DCs, frozen aliquots of M. tuberculosis were thawed to start cultures at a concentration of 2.5 × 106 bacteria/ml in liquid medium (7H9 Middlebrook medium; Difco). Bacterial cultures (5 to 7 days of culturing at 37°C in 5% CO2) were washed once, suspended in Dulbecco's minimal essential medium (DMEM; Life Technologies, Grand Island, N.Y.), and sonicated (in a cup-horn sonicator) for 20 s prior to the infection of cells.
IL-12 injections.
Recombinant murine IL-12 (batch Td23G5-7; bioactivity, 3.3 × 106 U/mg) was a generous gift of Genetics Institute, Cambridge, Mass. Mice were injected with 200 ng of recombinant murine IL-12 1 day prior to infection and every Monday, Wednesday, and Friday for 8 weeks. Control animals were injected with an equal volume (300 μl/injection) of PBS at the same time. The dose of 200 ng/mouse was selected based on prior work (9; J. L. Flynn, unpublished data).
CFU determination.
Bacterial burdens were determined by plating serial dilutions of lung homogenates onto 7H10 agar plates (Difco). Plates were incubated at 37°C in 5% CO2 for 21 days prior to counting of colonies.
Histology and immunohistochemistry.
For histological analysis, lungs were fixed in 4% paraformaldehyde and embedded in paraffin. Six-micrometer sections were stained with hematoxylin and eosin. For NOS2 immunohistochemistry, sections were deparaffinized, microwaved in citrate buffer for 10 min, and then stained with anti-NOS2 antibody (Transduction Laboratories, Cincinnati, Ohio) or a rabbit immunoglobulin G control (Accurate Chemical & Scientific Corp., Westbury, N.Y.) as previously described (40). For apoptosis immunohistochemistry, sections were deparaffinized, pretreated with proteinase K for 15 min at room temperature, and stained with terminal deoxynucleotidyl transferase or reaction buffer (ApopTag detection kit; Serologicals Corporation, Norcross, Ga.) for 1 h at 37°C. The sections were subsequently stained with an anti-digoxigenin peroxidase conjugate (ApopTag detection kit; Serologicals Corporation) for 30 min at room temperature. Staining was visualized by using 3,3′-diaminobenzidine (Sigma, St. Louis, Mo.) as a substrate, and cells were counterstained with methylene green.
MetaMorph analysis.
Hematoxylin-and-eosin-stained lung sections were digitally photographed at a magnification of ×2 with a Nikon Eclipse 800 microscope (Millburn, N.J.) and MagnaFire 2.0 software (Goleta, Calif.). Digital photographs were analyzed with MetaMorph Imaging System, version 6.0 (Universal Imaging Corp., Downingtown, Pa.). Each lung section was considered to be the threshold until all cells were highlighted; the total pixel area (excluding holes) was measured. The threshold was then reduced until only inflammatory and granulomatous areas were highlighted; this granulomatous pixel area (excluding holes) was recorded. The percentage of each lung comprised of granulomatous pathology was calculated by the equation % granuloma = granuloma threshold/total threshold × 100. To maintain consistency, we photographed lung sections from each experiment at the same 3-h session, and thresholds at each time point in each experiment were set at similar settings.
Flow cytometry.
Lung and lymph node cells were obtained from M. tuberculosis-infected mice at designated time points. Single-cell suspensions were prepared as described previously (44). Cells were stained with anti-CD4 (Cy-Chrome; clone RM4-5), anti-CD8 (Cy-Chrome; clone 53-6.7), anti-CD49b/Pan-NK (fluorescein isothiocyanate; clone DX5), anti-CD69 (phycoerythrin [PE]; clone H1.2F3), anti-CD11b (allophycocyanin; clone M1/70), anti-B220 (Cy-Chrome; clone RA3-6B2), anti-major histocompatibility complex class II (I-Ab; FITC; clone AF6-120), and anti-Ly-6G and -Ly-6C (PE; clones Gr-1 and RB6-8C5, respectively) fluorescently conjugated antibodies in PBS-20% mouse serum-0.1% bovine serum albumin (BSA)-0.1% sodium azide for 15 min at room temperature. All antibodies were used at 0.2 μg/106 cells and were purchased from BD Pharmingen (San Diego, Calif.). After incubation, cells were washed twice with PBS-0.1% BSA-0.1% sodium azide and fixed with 4% paraformaldehyde for 1 h. Cells were collected in a FACS Caliber instrument (Beckon Dickinson Immunocytometry Systems, San Jose, Calif.) and analyzed by CellQuest software (Becton Dickinson). Cells were gated on lymphocyte and monocyte populations, using forward and side scatter parameters.
Culturing of bone-marrow-derived DCs.
DCs were obtained by eluting the bone marrows of C57BL/6 mice. Bone-marrow-derived cells were washed once with DMEM, and red blood cells were lysed with an NH4-Tris solution at room temperature for 2 min. Bone marrow cells were then washed twice and cultured in NUNC LabTek plates (Fisher Scientific) in 20 ml of DC medium (DMEM, 10% fetal bovine serum [FBS], 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate) overnight to deplete adherent cells. Nonadherent cells were collected the following day and cultured at 106 cells/ml in DC medium supplemented with 1,000 U each of murine granulocyte-macrophage colony-stimulating factor and murine IL-4 (mIL-4)/ml (courtesy of Olivera Finn, University of Pittsburgh; also purchased from Peprotech, Rocky Hill, N.J.). After 3 days of culturing, cytokines were replenished (1,000 U/ml) and the original volume of medium was again added. After 5 to 6 days of culturing, cells were counted, resuspended at 106 cells/ml, and infected overnight with M. tuberculosis at a multiplicity of infection of 3 in the presence of 1,000 U each of murine granulocyte-macrophage colony-stimulating factor and mIL-4/ml.
ELIspot assays.
Millipore Multiscreen 96-well MAIPS4510 plates (Millipore Corp., Bedford, Mass.) were coated with an anti-IFN-γ capture antibody (clone R4-6A2; BD Pharmingen) in PBS at 10 μg/ml overnight at 4°C. Plates were washed the next day with PBS and were blocked with RPMI-15% FBS for 1 h at room temperature. Lung and lymph node single-cell suspensions were plated at 40,000 to 80,000 lung cells/well or 40,000 to 150,000 lymph node cells/well in T-cell medium (DMEM, 10% FBS, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 25 mM HEPES, 5 pM β-metacaptoperonol) supplemented with IL-2 at a final concentration of 20 U/ml. Lung and lymph node cells were either cultured in T-cell medium alone (negative control) or with concanavalin A (10 μg/ml [Sigma]) and uninfected or M. tuberculosis-infected WT DCs. DCs were incubated with T cells at a 1:2 ratio. Plates containing cells were incubated at 37°C in 5% CO2 for approximately 40 h. After incubation, plates were washed with PBS-0.1% Tween 20. A biotinylated anti-IFN-γ antibody (clone XMG 1.2; BD Pharmingen) was added at 5 μg/ml in PBS-0.5% BSA-0.1% Tween 20 and incubated at 37°C for 2 h. Plates were washed in PBS-0.1% Tween 20, and Avidin Peroxidase Complex (PK-6100; Vector Laboratories) was prepared (1 drop each of reagents A and B into 10 ml of PBS-0.1% Tween 20) and added to the plates at 100 μl/well. Plates were incubated for 1 h at room temperature. After incubation, plates were washed with PBS-0.1% Tween 20 and developed by the addition of Vectastain AEC substrate (SK-4200; Vector Laboratories) prepared according to the manufacturer's instructions. The spot-forming units (SFU) per well were counted in an ELIspot reader. The cutoff number of SFU that could be accurately measured by the ELIspot reader was 1,500 SFU/well.
RPAs.
RNase protection assays (RPAs) were performed as previously described (41). The custom-made template sets mck-2b (NOS2, IL-4, IL-12 p40, tumor necrosis factor alpha, IL-1β, IL-1α, and IFN-γ) and mck-1 (IL-4, IL-5, IL-10, IL-13, IL-15, IL-9, IL-2, IL-6, and IFN-γ) were used. Relative gene expression was quantified by use of a densitometer (ImageQuant Software; Molecular Dynamics, Sunnyvale, Calif.) and was compared to the abundance of the housekeeping gene L32.
Real-time reverse transcription-PCR (RT-PCR).
RNA extractions were performed from total lungs as described for RPA methods. cDNAs were synthesized from total lung RNAs. Probes and primers for IFN-γ were synthesized from published murine sequences (16). Taqman PCRs were prepared in 96-well microplates (Applied Biosystems) according to the manufacturer's instructions, with hypoxanthine phosphoribosyltransferase (HPRT) as an endogenous control. All samples were run in triplicate. An uninfected lung was used as a negative control. The reactions were performed in an ABI Prism 7700 SDS machine to generate cycles (Ct). The gene expression of IFN-γ was calculated as follows: 2−ΔΔCt, where ΔΔCt = ΔCt (of sample) − ΔCt (of uninfected lung) and ΔCt = Ct (of IFN-γ) − Ct (of HPRT). Efficiencies of the primer and probe sets for both IFN-γ and HPRT were >98%.
Statistics.
The results are given as means ± standard errors of the means. Statistical significance was calculated by using the student t test. For comparisons of CFU between mice, the raw data were transformed into log numbers prior to statistical analyses. P values of ≤0.05 or ≤0.01 were considered significant, as indicated.
RESULTS
IL-12 improved overall survival of CD4-T-cell deficient mice after aerosol infection with M. tuberculosis.
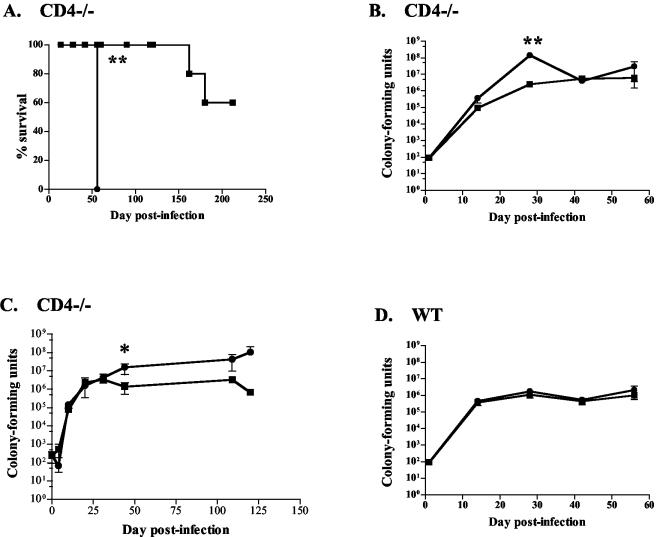
We examined the effect of IL-12 on CD4−/− mice infected with M. tuberculosis via aerosolization. There was no observed effect of short-term (1 week) IL-12 treatment on either WT C57BL/6 (WT) or CD4−/− mice (data not shown). A long-term treatment regimen was then used with WT and CD4−/− mice. IL-12 had no effect on the survival of WT mice; 90% of all mice survived the length of the experiment (data not shown). With IL-12 therapy, CD4−/− mice survived longer than their saline controls (Fig. 1A) in two of three experiments. This was surprising, since CD4−/− mice typically die from M. tuberculosis at 2 to 3 months postinfection due to an early (but transient) deficiency in IFN-γ production (4). In a third experiment, to our amazement no deaths occurred among the saline- or IL-12-treated CD4-T-cell-deficient mice after aerosol infection up to 120 days postinfection. Interestingly, these mice had received a high aerosol dose of M. tuberculosis (300 to 500 CFU compared to our target of 50 to 100 CFU per mouse) due to a temporary defect in our nebulizer. However, bacterial counts of the control mice were increased compared to those of the IL-12-treated group at the end of the experiment (Fig. 1C), supporting a beneficial effect of IL-12 in CD4−/− mice.
FIG. 1.
IL-12 improved survival and reduced bacterial loads of CD4-T-cell deficient mice after aerosol infection with M. tuberculosis, with no detriment to infected WT mice. Squares, IL-12 treatment; circles, PBS treatment. (A) CD4−/− mice treated with IL-12 for 8 weeks had increased survival compared with saline controls. Four to six mice per group were assessed for survival. Data are from one experiment which is representative of two experiments. (B and C) Reduction of pulmonary bacterial loads at 1 month postinfection was seen in CD4−/− mice with IL-12 therapy compared with saline controls. (D) WT mice had no difference in CFU. Each data point (except for survival graphs) represents three to four mice, and the bars represent standard errors. Graphs (except for survival curves) are representative of CD4−/− mice from three experiments and WT mice from four experiments. *, P ≤ 0.05; **, P ≤ 0.01.
By 1 month postinfection, bacterial numbers in the lungs of CD4-T-cell-deficient mice treated with IL-12 were ∼50-fold lower than those in saline controls (Fig. 1B and C), although there were no statistically significant differences at later time points. IL-12 had no apparent effect on bacterial loads in WT (C57BL/6) mice (Fig. 1D).
IL-12 reduced inflammation and granuloma formation in lungs.
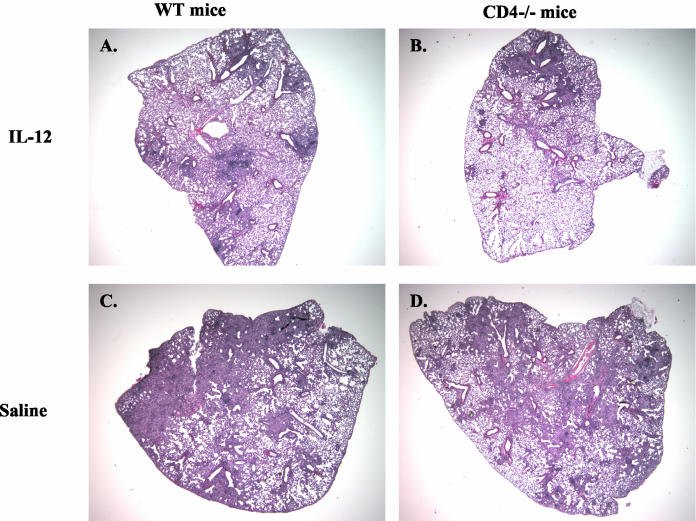
During the first 3 weeks postinfection, histologies and granuloma formation appeared similar for IL-12-treated and untreated groups of mice. However, by 1 month postinfection, lungs of both CD4−/− and WT mice given IL-12 had obvious differences from their saline-treated counterparts. IL-12-treated mice had smaller and fewer numbers of granulomas (Fig. 2), even though there were no differences in bacterial numbers in the lungs of WT mice (Fig. 1D). The lungs also displayed less cellular infiltration in the perivascular regions. A computer program (MetaMorph) was used to quantify infiltration in the lungs, using lung sections from treated and untreated mice. The percentage of lung tissue with heavy cellular infiltration and granulomas was lower for IL-12-treated animals than for saline controls by 4 to 6 weeks postinfection (Table 1), although the differences reached statistical significance only for the WT group.
FIG. 2.
Infected mice given IL-12 displayed less inflammation and fewer granulomas. Hematoxylin and eosin staining was performed on 6-μm-thick formalin-fixed tissue sections at each time point postinfection. Magnification, ×2. At day 44 postinfection, WT and CD4−/− mice treated with IL-12 (A and B) had less cellular infiltration and pathology than saline-treated mice (C and D). These sections are representative of CD4−/− mice from three experiments and WT mice from four experiments.
TABLE 1.
IL-12 reduces pathology in the lungs of infected micea
| TB-infected mice and treatment | % of granulomatous regions on day:
|
||
|---|---|---|---|
| 28 | 42 | 56 | |
| WT plus IL-12 | 40.3 ± 8.5 | 34.6 ± 3.9** | 66.4 ± 0.8** |
| WT plus saline | 49.6 ± 7.8 | 68.3 ± 3.4 | 78.1 ± 2.8 |
| CD4−/− plus IL-12 | 36.9 ± 6.9 | 53.6 ± 8.9 | 80.5 ± 1.4 |
| CD4−/− plus saline | 34.2 ± 13.0 | 65.8 ± 5.6 | 78.5 ± 4.4 |
MetaMorph analysis was performed on digital microscopy of hematoxylin-and-eosin-stained tissue sections (×2 magnification). Percentages were calculated by the equation [granulomatous regions (measured in pixels)/total lung area (in pixels)] × 100. Each value represents the mean of three or four mice ± the standard error. The data are representative of three experiments with CD4−/− mice and four experiments with WT mice. **, P < 0.01 when comparing treatments within WT or CD4−/− groups.
IL-12 reduced cell numbers in the lungs.
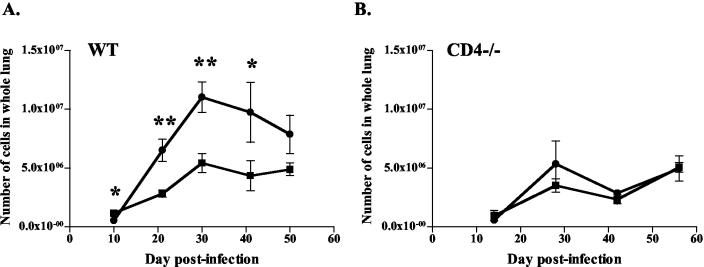
To address the histologic findings, we counted single-cell suspensions of the lungs at various time points postinfection. Significantly fewer cells were recovered from the lungs of IL-12-treated mice. For WT mice, the decrease in total cell numbers in the IL-12 group was seen by 3 weeks postinfection (Fig. 3A). The total cell numbers in the IL-12 groups averaged 56% of the those in the PBS group from 3 weeks postinfection until the end of IL-12 therapy (data not shown). In CD4−/− mice, modest or no differences in total cell numbers were observed (Fig. 3B). At later time points, less cellular debris (dead cells and extracellular material) was observed during cell enumeration of lungs from IL-12-treated mice (both CD4−/− and WT), suggesting a reduction of inflammation and necrosis in the lungs of IL-12-treated mice.
FIG. 3.
IL-12 administration reduced total numbers of immune cells in the lungs of TB-infected mice. Squares, IL-12 treatment; circles, PBS treatment. (A) IL-12 treatment reduced total cell numbers in the lungs of WT mice compared with saline controls. (B) Similar trends were seen in CD4−/− mice. Each data point represents three to four mice and the bars represent the standard errors. Graphs are representative of CD4−/− mice from three experiments and WT mice from four experiments. *, P ≤ 0.05; **, P ≤ 0.01.
T-cell populations were reduced in lungs of IL-12-treated mice.
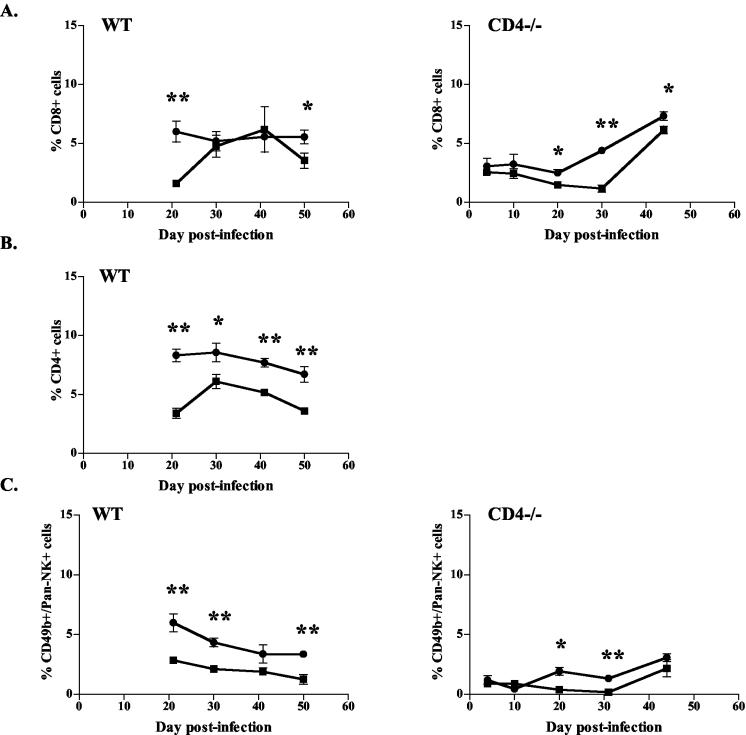
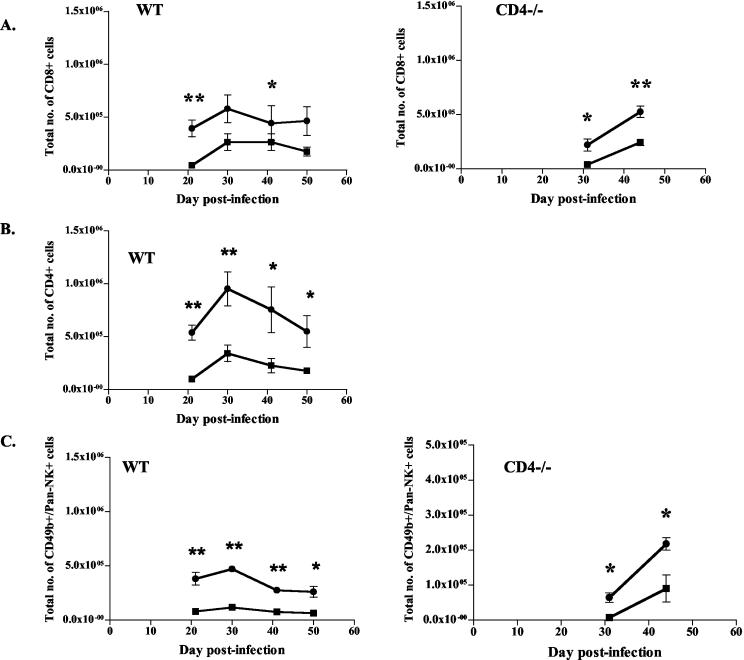
Given the reduced cell numbers seen with IL-12 treatment, we questioned whether certain subpopulations of immune cells were particularly affected. Flow cytometric analysis of the cellular composition of lungs of WT and CD4−/− mice revealed reductions in the frequency of CD8+ (Fig. 4A) and CD4+ (Fig. 4B, WT only) T cells in the IL-12-treated mice compared to saline-treated mice from 3 weeks postinfection until the end of therapy. This translates to an equivalent reduction in total numbers of CD8+ (39% ± 17%) and CD4+ (31% ± 13%) cells in the lungs of WT mice given IL-12 compared to those in the lungs of controls (Fig. 5A and B). No changes in the T-cell ratio of CD4/CD8 cells were noted between groups of IL-12- and PBS-treated WT mice, confirming that IL-12 did not preferentially affect either the CD8+ or CD4+ population (data not shown). During IL-12 therapy, numbers of NK cells were also reduced in the lungs of WT mice, and to a lesser extent, of CD4−/− mice (Fig. 4C and 5C). Given the reduction in total numbers of immune cells and the lower frequencies of specific lymphocyte populations, significant reductions in the numbers of CD8+, CD4+, and NK cells were observed in IL-12-treated mice (both CD4−/− and WT) compared to controls (Table 2 and Fig. 5). No differences in percentages of other immune cells (macrophages, neutrophils, and B cells) were noted, although the total numbers of these immune cells must have been decreased given the overall reduction in total immune cell numbers (Table 2).
FIG. 4.
IL-12 treatment decreased lymphocyte populations in lungs of TB-infected mice. Percentages of CD8+, CD4+, and NK+ (CD49b/Pan-NK) cells were determined by flow cytometry of total cells obtained from lung homogenates. Squares, IL-12 treatment; circles, PBS treatment. The percentages of CD8+ (A), CD4+ (B), and NK (C) cells in whole lungs were reduced in IL-12-treated mice compared to saline controls. Each data point represents three to four mice and the bars represent the standard errors. CD4+ and NK+ graphs are representative of CD4−/− mice from three experiments and WT mice from four experiments; CD8+ graphs are representative of CD4−/− mice from two experiments and WT mice from three experiments. *, P ≤ 0.05; **, P ≤ 0.01.
FIG. 5.
IL-12 treatment decreased total cell numbers in lungs of TB-infected mice. Total numbers of CD8+, CD4+, and NK+ (CD49b/Pan-NK) cells were determined by flow cytometry of total cells obtained from lung homogenates. Squares, IL-12 treatment; circles, PBS treatment. CD8+ (A), CD4+ (B), and NK (C) cell numbers in whole lungs were reduced in IL-12-treated mice compared to saline controls. Each data point represents three to four mice and the bars represent the standard errors. CD4+ and NK+ graphs are representative of CD4−/− mice from three experiments and WT mice from four experiments; CD8+ graphs are representative of CD4−/− mice from two experiments and WT mice from three experiments. In the above experiment with CD4−/− mice, total cell numbers from whole lungs were not available for early times postinfection. *, P ≤ 0.05; **, P ≤ 0.01.
TABLE 2.
Numbers of immune cells within the lungs at 44 days postinfectiona
| TB-infected mice and treatment | Total no. of cells (104) | No. of cells (104)
|
|||||
|---|---|---|---|---|---|---|---|
| CD8+ | CD4+ | NK+ | Neutrophils | Macrophages | IFN-γ+ | ||
| WT plus IL-12 | 313 ± 79 | 20.4 ± 7.7 | 18.0 ± 5.7 | 9.0 ± 2.8 | 6.1 ± 1.9 | 22.8 ± 7.8 | 1.1 ± 0.3 |
| WT plus saline | 973 ± 343 | 52.1 ± 13.8 | 74.8 ± 26.0* | 29.1 ± 7.8* | 16.4 ± 6.2 | 71.7 ± 26.5 | 4.1 ± 1.9 |
| CD4−/− plus IL-12 | 403 ± 66 | 24.3 ± 3.0 | NA | 9.0 ± 3.8 | 10.9 ± 3.3 | 26.8 ± 4.8 | 0.8 ± 0.4 |
| CD4−/− plus saline | 717 ± 54** | 52.6 ± 5.3** | NA | 21.8 ± 1.8* | 22.2 ± 2.1* | 55.7 ± 5.5** | 4.6 ± 0.6** |
Numbers of total cells, CD8+ lymphocytes, CD4+ lymphocytes, natural killer cells, neutrophils, macrophages, and IFN-γ producers at 44 days postinfection were calculated. Cell numbers are means ± standard errors. Each value represents the mean of three or four mice. The data are representative of three experiments with CD4−/− mice and four experiments with WT mice. For this experiment, B cells were not studied. *, P < 0.05; **, P < 0.01 when comparing treatments within WT or CD4−/− groups. NA, not applicable.
IL-12 decreased priming of T cells during M. tuberculosis infection.
We investigated possible mechanisms by which IL-12 therapy resulted in reduced cell numbers and pathology. Although multiple reports showed that IL-12 immunosuppression is mediated by an accumulation of nitric oxide (15, 26-28), no difference in NOS2 protein by immunohistochemistry of lung sections or NOS2 mRNA (by RPA) was seen with IL-12 therapy (data not shown). We tested whether IL-12 administration increased apoptosis in the lungs and observed no differences for in situ staining for apoptotic cells of lung sections from CD4−/− and WT mice. There was no difference in total immune cell numbers in mediastinal lymph nodes that would explain the subsequent difference in lung numbers during IL-12 therapy (data not shown).
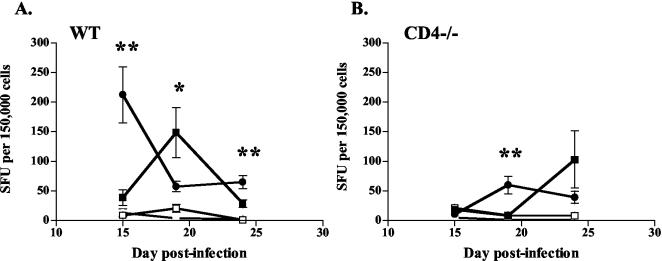
We studied whether reduced cell numbers in the lungs during IL-12 therapy resulted from decreased priming. For effector T cells to migrate to the lungs, they must be primed in secondary lymphoid tissues. After aerosol M. tuberculosis infection, this occurs in the mediastinal lymph nodes of mice; the peak of priming occurs between 2 and 3 weeks postinfection and is dependent on endogenous IL-12 production (5, 29). Priming can be assessed by measuring the numbers of IFN-γ-producing T cells in the lymph nodes (5, 29), which we did by ELIspot analysis. The peak of IFN-γ-producing T cells in the lungs occurred after the peak of priming, as cells migrated from the lymph nodes to the lungs. In WT and CD4−/− mice, the peak numbers of IFN-γ-producing T cells in the mediastinal lymph nodes were delayed by IL-12 treatment (Fig. 6). This suggests that although endogenous IL-12 is required for priming, excess IL-12, due to exogenous administration in this case, can delay the priming of T cells during M. tuberculosis infection.
FIG. 6.
A delay in T-cell priming during IL-12 therapy was seen in mediastinal lymph nodes. ELIspot assays were conducted, using 150,000 cells from mediastinal lymph nodes per well and uninfected (open symbols) or M. tuberculosis-infected (closed symbols) WT DCs as stimulators. Squares, IL-12 treatment; circles, PBS treatment. (A) IFN-γ production of T cells from lymph nodes was delayed in WT mice given IL-12 compared to saline controls. (B) Similar results were seen with CD4−/− mice. Mediastinal lymph nodes were unable to be grossly detected at days 5 and 10 postinfection. Each data point represents three or four mice and the bars represent the standard errors. Graphs represent two experiments with mediastinal lymph nodes from WT mice and one experiment with lymph nodes from CD4−/− mice. *, P ≤ 0.05; **, P ≤ 0.01.
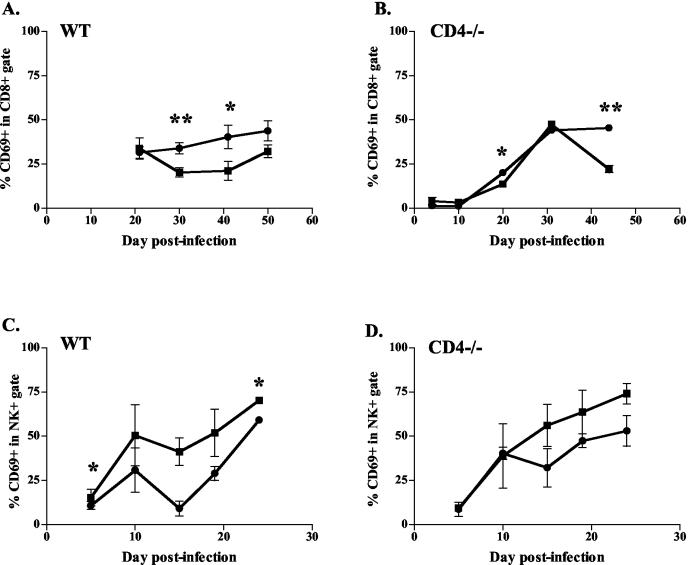
IL-12 increased activation of NK cells but decreased activation of CD8+ T cells.
To address the similar or improved clinical outcomes of mice in the face of fewer immune cells in the lungs, we studied whether IL-12 treatment increased the activation of lymphocytes and/or macrophages. CD69 was chosen as an activation marker for T and NK cells in the lungs (14, 43). The IL-12-treated group had fewer CD69+ cells within the CD8+ lymphocyte gate in the lungs than did PBS-treated controls for both WT and CD4−/− mice (Fig. 7A and B). No reproducible differences were seen in CD69 expression in the CD4+ lymphocyte population between the IL-12- and PBS-treated groups (data not shown). The number of CD69+ cells within the NK cell population increased with IL-12 administration (Fig. 7C and D). The presence of NOS2 protein in the lungs was used as a marker of macrophage activation. As discussed earlier, no differences by immunohistochemistry of NOS2 in lung sections were seen as a result of IL-12 therapy, nor were differences detected in the gene expression of NOS2 (data not shown).
FIG. 7.
IL-12-treated mice had reduced frequencies of activated cells within the CD8+-T-cell population, but increased activation of NK cells, compared with control mice. The specific lymphocyte subpopulation (either CD8+ or NK+ [CD49b/Pan-NK]) was gated, and percentages of CD69+ cells within the gate are depicted. Squares, IL-12 treatment; circles, PBS treatment. (A and B) Less activation of CD8+ T cells in WT and CD4−/− mice was seen during IL-12 treatment compared with saline controls. (C and D) More activation of NK cells in WT and CD4−/− mice was seen during IL-12 treatment compared with saline controls. Each data point represents three or four mice and the bars represent the standard errors. Graphs are representative of CD4−/− mice from two experiments and WT mice from three experiments. *, P ≤ 0.05; **, P ≤ 0.01.
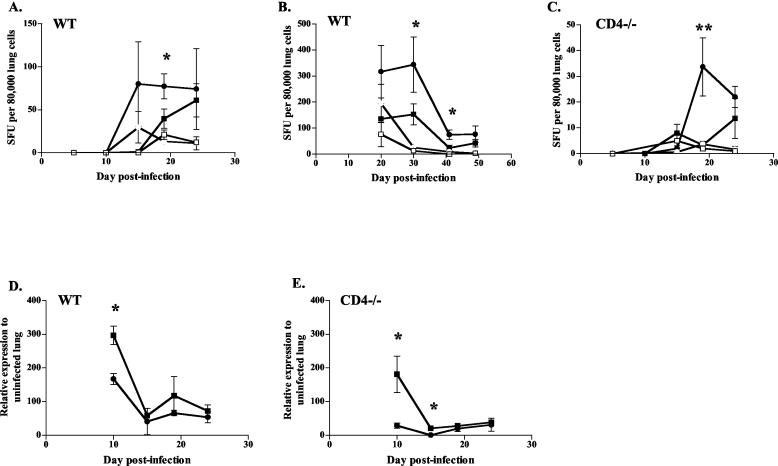
Early IFN-γ levels were enhanced by IL-12 treatment.
To determine the mechanism by which IL-12 improved the clinical outcome of CD4−/− mice despite a reduction in cell numbers, we investigated IFN-γ production. An ELIspot analysis was performed on lung cells to determine the frequency of T cells producing IFN-γ after IL-12 treatment. The lungs of IL-12-treated WT and CD4−/− mice had reduced frequencies of M. tuberculosis-specific, IFN-γ-producing T cells (Fig. 8A -C) at 2 weeks postinfection. No IFN-γ production was observed from lung T cells harvested at 5 and 10 days postinfection. Coupled with the lower overall cell numbers, this resulted in IL-12-treated animals (both CD4−/− and WT) having substantially fewer T cells producing IFN-γ in their lungs (Table 2).
FIG. 8.
IL-12 therapy decreased percentages of immune cells in lungs producing IFN-γ, but it may boost early production of IFN-γ from individual cells. ELIspot assays were conducted, using 80,000 lung cells per well and uninfected (open symbols) or M. tuberculosis-infected (closed symbols) WT DCs as stimulators. Squares, IL-12 treatment; circles, PBS treatment. Cells from WT mice (A and B) and CD4−/− mice (C) given IL-12 had lower frequencies of M. tuberculosis-specific cells producing IFN-γ than did saline controls. ELIspot graphs are representative of CD4−/− mice from two experiments and WT mice from three experiments. (D and E) mRNA expression of IFN-γ, measured by real time RT-PCR, was increased at day 10 postinfection in WT (D) and CD4−/− (E) mice treated with IL-12 compared with saline controls. mRNA results are representative of two experiments. In all graphs, each data point represents three or four mice and the bars represent the standard errors. *, P ≤ 0.05; **, P ≤ 0.01.
To determine the overall levels of IFN-γ in the lungs, including that from sources other than T cells, we assessed gene expression by real-time RT-PCR and RPA on lung RNAs from infected mice. There was a higher level of IFN-γ at day 10 in IL-12-treated WT and CD4−/− mice (Fig. 8D and E; data not shown). The increased production was not sustained, and IFN-γ levels in the IL-12-treated mice were at the level of the saline controls by 1 month postinfection. This early IFN-γ production is likely to be from cells other than T cells, since we rarely detect IFN-γ responses in the lungs by ELIspot prior to 2 weeks postinfection. From the results of our studies, the cells that are most likely to be responsible are NK cells, which were more activated in IL-12-treated mice (Fig. 7C and D).
DISCUSSION
The results presented here indicate that the administration of exogenous IL-12 to M. tuberculosis-infected mice (both CD4−/− and WT) has an anti-inflammatory effect, resulting in fewer immune cells in the lungs, with particular reductions in the frequencies of CD8+, CD4+, and NK cells. The reduction in cell numbers was observed in histologic sections of the lungs of IL-12-treated mice, which had smaller and fewer numbers of granulomas, and was confirmed by flow cytometry. The anti-inflammatory effects of exogenous IL-12 therapy during M. tuberculosis infection are reported here for the first time. Despite these reductions in both cell numbers and granuloma formation, IL-12 therapy did not have a detrimental effect on the mice. On the contrary, CD4−/− mice given IL-12 had an increased overall survival rate after M. tuberculosis infection, with a reduction in bacterial loads in the lungs.
The cornerstone of the control of TB remains antimycobacterial therapy. Immunomodulators are increasingly studied as potential adjuvants to therapy for immunosuppressed individuals infected with M. tuberculosis. IL-12 is the prototype cytokine of the IL-12 family (47), which includes recently discovered heterodimeric members IL-23 (composed of subunits p40 and p19) and IL-27 (composed of p28 and a p40-like subunit, EBI3) (47, 48). IL-12 drives Th0 cells toward the Th1 phenotype. The role of IL-12 is clearly beneficial in infectious models that require a Th1 profile to promote pathogen clearance. This was first reported for Leishmania (19, 46) and subsequently was reported for other intracellular pathogens, such as Toxoplasma gondii (13) and M. tuberculosis (9, 12). Our data indicate that although endogenous IL-12 is crucial for the priming of a T-cell response against M. tuberculosis (29), additional IL-12 delivered exogenously for an extended period of time, at least to resistant C57BL/6 mice, did not result in a stronger T-cell response. In fact, additional IL-12 had an anti-inflammatory effect in this model. The level of IL-12 in vivo appears to be an important determinant of the outcome of infection and the associated pathology. As a direct role for mycobacterial antigens in the induction of IL-12 in DCs was previously demonstrated (29), it is possible that various mycobacterial strains induce different amounts of IL-12, leading to differences in both the immune response and pathology.
There are multiple reports on the toxicity of IL-12 therapies, usually as a consequence of toxic levels of induced IFN-γ (3, 30). The use of IL-12 in the oncology, rheumatology, and infectious disease fields has shown another side effect of IL-12, namely a paradoxical immunosuppression (9, 27, 28, 33). In studies involving mice, very high doses of IL-12 (>500 ng/mouse) were used. Our results indicate that similar effects may be seen with the persistent low-dose administration of IL-12 in the setting of M. tuberculosis infections. We did not observe similar effects on cell numbers and pathology in infected mice treated for just 1 week with IL-12 (data not shown).
In different disease models, particular immune cell subsets may be affected by IL-12 therapy. Oncologic reports note the suppressive effects of IL-12 on CD8+ T cells (2, 32). IL-12 administration as an adjuvant during vaccination with various HIV T-cell epitopes results in a transient depletion of B cells, T cells, macrophages, and DCs in the spleen (37). In viral systems, IL-12 preferentially decreases the CD8+-T-cell population during murine lymphocytic choriomeningitis virus infection, while it decreases the CD4+-T-cell population during murine cytomegalovirus infection (33). It was hypothesized that IL-12 suppresses the cellular subpopulation that is most needed for resolution of the infection (33). We did not note any T-cell subset that was more profoundly affected by the administration of IL-12 during acute TB infections. In addition, no changes in the T-cell ratio of CD4 and CD8 numbers were noted.
Theories about the mechanism of cell suppression by exogenous IL-12 are contradictory and dependent on the disease model studied. Given that the benefits of IL-12 are often seen in the context of underlying immunosuppression, exogenous IL-12 given to an otherwise healthy host may cause a paradoxical immunosuppression. One published mechanism to describe this involves a negative feedback loop of increased IL-12 inducing the production of IFN-γ (15, 28, 47). This further activates macrophages to increase NOS2 production and subsequent reactive nitrogen intermediates, which can negatively inhibit T-lymphocyte proliferation. This mechanism did not explain our findings, since no differences in NOS2 were seen either qualitatively (in situ staining) or quantitatively (gene expression) during IL-12 treatment. Our findings are more in keeping with other reports of IL-12 immunosuppression without increases in reactive nitrogen intermediates (1, 39). Our report of IL-12 modulating pathology and cell numbers is also in concert with recent publications about other members of the growing IL-12 cytokine family. Mice lacking the IL-27 receptor WSX-1 are unable to downregulate protective T-cell responses to Toxoplasma gondii and Trypanosoma cruzi infections (18, 49), implying that IL-27 is important in moderating T-cell-dependent immune responses by limiting hyperactivity and IFN-γ levels (48).
We explored several possibilities to account for the reduced cell numbers in the lungs. The first possibility was that IL-12 increased the apoptosis of lung cells. Conflicting reports on IL-12 document that therapy may decrease (35) or increase (28) apoptosis. No qualitative differences were noted with in situ staining for apoptosis in lung sections. Since an accurate in vivo determination of apoptotic cells in the lungs is technically difficult and no obvious differences were seen for terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling of sections, we did not pursue this further. Another possibility was that IL-12 reduced total cell numbers in the mediastinal lymph nodes, resulting in fewer numbers in the lungs. We observed no difference in total cell numbers in lymph nodes between IL-12- and saline-treated animals (both CD4−/− and WT mice).
Interestingly, the priming of naïve T cells in the mediastinal lymph nodes obtained from CD4−/− and WT mice was delayed 4 to 5 days during IL-12 treatment. This is a potential cause of the delay of cellular infiltration in the lung and subsequent granuloma formation in IL-12-treated animals during TB infection. Although endogenous IL-12 augments priming (31, 42), no previous studies have shown that additional exogenous IL-12 delays T-cell priming. Interestingly, a recent study showed that activated NK cells may, in sufficient numbers, induce the death of DCs, particularly immature cells (14). The activation of NK cells by IL-12 may have the paradoxical effect of reducing antigen presentation. However, the cellular infiltration remained reduced throughout IL-12 treatment in our study, long after the initial peak in priming. Therefore, although the effects of IL-12 on priming IFN-γ responses may be responsible for reduced cellular migration, it is unlikely to be the only mechanism.
We are in the process of investigating other mechanisms by which IL-12 may mediate the inflammatory response. IL-10 is known to limit the effectiveness of the Th1 response (47) in many intracellular infectious models. Increased IL-10 in the supernatants of tumor-specific cytotoxic T lymphocytes (CTLs) cultured with a high dose of IL-12 has been reported (1). Although a well-described function of IL-12 is to augment the cytotoxic potential of CD8+ and NK cells (47), two studies reported that IL-12 administration suppressed their cytotoxic potential (1, 32). It would be interesting to determine the interaction and contribution of cytotoxicity with IL-12 in our TB model. IL-12 has been described to stabilize the Th1 phenotype and to increase the survival of T cells in leishmania and toxoplasmosis (36, 51). The persistent dosing in our experiments may have led to an increased longevity of cells and may explain why fewer T cells were needed at the site of infection. A study using a MethA sarcoma showed that IL-12 (and IFN-γ) induced a “later” population of CD4 T cells that were suppressive (32). Perhaps regulatory T cells play an important role in modulating the immune response-mediated pathology in chronic diseases such as TB. In addition, IL-12 may affect cellular recruitment by reducing the levels of chemokines (50) during acute TB infections.
Although histologically the granulomas of IL-12-treated mice (both CD4−/− and WT) were less numerous and smaller, they were still functional in controlling bacilli, since the bacterial loads in the WT mice given IL-12 were similar to those in saline controls, and CD4−/− mice actually had improved outcomes. The death of hosts suffering rapid fulminant TB occurs from the inability of the host to contain the pathogen or to control the pathology. For Listeria, Pneumocystis carinii, and M. tuberculosis infections, techniques or immunodeficiencies that reduce inflammation or cell infiltration can benefit the infected host (20, 38, 41). IL-12 therapy may moderate the early intense cellular inflammation that occurs after aerosol infection. Our group has previously published data showing that the immune response in C57BL/6 mice is overexuberant and that a reduced response can still control a low-dose infection (41). This reduction in pathology may explain in part the improved clinical outcome of CD4−/− mice with IL-12 therapy during M. tuberculosis infection.
In light of reduced cell numbers, it seemed logical to assume that IL-12 administration may increase lymphocytic activation; IL-12 p40−/− and IL-12 p35−/− M. tuberculosis-infected mice were reported to have fewer activated CD4 T cells in the lungs (7). Although we did not observe an increased activation of T cells, NK cells were more activated in IL-12-treated mice, as expected (25). In our model, IL-12 administration increased IFN-γ expression early after infection (day 10), but there was an overall reduction in the frequency and number of T cells producing IFN-γ from the lungs after 1 month of infection. We hypothesize that the enhanced IFN-γ production early in infection was related to the increase in activated NK cells. Other immune cells in the lungs, such as γδ cells, may also account (wholly or in part) for the early boost of IFN-γ. Since members of our laboratory previously reported that the early demise of CD4−/− mice infected with M. tuberculosis may be due to an early (but transient) decrease in IFN-γ production (4), an early boost in IFN-γ (from exogenous IL-12) may also be responsible for the improved outcome of CD4−/− mice that were treated with IL-12.
Previous reports of immunosuppression from IL-12 stemmed from infrequent or single-high-dose administration. We used a low dose of IL-12 to avoid toxicity from accumulated IFN-γ levels and administered it repetitively to accentuate its effects, assuming that in a human model of treatment, this would be a more palatable dosing regimen. This report shows that the persistent low-dose administration of IL-12 can have immunosuppressive effects in both CD4−/− and WT mice, with a modulation of pathology, which may not be detrimental to a competent host infected with M. tuberculosis and may still be beneficial to a CD4-deficient host.
Acknowledgments
We thank the Genetics Institute for providing recombinant murine IL-12. We are grateful to Vanja Lazarevic, Holly Scott, and the rest of the Flynn laboratory for helpful discussions and to Amy Myers and Carolyn Bigbee for technical assistance. We appreciate the help of Simon C. Watkins and members of his laboratory with digital microscopy and analysis.
This work was supported by a Pediatric Infectious Diseases Society fellowship and the Otis H. Childs Charitable Trust (D.N.) and by NIH grant RO1 AI37859 and American Lung Association grant CI-016-N (J.L.F.).
Editor: J. B. Bliska
REFERENCES
- 1.Asselin-Paturel, C., S. Megherat, I. Vergnon, H. Echchakir, G. Dorothee, S. Blesson, F. Gay, F. Mami-Chouaib, and S. Chouaib. 2001. Differential effect of high doses versus low doses of interleukin-12 on the adoptive transfer of human specific cytotoxic T lymphocyte in autologous lung tumors engrafted into severe combined immunodeficiency disease-nonobese diabetic mice: relation with interleukin-10 induction. Cancer 91:113-122. [PubMed] [Google Scholar]
- 2.Brunda, M. J., L. Luistro, R. R. Warrier, R. B. Wright, B. R. Hubbard, M. Murphy, S. F. Wolf, and M. K. Gately. 1993. Antitumor and antimetastatic activity of interleukin 12 against murine tumors. J. Exp. Med. 178:1223-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Car, B. D., V. M. Eng, B. Schnyder, M. LeHir, A. N. Shakhov, G. Woerly, S. Huang, M. Aguet, T. D. Anderson, and B. Ryffel. 1995. Role of interferon-gamma in interleukin 12-induced pathology in mice. Am. J. Pathol. 147:1693-1707. [PMC free article] [PubMed] [Google Scholar]
- 4.Caruso, A. M., N. Serbina, E. Klein, K. Triebold, B. R. Bloom, and J. L. Flynn. 1999. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J. Immunol. 162:5407-5416. [PubMed] [Google Scholar]
- 5.Chackerian, A. A., J. M. Alt, T. V. Perera, C. C. Dascher, and S. M. Behar. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70:4501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chehimi, J., S. E. Starr, I. Frank, M. Rengaraju, S. J. Jackson, C. Llanes, M. Kobayashi, B. Perussia, D. Young, E. Nickbarg, et al. 1992. Natural killer (NK) cell stimulatory factor increases the cytotoxic activity of NK cells from both healthy donors and human immunodeficiency virus-infected patients. J. Exp. Med. 175:789-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, A. M., A. Kipnis, J. Turner, J. Magram, J. Ferrante, and I. M. Orme. 2002. Mice lacking bioactive IL-12 can generate protective, antigen-specific cellular responses to mycobacterial infection only if the IL-12 p40 subunit is present. J. Immunol. 168:1322-1327. [DOI] [PubMed] [Google Scholar]
- 8.Cooper, A. M., J. Magram, J. Ferrante, and I. M. Orme. 1997. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J. Exp. Med. 186:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper, A. M., A. D. Roberts, E. R. Rhoades, J. E. Callahan, D. M. Getzy, and I. M. Orme. 1995. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology 84:423-432. [PMC free article] [PubMed] [Google Scholar]
- 10.Doherty, T. M., and A. Sher. 1998. IL-12 promotes drug-induced clearance of Mycobacterium avium infection in mice. J. Immunol. 160:5428-5435. [PubMed] [Google Scholar]
- 11.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. A. Stewart, and B. R. Bloom. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flynn, J. L., M. M. Goldstein, K. J. Triebold, J. Sypek, S. Wolf, and B. R. Bloom. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 155:2515-2524. [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 14.Gerosa, F., B. Baldani-Guerra, C. Nisii, V. Marchesini, G. Carra, and G. Trinchieri. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195:327-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gherardi, M. M., J. C. Ramirez, and M. Esteban. 2000. Interleukin-12 (IL-12) enhancement of the cellular immune response against human immunodeficiency virus type 1 env antigen in a DNA prime/vaccinia virus boost vaccine regimen is time and dose dependent: suppressive effects of IL-12 boost are mediated by nitric oxide. J. Virol. 74:6278-6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giulietti, A., L. Overbergh, D. Valckx, B. Decallonne, R. Bouillon, and C. Mathieu. 2001. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25:386-401. [DOI] [PubMed] [Google Scholar]
- 17.Greinert, U., M. Ernst, M. Schlaak, and P. Entzian. 2001. Interleukin-12 as successful adjuvant in tuberculosis treatment. Eur. Respir. J. 17:1049-1051. [DOI] [PubMed] [Google Scholar]
- 18.Hamano, S., K. Himeno, Y. Miyazaki, K. Ishii, A. Yamanaka, A. Takeda, M. Zhang, H. Hisaeda, T. W. Mak, A. Yoshimura, and H. Yoshida. 2003. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity 19:657-667. [DOI] [PubMed] [Google Scholar]
- 19.Heinzel, F. P., D. S. Schoenhaut, R. M. Rerko, L. E. Rosser, and M. K. Gately. 1993. Recombinant interleukin 12 cures mice infected with Leishmania major. J. Exp. Med. 177:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hori, S., T. L. Carvalho, and J. Demengeot. 2002. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur. J. Immunol. 32:1282-1291. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson, M. A., D. Hardy, E. Connick, J. Watson, and M. DeBruin. 2000. Phase 1 trial of a single dose of recombinant human interleukin-12 in human immunodeficiency virus-infected patients with 100-500 CD4 cells/microliter. J. Infect. Dis. 182:1070-1076. [DOI] [PubMed] [Google Scholar]
- 22.Jacobson, M. A., J. Spritzler, A. Landay, E. Chan, D. Katzenstein, B. Schock, L. Fox, J. Roe, S. Kundu, and R. Pollard. 2002. A phase I, placebo-controlled trial of multi-dose recombinant human interleukin-12 in patients with HIV infection. AIDS 16:1147-1154. [DOI] [PubMed] [Google Scholar]
- 23.Jouanguy, E., R. Doffinger, S. Dupuis, A. Pallier, F. Altare, and J. L. Casanova. 1999. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr. Opin. Immunol. 11:346-351. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi, K., J. Yamazaki, T. Kasama, T. Katsura, K. Kasahara, S. F. Wolf, and T. Shimamura. 1996. Interleukin (IL)-12 deficiency in susceptible mice infected with Mycobacterium avium and amelioration of established infection by IL-12 replacement therapy. J. Infect. Dis. 174:564-573. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170:827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koblish, H. K., C. A. Hunter, M. Wysocka, G. Trinchieri, and W. M. Lee. 1998. Immune suppression by recombinant interleukin (rIL)-12 involves interferon gamma induction of nitric oxide synthase 2 (iNOS) activity: inhibitors of NO generation reveal the extent of rIL-12 vaccine adjuvant effect. J. Exp. Med. 188:1603-1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurzawa, H., M. Wysocka, E. Aruga, A. E. Chang, G. Trinchieri, and W. M. Lee. 1998. Recombinant interleukin 12 enhances cellular immune responses to vaccination only after a period of suppression. Cancer Res. 58:491-499. [PubMed] [Google Scholar]
- 28.Lasarte, J. J., F. J. Corrales, N. Casares, A. Lopez-Diaz de Cerio, C. Qian, X. Xie, F. Borras-Cuesta, and J. Prieto. 1999. Different doses of adenoviral vector expressing IL-12 enhance or depress the immune response to a coadministered antigen: the role of nitric oxide. J. Immunol. 162:5270-5277. [PubMed] [Google Scholar]
- 29.Lazarevic, V., A. J. Myers, C. A. Scanga, and J. L. Flynn. 2003. CD40, but not CD40L, is required for the optimal priming of T cells and control of aerosol M. tuberculosis infection. Immunity 19:823-835. [DOI] [PubMed] [Google Scholar]
- 30.Leonard, J. P., M. L. Sherman, G. L. Fisher, L. J. Buchanan, G. Larsen, M. B. Atkins, J. A. Sosman, J. P. Dutcher, N. J. Vogelzang, and J. L. Ryan. 1997. Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production. Blood 90:2541-2548. [PubMed] [Google Scholar]
- 31.Manetti, R., F. Gerosa, M. G. Giudizi, R. Biagiotti, P. Parronchi, M. P. Piccinni, S. Sampognaro, E. Maggi, S. Romagnani, G. Trinchieri, et al. 1994. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J. Exp. Med. 179:1273-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noguchi, Y., E. C. Richards, Y. T. Chen, and L. J. Old. 1995. Influence of interleukin 12 on p53 peptide vaccination against established Meth A sarcoma. Proc. Natl. Acad. Sci. USA 92:2219-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orange, J. S., S. F. Wolf, and C. A. Biron. 1994. Effects of IL-12 on the response and susceptibility to experimental viral infections. J. Immunol. 152:1253-1264. [PubMed] [Google Scholar]
- 34.Ottenhoff, T. H., T. de Boer, C. E. Verhagen, F. A. Verreck, and J. T. van Dissel. 2000. Human deficiencies in type 1 cytokine receptors reveal the essential role of type 1 cytokines in immunity to intracellular bacteria. Microbes Infect. 2:1559-1566. [DOI] [PubMed] [Google Scholar]
- 35.Palmer, E. M., L. Farrokh-Siar, J. Maguire van Seventer, and G. A. van Seventer. 2001. IL-12 decreases activation-induced cell death in human naive Th cells costimulated by intercellular adhesion molecule-1. I. IL-12 alters caspase processing and inhibits enzyme function. J. Immunol. 167:749-758. [DOI] [PubMed] [Google Scholar]
- 36.Park, A. Y., B. D. Hondowicz, and P. Scott. 2000. IL-12 is required to maintain a Th1 response during Leishmania major infection. J. Immunol. 165:896-902. [DOI] [PubMed] [Google Scholar]
- 37.Peter, K., M. J. Brunda, and G. Corradin. 2002. IL-12 administration leads to a transient depletion of T cells, B cells, and APCs and concomitant abrogation of the HLA-A2.1-restricted CTL response in transgenic mice. J. Immunol. 169:63-67. [DOI] [PubMed] [Google Scholar]
- 38.Powrie, F., R. Correa-Oliveira, S. Mauze, and R. L. Coffman. 1994. Regulatory interactions between CD45RBhigh and CD45RBlow CD4+ T cells are important for the balance between protective and pathogenic cell-mediated immunity. J. Exp. Med. 179:589-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribas, A., S. N. Amarnani, G. M. Buga, L. H. Butterfield, V. B. Dissette, W. H. McBride, J. A. Glaspy, L. J. Ignarro, and J. S. Economou. 2002. Immunosuppressive effects of interleukin-12 coexpression in melanoma antigen gene-modified dendritic cell vaccines. Cancer Gene Ther. 9:875-883. [DOI] [PubMed] [Google Scholar]
- 40.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. L. Flynn. 2000. Depletion of CD4(+) T cells causes reactivation of murine persistent tuberculosis despite continued expression of interferon gamma and nitric oxide synthase 2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott, H. M., and J. L. Flynn. 2002. Mycobacterium tuberculosis in chemokine receptor 2-deficient mice: influence of dose on disease progression. Infect Immun. 70:5946-5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seder, R. A., R. Gazzinelli, A. Sher, and W. E. Paul. 1993. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc. Natl. Acad. Sci. USA 90:10188-10192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serbina, N. V., and J. L. Flynn. 2001. CD8(+) T cells participate in the memory immune response to Mycobacterium tuberculosis. Infect. Immun. 69:4320-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serbina, N. V., C. C. Liu, C. A. Scanga, and J. L. Flynn. 2000. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J. Immunol. 165:353-363. [DOI] [PubMed] [Google Scholar]
- 45.Silva, R. A., T. F. Pais, and R. Appelberg. 1998. Evaluation of IL-12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J. Immunol. 161:5578-5585. [PubMed] [Google Scholar]
- 46.Sypek, J. P., C. L. Chung, S. E. Mayor, J. M. Subramanyam, S. J. Goldman, D. S. Sieburth, S. F. Wolf, and R. G. Schaub. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 177:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 48.Trinchieri, G., S. Pflanz, and R. A. Kastelein. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19:641-644. [DOI] [PubMed] [Google Scholar]
- 49.Villarino, A., L. Hibbert, L. Lieberman, E. Wilson, T. Mak, H. Yoshida, R. A. Kastelein, C. Saris, and C. A. Hunter. 2003. The IL-27R (WSX-1) is re-quired to suppress T cell hyperactivity during infection. Immunity 19:645-655. [DOI] [PubMed] [Google Scholar]
- 50.Wang, J., E. Guan, G. Roderiquez, and M. A. Norcross. 1999. Inhibition of CCR5 expression by IL-12 through induction of beta-chemokines in human T lymphocytes. J. Immunol. 163:5763-5769. [PubMed] [Google Scholar]
- 51.Yap, G., M. Pesin, and A. Sher. 2000. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen Toxoplasma gondii. J. Immunol. 165:628-631. [DOI] [PubMed] [Google Scholar]